Abstract
Endocrine-disrupting chemicals are prevalent in the environment and can impair reproductive success by affecting the hypothalamic-pituitary-gonadal axis. The developing pituitary gland is sensitive to exposure to endocrine-disrupting chemicals, such as bisphenol A (BPA), and sex-specific effects can occur. However, effects on the critical window of neonatal pituitary gland development in mice have not been explored. Therefore, this study determined baseline gene expression in male and female pituitaries and consequences of environmental exposure to 17β-estradiol (E2) and BPA on transcription of genes exhibiting sex differences during the neonatal period. Through microarray and quantitative RT-PCR analysis of pituitaries at postnatal day (PND)1, 3 genes were differentially expressed between males and females: Lhb, Fshb, and intracellular adhesion molecule-5 (Icam5). To see whether E2 and BPA exposure regulates these genes, pituitaries were cultured at PND1 with 10−8M E2 or 4.4 × 10−6M BPA. E2 decreased expression of Lhb, Fshb, and Icam5 mRNA in females but only significantly decreased expression of Icam5 in males. BPA decreased expression of Icam5 similarly to E2, but it did not affect Lhb or Fshb. Importantly, in vivo exposure to 50-μg/kg · d E2 from PND0 to PND7 decreased expression of Lhb, Fshb, and Icam5 mRNA in both males and females, whereas 50-mg/kg · d BPA exposure during the same time frame decreased expression of Icam5 in females only. Overall, we have uncovered that genes differentially expressed between the sexes can be regulated in part by hormonal and chemical signals in vivo and directly at the pituitary and can be regulated in a sex-specific manner.
Endocrine-disrupting chemicals (EDCs) in the environment are correlated in humans with impaired reproductive health, including decreasing age of onset of puberty, infertility, miscarriage, and decreased sperm quality (1, 2). These disruptions may originate from alterations in development or function of the pituitary, which is a critical component of the hypothalamic-pituitary-gonadal (HPG) axis. Exposure to EDCs during developmental windows may be more detrimental as these periods can be more sensitive and disruptions in development and can lead to permanent effects later in life (3). Expansion of the pituitary gland occurs embryonically through the first 2 postnatal weeks in the mouse to create the hormone-secreting cell types including somatotropes, lactotropes, gonadotropes, corticotropes, thyrotropes, and melanotropes (4–6). The cells subsequently form functional networks through cell-cell contact, which are crucial in generating proper hormone release (7). The neonatal period of development is of particular interest because, unlike the embryonic period, the axes connecting the hypothalamus, pituitary, and target organs are established allowing for influence of circulating hormones. In terms of the HPG axis, the pituitary becomes responsive to GnRH at embryonic day 16, when the axons of GnRH neurons reach the median eminence (8, 9) and the testes can secrete testosterone in response to LH (9). Therefore, it is possible that circulating hormones, as well as EDC exposure, during the neonatal period could affect development of the pituitary, potentially having permanent effects. It is unknown whether there would be a difference between males and females with respect to pituitary development or response to EDCs during this time.
One prevalent EDC is bisphenol A (BPA), a plasticizer found in polycarbonate plastics, epoxy resins, and thermal paper (10), leading to daily exposure in humans. BPA's mode of action is considered to be estrogenic, despite its binding affinity to the classical estrogen receptors (ERs) (ERα and ERβ) being about 10 000-fold weaker than that of estradiol (11). BPA has a better binding affinity to the membrane G protein-coupled ER (GPER) (12). The pituitary expresses ERα and ERβ before birth (13, 14). GPER is expressed in the adult pituitary as well; however, expression in the developing pituitary is unknown (15). Because the pituitary possesses these ERs, it is possible that estrogens and BPA can affect neonatal pituitary development either directly or indirectly through the hypothalamus, which also expresses GPER, ERα, and ERβ (15–17).
In rodent models, developmental exposure to BPA adversely affects the HPG axis. For example, developmental BPA exposure can advance the age of vaginal opening and first estrus as well as cause various fertility problems (1, 18, 19). These effects have been correlated with elevated levels of Kiss1 and Gnrh mRNA in the hypothalamus and Fshb mRNA in the pituitary (20). Similarly, neonatal exposure to BPA alters estrous cyclicity and impairs LH release (21, 22). Thus, developmental time periods are sensitive to endocrine disruption, which can have lasting effects on function of the HPG axis. Despite the abundance of knowledge regarding the adverse effects of BPA exposure on the HPG axis, very few of these studies closely examined the pituitary or deciphered whether effects seen in vivo occurred at the pituitary itself.
BPA not only impacts the proper functioning of the HPG axis, but also has a number of sex-specific effects. The anteroventral periventricular nucleus is a sexually dimorphic nucleus important for generation of the LH surge in females. Estrogen, converted from testosterone via aromatase, is responsible for generation of the sex differences in the brain (23). Developmental BPA exposure alters the size of the anteroventral periventricular nucleus to make males and females more similar (24, 25) and alters sex differences in gene expression of Esr1, Esr2, Esrrg, and Kiss1 (26, 27). In the pituitary, embryonic exposure to BPA leads to an increase in gonadotrope number and altered gonadotrope-specific mRNA in females but not in males (28). However, it is unknown whether BPA would have a sex-specific effect on the pituitary during the critical neonatal period of development.
To understand why BPA has differing effects in males and females, it is important to characterize baseline differences between the sexes. Focusing on the pituitary, only 43 genes are differentially expressed in adults between males and females (29). No studies have been done to determine the sex differences during the neonatal period of development. However, it is known that serum levels of gonadotrope-derived LH and FSH are higher in females than males at the day of birth (30). It is also apparent that proper functioning of the HPG axis is essential for manifestation of this sex difference, because hypogonadal mice (hpg), which lack GnRH signaling, do not exhibit a sexual dimorphism in LH and FSH serum levels (30). Despite the differences in LH and FSH serum levels at birth and apparent dependence on GnRH, it is unknown what other differences exist in the pituitary during the neonatal period and whether there is any direct contribution of sex steroids to pituitary sexual dichotomy.
We hypothesize that there are sex differences in gene expression at the level of the pituitary during the neonatal period, and these genes can be regulated by hormones and EDCs. To test this hypothesis, we analyzed global mRNA expression in male and female CD-1 mouse pituitaries during the neonatal period. We found very few sex differences besides Lhb and Fshb during this neonatal period at the mRNA level. One novel gene uncovered was intracellular adhesion molecule-5 (Icam5). ICAM5 characteristically is expressed on dendritic filopodia of telencephalic neurons and is involved in dendritic shape changes during development (31–34). In the pituitary, we demonstrated that ICAM5 is detected predominantly in gonadotropes. We then showed that 17β-estradiol (E2) and BPA could disrupt gene expression of differentially expressed genes, including ICAM5, in the pituitary. These findings indicate the neonatal pituitary is sensitive to exogenous EDC exposure.
