Abstract
RNAs stored in the metaphase II-arrested oocyte play important roles in successful embryonic development. Their abundance is defined by transcriptional activity during oocyte growth and selective degradation of transcripts during LH-induced oocyte maturation. Our previous studies demonstrated that mRNA abundance is increased in mature ovulated oocytes collected from obese humans and mice and therefore may contribute to reduced oocyte developmental competence associated with metabolic dysfunction. In the current study mouse models of diet-induced obesity were used to determine whether obesity-dependent increases in proinflammatory signaling regulate ovarian abundance of oocyte-specific mRNAs. The abundance of oocyte-specific Bnc1, Dppa3, and Pou5f1 mRNAs as well as markers of proinflammatory signaling were significantly increased in ovaries of obese compared with lean mice which were depleted of fully grown preovulatory follicles. Chromatin-immunoprecipitation analyses also demonstrated increased association of phosphorylated signal transducer and activator of transcription 3 with the Pou5f1 promoter in ovaries of obese mice suggesting that proinflammatory signaling regulates transcription of this gene in the oocyte. The cecum microbial content of lean and obese female mice was subsequently examined to identify potential relationships between microbial composition and proinflammatory signaling in the ovary. Multivariate Association with Linear Models identified significant positive correlations between cecum abundance of the bacterial family Lachnospiraceae and ovarian abundance of Tnfa as well as Dppa3, Bnc1, and Pou5f1 mRNAs. Together, these data suggest that diet-induced changes in gut microbial composition may be contributing to ovarian inflammation which in turn alters ovarian gene expression and ultimately contributes to obesity-dependent reduction in oocyte quality and development of infertility in obese patients.
Obesity has been linked to abnormalities in embryo development in both rodent models and human studies (1–3). Although aneuploidy represents a major cause of increased miscarriage rates in women with advanced maternal age, elevated body mass index (>25) is associated with increased loss of normal karyotype embryos (4) suggesting that other mechanisms contribute to this loss. Recent studies indicate a correlation between excessive synthesis and storage of oocyte mRNAs and impaired preimplantation development (3, 5–7). Indeed, Park et al (5) showed that increased abundance of maternal transcripts in mature oocytes interferes with their degradation during mammalian embryonic development and also impairs zygotic genome activation. Likewise, Giraldez et al (6) demonstrated that persistence of maternal RNAs interferes with the developmental program of zebrafish embryos. Taken together, these data suggest that abnormal regulation of mRNA synthesis and/or storage may be one obesity-dependent mechanism that reduces the developmental competence of the oocyte.
Transcription in the oocyte is restricted to the growth phase with transcriptional quiescence coinciding with chromatin condensation just before oocyte maturation and persisting through fertilization (8, 9). During the growth phase, the rate of transcription is high and the half-life of most mRNAs is long resulting in the accumulation of transcripts in the oocyte cytoplasm (10, 11). Regulation of oocyte gene transcription is also unique compared with most somatic cells with the use of alternative promoters, transcriptional start sites (TSSs), and core transcription factors (12). For example, the core transcription factor TATA-binding protein 2 (TBP2) rather than TBP is preferentially expressed during oocyte growth (13). In addition, folliculogenesis specific bHLH transcription factor (FIGLA), newborn ovary homeobox (NOBOX), and skin-, embryo-, brain-, and oocyte-specific homeobox (SEBOX) are transcription factors which are specifically expressed in oocytes and directly regulate expression of known oocyte genes (eg, developmental pluripotency-associated 3 [Dppa3] and pluripotency factor POU domain, class 5, transcription factor 1 [Pou5f1]) (5, 14).
One important population of mRNAs expressed in the oocyte are maternal effect gene transcripts which are synthesized and stored during oocyte growth with a significant amount of their translation into functional protein occurring after ovulatory stimulation and/or fertilization (15–17). Thus, the physiological importance of maternal effect gene proteins is during the earliest stages of embryonic development despite the fact that their mRNAs are expressed during oocyte growth. It is well documented that loss of specific maternal effect genes results in termination of embryonic development with no impact on oocyte growth, maturation, or fertilization. For example, loss of maternally derived Dppa3 (also known as Stella or PGC7), which binds to methylated histones to protect against DNA demethylation (18, 19) results in arrested development at the zygote, 2-cell, and morula stages (20). Conditional loss of oocyte basonuclin 1 (Bnc1), a transcription factor that regulates ribosomal RNA transcription in the oocyte (21), also results in arrested embryonic development at the 2-cell stage despite normal growth, maturation, and ovulation of oocytes (22). Likewise, there is growing evidence that maternal expression of the Pou5f1 (also known as octamer-binding transcription factor 4, Oct-4) is required for normal preimplantation development of the embryo (23, 24). Our previous studies showed increased abundance of these and other maternal effect genes in mature ovulated oocytes obtained from mouse models of obesity and suggested that excess abundance of these mRNAs was potentially detrimental to embryonic development (3). However, obesity-dependent mechanisms of increased mRNA levels in these oocytes were not determined.
Chronic, low-grade inflammation, a common phenotype of obesity, is characterized by elevated systemic levels of proinflammatory cytokines including TNFα and IL-6 (25). Common signaling pathways activated by IL-6 and/or TNFα are Janus kinase-signal transducer and activator of transcription 3 (JAK-STAT3) and nuclear factor κB (NFκB) (26, 27). The end result of activation of these pathways is phosphorylation of STAT3 and NFκB p65, respectively, which results in their translocation to the nucleus where they regulate transcription of cytokine, antiapoptotic, and cell survival genes (28). Although these studies have typically been performed using metabolic or immune cells, it is important to note that the ovary is infiltrated with immune cells (eg, macrophages), which play important roles in its normal physiological function (29). Furthermore, there is growing evidence that chronic inflammation associated with obesity leads to ovarian dysfunction which may be mediated, in part, by these immune cells (30, 31). However, despite demonstration that NFκB p65 and STAT3 are expressed in the ovary (30, 32), little is known about their transcriptional regulation of genes in oocytes and/or somatic cells of the ovary.
In addition to chronic inflammation, gut dysbiosis represents another characteristic of obesity (33). This term refers to disruptions in the symbiotic relationship between gut microbial populations and the luminal cells of the large and small intestines (33, 34). These changes in gut microbial populations, which include a decrease in the ratio of Bacteroidetes to Firmicutes in the cecum, have been correlated with increased permeability of the gut epithelium and increased intestinal and systemic inflammation (35–38). Interestingly, Tremellen and Pearce (39) recently proposed that obesity-dependent gut dysbiosis is a contributing factor to the development of ovarian dysfunction associated with metabolic syndrome. In the current study we provide evidence of a direct relationship between ovarian inflammation and the synthesis of specific oocyte mRNAs. Furthermore, we demonstrate positive correlations between obesity-dependent changes in ovarian inflammation, oocyte gene expression and relative populations of gut microbes.
Materials and Methods
Animals
Five-week-old C57BL/6 female mice (Charles River Laboratory) were randomly assigned to receive normal rodent chow diet (ND) (T.2918.15), or high-fat diet containing 45% of its kilocalories from fat (45HFD) (TD.0615) or 60HFD (TD.06414) (Harlan Laboratories). After 12 weeks on their respective diet regimen, all mice received an ip injection of 5-IU equine chorionic gonadotropin (CG) 48 hours before ip administration of 5-IU human CG. Sixteen hours after human CG, mice were euthanized, body weight was recorded, and whole ovary and abdominal adipose tissue were removed, weighed, and flash frozen or fixed with Bouin's solution. At the same time, ovulated oocytes and their associated cumulus cells were retrieved from the oviduct of each animal and counted. In separate experiments, cumulus-oocyte complexes (COCs) were collected via follicle puncture from the ovaries of CD1 outbred female mice (Charles River Laboratory) 48 hours after stimulation with 5-IU equine CG. All animal procedures were reviewed and approved by the University of Nebraska-Lincoln Institutional Animal Care and Use Committee.
Ovary histology and follicle counting
One whole ovary from each female (ND, n = 5; 45HFD, n = 6; 60HFD, n = 5) was fixed in Bouin's solution, paraffin embedded, and sectioned (7 μm) as described (40). Serial sections were placed on microscope slides and the middle slide from each ovary, which included 18 serial sections, was stained with hemotoxcylin and eosin for examination of morphology and follicle counting as described (40). Only follicles which contained an oocyte with a clearly visible nucleus were counted. Follicle classification as primordial, primary, secondary, tertiary, or antral was performed according to criteria established by Parrott and Skinner (41). Follicle counting was carried out by 2 independent individuals and the resulting follicle numbers averaged.
