Abstract
Consumption of a diet high in fat and refined carbohydrates (Western diet [WD]) is associated with obesity and insulin resistance, both major risk factors for cardiovascular disease (CVD). In women, obesity and insulin resistance abrogate the protection against CVD likely afforded by estrogen signaling through estrogen receptor (ER)α. Indeed, WD in females results in increased vascular stiffness, which is independently associated with CVD. We tested the hypothesis that loss of ERα signaling in the endothelium exacerbates WD-induced vascular stiffening in female mice. We used a novel model of endothelial cell (EC)-specific ERα knockout (EC-ERαKO), obtained after sequential crossing of the ERα double floxed mice and VE-Cadherin Cre-recombinase mice. Ten-week-old females, EC-ERαKO and aged-matched genopairs were fed either a regular chow diet (control diet) or WD for 8 weeks. Vascular stiffness was measured in vivo by pulse wave velocity and ex vivo in aortic explants by atomic force microscopy. In addition, vascular reactivity was assessed in isolated aortic rings. Initial characterization of the model fed a control diet did not reveal changes in whole-body insulin sensitivity, aortic vasoreactivity, or vascular stiffness in the EC-ERαKO mice. Interestingly, ablation of ERα in ECs reduced WD-induced vascular stiffness and improved endothelial-dependent dilation. In the setting of a WD, endothelial ERα signaling contributes to vascular stiffening in females. The precise mechanisms underlying the detrimental effects of endothelial ERα in the setting of a WD remain to be elucidated.
Stiffness of the vasculature is a naturally occurring phenomenon related to aging, and conditions of insulin resistance such as obesity and type 2 diabetes mellitus (T2DM) accelerate its development (1, 2). Vascular stiffness decreases after puberty (3) and increases after menopause (4). Females are particularly predisposed to the appearance of vascular stiffness as they age and under conditions of insulin resistance (5). Postmenopausal women have greater aortic stiffness as measured by pulse wave velocity (PWV) when compared with premenopausal women, independently of body weight and blood pressure (4). Importantly, increased vascular stiffness is a biomarker that strongly correlates with increased cardiovascular disease (CVD) (6, 7). Furthermore, women with T2DM are at especially high risk for CVD, with a 50% increased risk of death from coronary artery disease (8), and 27% increased relative risk of stroke relative to men (9). Therefore, it is likely that the increased incidence and severity of CVD in diabetic women can be partially related to increased vascular stiffness (6). We have previously shown that consumption of a diet high in fat and refined carbohydrates (Western diet [WD]) results in whole-body insulin resistance and cardiac stiffness earlier and to a greater degree in young females than in age-matched males (10). Moreover, recently we also demonstrated that WD leads to aortic and femoral stiffness in females (11). The role of estrogen in the regulation of vascular stiffness is highlighted by variations seen in vascular stiffness throughout the lifespan of females (4, 6).
The actions of estrogen in the vasculature are mediated primarily via signaling through the estrogen receptor (ER)α (12–14). Under physiologic conditions, signaling via ERα increases endothelial nitric oxide (NO) bioavailability and promotes an antiinflammatory vascular phenotype (12–14). Importantly, ERα effects are not limited to the endothelium and recently the role of ERα in vascular smooth muscle cells has been highlighted in a model of vascular injury (15). Thus, healthy premenopausal women are naturally protected from CVD, partially via ERα signaling in the vasculature (16).
Nevertheless, the beneficial effects of ERα signaling are blunted in conditions of insulin resistance and obesity (3, 5). Even before the appearance of hyperglycemia or hypertension, the presence of insulin resistance has been correlated with increased vascular stiffness (17). Insulin resistance in the vasculature is manifested by impaired endothelial-dependent vasodilatation and increased remodeling (18). The mechanisms underlying the abrogation of the beneficial vascular effects of ERα signaling in conditions of systemic and vascular insulin resistance have not been explored. In the present investigation, we examined the role of the endothelial ERα in the genesis of vascular stiffness in a female model of selective knockout (KO) of the ERα in endothelial cells (ECs) and insulin resistance induced by WD. Specifically, we hypothesize that loss of ERα signaling in the endothelium exacerbates WD-induced vascular stiffening.
Materials and Methods
Animals
All animal procedures were performed in accordance with the Animal Use and Care Committee at the University of Missouri-Columbia and National Institutes of Health guidelines. We studied females as we have previously shown that they are particularly predisposed to the appearance of cardiovascular stiffness when fed the WD (10). We used the ERα-specific endothelial KO mouse model and control genopairs (GP). To generate these mice, the genomic region encompassing exon 3 of the ERα gene was flanked by loxP sites via homologous recombination in embryonic stem cells (19, 20). These ERα floxed (ERαf/f) mice were kindly provided by Dr Pierre Chambon (Institute for Genetics and Cellular and Molecular Biology, University of Strasbourg, France). After sequential crossing with the Cad-Cre positive mice (VE-Cadherin promoter driving expression of Cre-recombinase) according to standard procedures, ECs from ERαKO mice were generated (EC-specific ERαKO [EC-ERαKO]) (21). As controls, we used the ERαf/f mice (GP). Beginning at 8–10 weeks of age, mice were fed a WD consisting of high fat (46%) and high carbohydrate as sucrose (17.5%) and high fructose corn syrup (17.5%) for 8 weeks (Testdiet APC). Parallel groups of age-matched control mice were fed a control diet (CD) (Lab Diet 5008). We have previously shown that this 8-week WD feeding paradigm results in whole-body insulin resistance when assessed using hyperinsulinemic-euglycemic clamps (10). All the cohorts were provided water ad libitum while housed in pairs under a 12-hour light, 12-hour dark cycle.
Body weight, retroperitoneal fat, and cholesterol measurements
Mice were weighted immediately before killing. During killing, the retroperitoneal fat pad was isolated and weighted (22). Total cholesterol was determined by an automated analyzer as previously reported (23).
PCR for genomic DNA
DNA from mouse punches was lysed by DirectPCR DNA Extraction System (Viagen Biotech, Inc) rotating at 55°C for overnight and 85°C for 45 minutes. One-microliter lysates, 1-μL primers, and 23-μL Platinum Blue PCR SuperMix (Invitrogen) were mixed for PCR genotyping. The primer sequences used were for Cre, 5′-GAACCTGATGGACATGTTCAGGGA-3′ and Reverse 5′-CAGAGTCATCCTTAGCGCCGTAAA-3′; ERα, 5′-TTGCCCGATAACAATAACAT-3′ and Reverse 5′-TGCAGCAGAAGGTATTTGCCTGTTA-3′.