Materials and Methods
Mice
CD-1 mice were bred in house. Sex was confirmed by visual inspection and SRY genotyping using the primer sequences SRY forward 5′ to 3′ TGCAGCTCTACTCCAGTCTTG and reverse 5′ to 3′ GATCTTCATTTTTAGTGTTC (Life Technologies). The University of Illinois Urbana-Champaign Institutional Animal Care and Use Committee approved all procedures.
Microarray
Control postnatal day (PND)1 male and female pituitaries were collected and stored in RNAlater (Ambion) at −20°C. Two pituitaries of same sex were then pooled, and 4 independent pools were made for each sex. RNA was isolated using the RNAqueous-Micro kit (Ambion). RNA was sent to Roy G. Carver Biotechnology Center (University of Illinois at Urbana-Champaign). Agilent Mouse 4 × 44K expression microarrays were used to examine pituitary gene expression. Total RNA (200 ng per sample) was reverse transcribed and linear-amplified according to the manufacturer's instructions using the Agilent Low Input QuickAmp labeling kit (Agilent Technologies) and labeled with either Cy3 or Cy5 cyanine dyes. One of the male samples failed to label well so the other sample in the same dye was used twice. Samples (2 per array) were hybridized on the microarray slide and washed according to the Agilent protocol. Slides were scanned using an Axon 4000B scanner, and images were analyzed with genepix 6.1 software (Molecular Devices).
Quantitative RT-PCR (qRT-PCR)
For sex difference RNA analysis, 2 litters were combined and analyzed at PND0 (n = 10–15), PND4 (n = 7–11), PND9 (n = 10–11), and adult (n = 7). One litter was analyzed at PND20 (n = 6–7). RNA was processed as previously described (35). Gapdh, Lhb, and Fshb primers were used previously in our lab (28). For Icam5, the sequences used were forward 5′ to 3′: CCAAACAGCTGATGTGCGTC and reverse 5′ to 3′: AGAGGAGTCGGGAAGCTGTA. For Eif2s3x the sequences used were forward 5′ to 3′: GGGACCAAAGGGAACAAG and reverse 5′ to 3′: AGCATCCTAGCCATCAAAATATCA (Life Technologies). Data were analyzed using the standard comparative Δcycle threshold value method as previously described (36). The error bars in the figures represent the SEM of the relative fold change for each group.
Immunohistochemistry
LHβ staining was performed on paraffin sections as previously described (28) on PND0 (day of birth). Three slides evenly spaced spanning the entire pituitary from 4 male and female CD1 pups were stained with anti-LHβ antibody. The number of LHβ-positive cells were counted and divided by the total number of cells in the anterior lobe. Sections on a slide, as well as slides per animal, were averaged together to obtain the mean percent positive LHβ cells.
For ICAM5 staining, pituitaries from 3 male and female PND7–PND9 mice were fixed in 3.7% paraformaldehyde, cryoprotected in 30% sucrose/PBS solution, then frozen in Optimal Cutting Temperature compound (Electron Microscopy Sciences). The ICAM5 antibody was applied on 10-μm sections (37). For double stains with ICAM5, 3 female pituitaries were stained at PND9 for LHβ, TSHβ, ACTH, and GH and at PND75 for PRL and LHβ. All antibodies are listed in Table 1. All experiments contained a slide without any primary antibodies to ensure specificity. Slides were counterstained with 4′,6-diamidino-2-phenylindole (1:1000; Sigma) and visualized and processed as previously described (28).
Table 1.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used |
|---|---|---|---|---|---|
| Rat βLH | N/A | Anti-rβLH-IC | National Hormone and Peptide Program (NHPP) Dr A. F. Parlow | Rabbit; polyclonal | Paraffin 1:1000 |
| Frozen 1:50 | |||||
| Mouse ICAM5 (TLCN) | AEGGAETPGTAESPADGEVFAIQLTSS | Anti-TLCN-C | Dr Yoshihiro Yoshihara | Rabbit; polyclonal | 1:4000 |
| Rat βTSH | N/A | Anti-rβTSH-IC-1 | National Hormone and Peptide Program (NHPP) Dr A. F. Parlow | Rabbit; polyclonal | 1:1000 |
| Rat adrenocorticotropic hormone | N/A | Anti-rACTH | National Hormone and Peptide Program (NHPP) Dr A. F. Parlow | Rabbit; polyclonal | 1:1000 |
| Rat GH | N/A | Anti-rGH | National Hormone and Peptide Program (NHPP) Dr A. F. Parlow | Rabbit; polyclonal | 1:1000 |
| Rat prolactin | N/A | Anti-rPRL | National Hormone and Peptide Program (NHPP) Dr A. F. Parlow | Rabbit; polyclonal | 1:1000 |
Antibodies used in this study.
In situ hybridization (ISH)
An antisense probe for Icam5 was prepared from mouse cDNA using sequence-specific primers, forward primer 5′-3′ ATGACTTGGATTGTCCCAGAAG and reverse primer 5′-3′ CAGGTTACCAGGGGTCATAGAG. The resulting PCR product was cloned into pGEM-T easy vector following the manufacturer's guidelines (Promega). The probe was linearized and transcribed with T7 polymerase (Promega) in the presence of digoxigenin-labeled nucleotides (Roche). ISH was performed on 3 PND9–PND11 male and female pituitaries prepared as for ICAM5 immunohistochemistry above. For ISH, pituitaries were thawed and fixed with 3.7% paraformaldehyde, permeabilized with proteinase K (0.1 μg/mL) (Life Technologies), and incubated with the Icam5 probe at 55°C overnight. Probe detection was carried out as previously described (35). Slides were then counterstained with methyl green (National Diagnostics) and mounted with Permount Mounting Medium (Fisher).