Ovarian, oocyte, and cumulus cell RNA extraction, reverse transcription, and quantitative real-time RT-PCR (qPCR)
Total RNA was extracted from one whole ovary from each female mouse (ND, n = 12; 45HFD, n = 12; 60HFD, n = 12) using Tri-Reagent (Sigma-Aldrich) and from oocytes and cumulus cells using the Ambion Ribopure kit (Life Technologies) per manufacturers' instructions. RNA was quantified and reverse transcribed to generate cDNA as described (42). Primers (Supplemental Table 1) were designed and empirically tested as described. Primers and equivalent dilutions of each cDNA sample were used to perform qRT-PCRs (42). The resulting data for each target gene were normalized using the geometric mean of Gapdh and Actb, which were the most stable housekeeping genes as determined by Normfinder (43). The normalized abundance of each candidate gene in 45HFD- and 60HFD-derived samples was subsequently compared with the corresponding mean-normalized mRNA abundance in ND-derived samples, and expressed as a fold change.
Western blotting
Protein extracts from one whole ovary from each female mouse (ND, n = 6; 45HFD, n = 6; 60HFD, n = 6) were isolated using ice-cold radioimmunoprecipitation assay buffer (RIPA) (65mM Tris-HCl [pH 7.4], 115mM NaCl, 1mM Na2EDTA, 1mM EGTA, and 1% Triton X-100; Fisher Scientific) containing Halt Protease inhibitor cocktail (Sigma-Aldrich). Protein concentration was determined using bicnichoninic acid assay reagent according to the manufacturer's directions (Thermo Fisher). Equivalent amounts of protein (30 μg) were separated, transferred to polyvinylidene fluoride membranes (Millipore), and blocked with 5% nonfat milk in Tris-buffered saline containing 0.05% Tween 20. Membranes were incubated with primary antibodies against phosphorylated proteins (Table 1) overnight at 4°C. Bound antibodies were detected with a horseradish peroxidase-conjugated secondary antibody (Table 1) and Femto Western Blotting Detection kit (Thermo Scientific). After detection, blots were stripped with Restore Western Blot Stripping buffer (Pierce), reblocked, and reprobed with antibody for total protein or beta-actin (ACTB) (Table 1), which was used as a loading control. Quantification of protein signal was carried out as previously described (42). Briefly, target protein band pixel number and density was determined for each sample using Photoshop Elements 11. The density of the protein-specific band was normalized to either the density of the total protein or ACTB band. The normalized values for each protein target were represented as fold changes relative to ND control.
Table 1.
Antibody Table
| Antibodies | Species Raised in | Manufacturer (Product Number) | Dilution Used | Application (Size in kDa) |
|---|---|---|---|---|
| Primary antibodies | ||||
| DPPA3 | Rabbit | Abcam (ab19878) | 1:8000 | WB (∼23) |
| POU5F1 | Rabbit | Abcam (ab19857) | 1:8000, 1:100 | WB (∼43), ChIP |
| BNC1 | Rabbit | Bioss antibodies (bs-9292R) | 1:8000 | WB (∼111) |
| P-STAT3 | Rabbit | Cell Signaling (9145S) | 1:10 000, 1:400, 1:100 | WB (∼86), IHC, ChIP |
| STAT3 | Rabbit | Cell Signaling (4904) | 1:10 000, 1:400 | WB (∼86), IHC |
| P-P65 | Rabbit | Cell Signaling (3033S) | 1:10 000, 1:400, 1:100 | WB (∼65), IHC, ChIP |
| P65 | Rabbit | Cell Signaling (3034) | 1:10 000, 1:400 | WB (∼65), IHC |
| P-P42/44 | Rabbit | Cell Signaling (9102S) | 1:10 000 | WB (∼42 and 44) |
| 42/44 | Rabbit | Cell Signaling (9106S) | 1:10 000 | WB (∼42 and 44) |
| P-AKT | Rabbit | Cell Signaling (4060S) | 1:10 000 | WB (∼60) |
| AKT | Rabbit | Cell Signaling (9272S) | 1:10 000 | WB (∼60) |
| P-SAPK/JNK | Rabbit | Cell Signaling (4668S) | 1:10 000 | WB (∼54 and 46) |
| SAPK/JNK | Rabbit | Cell Signaling (9252) | 1:10 000 | WB (∼54 and 46) |
| ACTB | Rabbit | Cell Signaling (4967S) | 1:20 000 | WB (∼43) |
| Rabbit IgG | Rabbit | Millipore (24982) | 1:100 | ChIP |
| Secondary antibodies | ||||
| HRP-rabbit IgG | Rabbit | Cell Signaling (7074) | 1:20 000 | WB |
| Rabbit IgG | Goat | Abcam (ab150077) | 1:800 | IHC |
Antibodies for Western Blottings, IHC, and ChIP.
Abbreviations: ACTB, beta actin; AKT, protein kinase b; IgG, immunoglobin; P42/44, p42/44 mitogen activated protein kinase; P-AKT, phosphorylated protein kinase b; P-P42/44, phosphorylated p42/44 mitogen activated protein kinase; P-SAPK/JNK, phosphorylated stress-activated protein kinase/jun amino terminal kinase; SAPK/JNK, stress-activated protein kinase/jun amino terminal kinase.
Immunofluorescence (IF)
Ovarian sections were deparaffinized, rehydrated, and antigen retrieval performed followed by blocking with 3% goat serum. The COCs were fixed in 4% paraffin-formaldehyde, washed, permeablized, and protease treated followed by blocking with 3% goat serum. Primary antibodies (Table 1) were diluted in antibody diluent (5% BSA in PBS-Tween), added to each section or group of COCs and incubated at 4°C in a humidified chamber overnight. For negative control, ovarian sections or COCs were incubated with antibody diluent only. All ovarian sections and COCs were subsequently incubated with Alexa Fluor 488 conjugated to antirabbit IgG (Table 1) which was diluted in antibody diluent followed by counterstaining with 4′, 6-diamidino-2-phenylindole (Vector Laboratories, Inc). Coverslips were mounted using Prolong Gold antifade reagent (Life Technologies) and slides were imaged using an IX71 Olympus Inverted Brightfield and Fluorescence Microscope (Hitschfel Instruments, Inc).
Chromatin immunoprecipitation (ChIP)
One whole ovary from each female (ND, n = 7; 45HFD, n = 7; 60HFD, n = 7) was suspended in 20mM butyrate before cross-linking of proteins to chromatin using 1% formaldehyde. The cross-linking reaction was terminated by the addition of 2.5M glycine. Cross-linked tissue was placed in lysis buffer (50mM Tris-HCl [pH 8], 10mM EDTA, and 1% sodium dodecyl sulfate) containing 20mM butyrate, 1mM phenylmethylsulfonyl fluoride, and 1mM protease inhibitor (Pierce) and sonicated (Fisher Scientific) to produce approximately 500-bp DNA fragments. Supernatant was removed, and RIPA ChIP buffer (25mM Tris-HCl [pH 7.6], 150mM NaCl, 1% NP-40, 1% sodium deoxycholate, and 0.1% sodium dodecyl sulfate) containing 20mM butyrate, 1mM phenylmethylsulfonyl fluoride, and 1mM protease inhibitor (Pierce) were added to the cell pellet. The resulting cross-linked protein-chromatin complexes (ie, input) were used for immunoprecipitation. Briefly, protein A DynaBeads (Life Technologies) were washed with RIPA buffer and conjugated with protein-specific antibody (Table 1) or normal rabbit IgG (negative control). Input was applied to the antibody-conjugated beads and incubated overnight at 4°C. Retained chromatin DNA was extracted from the beads via incubation with 10% (wt/vol) Chelex-100 and protease K (20 mg/mL). To detect and quantitate pull-down of gene-specific proximal promoters (0–1500 bp upstream of the TSS) in the immunoprecipitated chromatin, primers (Supplemental Table 2) were designed (Primer Express 3.0; Applied Biosystems) and synthesized (Integrated DNA Technologies). Each gene's presumptive TSS was determined using the eukaryotes promoter database (44). qPCRs were performed as described above using antibody-specific precipitated DNA, input DNA or IgG precipitated DNA. Specificity of the immunoprecipitation was confirmed by detection of only background amplification (Ct > 38) of IgG-precipitated DNA. Relative quantity of DNA at each promoter site was determined for each sample using delta delta Ct (ddCt) analysis. Specifically, each immunoprecipitated sample was normalized using the threshold cycle (Ct) value from its corresponding input DNA (immunoprecipitated ND/45HFD/60HFD Ct − input ND/45HFD/60HFD Ct). The average normalized Ct value for ND samples was determined and subsequently subtracted from the normalized Ct for each experimental sample. This resulting ddCt value which is on the normal log scale was converted to a linear number (2−ddCt) for each sample and represents the relative fold difference in DNA for each sample.