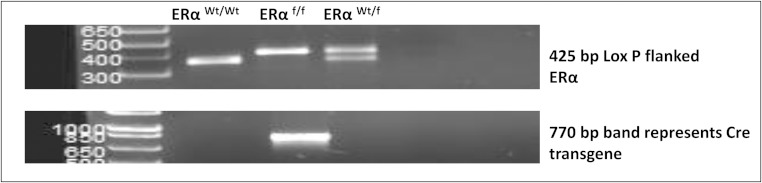
The PCR cycling conditions used were 4 minutes at 94°C for initial denaturation, 35 cycles of 30 seconds at 94°C, 30 seconds at 55°C, and 1 minute at 72°C. Extension for 7 minutes at 72°, then held at 4°C. Amplification products were analyzed by 1.5% agarose gel electrophoresis. Band sizes: wild type (Wt), 366 bp; ERαf/f, 425 bp; and heterozygote (Wt/f), 366/425 (Figure 1).
Figure 1.
Genomic DNA results. Samples obtained by ear punch after weaning. Band sizes: wild type (Wt), 366 base pairs (bp); ERαf/f, 425 bp; heterozygote (Wt/f), 366/425 bp.
Assessment of whole-body insulin sensitivity
Intraperitoneal glucose tolerance test (IPGTT)
IPGTT was performed in the 4 different cohorts after a 5-hour fast as previously described (24). Briefly, dextrose (1.5 g/kg) was injected ip, and the glucose excursion was monitored over a 2-hour time course and compared between treatment groups. Blood samples were analyzed for glucose (AlphaTRACK) at time 0 and 15, 30, 45, 60, and 120 minutes after dextrose injection. The cumulative glycemic excursion was evaluated as the area under the curve (AUC) (25).
Aortic stiffness by in vivo PWV
To evaluate PWV, the gold standard technique for in vivo determination of arterial stiffness, Doppler ultrasound (Indus Mouse Doppler System) measurements were made on the different mice cohorts according to a previously established protocol (26). PWV calculations were based on the transit time method utilized to determine the difference in arrival times of a Doppler pulse wave at 2 locations along the aorta at a known distance apart as previously described (11). Arrival time of each pulse wave was measured as the time from the peak of the electrocardiogram R-wave to the leading foot of the pulse wave at which time velocity begins to rise at the start of systole (11). The distance between the 2 locations along the aorta is divided by the difference in arrival times and is expressed in m/s. All Doppler procedures were performed on isoflurane-anesthetized mice (1.75% in 100% oxygen stream) (11).
Preparation of ex vivo aortic explants for atomic force microscopy (AFM)
To evaluate the stiffness of ECs in enface aortic preparations, a 2 × 2-mm segment of the thoracic aorta was obtained from mice after killing as previously described (11). The aorta was opened longitudinally and the adventitial surface of each explant was fastened to a glass cover slip using cell tak. Stiffness (elastic modulus) of the EC surface was measured by AFM using a cell nano-indentation protocol as previously described (11).
Assessment of arterial vasomotor responses ex vivo
Aorta
A 2-mm segment of thoracic aorta was collected immediately after euthanasia and placed in ice-cold physiological salt solution (PSS) containing 119mM NaCl, 4.7mM KCl, 2.5mM CaCl, 1.18mM KH2PO4, 1.17mM MgSO4, 0.027mM EDTA, 5.5mM glucose, and 25mM NaHCO3 (pH 7.4). For assessment of vasomotor responses, arterial rings were mounted on a wire myograph. Before experimentation, the aortic contractile state was ascertained by KCl (80mM L−1). Aortas were preconstricted with U46619 (100nM). Relaxation responses of arterial rings to acetylcholine (Ach) (1nM–100μM) and the NO-donor sodium nitroprusside (SNP) (1nM–100μM) were assessed by cumulative addition of agonist to the vessel bath.
Aortic relaxation responses are presented as percent maximal relaxation, calculated as [(Fb − Fd)/(Fb − Fmin)] × 100, where Fd is force after a drug intervention, Fb is baseline force, and Fmin is force before the intervention. In addition, EC50s for Ach were calculated using R Software version 3.2.2.
Femoral arteries
The proximal femoral artery was isolated and cannulated onto glass micropipettes, pressurized at 70 mm Hg without flow, and warmed to 37°C in commercial myograph chambers (Living Systems Instrumentation) as previously described (27, 28). To test for viability, the cannulated arteries were allowed to stabilize for 40 minutes and then exposed to PSS in which NaCl was equimolarly substituted with 80mM KCl. Only arteries that constricted more than 20% to this 80mM K+ solution were used in the analyses. After the exposure to high K+, the arteries were washed 3 times with fresh PSS. Vessels were then placed in Ca2+-free PSS and exposed to consecutive changes in intraluminal pressure from 5 to 120 mm Hg while under passive conditions to determine the elastic properties of the arteries as previously reported (29–31). Throughout the experiment, chambers were mounted on inverted microscopes with charge-couple device cameras. Luminal diameter and wall thicknesses were recorded using a video caliper (Living Systems Instrumentation) and a Powerlab data acquisition system (ADInstruments, Inc).
Immunoblotting
Aorta was collected and lysed as previously described (11). Protein concentration of the lysate was determined by commercial protein assay. Proteins were resolved by SDS-PAGE and transferred to nitrocellulose membranes. Nonspecific proteins were blocked by incubation in blocking buffer and the membranes were incubated overnight at 4°C with blocking buffer containing antibodies to 3-nitrotyrosine (ab5411; Millipore) and TGFβ (ab92486; Abcam) (Table 1).