E2 and BPA culture experiments
PND1 pituitaries were cultured overnight on Millicell CM membranes (Millipore) in a 24-well plate. The culture media consisted of phenol red-free DMEM/F12 (Fisher), supplemented with 10% charcoal-stripped fetal bovine serum (Sigma) and 100× penicillin-streptomycin solution (Fisher). A dose-response curve for BPA (Aldrich ≥ 99% purity) was performed, including vehicle control (0.1% ethanol), 4.4 × 10−8M BPA, 4.4 × 10−7M BPA, 4.4 × 10−6M BPA, and 4.4 × 10−5M BPA. Male and female pituitaries were pooled with 4–5 pituitaries in each group. Assuming that all BPA in an in vivo treatment paradigm reaches the pituitary, a dose of the lowest observed adverse effect level (50 mg/kg · d) would equate to 50 μg/mL or 2.106 × 10−4M (38, 39). Therefore, the in vitro dose response corresponds to in vivo doses ranging from 10 μg/kg · d to 10 mg/kg · d, which encompasses the oral reference dose of 50 μg/kg · d. For subsequent cultures comparing the effects of E2 and BPA in combination with the estrogen receptor antagonist, ICI 182780 (Tocris), PND1 pituitaries were cultured as described above. Pituitaries were placed in 1 of 7 treatment groups; control (0.2% ethanol), E2 (10−8M), E2+ICI (10−8M + 10−5M), BPA (4.4 × 10−6M), BPA+ICI (4.4 × 10−6M + 10−5M), and ICI (10−5M). Four individual experiments were conducted consisting of control, E2, and E2+ICI for a total of 6–9 pituitaries for each male and female group. Five individual experiments were conducted consisting of control, BPA, BPA+ICI, and ICI for a total of 6–9 pituitaries for each male and female group. Cultures were harvested 48 hours after treatment for analysis. This time was chosen to maximize the possibility of seeing changes in genes that were directly or indirectly regulated by E2 or BPA.
E2 and BPA dosing experiments
Neonatal CD-1 mice were dosed orally with 50 μg/kg · d of E2 (Tocris) dissolved in tocopherol-stripped corn oil (MP Biomedicals) or 0.1% ethanol in corn oil as a control. Pups were dosed orally once daily from PND0 to PND7 and pituitaries were collected on the morning of PND7, 1 hour after the last treatment. A total of 6–8 pituitaries spanning 3 litters for each group were analyzed. For BPA dosing experiments, mice were dosed during the same period with either 50-mg/kg · d BPA (Aldrich ≥ 99% purity) or 0.5% ethanol in corn oil. Two liters were individually dosed with control and 2 with BPA, giving 7–14 pituitaries for each group.
Statistical analysis
Statistical significance was determined using a 2-tailed t test in Microsoft Excel for qRT-PCR and cell counting. P < .05 was considered statistically significant. For culture experiments, a one-way ANOVA was performed and groups were compared via Tukey honestly significant difference test.
Results
Lhb and Fshb mRNA levels are higher in neonatal females than males
In this study, we sought to determine the baseline differences between males and females during the critical neonatal period of pituitary development before exploring hormonal regulation of pituitary gene expression. To address this question, pituitaries were collected on the day of birth (PND0) for RNA analysis and histology from CD-1 mice. At PND0, mRNA levels for LHβ (Lhb) and FSHβ (Fshb) were significantly higher in females than in males as assessed by qRT-PCR (Figure 1A). We further characterized the expression of other gonadotrope-specific genes in males and females at this age, such as GnRH receptor (Gnrhr) as well as transcription factors necessary for the expression of Lhb and Fshb: forkhead box L2 (Foxl2), early growth response 1 (Egr1), and steroidogenic factor 1 (Nr5a1). Expression levels of Foxl2, Erg1, and Nr5a1 were not significantly different between the sexes; however, the level of Gnrhr in females was slightly lower than in males. Thus, mRNA levels of these genes did not explain why Lhb and Fshb mRNA was higher in females. Next, to see whether gonadotrope number was different in males and females, as a possible explanation for the difference in Lhb and Fshb mRNA, we performed immunohistochemistry for LHβ in PND0 males (Figure 1B) and females (Figure 1C) and counted the number of cells, which are represented as the percent positive cells (Figure 1D). There was no sexual dimorphism in the number of gonadotrope cells at PND0.
Figure 1.
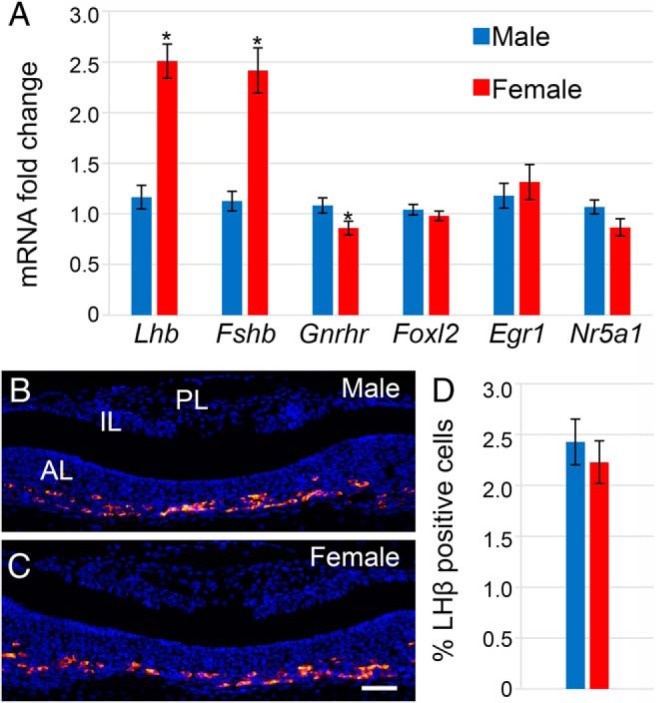
Lhb and Fshb mRNA levels were higher in females than males at PND0, but gonadotrope cell number was the same. Females showed elevated levels of Lhb and Fshb compared with males, but levels of transcription factors Foxl2, Egr1, and Nr5a1 were the same between sexes, and there was a significant but small difference in Gnrhr (n = 10–15) (A). Immunohistochemistry at PND0 with LHβ marked gonadotrope cells (magnification, ×200) in males (B) and females (C). Cell counts showed that there were no differences in gonadotrope cell number (n = 4) (D). Scale bar, 50 μm. *, P = .05.