Cecum microbiota sequencing, correlation with ovarian gene expression, and qPCR validation
16S rRNA gene amplicon libraries of the variable 3 (V3) region were prepared using custom barcoded universal fusion primers as described (45) using a barcoded 518R primer. Cecum DNA was extracted using Mo Bio PowerSoil DNA Isolation kit (Mo Bio Laboratories). A 15-μL PCR contained 0.5 U of Terra DNA polymerase (Clontech Laboratories), 1× reaction buffer, 200μM deoxyribose nucleotid triphosphates (New England Biolabs), 200nM each primer, 0.1-μg/μL BSA (New England Biolabs), and 50–100 ng of nucleic acid template or no-template control. Amplified PCR products were normalized using SequalPrep Normalization Plate kit (Invitrogen) and pooled at equal concentration to obtain equal depth of sequencing. The pooled libraries were further purified on a 2.0% E-Gel SizeSelect Gel (QIAGEN), and quantified using a DNA1000 LabChirp (Agilent Bioanalyzer 2100). Sequencing of the 16S rRNA gene fragments was carried out using the Ion Torrent Personal Genome Machine using a 200-bp Sequencing kit v2 on a 316 chip according to manufacturer's protocols. Methods used for emulsion PCR, bead deposition, and sequencing on the Personal Genome Machine were as described by the manufacturer. Initial quality control of sequences generated was performed using the Torrent Suite Software version 3.6.2, which included trimming of the 3′ end of sequences that dropped below the average Q15 score over a 30-bp window and removing sequences with unidentified bases (N). The resulting sequences and a script to recreate the analysis below can be found at https://github.com/FernandoLab/2016_Xie_et_al. The sequences were downloaded from the Torrent Suite and demultiplexed within the QIIME software package (46). Sequences were allowed a default mismatch of 1.5 errors to the barcode. After demultiplexing, universal primers used for sequencing were removed, allowing 1 mismatch in the 5′ (518R) primer and 2 in the 3′ reverse primer (341F). Sequences shorter than 130bp were removed and remaining sequences were trimmed to a fixed length of 130 bp. Quality trimmed sequences were reverse complemented for community analysis. The UPARSE pipeline (USEARCH v7.0.1090) (47), was used to pick operational taxonomic units (OTUs) for downstream analysis, which are the classification of specific subset of phylum, class, order, family, and genus. Singleton reads were removed and both de novo and reference based methods were utilized to remove chimeras. Taxonomy was assigned to OTU representative sequences using the uclust consensus taxonomy assigner (QIIME default) (47) with the Greengenes (48) taxonomy release (database 12_10) within the QIIME package (46). Representative sequences were aligned using the RDP Aligner tool (49). The generated alignment was filtered to remove OTUs that produced an alignment outside the V3 region. The resulting alignment was used to generate a phylogenetic tree in clearcut (50) in mothur (v.1.35.1) (51) Samples with less than 10 000 sequences were removed and β-diversity was calculated using unweighted UniFrac phylogenetic distance (52) in QIIME. Multivariate linear regressions analysis was performed using Multivariate Association with Linear Models (MaAsLin) Galaxy Module based on relative abundance of ovarian expressed Bnc1, Dppa3, Pou5f1, or Tnfa, cage identifier, diet, and relative OTU abundance.
To validate the abundance of the Lachnospiraceae family in cecum contents of ND, 45HFD, and 60HFD female mice, isolated DNA (described above) was combined with Lachnospiraceae-specific (53) or rpoB-specific (54, 55) primers and qPCR performed (described above). The relative abundance of Lachnospiraceae DNA in each sample was determined using ddCt analysis (described above) with Ct values for rpoB, which is a universally conserved single copy microbial gene, used as the normalizer for each sample.
Statistical analyses
All statistics (except microbial community regression analyses which were performed using MaAsLin Galaxy Module as described above) were performed using GraphPad Prism 5.0 (GraphPad Software). For each comparison between experimental groups (ND, 45HFD, and 60HFD), one-way ANOVA was performed followed by Tukey's multiple comparison post hoc test. Bartlett's test for equal variances was used to determine whether data required log-transformation before ANOVA analysis. Data were presented as mean ± SEM for each experimental group. Differences between experimental groups in all analyses were considered significant at P < .05.
Results
Diet-induced obesity increases the abundance of Dppa3, Pou5f1, and Bnc1 in growing oocytes
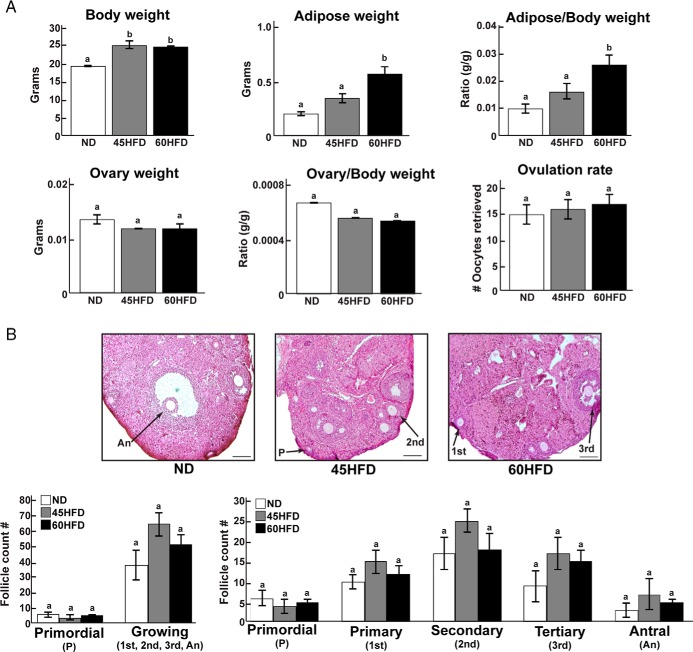
To induce an obese phenotype, female mice were randomly assigned to receive ND or 45HFD or 60HFD. After 12 weeks on their respective diet (17 wk of age), body weight was significantly increased in 45HFD and 60HFD compared with ND female mice, whereas adipose tissue weight was significantly higher in 60HFD vs ND and 45HFD female mice (Figure 1A). There were no differences in ovarian weight between experimental groups. Likewise, stimulation with exogenous gonadotropins resulted in similar numbers of ovulated oocytes (Figure 1A). Histological analyses showed that ovaries from ND, 45HFD, and 60HFD mice were morphologically similar (Figure 1B). Due to the ovulatory stimulation before collection, we did not detect any preovulatory follicles on any of the ovaries we examined indicating that the ovaries predominately contained follicles with growing, transcriptionally active oocytes. Furthermore, there were similar numbers of primordial and growing (sum of primary, secondary, tertiary, and antral) follicles in the ovaries from each experimental group (Figure 1B).
Figure 1.
Phenotype and ovarian morphology of mice fed ND, 45HFD, or 60HFD. A, Body weight, adipose tissue weight, and the ratio of adipose tissue to body weight were documented for each ND (n = 18), 45HFD (n = 18), and 60HFD (n = 18) female mouse at 120d of age. Likewise, combined ovary weight and the ratio of ovary weight to body weight were determined. The number of oocytes recovered from the oviducts of each mouse was counted and represents the ovulation rate for each mouse in each experimental group. Differences were considered statistically significant at P < .05 and are indicated by different letters. B, Representative ovarian sections that were stained with hematoxylin and eosin to visualize morphological structures in ND (n = 5), 45HFD (n = 6), and 60HFD (n = 5) female mice (scale bars, 50 μm). Follicles were counted and classified as primordial (P), primary (1st), secondary (2nd), tertiary (3rd), or antral (An) as indicated in Materials and Methods. There were no statistical differences in the number of follicles at any stage between the experimental groups.