Table 1.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog Number | Species Raised in; Monoclonal or Polyclonal | Dilution Used |
|---|---|---|---|---|---|
| TGFβ | N/A | Anti-TGF β 1 antibody | Abcam, ab92486 | Rabbit; polyclonal | 1:500 |
| Nitrotyrosine | N/A | Nitrotyrosine antibody | Millipore, ab5411 | Rabbit; polyclonal | 1:1000 |
Immunohistochemistry
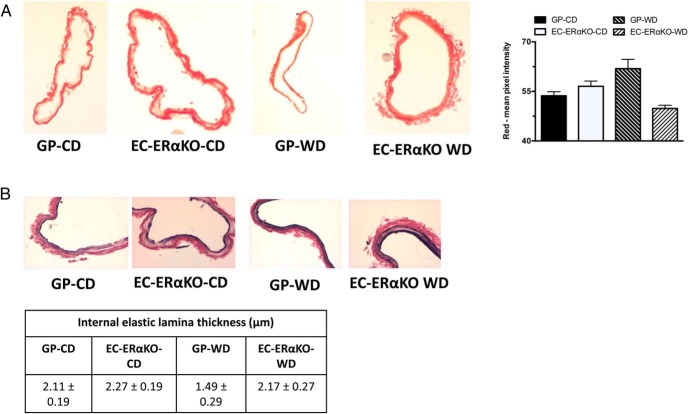
A segment of femoral artery was immersed fixed in 3% paraformaldehyde, dehydrated in ethanol, paraffin embedded, and transversely sectioned in 5-μm slices. Four sections each for 4 mice per group were examined. To evaluate remodeling as previously described, sections were stained with picrosirius red and Verhoeff-von Gieson (VVG) (11). Picrosirius red was quantified as the average percent intensity of staining in 5 images per mouse femoral artery. VVG staining was used to quantify the thickness of the internal elastic lamina (IEL). These measurements were obtained at ×40 magnification using the ImageJ/Fiji image analysis platform.
Statistical analysis
Data are reported as the mean ± SEM. Differences in outcomes were determined using a two-way (genotype by diet) ANOVA and post hoc tests (Tukey) to examine differences in outcomes between GP and KO mice fed with a WD or a CD (Sigma Plot 13.0; Systat Software).
Results
Endothelial specific ERKO does not result in greater adiposity after 8 weeks of WD feeding
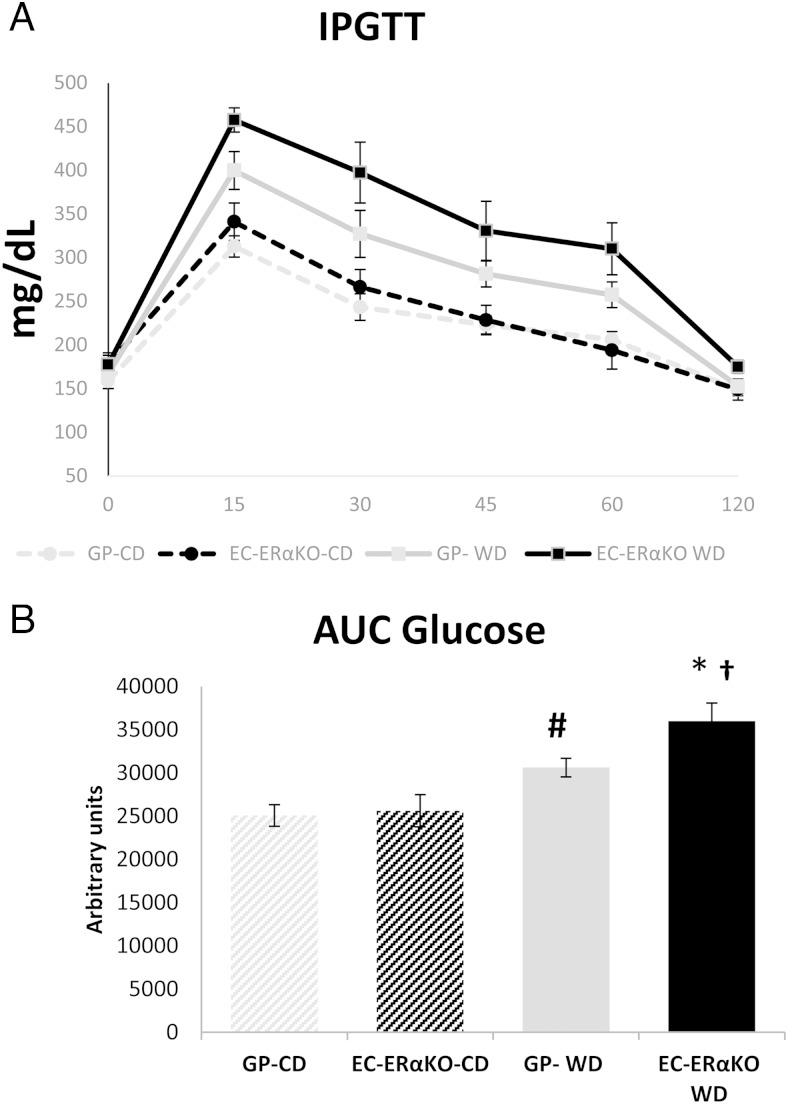
We have previously shown that global ERαKO results in early onset obesity and whole-body insulin resistance (25). In the present investigation, there was no significant difference in body weight at the time of killing between the EC-ERαKO and GP, regardless of the feeding paradigm (Table 2). However, retroperitoneal fat (a surrogate of visceral fat) was significantly elevated after 8 weeks of WD in both the GP and EC-ERαKO cohorts (Table 2). Whole-body glucose homeostasis was evaluated via IPGTT in the different groups (Figure 2). Eight weeks of WD resulted in a significant increment of the AUC in both the GP-WD and the EC-ERαKO-WD when compared with their respective CD-fed controls (Figure 2). In addition, post hoc analysis showed a significant difference between the GP-WD and EC-ERαKO-WD with the latter having a higher AUC (Figure 2). These results demonstrate that 8 weeks of WD feeding result in insulin resistance and impaired glucose homeostasis with a greater impact in the EC-ERαKO-WD cohort.
Table 2.
Body Weight, Retroperitoneal Fat, and Cholesterol Concentrations at the Time of Killing of the Different Cohorts
| Body Weight (g) | Retroperitoneal Fat (g) | Total Cholesterol (mg/dL) | |
|---|---|---|---|
| EC-ERαKO-CD | 20.95 ± 0.40 | 101.26 ± 19.67 | 54 ± 5.99 |
| GP-CD | 21.72 ± 0.73 | 87.3 ± 26.63 | 55.25 ± 8.47 |
| EC-ERαKO-WD | 22.26 ± 1.14 | 147.86 ± 26.63a | 97.4 ± 7.57a,b |
| GP-WD | 22.03 ± 1.03 | 141.87 ± 23.07a | 75.714 ± 6.40c |
Values are presented as mean ± SE. For body weight, n = 5–10; for retroperitorneal fat, n = 6–10; for total cholesterol, n = 4–8.