Transcriptional profiling identifies Icam5 as a differentially expressed gene between males and females in the pituitary
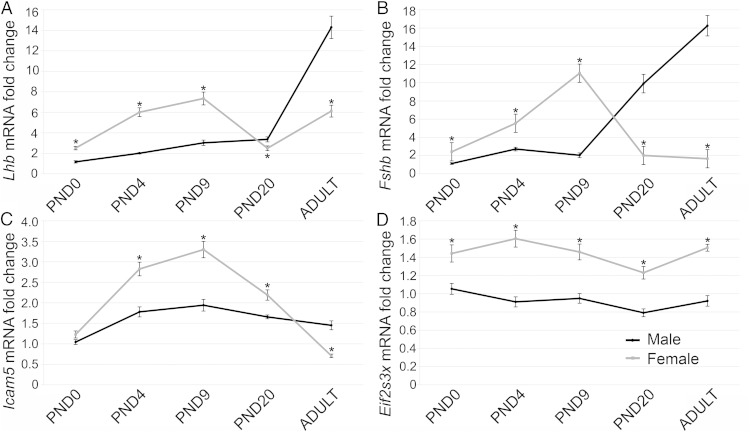
To determine what other differences exist between male and female mouse pituitaries at the mRNA level that may explain variations in levels of Lhb and Fshb, we performed a microarray analysis of male and female PND1 pituitaries. Table 2 shows the differentially expressed genes with a P ≤ .06. Only 8 genes were found to be significantly different by the microarray, most of which are sex-chromosome linked genes. We found only 2 somatic chromosomal genes that exhibited significant differences between the sexes: Lhb and Icam5. A difference in Fshb expression was near significance. Therefore, we chose Lhb, Fshb, Eif2s3x, and Icam5 for further analysis. These genes were compared in males and females, by qRT-PCR at PND0, PND4, PND9, PND20, and adult (6 wk old, females in diestrus). Lhb (Figure 2A) and Fshb (Figure 2B) followed similar expression patterns over the postnatal period with higher expression in females than males from PND0 to PND9, but lower expression in females at PND20 and the adult. The differences between male and female Lhb and Fshb mRNA levels in the adult appear to be due to both increasing levels of Lhb and Fshb in males and decreasing Fshb in females. These mRNA expression patterns of Lhb and Fshb correlate to LH and FSH serum levels over the postnatal period (40). Icam5 expression was not different between males and females at PND0 but was higher in females from PND4 to PND20 and lower in females at the adult time point (Figure 2C). Expression of Icam5 peaks at PND9 in both males and females. The X-linked gene Eif2s3x was always more highly expressed in the female and expression of this gene did not change greatly over time (Figure 2D). Taken together, these data demonstrate that Icam5 mRNA is dynamically regulated over time in a sex-specific manner.
Table 2.
Few Sex Differences Discovered in the Pituitary at Postnatal Day 1 by Microarray
| Gene | Fold Change | P Value |
|---|---|---|
| Higher in males | ||
| Ddx3y | 11.6176 | 4.3006E-11 |
| Eif2s3y | 10.2549 | 6.8983E-05 |
| Uty | 1.6276 | 0.0142 |
| Higher in females | ||
| Xist | 5.1038 | 1.6954E-07 |
| Xist | 2.9461 | 5.5872E-06 |
| Lhb | 3.7814 | 1.5293E-04 |
| Eif2s3x | 1.5297 | .0218 |
| Icam5 | 1.4555 | .0290 |
| Fshb | 2.5616 | .0561 |
Few sex differences discovered in the pituitary at postnatal day 1 by microarray. Microarray analysis of PND1 control pituitaries showed few genes to be differentially expressed between males and females, of which only 3 are not sex chromosome linked: Lhb, Fshb, and Icam5.
Figure 2.
Lhb, Fshb, and Icam5 demonstrated fluctuations in expression over the postnatal period. Lhb, Fshb, Icam5, and Eif2s3x were examined at postnatal day (PND) 0 (n = 10–15), PND4 (n = 7–12), PND9 (n = 10–11), PND20 (n = 6–7), and 6-week-old adults, with females taken in diestrus (n = 7). Lhb (A) and Fshb (B) were more highly expressed in females than males from PND0 to PND9 and less expressed in females by PND20 and in adulthood. Icam5 was not different between males and females at PND0, was higher in females from PND4 to PND20, and was lower in females in the adult (C). Eif2s3x was higher in females than males at all time points examined (D). *, P = .05.
Icam5 is expressed in the anterior pituitary of neonatal mice, mainly in gonadotropes
Icam5 (telencephalin) has been reported as a telecephalon-specific adhesion molecule and its expression in the pituitary has never been documented. Therefore, we performed ISH to elucidate the expression pattern of this gene in the anterior pituitary at PND9–PND11 when the expression levels of Icam5 were highest and most different between males and females (Figure 2C). We observed that Icam5 localized to isolated cells in the anterior lobe in males (Figure 3A) and more strongly in females (Figure 3B), which correlates well with the qRT-PCR data (Figure 2C). Similarly, ICAM5 protein was expressed in the anterior lobe of the pituitary and it appeared to be more highly expressed in the female (Figure 3D) than the male (Figure 3C). Next, immunohistochemistry was used to colocalize ICAM5 with pituitary hormones. At PND9, ICAM5 is rarely expressed in TSHβ-expressing thyrotopes (Figure 3E) and ACTH-expressing corticotropes (Figure 3F). Although occasional GH-expressing somatotropes were double labeled (Figure 3G), many, but not all, LHβ-expressing gonadotrope cells (Figure 3H) contain ICAM5. In the adult, most ICAM5 positive cells were gonadotropes (Figure 3J). Prolactin colocalization was also performed in the adult due to low detection levels neonatally and was rarely colocalized with ICAM5 (Figure 3I). Therefore, the main cell type that ICAM5 is expressed in is the gonadotrope population. Importantly, Icam5 mRNA was also expressed in the human brain and pituitary, as assessed by RT-PCR analysis (data not shown), indicating that expression and regulation of this gene could be important in human physiology.
Figure 3.