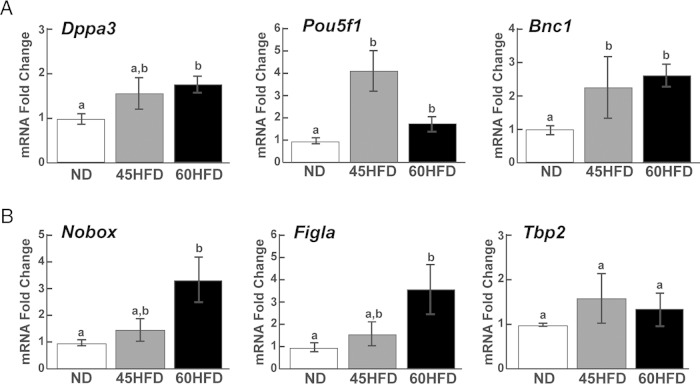
We previously demonstrated that the mRNA abundance of the maternal effect genes Dppa3, Pou5f1, and Bnc1 were increased in mature ovulated oocytes collected from obese females (3). To determine whether these obesity-induced increases in mRNA abundances could be due to transcriptional increases during oocyte growth, qPCR was performed using whole ovaries of ND, 45HFD, and 60HFD mice after ovulatory stimulation. Oocyte-specific expression of Dppa3, Pou5f1, and Bnc1 was confirmed by qPCR analysis of mature oocytes vs cumulus granulosa cells (Supplemental Figure 1A), immunohistochemical analysis of whole ovary (Supplemental Figure 1B), and immunofluorescent analysis of isolated COCs (Supplemental Figure 1B). The mRNA abundance of Pou5f1 and Bnc1 was increased 2- to 4-fold in the ovaries of 45HFD and 60HFD compared with ND mice (Figure 2). Furthermore, Dppa3 mRNAs were 1.8-fold higher in 60HFD compared with ND ovaries. Increased mRNA abundance of Pou5f1 resulted in increased POU5F1 protein in the ovaries of mice fed 60HFD. However, ovarian DPPA3 and BNC1 protein levels were not different between the experimental groups (Supplemental Figure 2). In addition to these maternal effect genes, the mRNA abundance of oocyte-specific transcription factors were also determined using qPCR. Both Figla and Nobox mRNAs were increased approximately 3-fold in ovaries of 60HFD compared with ND mice with levels of both mRNAs in 45HFD ovaries intermediate between ND and 60HFD ovaries (Figure 2). Conversely, there was no difference in Tbp2 abundance between the 3 experimental groups (Figure 2).
Figure 2.
Effect of high-fat diet on maternal effect gene and oocyte-specific transcription factor mRNA levels. Abundance of Dppa3, Pou5f1, and Bnc1 transcripts (A) and Nobox, Figla, and Tbp2 mRNAs (B) were determined by qPCR analysis of ovarian cDNA from ND (n = 12), 45HFD (n = 9–10), and 60HFD (n = 11) mice. Fold differences (y-axis) in mRNA abundance were calculated based on the average abundance in ND ovaries. Differences between experimental groups were determined and considered statistically significant at P < .05, which is represented by different letters.
Diet-induced obesity increases inflammatory signaling including phosphorylation of the transcription factors STAT3 and NFκB p65 in ovary
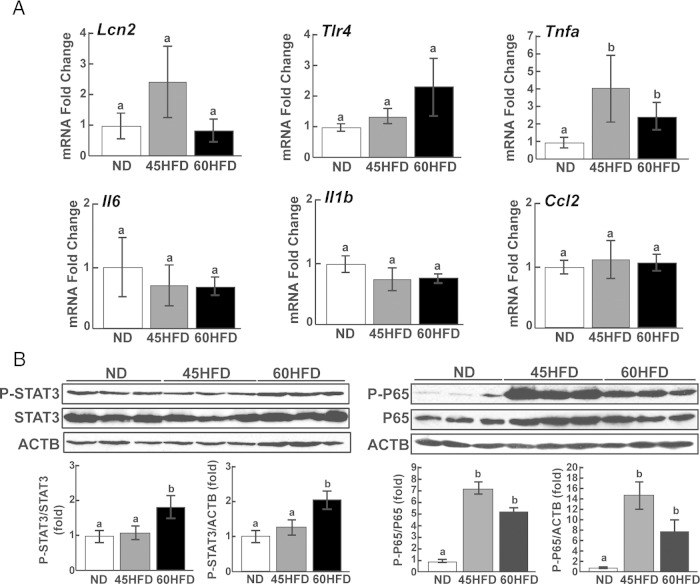
To determine whether the phenotype of 45HFD and 60HFD female mice included ovarian inflammation, qPCR was carried out to measure the mRNA abundance of proinflammatory factors. The relative abundance of TNFα (Tnfα) was increased 3- to 4-fold in ovaries from 45HFD and 60HFD mice; however, none of the other markers were significantly different between experimental groups (Figure 3A). To determine whether the activity of proinflammatory signaling pathways were altered in the ovaries of 45HFD and/or 60HFD mice, Western blot analyses of the JAK-STAT and NFκB signaling pathways were performed. Phosphorylated NFκB p65 (P-P65) was increased 6- to 14-fold in ovaries collected from 45HFD and 60HFD mice (Figure 3B). P-STAT3 protein expression was also increased 2-fold in 60HFD compared with ND ovaries but showed no increase in 45HFD ovaries mice (Figure 3B). Western blot analyses of common metabolic signaling pathways were also carried out. There was no diet-induced effect on P-p42/44 mitogen activated kinase (MAPK) or stress activated protein kinase/jun amino terminal kinase (P-SAPK/JNK) levels (Supplemental Figure 3). Conversely, protein kinase B (P-AKT) showed modest but significant increases in ovaries from 60HFD mice (Supplemental Figure 3).
Figure 3.
Expression of proinflammatory markers in ovaries of ND, 45HFD, and 60HFD Mice. A, The mRNA abundances of Lcn2 (lipocalin 2), Tlr4 (toll-like receptor 4), Tnfa, Il6 (interleukin 6), Il-1b (interleukin 1b), and Ccl2 (chemokine ligand 2) were determined by qPCR analyses of ovarian cDNA from ND (n = 9–12), 45HFD (n = 7–11), and 60HFD (n = 9–12) mice. Fold differences (y-axis) in mRNA abundance were calculated based on the average abundance in ND ovaries. Differences between experimental groups were determined and considered statistically significant at P < .05, which is represented by different letters. B, Representative Western blottings show detection of P-STAT3 and P-P65 in ovarian protein extracts from ND, 45HFD, and 60HFD mice. Band density was determined for each protein and normalized to total STAT3 (P-STAT3/STAT3), total P65 (P-P65/P65) or ACTB (P-STAT3/ACTB, P-P65/ACTB) band density in each sample. Fold differences (y-axis) in protein abundance from ND (n = 6–7), 45HFD (n = 6–7), and 60HFD (n = 6–7) ovaries were calculated based on the average abundance in ND ovaries. Differences between experimental groups were determined and considered statistically significant at P < .05, which is represented by different letters.
P-STAT3 was increased at the Pou5f1 promoter region in the ovary of obese female mice
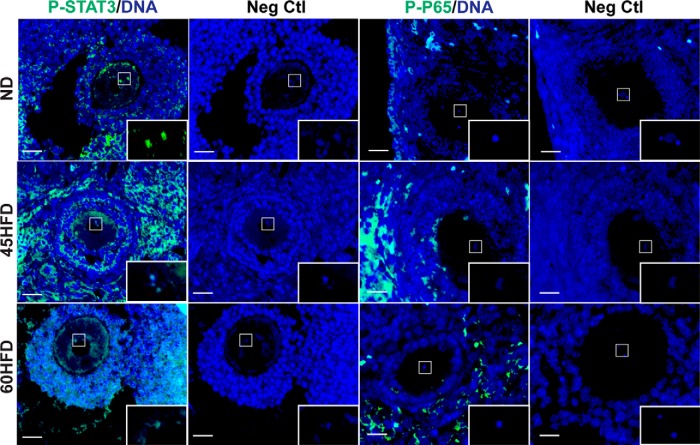
Given that STAT3 and P65 are transcription factors, we next investigated their potential interaction with the Dppa3, Pou5f1, and Bnc1 promoters in oocytes. IF was first carried out to determine ovarian cellular localization of P-STAT3 and P-P65. As expected, the fluorescence intensity of P-STAT3 and P-P65 appeared increased in ovaries of 45HFD and 60HFD mice (Figure 4). Furthermore, the IF assays demonstrated P-STAT3 localization to oocytes including colocalization with oocyte chromatin (Figure 4, increased magnification in box) as well as other somatic cells of the ovary. Conversely, P-P65 was only localized to somatic cells, including stroma and granulosa cells (Figure 4).
Figure 4.
Ovarian localization of P-STAT3 and P-P65. Representative immunohistochemical images show localization of P-STAT3 and P-P65 in ovarian sections from ND, 45HFD, or 60HFD. Primary antibodies were hybridized with ovarian sections (ND, n = 3–4; 45HFD n = 3–4; 60HFD, n = 3–4) followed by detection with FITC-conjugated secondary antibody (green). Additional serial sections were hybridized with only FITC-conjugated secondary antibody and served as negative controls (Neg Ctl). Each section was counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). The oocyte chromatin region in each section was magnified in the box in the lower right corner of each image. Scale bar, 50 μm.