P < .05 CD vs WD.
P < .05, EC-ERαKO-CD vs EC-ERαKO-WD.
P = .068 GP-CD vs GP-WD.
Figure 2.
Lack of ERα does not impair glucose homeostasis under CD feeding conditions. Whole-body glucose homeostasis was assessed using an IPGTT. Time course of glucose levels (A) and AUC values in the different cohorts (B). Values are presented as mean ± SE. Post hoc comparisons within different groups; n = 4–6; *, P < .05 EC-ERαKO-CD vs EC-ERαKO WD; # GP-WD vs GP-CD; † P < .05 GP-WD vs EC-ERαKO WD.
We also assessed total cholesterol in the different groups as cholesterol byproducts have been involved in the genesis of CVD in conditions of a high-fat diet feeding (32). There was a significant increase in total cholesterol concentration upon WD, with post hoc analysis showing a significant effect in the EC-ERαKO group. Similarly, total cholesterol tended to be increased in the GP-WD vs GP-CD (Table 2).
Effect of EC-ERα deletion on vascular stiffness induced by WD
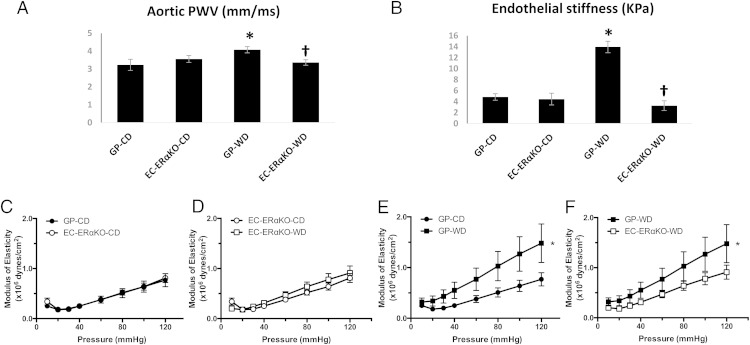
We have previously shown that female mice fed a WD develop vascular stiffness (11). In the present study, we assessed the effect of WD on arterial stiffness in the setting of EC-specific deletion of ERα. In vivo evaluation of aortic stiffness with PWV did not reveal differences between the EC-ERαKO and GP-CD-fed cohorts (3.56 ± 0.18 vs 3.23 ± 0.26 m/s, respectively) (Figure 3A). On the other hand, WD feeding resulted in a significant increase in PWV in the GP-WD group when compared with both the GP-CD (4.08 ± 0.18 vs 3.23 ± 0.26 m/s, P < .05) and the EC-ERαKO-WD group (4.08 ± 0.18 vs 3.37 ± 0.18, P < .05). Similarly, ex vivo assessment of EC surface stiffness in aortic explants using AFM was not different between EC-ERαKO-CD and GP-CD mice (4.46 ± 1.07 vs 4.84 ± 1.07 kPa, respectively) (Figure 3B), whereas WD feeding caused a significant increase in EC stiffness in the GP-WD cohort (Figure 3B).
Figure 3.
Lack of endothelial ERα abrogates the increased in arterial stiffness induced by WD feeding. A, PWV values obtained before killing. B, Force measurements were acquired by interaction between a cantilever tip and the EC surface of aortic explants from mice. C–F, Femoral artery modulus of elasticity. Values are mean ± SE. n = 5–8 per group. Post hoc comparisons within different groups; *, P < .05 GP-CD vs GP-WD; †, P < .05 GP-WD vs EC-ERαKO-WD.
Indices of arterial stiffness were also examined ex vivo in femoral arteries. The modulus of elasticity, a measurement of arterial stiffness (30), was not significantly different among CD-fed cohorts (Figure 3C), but it was significantly elevated in the GP-WD group relative to both the GP-CD and the EC-ERαKO groups (Figure 3, D–F). These results suggest that lack of ERα protects females from WD-induced arterial stiffness.
Absence of EC-ERα does not result in impaired vasodilation
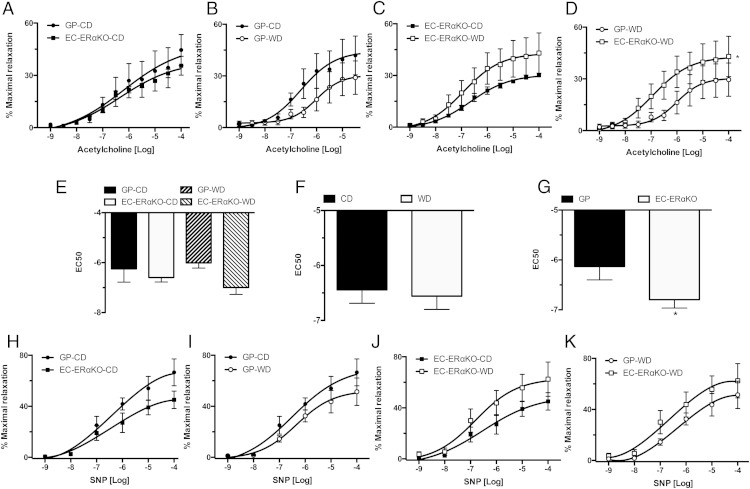
Previous work from our laboratory has shown that WD feeding results in abnormal endothelial-dependent and independent vasorelaxation in female mice (11). Because endothelial-dependent dilation correlates with the presence of arterial stiffness (11), we investigated whether the changes seen in vascular stiffness were related to changes in vasodilatory responses in the EC-ERαKO model. There was not a significant difference in the dose-response curves to Ach between the CD-fed cohorts (Figure 4A), the GP-CD and GP-WD groups (Figure 4B), or the EC-ERαKO cohorts (Figure 4C). However, there was a significant difference between the dose response to Ach in the GP-WD and the EC-ERαKO-WD group, with the GP cohort having a reduced response (Figure 4D). Calculation of EC50 to Ach revealed a significant strain difference between KO mice and GP (Figure 4, E–G) (Table 3). In addition, there was no significant difference in the response to SNP among the different groups (Figure 4, H–K). These data suggest that abrogation of ERα signaling in ECs in conditions of a WD feeding results in decreased vascular stiffness and preserved endothelial-dependent vasodilation.