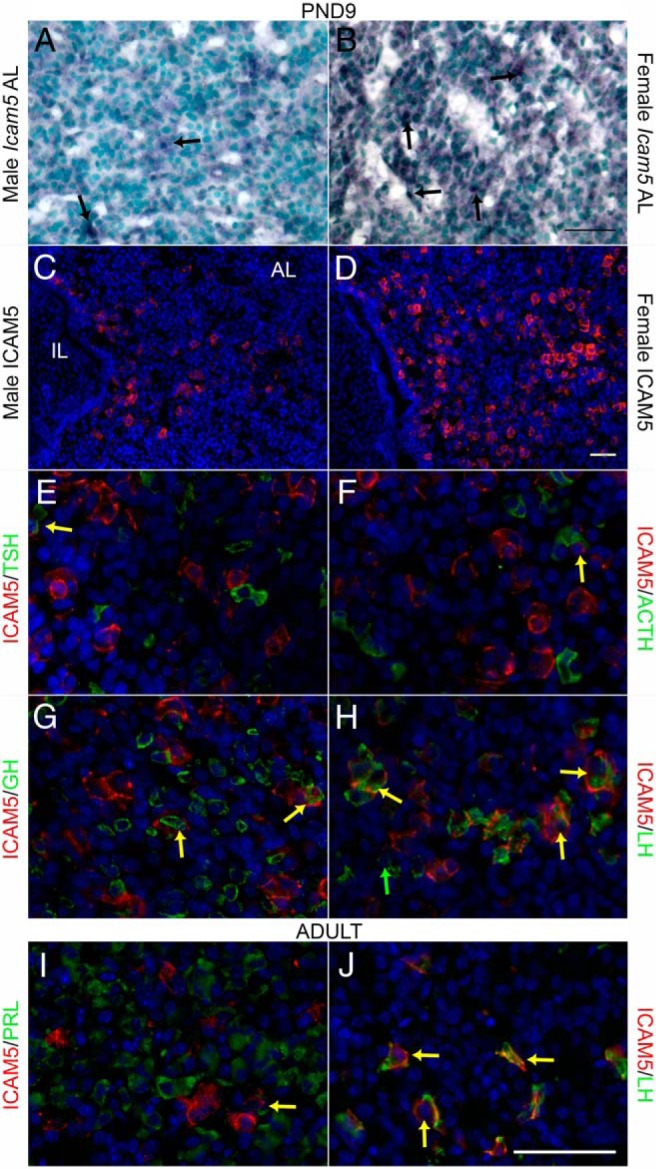
Icam5 was expressed more strongly in the anterior pituitary of females than males at postnatal day (PND) 9 and mainly expressed in gonadotrope cells. ISH shows expression of Icam5 mRNA in isolated cells in the anterior lobe (AL) of males (A) and stronger expression in females (B) at PND9 (n = 3). Arrows denote positive cells. Immunohistochemistry for ICAM5 shows similar expression in the anterior lobe of males (C) and stronger expression in females (D) at PND9 (n = 3). Double IHC shows ICAM5 is rarely expressed in thyrotropes (E) and corticotropes (F), occasionally expressed in somatotropes (G) and mainly expressed in gonadotrope cells in PND9 females (H). In the adult, ICAM5 is rarely expressed in lactotrope cells (I) and continues to be mainly expressed in gonadotrope cells (J) of randomly cycling adult females. Double labeled cells marked with yellow arrow. Scale bar, 50 μm.
Regulation of Icam5 may occur directly at the level of the pituitary
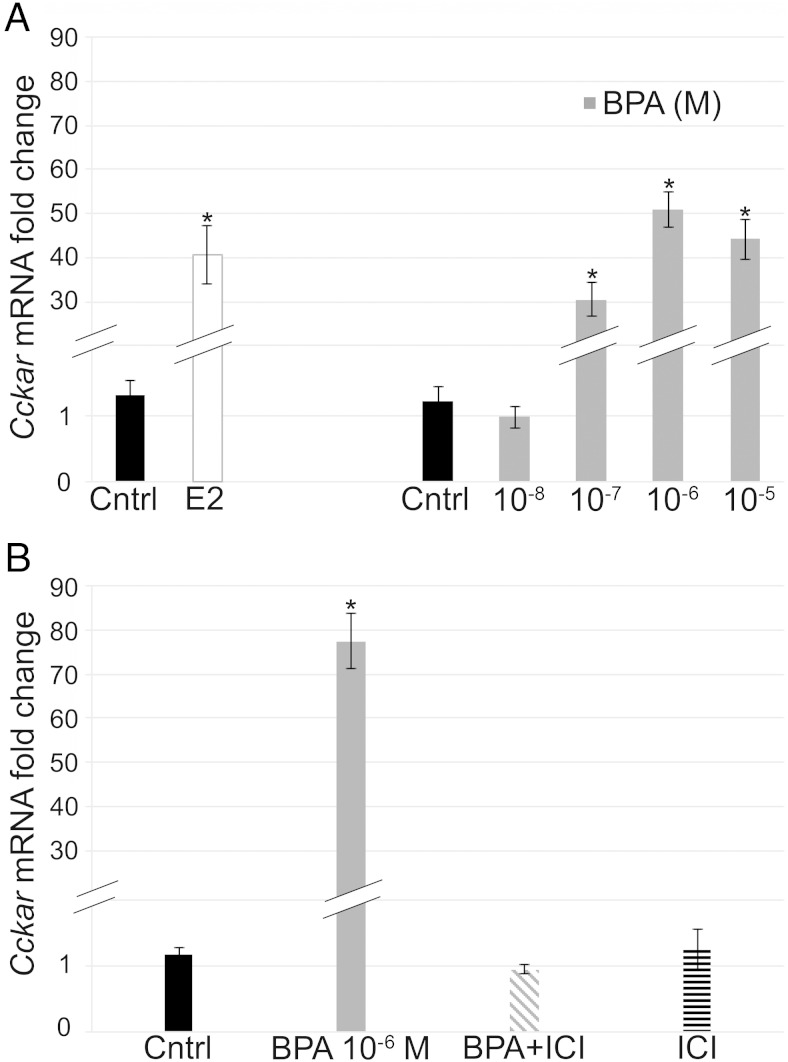
To see whether exposure to exogenous estrogenic compounds could alter expression of sexually dichotomous genes, we isolated pituitaries at PND1, which is a time point at which expression of these genes is sexually dichotomous in vivo, and treated them with E2 and BPA. First, to determine whether the pituitary would be responsive to E2 in culture, we looked at the ability of E2 to regulate expression of cholecystokinin A receptor (Cckar), a known estrogen-responsive gene (41). E2 increased Cckar mRNA levels (Figure 4A), indicating that the cultured pituitary is able to respond to estrogenic stimuli in vitro. To see whether the xenoestrogen, BPA, would similarly increase Cckar mRNA and to determine the dose of BPA inducing the strongest estrogenic effect, we performed a dose response for BPA and observed that 4.4 × 10−6M BPA induced Cckar mRNA to the greatest extent (Figure 4A). Therefore, we chose this dose for all further BPA cultures. To determine whether BPA is acting through ERs, ERα, or ERβ, pituitaries were cotreated with the ER antagonist ICI 182780, which blocks signaling through ERα and ERβ (42). However, this compound has also been shown to activate the GPER (43). Expression of Cckar mRNA was examined in pituitary explants treated with control, BPA, BPA+ICI, and ICI alone (Figure 4B). BPA increased Cckar mRNA levels, ICI was able to block this increase, and ICI alone had no effect on gene expression. These data show that the in vitro whole organ pituitary explants are a good system to study the regulation of estrogen-responsive gene expression.