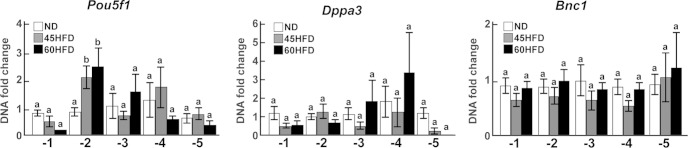
To determine whether P-STAT3 was bound to the promoter region of the Dppa3, Pou5f1, and Bnc1 genes, ovarian chromatin chemically cross-linked with bound proteins was immunoprecipitated using an antibody to P-STAT3. The immunoprecipitated DNA was isolated and, subsequently used for qPCR analyses of 5 regions upstream of the TSSs for Dppa3, Pou5f1, and Bnc1, which were representative of each gene's proximal promoter (within 1500 bp of the TSS) (Supplemental Table 2). The eukaryotes promoter database which identifies TATA box, GC box, and CAATT box sequences was used to determine the predicted site of transcription initiation for each gene (44). A 2.5-fold increased association of P-STAT3 with region 2 of the Pou5f1 promoter, which was 300–500 bp upstream of the TSS, was detected when chromatin was immunoprecipitated from 45HFD and 60HFD ovaries (Figure 5). However, there was no detected change in P-STAT3 association with any other region within the Pou5f1 proximal promoter (Figure 5, regions 1, 3, 4, or 5). Likewise, there were no differences in association of P-STAT3 with any of the sites examined within the Dppa3 or Bnc1 proximal promoters (Figure 5, regions 1–5). Given the lack of P-P65 expression in the oocyte, ChIP coupled to qPCR did not detect any association of P-P65 with the Dppa3, Pou5f1, or Bnc1 promoters (data not shown).
Figure 5.
Association of phosphorylated STAT3 with Dppa3, Pou5f1, and Bnc1 proximal promoters. Cross-linked protein-chromatin fragments from ND (n = 7, white bars), 45HFD (n = 7, gray bars), and 60HFD (n = 7, black bars) ovaries were immunoprecipitated using the P-STAT3 antibody. Quantitative PCR was performed using immunoprecipitated DNA and primers designed to 5 regions (−1 to −5) (Supplemental Table 2) upstream of the TSS of Pou5f1, Dppa3, or Bnc1. Threshold cycle for immunoprecipated DNA was normalized using the threshold cycle for input DNA in each sample. The fold change (y-axis) at each promoter region (x-axis) was calculated using the average normalized threshold cycle for ND. Differences between experimental groups were determined and considered statistically significant at P < .05, which is represented by different letters.
Increased ovarian Dppa3, Pou5f1, Bnc1, and Tnfa mRNA was positively correlated with increased Lachnospiraceae in cecum contents
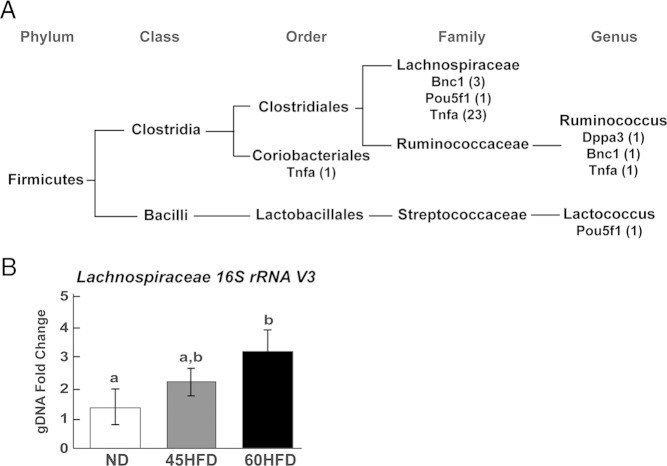
To determine whether gut microbes contribute towards ovarian inflammation and increased ovarian mRNA abundances in 45HFD and 60HFD mice, the cecum microbial community composition of each mouse was evaluated. From each cecum content sample, DNA was extracted and sequencing of the 16S rRNA V3 region was performed. This sequencing produced OTU sequences that were annotated to specific subsets of bacterial phylum, class, order, family, or genus. Multivariate linear regressions analysis was subsequently carried out using MaAsLin Galaxy Module based on relative abundance of ovarian expressed Bnc1, Dppa3, Pou5f1, or Tnfa, cage identifier, diet, and relative OTU abundance. All of the resulting sequence reads and a script to recreate the complete analysis can be found at https://github.com/FernandoLab/2016_Xie_et_al. MaAsLin identified significant, positive correlations between the relative abundance of specific OTUs and the relative ovarian abundance of Bnc1, Dppa3, Pou5f1, and Tnfa. Specifically, Bnc1 mRNA abundance was positively correlated with 4 OTUs, Dppa3 mRNA was positively correlated with 1 OTU, Pou5f1 mRNA was positively correlated with 2 OTUs, and Tnfa mRNA was positively correlated with 25 OTUs (Table 2). Classification of these OTUs using the uclust consensus taxonomy assigner, in which the most detailed lineage description is shared by 90% or more of the sequences within an OTU, showed that all 32 of the OTUs belonged to the phylum Firmicutes and 27 of the 32 OTUs belonged to the family Lachnospiraceae (Figure 6A and Table 2). Three OTUs from the Ruminococcaceae family and Ruminococcus genus were also correlated with ovarian expressed genes (Dppa3, Bnc1, and Tnfa). Despite these positively identified correlations between cecum microbial composition and ovarian gene expression, there were no significant correlations identified based on mouse diet which may have been due to cage affects associated with cohousing of mice and the small sample size.
Table 2.
Gut Microbial OTUs Positively Correlated With Relative Ovarian mRNA Abundances
| Variable | Phylum | Class | Order | Family | Genus | Feature | P Value | Q Value |
|---|---|---|---|---|---|---|---|---|
| Bnc1 | Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | Ruminococcus | OTU1817 | .00002 | .000568937 |
| Bnc1 | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | OTU1655 | .000112054 | .00229515 | |
| Bnc1 | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | OTU2638 | .002683163 | .028978418 | |
| Bnc1 | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | OTU1022 | .004744518 | .045176941 | |
| Dppa3 | Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | Ruminococcus | OTU2821 | .003281158 | .033634121 |
| Pou5f1 | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | OTU328 | .000381008 | .006206844 | |
| Pou5f1 | Firmicutes | Bacilli | Lactobacillales | Streptococcaceae | Lactococcus | OTU2164 | .003950931 | .039110543 |
| Tnfα | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | OTU919 | .00000155 | .0000734 | |
| Tnfα | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | OTU456 | .0000484 | .001154926 | |
| Tnfα | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | OTU1770 | .0000977 | .002051789 | |
| Tnfα | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | OTU425 | .000123851 | .002493416 | |
| Tnfα | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | OTU2611 | .000141149 | .002766935 | |
| Tnfα | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | OTU1767 | .000405452 | .006477669 | |
| Tnfα | Firmicutes | Clostridia | Coriobacteriales | OTU917 | .000421166 | .006666817 | ||
| Tnfα | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | OTU2638 | .000428907 | .006774971 | |
| Tnfα | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | OTU6 | .000446279 | .00695295 | |
| Tnfα | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | OTU332 | .000498264 | .007617689 | |
| Tnfα | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | OTU186 | .000549956 | .00830579 | |
| Tnfα | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | OTU1746 | .00082553 | .011513781 | |
| Tnfα | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | OTU1599 | .001257332 | .016097736 | |
| Tnfα | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | OTU751 | .001380733 | .01729163 | |
| Tnfα | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | OTU1487 | .001393948 | .017379281 | |
| Tnfα | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | OTU1290 | .001451654 | .017869649 | |
| Tnfα | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | OTU1925 | .001674824 | .020000422 | |
| Tnfα | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | OTU257 | .001695527 | .020215275 | |
| Tnfα | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | OTU2840 | .001920991 | .022251062 | |
| Tnfα | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | OTU1308 | .00200784 | .023115474 | |
| Tnfα | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | OTU1051 | .002436828 | .026982444 | |
| Tnfα | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | OTU1167 | .003116615 | .032453137 | |
| Tnfα | Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | Ruminococcus | OTU2718 | .003637947 | .036505142 |
| Tnfα | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | OTU676 | .004388051 | .042487286 | |
| Tnfα | Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | OTU1655 | .005092514 | .047758601 |
Figure 6.