Figure 4.
Endothelial-dependent and independent dilation is intact in the EC-ERαKO mice. Vasodilator responses of isolated aortic rings to the endothelium-dependent dilator, Ach (A) CD-fed cohorts; (B) GP-CD vs GP-WD; (C) EC-ERαKO cohorts; (D) WD cohorts; (E) EC50 to Ach for the different cohorts; (F) diet effect on EC50; (G) effect of the ER KO on EC50; vasodilator responses to the endothelium-independent dilator, SNP (H) CD-fed cohorts; (I) GP-CD vs GP-WD; (J) EC-ERαKO cohorts; and (K) WD cohorts. Values are mean ± SE; n = 5–6 for all groups. Post hoc comparisons within the different feeding and KO paradigms. *, P < .05.
Table 3.
EC50 to Ach and Maximal Responses to Ach and SNP
| GP-CD | EC-ERαKO-CD | GP-WD | EC-ERαKO-WD | |
|---|---|---|---|---|
| EC50 Ach (log M) | −6.25 ± 0.53a | −6.60 ± 0.18a | −6.01 ± 0.21 | −6.70 ± 0.28 |
| Rmax Ach (%) | 46.05 ± 11.27 | 30.12 ± 0.85 | 39.58 ± 9.65 | 43.01 ± 11.71 |
| Rmax SNP (%) | 66.63 ± 10.58 | 45.09 ± 6.81 | 51.41 ± 10.75 | 62.50 ± 13.48 |
Values are mean ± SE; n = 5–6 for all groups.
P < .05 GP vs EC-ERαKO, no significant interaction effect.
EC-ERαKO results in decreased femoral artery wall material
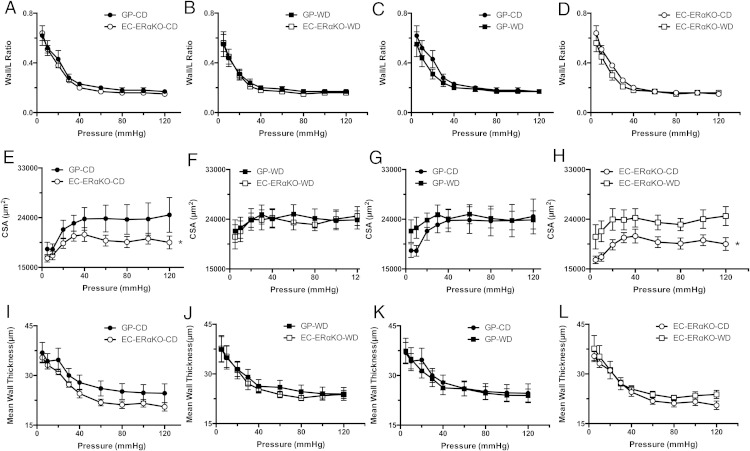
We explored changes in vascular remodeling as a potential mechanism for the observed changes in arterial stiffness. There were no significant differences in wall/lumen ratio among the different study groups (Figure 5, A–D). The femoral arteries from EC-ERαKO-CD mice exhibited significantly lower cross-sectional area (CSA) than the GP-CD (Figure 5E). This difference was not present in the WD-fed cohorts (Figure 5F). There were not significant differences between the GP cohorts (Figure 5G). Moreover, there was a significant increase in the CSA after WD in EC-ERαKO (Figure 5H). Mean wall thickness among the different cohorts was not different (Figure 5, I–L). These results demonstrated that the endothelial-specific KO was associated with a significant reduction in the total amount of wall material, which was characterized by a decline in mean CSA and this change was reversed by WD-feeding. To further explore these morphological differences, we performed immunohistochemistry analysis of picrosirus red staining in femoral arteries which did not reveal a significant change in collagen content in the WD-fed cohorts (Figure 6A). In addition, we also assessed the thickness of the IEL via VVG staining and found no significant differences (Figure 6B).
Figure 5.
Endothelial-specific deletion of ERα results in decreased femoral artery CSA. A–D, Wall to lumen. E–H, CSA. I–L, Mean wall thickness. Values are mean ± SE; n = 5–6 for all groups. Values are mean ± SE; n = 6 for all groups. Post hoc comparisons within the different feeding and KO paradigms. *, P < .05.
Figure 6.
Endothelial ERαKO mice do not exhibit changes in femoral artery collagen content or IEL thickness. A, Representative micrographs (×10) of picrosirus red immunostaining and its quantification. B, Representative micrographs (×40) of Verhoeff-Van Gieson staining and correspondent IEL measurements. Values are mean ± SE; n = 5–9 for all groups.
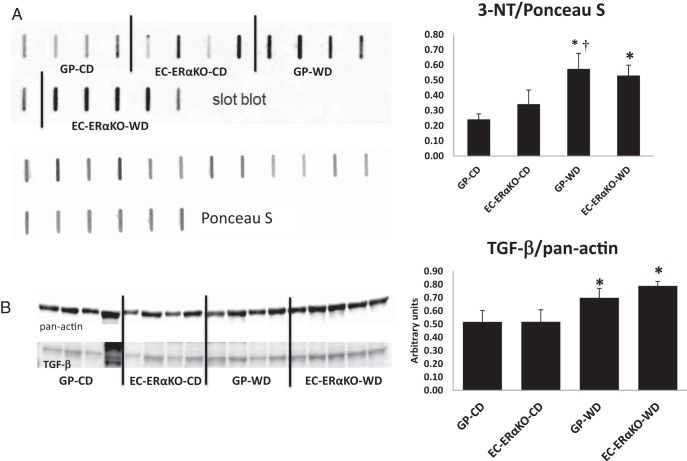
We have previously reported that WD feeding is associated with increased oxidative stress and TGFβ expression in the vasculature (11, 29). Consequently, we explored the role of oxidative stress and TGFβ in our current model. There was a significant increase in the presence 3-nitrotyrosine, assessed via immunoblotting, in the aorta of the GP-WD model without a significant effect of the EC-ERα deletion (Figure 7A). In addition, the expression of TGFβ in the aorta, one of a myriad of profibrotic stimuli, was significantly increased upon WD feeding (Figure 7B). These data suggest that in the EC-ERαKO model the decreased vascular stiffness and vascular remodeling implicates mechanisms not dependent on increased oxidative stress and/or TGFβ expression.