Figure 4.
E2 and BPA increased expression of Cckar, in vitro. Whole organ pituitary cultures were treated with 10−8M E2 and doses of BPA from 4.4 × 10−8M to 4.4 × 10−5M. Cckar expression was significantly increased with E2, BPA 10−7M, BPA 10−6M, and BPA 10−5M with the highest induction by BPA at the 10−6M concentration (A). In whole organ pituitary cultures treated with BPA, ICI, or a combination of the 2, BPA (10−6M) increased expression of Cckar, cotreatment of BPA+ICI blocked this increase, and ICI alone had no effect (B) (n = 4–5). *, P = .05.
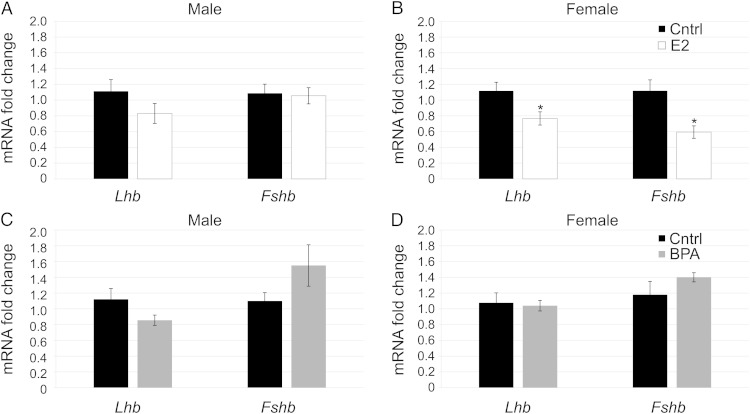
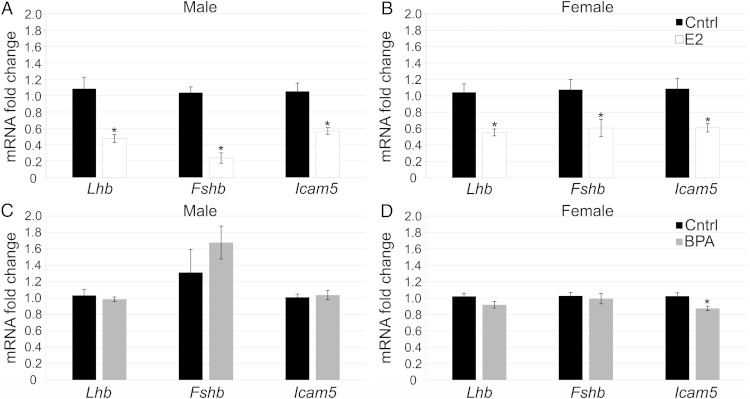
Next, to see whether estrogen could regulate expression of sexually dichotomous genes directly at the pituitary, we treated pituitaries at PND1 with E2, BPA, ICI, or a combination of these compounds. In the male, E2 was unable to alter levels of Lhb and Fshb (Figure 5A). In contrast, in the female, E2 significantly decreased expression of Lhb and Fshb (Figure 5B). This indicates that there is a difference in the way male and female pituitaries respond to E2 in vitro. Interestingly, treatment with BPA was unable to alter expression of Lhb or Fshb in males (Figure 5C) or females (Figure 5D), showing that BPA does not act exactly like E2 in regulation of Lhb or Fshb, despite its ability to alter Cckar expression.
Figure 5.
E2 and BPA had different effects on Lhb and Fshb mRNA levels in vitro. Pituitaries treated in vitro with E2 (10−8M) showed no significant change in Lhb or Fshb levels compared with controls in males (A); however, E2 decreased expression of Lhb and Fshb significantly in females (B) (n = 6–9). Pituitaries treated in vitro with BPA (4.4 × 10−6M) showed no significant change in Lhb or Fshb in males (C) or females (D) (n = 6–9). *, P = .05.
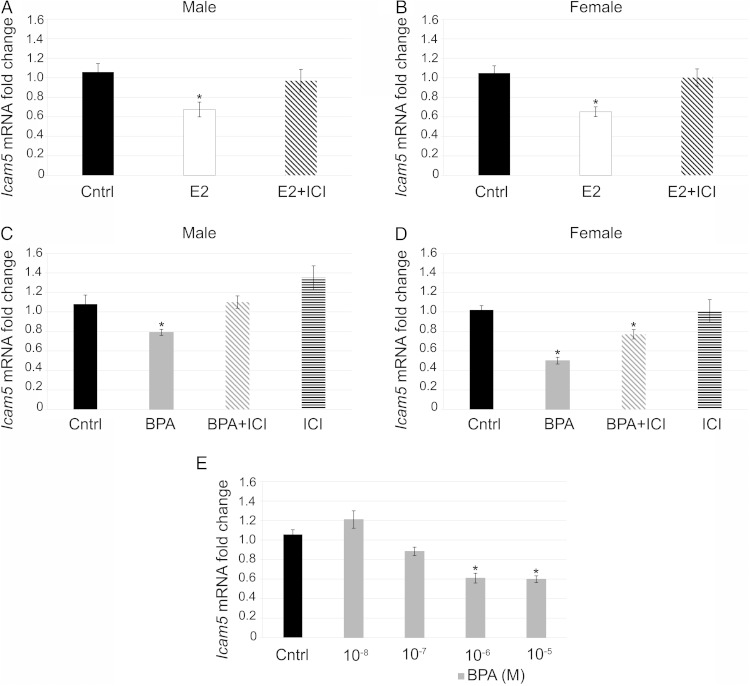
Lastly, we examined the direct effect of E2 and BPA on the expression of Icam5 in the pituitary. E2 decreased expression of Icam5 and cotreatment with ICI blocked this effect in males (Figure 6A) and in females (Figure 6B). BPA treatment also decreased expression of Icam5 in both males (Figure 6C) and females (Figure 6D) similarly to E2 and cotreatment with ICI blocked the decrease in males (Figure 6C) but only partially blocked the decrease in females (Figure 6D). ICI alone had no effect in males or females (Figure 6, C and D). Finally, to show that suppression of Icam5 by BPA occurs in a dose-dependent manner, we examined Icam5 mRNA levels in the samples used for the Cckar dose-response curve (Figure 4A). BPA decreased expression of Icam5 at the 4.4 × 10−6M and 4.4 × 10−5M concentrations, but did not significantly reduce expression at the 4.4 × 10−7M or 4.4 × 10−8M concentrations, demonstrating a dose-dependent effect of BPA on Icam5 expression (Figure 6E). Taken together, these data demonstrate that E2 and BPA can both decrease expression of Icam5 mRNA directly at the pituitary in males and females; however, some of this suppression may come from a pathway other than an ER-mediated pathway. This is in contrast to Lhb and Fshb that were only significantly regulated by E2 at the level of the pituitary in females and were unaffected by BPA. Thus, some gene expression can be regulated directly at the level of the pituitary in a sexually dichotomous manner and BPA does not have identical effects to that of E2.