Correlation between gut microbial OTUs and relative abundances of ovarian mRNAs. A, Relative ovarian abundance of Tnfa, Dppa3, Pou5f1, and Bnc1 transcripts were positively correlated to cecum microbial OTUs (number of OTUs shown in parentheses). Each OTU was classified into a phylum, class, order, family, and/or genus taxa as indicated. B, The relative abundance of Lachnospiraceae genomic DNA was determined by qPCR using primers that target the 16S rRNA V3 region common to all members of the Lachnospiraceae family. DNA was extracted from the cecum contents of ND (n = 12), 45HFD (n = 10), and 60HFD (n = 11) female mice. Threshold cycle for V3 gDNA was normalized using the threshold cycle for rpoB (bacterial RNA polymerase beta subunit) in each sample. The fold change (y-axis) was calculated using the average normalized threshold cycle for ND. Differences between experimental groups were determined and considered statistically significant at P < .05, which is represented by different letters.
OTUs are short DNA reads from high throughput sequencing; therefore, qPCR analysis was performed to validate diet-induced changes in bacterial abundance within the cecum contents from ND, 45HFD, and 60HFD female mice. Because OTUs positively correlated to ovarian expressed genes were predominately annotated as Lachnospiraceae, qPCR primers were designed to a region of the 16S rRNA V3 which is common to all members of the Lachnospiraceae family (53). Analysis of the resulting qPCR data showed a significant 3.5-fold increase in Lachnospiraceae in the cecum contents of 60HFD compared with ND female mice (Figure 6B). Although not significantly different compared with ND or 60HFD, there was a 2-fold numerical increase in Lachnospiraceae in the cecum contents of 45HFD female mice.
Discussion
The focus of the current study was to address how chronic inflammation associated with obesity impacts transcriptional regulation of mRNAs in the oocyte and thereby contributes to increased maternal mRNAs in mature ovulated oocytes. During oocyte growth, chromatin is decondensed (ie, nonsurrounded nucleolus [SN]) and mRNA synthesis rates are high (8, 11). This is in contrast to healthy, fully grown oocytes which exhibit chromatin condensation demarcated by a SN and transcriptional quiescence (8, 9). A well described and commonly used mouse model of diet-induced obesity was used for the study with animals randomly assigned to diet at postnatal d 35 (35d) and terminated at 120d. Abe et al (56) showed that the number of transcriptionally active, non-SN oocytes that can be isolated from a female mouse peaks at 12d of age. Furthermore, the percentage of SN oocytes is increased in antral follicles and approaches 100% in large antral follicles (57). Due to the age of the mice used in our study (120d) and the difficulty in isolating oocytes free from granulosa cells from preantral follicles, whole ovaries were collected from the adult (120d) mice 16 hours after ovulatory stimulation for the described experiments. This time point was chosen to eliminate most medium and large antral follicles which contain predominately SN oocytes and thereby increase the relative proportion of preantral follicles in the adult ovary. Using this experimental design, we demonstrated similar numbers of primordial and preantral follicles in lean and obese female mice after ovulatory stimulation with exogenous gonadotropins (Figure 1B). Furthermore, we did not detect any large preovulatory follicles in any of the ovaries examined histologically and similar numbers of ovulated oocytes were observed in the oviducts of ND, 45HFD, and 60HFD mice (Figure 1A) Thus, this design provided us with ovarian tissue which contained similar numbers of predominately preantral follicles in all 3 experimental groups that was subsequently used to examine the abundance and potential regulation of oocyte expressed mRNAs.
To begin to assess the impact of diet-induced obesity on oocyte gene expression, the abundance of oocyte-specific transcription factors and maternal effect genes were examined. Transcript levels of the oocyte-specific core transcription factor Tbp2 was not different between ovaries from ND, 45HFD, and 60HFD mice (Figure 2) suggesting gene-specific rather than global increases in mRNA synthesis in the ovary of obese female mice. Conversely, we demonstrated increased expression of Figla in whole ovary of 60HFD mice (Figure 2) and ovulated oocytes from 45HFD mice (3). Likewise, mRNA abundance of Nobox was also increased in the whole ovary of 60HFD mice (Figure 2). Interestingly, loss of FIGLA in the newborn ovary results in decreased expression of Dppa3 and Pou5f1 mRNAs (58), whereas newborn ovaries lacking NOBOX exhibit decreased expression of Pou5f1 (59, 60). Furthermore, Choi and Rajkovic (61) demonstrated NOBOX binding to and increased transcription of the Pou5f1 promoter. Given that both Dppa3 and Pou5f1 transcripts were increased in the whole ovary of 45HFD and 60HFD (Figure 2), these data suggest a potential mechanism of obesity-dependent increases in the expression of FIGLA and NOBOX which subsequently increases Pou5f1 and Dppa3 transcription. POU5F1 and BNC1 are also both transcription factors. Although mRNA abundance for both Pou5f1 and Bnc1 were increased upon diet-induced obesity, only POU5F1 protein was increased in ovaries of 60HFD mice (Supplemental Figure 2). POU5F1 has been shown to regulate the expression of Dppa3 in developmentally competent oocytes (62) and embryonic stem (ES) cells (63). Okumura-Nakanishi et al (64) showed that POU5F1 also regulates its own gene expression in ES cells. However, we did not detect increased POU5F1 association with the proximal promoter of Dppa3, Pou5f1, or Bnc1 in ovaries from 45HFD or 60HFD mice (data not shown). It should be noted that POU5F1 interaction with the Dppa3 and Pou5f1 genes in ES cells is at distal enhancer elements and this may be the case in the oocyte as well.
A hallmark characteristic of obesity is chronic, low-grade inflammation. Indeed, female obesity and ovarian inflammation have been correlated (30, 31, 65). Ovaries from 45HFD and 60HFD mice had increased Tnfa transcript levels (Figure 3A). Furthermore, there was increased phosphorylation of STAT3 and P65 in the ovaries of 60HFD or 45HFD and 60HFD mice, respectively (Figures 3B and 4). Although the changes were modest, these data are indicative of a chronic, low-grade inflammatory response in the ovary of 45HFD and 60HFD mice consistent with an obese phenotype. Phosphorylation of STAT3 and P65 results in their translocation to the nucleus where they interact with the promoter of target genes. Furthermore, activation of the JAK-STAT3 pathway in ES cells increases expression of pluripotency genes including Nanog, Sox2, and Pou5f1 (66, 67) suggesting a possible mechanism for increased oocyte expression of maternal effect genes. Indeed, we demonstrated oocyte localization of P-STAT3 (Figure 4) and increased association of P-STAT3 with the Pou5f1 proximal promoter in ovaries from 45HFD and 60HFD mice (Figure 5). Although oocyte-specific loss of STAT3 does not impair oocyte developmental competence, increased STAT3 in oocytes and embryos is associated with reduced developmental success (68, 69). Thus, our data suggests that increases in P-STAT3potentially cause decreases in oocyte quality due to its role in increased transcription of Pou5f1. Interestingly, Joshi et al (58) demonstrated that FIGLA increases Stat3 expression in the newborn ovary and therefore these data also suggest a potential mechanistic link between FIGLA and STAT3 regulation of oocyte genes. Conversely, NFκB p65 protein was only detected in somatic cells of the ovary. The inability to detect any association of P-P65 with the Bnc1, Dppa3, and Pou5f1 promoters (data not shown) confirmed its absence in the oocyte and points to an indirect mechanism of action for this signaling pathway. For example, increases in the oxidative stress marker superoxide dismutase 1 (Sod1) are stimulated by NFκB p65 in the cumulus cell of female patients with endometriosis which subsequently impairs oocyte meiotic maturation (70).
Recent studies of the gut microbiota have implicated its role in host metabolism and immune function (34, 71, 72). Many studies have demonstrated shifts in the microbiome of diet-induced obese rodent models and obese humans which include decreased species diversity and a higher ratio of Firmicutes compared with Bacteroidetes in the cecum (33, 35, 72). Therefore, we investigated the possible associations between gut microbiota species composition and ovarian mRNA expression to determine whether the gut microbiome in obese mice may contribute to reduced oocyte quality. Microbial analysis using 16S rRNA tag sequencing identified positive correlations between relative ovarian abundance of the oocyte-specific Dppa3, Pou5f1, and Bnc1 transcripts and cecum microbiota composition. The relative abundance of many OTUs belonging to Lachnospiraceae, were positively correlated with these oocyte-specific mRNAs (Figure 6A). In addition, a positive correlation between the abundance of Lachnospiraceae in the cecum and ovarian abundance of Tnfα was identified and suggests that Lachnospiraceae may indirectly influence ovarian inflammation. Previous studies have demonstrated an increase in Lachnospiraceae in mice fed a high-fat diet (35, 73). However, our regression analysis did not identify a positive correlation between Lachnospiraceae and diet (https://github.com/FernandoLab/2016_Xie_et_al), which may have been a result of a strong cage effect due to our experimental design. Specifically, mice on each diet were cohoused, and we were using a small sample size for each diet. Previous investigations typically utilized male mice for their studies and therefore our results may also represent gender-dependent differences in diet-induced changes to the gut microbial community. The diet-induced effect may also be muted as we did detect increased abundance of Lachnospiraceae in the cecum contents of 60HFD mice using qPCR.