Figure 7.
Neutral impact of EC-ERαKO on aortic oxidative stress and TGFβ expression in conditions of WD feeding. A, 3-nitrotyrosine (3-NT) in aortic lysates; representative blots with corresponding quantitative analysis. Two-way ANOVA analysis resulted a P = .07 for a diet effect. B, TGFβ expression in aortic lysates; representative blots with corresponding quantitative analysis. *, P < .05 for a diet effect; †, P < .05 GP-CD vs GP-WD. Values are mean ± SE; n = 4–5 for all groups.
Discussion
Chronic overnutrition is the driving force behind the alarming prevalence of obesity and T2DM (33). Obese, insulin resistant and diabetic women develop elevations in vascular stiffness, which is a strong independent risk factor for chronic kidney disease and CVD including stroke, hypertension, and diastolic heart failure (5, 8, 9, 34). Premenopausal women, classically considered protected against CVD, lose this protection if obese or type 2 diabetic (9). In the present study we utilize a novel experimental model to clarify the role of endothelial ERα signaling in the pathogenesis of vascular stiffness in females. Our main finding is that abrogation of ERα signaling in the endothelium protects female mice against WD-induced vascular stiffness both in vivo and ex vivo and results in improvements in endothelial-dependent vasodilation.
We targeted the expression of ERα in the endothelium as multiple beneficial effects of estrogen are mediated via signaling through this receptor (16). These actions include increased NO and prostacyclin production, decreased endothelial permeability and activation, and increased endothelial progenitors cells (16, 35). Different models of ERαKO have been developed with varying impact on the vascular effects of estrogen (35). In the present investigation, we used a model where the exon 3 of the ERα gene is floxed (21). This site has been documented as essential for the vascular effects of estrogens (36). The specificity to EC of the KO was provided by the use of a Cre-recombinase under the control of the VE-Cadherin promoter, where the expression of the promoter is limited to endothelium in adult life (37, 38).
Global ERαKO models manifest obesity and insulin resistance early in life (25). In our study, EC-ERαKO female mice fed a CD did not exhibit differences in body weight, insulin sensitivity, glucose homeostasis when compared with GP controls at 4 months of age. However, WD feeding for 8 weeks resulted in insulin resistance and impaired glucose tolerance in EC-ERαKO mice. This finding supports a critical role for ERα signaling in endothelium in mediating insulin actions and sensitivity across different tissues.
Previous studies have examined the effect of estrogen depletion on vascular stiffness, and have shown consistently that estrogen treatment decreases vascular stiffness in postmenopausal women (39, 40). However, the exact role of ERα signaling has not been fully characterized. Analysis of polymorphisms of the ERα gene in a cohort of older adults found a positive correlation between arterial stiffness and certain polymorphisms in women but not in men (41). Similarly, examination of the Framingham Heart Study Offspring cohort showed that variations in the ERα and ERβ genes carry increase risk of vascular stiffness (42). In the present investigation we did not find significant impairments of the endothelial-dependent and -independent relaxation in aortic rings in the CD-fed EC-ERαKO mice. Similarly, EC-ERαKO mice fed a CD did not exhibit differences in in vivo or ex vivo measures of vascular stiffness. A previous investigation by Muller-Delp et al has shown that endothelial mediated vasodilatory responses can be intact in a model of global absence of ERα with up-regulation of antioxidant systems (14). These data suggest that under normal conditions, ERα signaling in the endothelium is not involved in the regulation of vascular function and stiffness in female mice.
We further examined the vascular effects of WD in our model. This is a translational and relevant approach as it mimics the diet consumed in many westernized societies and women are more prone to developing CVD in conditions of insulin resistance relative to men (33). Our group has previously shown that WD feeding results in increased vascular stiffness in female mice and impaired endothelial dependent relaxation (11). Despite impaired whole-body insulin sensitivity in the EC-ERαKO-WD-fed cohort, abrogation of endothelial ERα signaling in WD-fed mice resulted in a decrease in vascular stiffness when assessed via aortic PWV, AFM indentation of endothelial surface of the aorta and modulus of elasticity of femoral vessels. Thus, we have demonstrated that reduction in stiffness is not limited to isolated vascular beds but is a generalized phenomenon in conductance vessels.
To the best of our knowledge, the current investigation is the first to examine the role of endothelial ERα in the genesis of vascular stiffness in conditions of overnutrition and insulin resistance. The relative vascular protection afforded by the absence of endothelial ERα in our model is in line with the clinical findings indicating a lack of clear cardiovascular benefits from estrogen therapy (43). The specific role of the endothelium in the genesis of vascular stiffness is still a matter of active investigation. In the present work although no statistical significance was reached, there is an apparent reduction in Ach relaxation in mice fed a WD. However, EC-ERαKO significantly preserved the relaxation response in animals fed a WD. This resulted in a difference between the EC-ERαKO-WD and GP-WD groups. This is indicative of a preserved and even increased availability of NO in the EC-ERαKO under WD conditions. This result suggests preservation of NO-mediated vasodilatory responses that parallels the resistance toward developing vascular stiffness. The preserved bioavailability of NO in turn can result in increased or preserved EC compliance, reducing the stiffening effects of a WD (11, 29). As we have previously reported increased vascular oxidative stress in WD females in concert with impaired endothelial dependent vasodilatory responses (11), we examined the presence of aortic oxidative stress in our WD model and found no definitive effect of the endothelial-specific lack of ERα on it.
Another potential mechanism of augmented stiffness is vascular remodeling with changes in the cytoskeleton and extracellular matrix composition (eg, actin, elastin, and collagen). Even though we did not find a significant difference in the collagen deposition or in the IEL thickness, we cannot rule out in our model that changes in the number and size of fenestrae of the IEL or changes in other extracellular matrix characteristics are present as those changes have been associated with vascular stiffness in conductance vessels (29). A significant finding in our present investigation is the decreased CSA of our KO model in CD conditions. This finding likely reflects a reduction in the total amount of wall material. The impact that this outcome has on the overall lesser vascular stiffness seen in the KO when compared with the control under WD feeding conditions will be an area of future investigations.