Figure 6.
E2 and BPA decreased expression of Icam5 in vitro. Pituitaries treated in vitro with E2 (10−8M) showed decreased expression of Icam5, this decrease was inhibited by cotreatment of E2+ICI in males (A) and females (B) (n = 6–9). Pituitaries treated with BPA (4.4 × 10−6M) demonstrated decreased expression of Icam5, which was blocked fully by cotreatment of BPA+ICI in males (C) but only partially blocked the decrease in females (BPA+ICI significantly lower than control in females) (D). ICI alone had no effect in males (C) or females (D) (n = 6–9). A dose-response curve showed that BPA decreased expression of Icam5 at the 10−6M and 10−5M concentrations but had no effect at lower concentrations in vitro (n = 4–5) (E). *, P = .05.
Neonatal exposure to E2 or BPA can influence the levels of sexually dichotomous genes in vivo
Because E2 and BPA were able to regulate expression of sexually dichotomous genes in vitro, we next determined whether these effects were translatable to an in vivo scenario. Neonatal mice were orally dosed for 1 week (PND0–PND7) with 50 μg/kg · d of E2, which is a dose sufficient to regulate expression of estrogen-responsive genes: progesterone receptor (Pgr) and Cckar (data not shown) or 50-mg/kg · d BPA, which is the lowest observed adverse effect level for BPA and was similar to the effective dose used in vitro. E2 was sufficient to suppress levels of Lhb and Fshb mRNA, which are genes known to be regulated by E2 in the adult (44) in both males (Figure 7A) and females (Figure 7B). Interestingly, we found that E2 treatment also reduced levels of Icam5 in both males and females (Figure 7, A and B), demonstrating that E2 may play a role in regulation of Icam5 transcription in vivo. Exposure to BPA during the neonatal period had a sex-specific effect. BPA significantly decreased the levels of Icam5 in females (Figure 7D); however, males were unaffected by exposure (Figure 7C). Lhb and Fshb were not significantly affected by BPA during this time period in males (Figure 7C) or females (Figure 7D), which is similar to in vitro data (Figure 5, C and D). Thus, Icam5 can be regulated in part by estrogenic exposure during the critical neonatal period and BPA can alter its expression in a sex-specific manner.
Figure 7.
E2 decreased expression of Lhb, Fshb, and Icam5 in vivo and BPA decreased expression of Icam5 in females only. Mice dosed from PND0–PND7 with 50-μg/kg · d E2 showed lower expression of Lhb, Fshb, and Icam5 in males (A) and females (B) at PND7 compared with control (n = 6–8). Mice dosed with 50-mg/kg · d BPA showed no change in Lhb, Fshb, or Icam5 in males (C) but showed a slight repression of Icam5 in females (D). *, P = .05.
Discussion
BPA is thought to cause adverse reproductive health effects due to HPG axis disruption and mediates numerous sex-specific outcomes. However, little is known about any potential sexual dichotomy at the level of the pituitary gland. Therefore, we documented sex differences in gene expression during the critical neonatal period of pituitary development and determined whether E2 and BPA could alter expression of genes expressed differently between the sexes. In this study, we found a few genes to be differentially expressed between male and female pituitaries at PND1. These genes were Lhb, Fshb, and a novel gene to the pituitary, Icam5. E2 attenuated Lhb and Fshb gene expression in females both in vitro and in vivo but only suppressed these genes in vivo in males. In contrast, Icam5 was suppressed in males and females both in vitro and in vivo by E2. BPA was unable to suppress Lhb and Fshb mRNA but did suppress Icam5 similarly to E2 in both sexes in vitro. In vivo, BPA only suppressed Icam5 in females. Thus, the neonatal pituitary is sensitive to hormonal and chemical stimuli that can potentially disrupt expression of sexually dichotomous genes within the pituitary, potentially contributing to negative effects on reproduction seen with BPA treatments (1, 18).
Gonadotrope subunit mRNAs represent the main sex difference in the neonatal pituitary. The higher levels of Lhb and Fshb mRNA observed in females correspond with previous studies showing higher LH and FSH serum levels in females (30). However, the differences in Lhb and Fshb mRNA does not seem to be due to mRNA levels of other transcription factors regulating these genes, indicating the difference likely exists at the level of the hypothalamus or in protein levels not examined in our study. Additionally, at PND1 gonadotrope cell number between males and females was similar, which is in agreement with previous studies (45, 46). However, another study did indicate that there are more gonadotrope cells in females at PND5 (47). This discrepancy may be due to the age of the mice or the method of staining (colormetric vs fluorescence). If it is the age, it is possible that sex differences in cell number do not develop till a later time point. It is somewhat surprising that we uncovered few other sex differences in the neonatal pituitary via microarray analysis. In the adult pituitary more differences were found via serial analysis gene expression (29). This discrepancy may be due to age of the pituitary or technique used to probe gene expression. Furthermore, the pituitary consists of a very heterogeneous population of cells. It may be difficult to detect changes that occur only in 1 small population of cells within the pituitary.
Gonadotropin subunit mRNAs can be regulated by E2 throughout the lifespan. Our data show that, at the level of the pituitary, E2 decreased expression of Lhb and Fshb in females although not in males. This sex-specific effect in vitro may suggest that the neonatal male pituitary is programmed in such a way to make it unable to respond to E2 directly at the level of the pituitary. Males are exposed to greater hormonal stimuli during the perinatal testosterone surge. This surge has been shown to cause epigenetic changes in the brain possibly leading to permanent modifications, such as sexually dichotomous expression of ERα mRNA (48) and sex differences in the DNA methylation pattern of the ERα promoter (49, 50). Our data suggest that the pituitary also experiences gender-specific changes that modify the direct response of the pituitary to E2. These modifications are not necessarily permanent and can be reprogrammed across the lifespan (51, 52); therefore, it is possible that a male pituitary could directly respond to E2 at a different age.