To our knowledge, this is the first report demonstrating a significant positive correlation between Lachnospiraceae abundance and ovarian transcription using a mouse model of obesity. Furthermore, we have demonstrated not only a significant correlation between changes in the cecum microbial composition and a marker of ovarian inflammation but also a potential mechanistic link between ovarian inflammation and oocyte gene expression. Together, these data suggest that manipulation of the gut microbiome may represent a new therapeutic strategy to reverse ovarian inflammation and its associated effect on reduced oocyte quality and thereby improve obesity-dependent contributions to female infertility.
Acknowledgments
We thank Dr Andrea Cupp for critical reading of this manuscript.
This work was supported by University of Nebraska-Lincoln Hatch Funds (NEB-26-198 and NEB-26-206) and a University of Nebraska-Lincoln Research Council Seed Grant (J.R.W.) and Nebraska Center for the Prevention of Obesity Diseases National Institutes of Health Centers for Biomedical Research Excellence funds (P20GM104320) (to S.C.F.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AKT
- protein kinase B
- Bnc1
- basonuclin 1
- CG
- chorionic gonadotropin
- ChIP
- chromatin immunoprecipitation
- COC
- cumulus-oocyte complex
- 35d
- postnatal day 35
- ΔΔCt
- delta delta cycle threshold
- Dppa3
- developmental pluripotency-associated 3
- ES
- embryonic stem
- FIGLA
- folliculogenesis specific bHLH transcription factor
- 45HFD
- high-fat diet containing 45% of its kilocalories from fat
- IF
- immunofluorescence
- JAK-STAT3
- Janus kinase-signal transducer and activator of transcription 3
- MaAsLin
- Multivariate Association with Linear Models
- ND
- normal rodent chow diet
- NFκB
- nuclear factor κB
- NOBOX
- newborn ovary homeobox
- OTU
- operational taxonomic unit
- Pou5f1
- pluripotency factor POU domain, class 5, transcription factor 1
- P-P65
- Phosphorylated NFκB p65
- qPCR
- quantitative real-time RT-PCR
- RIPA
- radioimmunoprecipitation assay buffer
- SAPK/JNK
- stress-activated protein kinase/jun amino terminal kinase
- SN
- surrounded nucleolus
- TSS
- transcriptional start site
- V3
- variable 3.
References
- 1. Luzzo KM, Wang Q, Purcell SH, et al. High fat diet induced developmental defects in the mouse: oocyte meiotic aneuploidy and fetal growth retardation/brain defects. PLoS One. 2012;7(11):e49217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Minge CE, Bennett BD, Norman RJ, Robker RL. Peroxisome proliferator-activated receptor-γ agonist rosiglitazone reverses the adverse effects of diet-induced obesity on oocyte quality. Endocrinology. 2008;149(5):2646–2656. [DOI] [PubMed] [Google Scholar]
- 3. Pohlmeier WE, Xie F, Kurz SG, Lu N, Wood JR. Progressive obesity alters the steroidogenic response to ovulatory stimulation and increases the abundance of mRNAs stored in the ovulated oocyte. Mol Reprod Dev. 2014;81(8):735–747. [DOI] [PubMed] [Google Scholar]
- 4. Landres IV, Milki AA, Lathi RB. Karyotype of miscarriages in relation to maternal weight. Hum Reprod. 2010;25(5):1123–1126. [DOI] [PubMed] [Google Scholar]
- 5. Park MW, Kim KH, Kim EY, Lee SY, Ko JJ, Lee KA. Associations among Sebox and other MEGs and its effects on early embryogenesis. PLoS One. 2015;10(2):e0115050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Giraldez AJ, Mishima Y, Rihel J, et al. Zebrafish MiR-430 promotes deadenylation and clearance of maternal mRNAs. Science. 2006;312(5770):75–79. [DOI] [PubMed] [Google Scholar]
- 7. Wood JR, Dumesic DA, Abbott DH, Strauss JF., 3rd Molecular abnormalities in oocytes from women with polycystic ovary syndrome revealed by microarray analysis. J Clin Endocrinol Metab. 2007;92(2):705–713. [DOI] [PubMed] [Google Scholar]
- 8. De La Fuente R, Eppig JJ. Transcriptional activity of the mouse oocyte genome: companion granulosa cells modulate transcription and chromatin remodeling. Dev Biol. 2001;229(1):224–236. [DOI] [PubMed] [Google Scholar]
- 9. Bouniol-Baly C, Hamraoui L, Guibert J, Beaujean N, Szöllösi MS, Debey P. Differential transcriptional activity associated with chromatin configuration in fully grown mouse germinal vesicle oocytes. Biol Reprod. 1999;60(3):580–587. [DOI] [PubMed] [Google Scholar]
- 10. Brower PT, Gizang E, Boreen SM, Schultz RM. Biochemical studies of mammalian oogenesis: synthesis and stability of various classes of RNA during growth of the mouse oocyte in vitro. Dev Biol. 1981;86(2):373–383. [DOI] [PubMed] [Google Scholar]
- 11. Gosden R, Lee B. Portrait of an oocyte: our obscure origin. J Clin Invest. 2010;120(4):973–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. DeJong J. Basic mechanisms for the control of germ cell gene expression. Gene. 2006;366(1):39–50. [DOI] [PubMed] [Google Scholar]
- 13. Gazdag E, Rajkovic A, Torres-Padilla ME, Tora L. Analysis of TATA-binding protein 2 (TBP2) and TBP expression suggests different roles for the two proteins in regulation of gene expression during oogenesis and early mouse development. Reproduction. 2007;134(1):51–62. [DOI] [PubMed] [Google Scholar]
- 14. Jagarlamudi K, Rajkovic A. Oogenesis: transcriptional regulators and mouse models. Mol Cell Endocrinol. 2012;356(1–2):31–39. [DOI] [PubMed] [Google Scholar]
- 15. Kim KH, Lee KA. Maternal effect genes: findings and effects on mouse embryo development. Clin Exp Reprod Med. 2014;41(2):47–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li L, Zheng P, Dean J. Maternal control of early mouse development. Development. 2010;137(6):859–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bachvarova R, De Leon V. Polyadenylated RNA of mouse ova and loss of maternal RNA in early development. Dev Biol. 1980;74(1):1–8. [DOI] [PubMed] [Google Scholar]
- 18. Nakamura T, Liu YJ, Nakashima H, et al. PGC7 binds histone H3K9me2 to protect against conversion of 5mC to 5hmC in early embryos. Nature. 2012;486(7403):415–419. [DOI] [PubMed] [Google Scholar]
- 19. Nakamura T, Arai Y, Umehara H, et al. PGC7/Stella protects against DNA demethylation in early embryogenesis. Nat Cell Biol. 2007;9(1):64–71. [DOI] [PubMed] [Google Scholar]
- 20. Payer B, Saitou M, Barton SC, et al. Stella is a maternal effect gene required for normal early development in mice. Curr Biol. 2003;13(23):2110–2117. [DOI] [PubMed] [Google Scholar]
- 21. Tian Q, Kopf GS, Brown RS, Tseng H. Function of basonuclin in increasing transcription of the ribosomal RNA genes during mouse oogenesis. Development. 2001;128(3):407–416. [DOI] [PubMed] [Google Scholar]
- 22. Ma J, Zeng F, Schultz RM, Tseng H. Basonuclin: a novel mammalian maternal-effect gene. Development. 2006;133(10):2053–2062. [DOI] [PubMed] [Google Scholar]
- 23. Foygel K, Choi B, Jun S, et al. A novel and critical role for Oct4 as a regulator of the maternal-embryonic transition. PLoS One. 2008;3(12):e4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zuccotti M, Merico V, Sacchi L, et al. Maternal Oct-4 is a potential key regulator of the developmental competence of mouse oocytes. BMC Dev Biol. 2008;8:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Piya MK, McTernan PG, Kumar S. Adipokine inflammation and insulin resistance: the role of glucose, lipids and endotoxin. J Endocrinol. 2013;216(1):T1–T15. [DOI] [PubMed] [Google Scholar]
- 26. Imada K, Leonard WJ. The Jak-STAT pathway. Mol Immunol. 2000;37(1–2):1–11. [DOI] [PubMed] [Google Scholar]
- 27. Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-κB signaling pathways. Nat Immunol. 2011;12(8):695–708. [DOI] [PubMed] [Google Scholar]
- 28. Grivennikov SI, Karin M. Dangerous liaisons: STAT3 and NF-κB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010;21(1):11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wu R, Van der Hoek KH, Ryan NK, Norman RJ, Robker RL. Macrophage contributions to ovarian function. Hum Reprod Update. 2004;10(2):119–133. [DOI] [PubMed] [Google Scholar]