Other potential mechanisms that could explain our findings can be attributed to binding of circulating oxysterols, such as 27-hydroxycholesterol, to ERα (44). 27-hydroxycholesterol has been postulated as a potential blocker of the beneficial effects of estrogen in the vasculature (44). Recent findings indicate that binding of 27-hydroxycholesterol to the ERα results in vascular inflammation in a model of high-fat feeding (32). WD feeding used in the present investigation resulted in increased total cholesterol (23); however, 27-hydroxycholesterol signaling through the ERα in ECs contribution to vascular stiffness is an attractive hypothesis that needs to be tested in future studies. Another potential mechanism that can contribute to the paradoxical effects seen in our model lies in the estrogenic effect on epithelial Na(+) channel (ENaC), because estrogen is known to regulate ENaC expression in epithelial cells (45). Importantly, ENaC expression and activity correlates with endothelial function and cell stiffness (46). This alternative hypothesis also needs to be explored in future studies. Finally, because estrogen is still present, it would be of special interest to examine the role of estrogen signaling through ERβ and G protein-coupled receptor 30 on the modulation of vascular stiffness. In fact, increased expression of ERβ has been reported after vascular injury (47).
In conclusion, we demonstrate in a model of chronic overnutrition and insulin resistance that endothelial ERα signaling promotes stiffness in different vascular beds. Further studies are needed to clarify the molecular pathway responsible for this clinically relevant findings and its potential impact in the treatment of CVD in women.
Acknowledgments
We thank Brenda Hunter for editorial assistance. ERα floxed mice (ERαf/f) were kindly provided by Dr Pierre Chambon (Institute for Genetics and Cellular and Molecular Biology, University of Strasbourg, France) and Dr Richard Karas (Tufts University, Medford, MA).
This work was supported by National Institutes of Health Grants 1K08HL129074-01 (to C.M.), R01-HL073101 and R01-HL107910 (to J.R.S.), K01HL125503 (to J.P.), and VA I01 BX001981/BX/BLRD (to J.R.S.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AFM
- atomic force microscopy
- Ach
- acetylcholine
- AUC
- area under the curve
- CD
- control diet
- CSA
- cross-sectional area
- CVD
- cardiovascular disease
- EC
- endothelial cell
- EC-ERαKO
- EC-specific ERαKO
- ENaC
- epithelial Na(+) channel
- ER
- estrogen receptor
- ERαf/f
- ERα floxed
- GP
- genopairs
- IEL
- internal elastic lamina
- IPGTT
- intraperitoneal glucose tolerance test
- KO
- knockout
- NO
- nitric oxide
- PSS
- physiological salt solution
- PWV
- pulse wave velocity
- SNP
- sodium nitroprusside
- T2DM
- type 2 diabetes mellitus
- VVG
- Verhoeff-von Gieson
- WD
- Western diet
- Wt
- wild type.
References
- 1. Webb DR, Khunti K, Silverman R, et al. Impact of metabolic indices on central artery stiffness: independent association of insulin resistance and glucose with aortic pulse wave velocity. Diabetologia. 2010;53:1190–1198. [DOI] [PubMed] [Google Scholar]
- 2. Stehouwer CD, Henry RM, Ferreira I. Arterial stiffness in diabetes and the metabolic syndrome: a pathway to cardiovascular disease. Diabetologia. 2008;51:527–539. [DOI] [PubMed] [Google Scholar]
- 3. Ahimastos AA, Formosa M, Dart AM, Kingwell BA. Gender differences in large artery stiffness pre- and post puberty. J Clin Endocrinol Metab. 2003;88:5375–5380. [DOI] [PubMed] [Google Scholar]
- 4. Staessen JA, van der Heijden-Spek JJ, Safar ME, et al. Menopause and the characteristics of the large arteries in a population study. J Hum Hypertens. 2001;15:511–518. [DOI] [PubMed] [Google Scholar]
- 5. Lehmann ED, Hopkins KD, Gosling RG. Increased aortic stiffness in women with NIDDM. Diabetologia. 1996;39:870–871. [DOI] [PubMed] [Google Scholar]
- 6. Coutinho T. Arterial stiffness and its clinical implications in women. Can J Cardiol. 2014;30:756–764. [DOI] [PubMed] [Google Scholar]
- 7. Ben-Shlomo Y, Spears M, Boustred C, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63:636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee WL, Cheung AM, Cape D, Zinman B. Impact of diabetes on coronary artery disease in women and men: a meta-analysis of prospective studies. Diabetes Care. 2000;23:962–968. [DOI] [PubMed] [Google Scholar]
- 9. Peters SA, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet. 2014;383:1973–1980. [DOI] [PubMed] [Google Scholar]
- 10. Manrique C, DeMarco VG, Aroor AR, et al. Obesity and insulin resistance induce early development of diastolic dysfunction in young female mice fed a Western diet. Endocrinology. 2013;154:3632–3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DeMarco VG, Habibi J, Jia G, et al. Low-dose mineralocorticoid receptor blockade prevents Western diet-induced arterial stiffening in female mice. Hypertension. 2015;66:99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deroo BJ, Korach KS. Estrogen receptors and human disease. J Clin Invest. 2006;116:561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Prabhushankar R, Krueger C, Manrique C. Membrane estrogen receptors: their role in blood pressure regulation and cardiovascular disease. Curr Hypertens Rep. 2014;16:408. [DOI] [PubMed] [Google Scholar]
- 14. Muller-Delp JM, Lubahn DB, Nichol KE, et al. Regulation of nitric oxide-dependent vasodilation in coronary arteries of estrogen receptor-α-deficient mice. Am J Physiol Heart Circ Physiol. 2003;285:H2150–H2157. [DOI] [PubMed] [Google Scholar]
- 15. Smirnova NF, Fontaine C, Buscato M, et al. The activation function-1 of estrogen receptor α prevents arterial neointima development through a direct effect on smooth muscle cells. Circ Res. 2015;117:770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murphy E. Estrogen signaling and cardiovascular disease. Circ Res. 2011;109:687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Park JS, Nam JS, Cho MH, et al. Insulin resistance independently influences arterial stiffness in normoglycemic normotensive postmenopausal women. Menopause. 2010;17:779–784. [DOI] [PubMed] [Google Scholar]
- 18. Manrique C, Lastra G, Sowers JR. New insights into insulin action and resistance in the vasculature. Ann NY Acad Sci. 2014;1311:138–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vestweber D. VE-cadherin: the major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arterioscler Thromb Vasc Biol. 2008;28:223–232. [DOI] [PubMed] [Google Scholar]
- 20. Metzger D, Clifford J, Chiba H, Chambon P. Conditional site-specific recombination in mammalian cells using a ligand-dependent chimeric Cre recombinase. Proc Natl Acad Sci USA. 1995;92:6991–6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors α (ERα) and β (ERβ) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. [DOI] [PubMed] [Google Scholar]
- 22. Johnson PR, Hirsch J. Cellularity of adipose depots in six strains of genetically obese mice. J Lipid Res. 1972;13:2–11. [PubMed] [Google Scholar]
- 23. Aroor AR, Habibi J, Ford DA, et al. Dipeptidyl peptidase-4 inhibition ameliorates Western diet-induced hepatic steatosis and insulin resistance through hepatic lipid remodeling and modulation of hepatic mitochondrial function. Diabetes. 2015;64:1988–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lastra G, Habibi J, Whaley-Connell AT, et al. Direct renin inhibition improves systemic insulin resistance and skeletal muscle glucose transport in a transgenic rodent model of tissue renin overexpression. Endocrinology. 2009;150:2561–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Manrique C, Lastra G, Habibi J, Mugerfeld I, Garro M, Sowers JR. Loss of estrogen receptor α signaling leads to insulin resistance and obesity in young and adult female mice. Cardiorenal Med. 2012;2:200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hartley CJ, Reddy AK, Madala S, Entman ML, Michael LH, Taffet GE. Doppler velocity measurements from large and small arteries of mice. Am J Physiol Heart Circ Physiol. 2011;301:H269–H278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martinez-Lemus LA. Persistent agonist-induced vasoconstriction is not required for angiotensin II to mediate inward remodeling of isolated arterioles with myogenic tone. J Vasc Res. 2008;45:211–221. [DOI] [PubMed] [Google Scholar]
- 28. Ramirez-Perez FI, Schenewerk AL, Coffman KL, et al. Effects of the use of assisted reproductive technologies and an obesogenic environment on resistance artery function and diabetes biomarkers in mice offspring. PLoS One. 2014;9:e112651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bender SB, Castorena-Gonzalez JA, Garro M, et al. Regional variation in arterial stiffening and dysfunction in western diet-induced obesity. Am J Physiol Heart Circ Physiol. 2015;309:H574–H582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Castorena-Gonzalez JA, Staiculescu MC, Foote C, Martinez-Lemus LA. Mechanisms of the inward remodeling process in resistance vessels: is the actin cytoskeleton involved? Microcirculation. 2014;21:219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Castorena-Gonzalez JA, Staiculescu MC, Foote CA, Polo-Parada L, Martinez-Lemus LA. The obligatory role of the actin cytoskeleton on inward remodeling induced by dithiothreitol activation of endogenous transglutaminase in isolated arterioles. Am J Physiol Heart Circ Physiol. 2014;306:H485–H495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Umetani M, Ghosh P, Ishikawa T, et al. The cholesterol metabolite 27-hydroxycholesterol promotes atherosclerosis via proinflammatory processes mediated by estrogen receptor α. Cell Metab. 2014;20:172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Johnson RJ, Nakagawa T, Sanchez-Lozada LG, et al. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes. 2013;62:3307–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schram MT, Kostense PJ, Van Dijk RA, et al. Diabetes, pulse pressure and cardiovascular mortality: the Hoorn Study. J Hypertens. 2002;20:1743–1751. [DOI] [PubMed] [Google Scholar]
- 35. Arnal JF, Fontaine C, Billon-Galés A, et al. Estrogen receptors and endothelium. Arterioscler Thromb Vasc Biol. 2010;30:1506–1512. [DOI] [PubMed] [Google Scholar]
- 36. Pare G, Krust A, Karas RH, et al. Estrogen receptor-α mediates the protective effects of estrogen against vascular injury. Circ Res. 2002;90:1087–1092. [DOI] [PubMed] [Google Scholar]
- 37. Alva JA, Zovein AC, Monvoisin A, et al. VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Dev Dyn. 2006;235:759–767. [DOI] [PubMed] [Google Scholar]
- 38. Mueller KB, Bender SB, Hong K, et al. Endothelial mineralocorticoid receptors differentially contribute to coronary and mesenteric vascular function without modulating blood pressure. Hypertension. 2015;66:988–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nagai Y, Earley CJ, Kemper MK, Bacal CS, Metter EJ. Influence of age and postmenopausal estrogen replacement therapy on carotid arterial stiffness in women. Cardiovasc Res. 1999;41:307–311. [DOI] [PubMed] [Google Scholar]
- 40. Moreau KL, Donato AJ, Seals DR, DeSouza CA, Tanaka H. Regular exercise, hormone replacement therapy and the age-related decline in carotid arterial compliance in healthy women. Cardiovasc Res. 2003;57:861–868. [DOI] [PubMed] [Google Scholar]
- 41. Hayashi K, Maeda S, Iemitsu M, et al. Sex differences in the relationship between estrogen receptor α gene polymorphisms and arterial stiffness in older humans. Am J Hypertens. 2007;20:650–656. [DOI] [PubMed] [Google Scholar]
- 42. Peter I, Kelley-Hedgepeth A, Huggins GS, et al. Association between arterial stiffness and variations in oestrogen-related genes. J Hum Hypertens. 2009;23:636–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Anderson GL, Limacher M, Assaf AR, et al. ; The Women's Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. [DOI] [PubMed] [Google Scholar]
- 44. Umetani M, Domoto H, Gormley AK, et al. 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nat Med. 2007;13:1185–1192. [DOI] [PubMed] [Google Scholar]
- 45. Yusef YR, Thomas W, Harvey BJ. Estrogen increases ENaC activity via PKCδ signaling in renal cortical collecting duct cells. Physiol Rep 2014;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jeggle P, Callies C, Tarjus A, et al. Epithelial sodium channel stiffens the vascular endothelium in vitro and in Liddle mice. Hypertension. 2013;61:1053–1059. [DOI] [PubMed] [Google Scholar]
- 47. Lindner V, Kim SK, Karas RH, Kuiper GG, Gustafsson JA, Mendelsohn ME. Increased expression of estrogen receptor-β mRNA in male blood vessels after vascular injury. Circ Res. 1998;83:224–229. [DOI] [PubMed] [Google Scholar]