- 30. Nteeba J, Ganesan S, Keating AF. Progressive obesity alters ovarian folliculogenesis with impacts on pro-inflammatory and steroidogenic signaling in female mice. Biol Reprod. 2014;91(4):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Robker RL, Wu LL, Yang X. Inflammatory pathways linking obesity and ovarian dysfunction. J Reprod Immunol. 2011;88(2):142–148. [DOI] [PubMed] [Google Scholar]
- 32. Murphy K, Carvajal L, Medico L, Pepling M. Expression of Stat3 in germ cells of developing and adult mouse ovaries and testes. Gene Expr Patterns. 2005;5(4):475–482. [DOI] [PubMed] [Google Scholar]
- 33. Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bäckhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101(44):15718–15723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hamilton MK, Boudry G, Lemay DG, Raybould HE. Changes in intestinal barrier function and gut microbiota in high-fat diet-fed rats are dynamic and region dependent. Am J Physiol Gastrointest Liver Physiol. 2015;308(10):G840–G851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ding S, Chi MM, Scull BP, et al. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS One. 2010;5(8):e12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. [DOI] [PubMed] [Google Scholar]
- 38. Kim KA, Gu W, Lee IA, Joh EH, Kim DH. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS One. 2012;7(10):e47713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tremellen K, Pearce K. Dysbiosis of gut microbiota (DOGMA)–a novel theory for the development of polycystic ovarian syndrome. Med Hypotheses. 2012;79(1):104–112. [DOI] [PubMed] [Google Scholar]
- 40. McFee RM, Artac RA, McFee RM, et al. Inhibition of vascular endothelial growth factor receptor signal transduction blocks follicle progression but does not necessarily disrupt vascular development in perinatal rat ovaries. Biol Reprod. 2009;81(5):966–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Parrott JA, Skinner MK. Kit-ligand/stem cell factor induces primordial follicle development and initiates folliculogenesis. Endocrinology. 1999;140(9):4262–4271. [DOI] [PubMed] [Google Scholar]
- 42. Mack EM, Smith JE, Kurz SG, Wood JR. cAMP-dependent regulation of ovulatory response genes is amplified by IGF1 due to synergistic effects on Akt phosphorylation and NF-κB transcription factors. Reproduction. 2012;144(5):595–602. [DOI] [PubMed] [Google Scholar]
- 43. Vandesompele J, De Preter K, Pattyn F, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dreos R, Ambrosini G, Cavin Perier R, Bucher P. EPD and EPDnew, high-quality promoter resources in the next-generation sequencing era. Nucleic Acids Res. 2013;41(Database issue):D157–D164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Whiteley AS, Jenkins S, Waite I, et al. Microbial 16S rRNA Ion Tag and community metagenome sequencing using the Ion Torrent (PGM) Platform. J Microbiol Methods. 2012;91(1):80–88. [DOI] [PubMed] [Google Scholar]
- 46. Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10(10):996–998. [DOI] [PubMed] [Google Scholar]
- 48. DeSantis TZ, Hugenholtz P, Larsen N, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72(7):5069–5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cole JR, Wang Q, Cardenas E, et al. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009;37:D141–D145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Evans J, Sheneman L, Foster J. Relaxed neighbor joining: a fast distance-based phylogenetic tree construction method. J Mol Evol. 2006;62(6):785–792. [DOI] [PubMed] [Google Scholar]
- 51. Schloss PD, Westcott SL, Ryabin T, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75(23):7537–7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lozupone C, Lladser ME, Knights D, Stombaugh J, Knight R. UniFrac: an effective distance metric for microbial community comparison. ISME J. 2011;5(2):169–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104(34):13780–13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dahllöf I, Baillie H, Kjelleberg S. rpoB-based microbial community analysis avoids limitations inherent in 16S rRNA gene intraspecies heterogeneity. Appl Environ Microbiol. 2000;66(8):3376–3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fernando SC, Purvis HT, 2nd, Najar FZ, et al. Rumen microbial population dynamics during adaptation to a high-grain diet. Appl Environ Microbiol. 2010;76(22):7482–7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Abe K, Inoue A, Suzuki MG, Aoki F. Global gene silencing is caused by the dissociation of RNA polymerase II from DNA in mouse oocytes. J Reprod Dev. 2010;56(5):502–507. [DOI] [PubMed] [Google Scholar]
- 57. Tan JH, Wang HL, Sun XS, Liu Y, Sui HS, Zhang J. Chromatin configurations in the germinal vesicle of mammalian oocytes. Mol Hum Reprod. 2009;15(1):1–9. [DOI] [PubMed] [Google Scholar]
- 58. Joshi S, Davies H, Sims LP, Levy SE, Dean J. Ovarian gene expression in the absence of FIGLA, an oocyte-specific transcription factor. BMC Dev Biol. 2007;7:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Choi Y, Qin Y, Berger MF, Ballow DJ, Bulyk ML, Rajkovic A. Microarray analyses of newborn mouse ovaries lacking Nobox. Biol Reprod. 2007;77(2):312–319. [DOI] [PubMed] [Google Scholar]
- 60. Rajkovic A, Pangas SA, Ballow D, Suzumori N, Matzuk MM. NOBOX deficiency disrupts early folliculogenesis and oocyte-specific gene expression. Science. 2004;305(5687):1157–1159. [DOI] [PubMed] [Google Scholar]
- 61. Choi Y, Rajkovic A. Characterization of NOBOX DNA binding specificity and its regulation of Gdf9 and Pou5f1 promoters. J Biol Chem. 2006;281(47):35747–35756. [DOI] [PubMed] [Google Scholar]
- 62. Zuccotti M, Merico V, Sacchi L, et al. Oct-4 regulates the expression of Stella and Foxj2 at the Nanog locus: implications for the developmental competence of mouse oocytes. Hum Reprod. 2009;24(9):2225–2237. [DOI] [PubMed] [Google Scholar]
- 63. Levasseur DN, Wang J, Dorschner MO, Stamatoyannopoulos JA, Orkin SH. Oct4 dependence of chromatin structure within the extended Nanog locus in ES cells. Genes Dev. 2008;22(5):575–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Okumura-Nakanishi S, Saito M, Niwa H, Ishikawa F. Oct-3/4 and Sox2 regulate Oct-3/4 gene in embryonic stem cells. J Biol Chem. 2005;280(7):5307–5317. [DOI] [PubMed] [Google Scholar]
- 65. Nteeba J, Ortinau LC, Perfield JW, 2nd, Keating AF. Diet-induced obesity alters immune cell infiltration and expression of inflammatory cytokine genes in mouse ovarian and peri-ovarian adipose depot tissues. Mol Reprod Dev. 2013;80(11):948–958. [DOI] [PubMed] [Google Scholar]
- 66. Do DV, Ueda J, Messerschmidt DM, et al. A genetic and developmental pathway from STAT3 to the OCT4-NANOG circuit is essential for maintenance of ICM lineages in vivo. Genes Dev. 2013;27(12):1378–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tang Y, Tian XC. JAK-STAT3 and somatic cell reprogramming. JAKSTAT. 2013;2(4):e24935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Robker RL, Watson LN, Robertson SA, Dunning KR, McLaughlin EA, Russell DL. Identification of sites of STAT3 action in the female reproductive tract through conditional gene deletion. PLoS One. 2014;9(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shirazi A, Heidari M, Shams-Esfandabadi N, Momeni A, Derafshian Z. Overexpression of signal transducers and activators of transcription in embryos derived from vitrified oocytes negatively affect E-cadherin expression and embryo development. Cryobiology. 2015;70(3):239–245. [DOI] [PubMed] [Google Scholar]
- 70. Donabela FC, Meola J, Padovan CC, de Paz CC, Navarro PA. Higher SOD1 gene expression in cumulus cells from infertile women with moderate and severe endometriosis. Reprod Sci. 2015;22:1452–1460. [DOI] [PubMed] [Google Scholar]
- 71. Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. [DOI] [PubMed] [Google Scholar]
- 72. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. [DOI] [PubMed] [Google Scholar]
- 73. de La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, Raybould HE. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;299(2):G440–G448. [DOI] [PMC free article] [PubMed] [Google Scholar]