Surprisingly, exposure to BPA did not affect transcription of Lhb or Fshb in neonatal males or females. Embryonic BPA exposure has been shown to alter expression of Lhb and Fshb in vivo (20, 28); therefore, it could be that the embryonic period is more sensitive to exposure to BPA. However, neonatal BPA exposure has been shown to decrease GnRH induced LH serum levels, indicating that BPA may be affecting LH release during this time period in concert with GnRH stimulation (22). Prolactin, another important pituitary derived hormone, exhibits increases in expression and release by exposure to both E2 and BPA in the adult (53). In a previous study, embryonic exposure to BPA did not increase expression of prolactin at birth, which is the time point at which we are isolating pituitaries for culture analysis (28). Although we have not analyzed prolactin expression because it was not detected as sexually dichotomous at this time point on our array, we cannot rule out that it would not be regulated by E2 or BPA neonatally. Taken together, there are age dependent effects of environmental exposures on several pituitary hormones.
One of the most significant findings of our study is the sex-specific expression pattern of Icam5 mRNA and protein in the pituitary. ICAM5 is characterized as a telencephalon-specific cell adhesion molecule (37). It is expressed in the soma-dendritic membrane of spiny neurons in the mammalian telencephalon, but not the axonal membrane and is absent from other brain regions and the spinal cord (37, 54–56). Our data indicate that ICAM5 is also expressed in the anterior pituitary gland, and its expression appears greater in females than males (Figure 3). The cell type where we see the greatest expression of ICAM5 is the gonadotrope cells, possibly indicating that this adhesion molecule could be contributing to differences in LH and FSH serum levels between males and females (30). Icam5 mRNA is not readily expressed in the embryo, but begins to be expressed at birth and reaches maximal levels at PND10/PND9 in the brain (37) and the pituitary (Figure 2C). This timing corresponds with the timing of dendritic development and synapse formation in the brain (57, 58) and active pituitary gland expansion (6, 59). The dynamic expression of Icam5 mRNA over time, especially in females, may indicate hormonal regulation similar to Lhb and Fshb. The ability of E2 to decrease Icam5 expression was confirmed in both males and females, and the regulation appears to occur at the level of the pituitary. The mechanism of action likely takes place through an ERα- or ERβ-mediated pathway as cotreatment with ICI inhibited this decrease in both males and females (42). This shows that Icam5 expression can be regulated by classical estrogen signaling pathways that are very important for proper transcription and hormone release within the pituitary.
The xenoestrogen BPA is also capable of decreasing Icam5 expression in both males and females at the level of the pituitary. However, ICI did not fully block this effect in females, suggesting that Icam5 can potentially be regulated by BPA via mechanisms other than ERα or ERβ. One potential pathway would be through the G protein-coupled receptor, GPER (12). Additionally, BPA has also been shown to act via androgen receptor, thyroid hormone receptor, estrogen-related receptor-γ, and aryl hydrocarbon receptor (60–63). However, we do know that BPA can act at ERα in the pituitary because BPA induced expression of Cckar (Figure 4), which is an ERα regulated gene (41) and this increase was fully blocked by ICI. Therefore, BPA likely has multiple pathways of action within the pituitary, as well as actions in the HPG axis that will subsequently influence the pituitary. BPA in vivo was only able to decrease Icam5 in females and not males. This is similar to the sex-specific effects seen with embryonic BPA treatment (28). Because BPA was able to decrease Icam5 in vitro in males but not in vivo, it is possible that signals from the hypothalamus may either protect the male pituitary or counteract the actions of BPA. It is also possible that because levels of Icam5 are lower in males in vivo than females that BPA is unable to reduce Icam5 further. Therefore, it is possible that BPA can regulate LH and FSH levels by changing Icam5 expression and possibly interfering with either response to GnRH or release of LH and FSH.
Although the function of ICAM5 in the pituitary is unknown, its roles in the brain as a cell adhesion molecule have been extensively studied. ICAM5 is abundantly expressed in the dendritic filopodia and plays an important role in synapse formation with presynaptic axons, via the extracellular matrix molecule, vitronectin (64), and/or β1 integrins (32, 65). Once these connections are established, the extracellular domain of ICAM5 is cleaved, disrupting the cytoplasmic actin cytoskeleton through to α-actinin or ezrin/radixin/moesin proteins promoting reorganization to induce spine maturation (31–34, 34, 66, 67). Based on the role of ICAM5 in cell adhesion and its ability to alter the actin cytoskeleton, we propose that ICAM5 may play a role in structural organization of functional homotypic cell networks within the pituitary. These cell networks in the pituitary are important because they set up the ability of the pituitary cells to have proper hormone release (7, 68, 69). Cells and cell networks can also adjust in response to external stimuli. For example, GnRH signaling in gonadotrope cells can cause actin reorganization, resulting in membrane projections and cellular migration towards blood vessels (70). Interestingly, pituitary cell networks can also exhibit sex differences. For example, a higher proportion of somatotrope cells respond to GHRH in males than in females (71). It is possible that something similar may be happening with the gonadotrope population due to expression of ICAM5. Also, cell networks can be regulated by hormonal mechanisms because gonadectomy alters the responsiveness of cells to GHRH, including hormone release and cell motility (71, 72). In gonadotropes, GnRH treatment leads to an increase in process extension, which is enhanced by previous E2 exposure (73). Therefore, it is evident that sex differences can exist in pituitary networks and these networks can be regulated by gonadal hormones. However, the mechanism behind these observations has yet to be elucidated. Our data provide one potential adhesion and cytoskeletal modifying molecule that is regulated by E2 and BPA in the pituitary, ICAM5. Neonatal exposure to BPA caused decreased basal LH secretion as well as decreased amount of LH release in response to GnRH (22). Thus, if ICAM5 is important in the function of pituitary networks, which are necessary for proper response to signals from the hypothalamus and subsequent hormone release, disruption of ICAM5 via BPA could be responsible for negative impacts on reproductive health directly at the pituitary. Further investigation into the role of ICAM5 in the pituitary is warranted.
Acknowledgments
We thank Ms Sachiko Mitsui (RIKEN Brain Science Institute) for technical assistance; Mary Majewski and Dr Mark Band of the Functional Genomics Unit of the Carver Biotechnology Center, University of Illinois, for help with the microarray hybridizations; Dr Jenny Drnevich of the High Performance Biological Computing (HPCBio) group of the Carver Biotechnology Center for help with the statistical analysis; and Dr Jodi Flaws (University of Illinois at Urbana-Champaign) for providing the mice for the pituitaries used in the microarray experiment.
This work was supported by National Institutes of Health Grants R01 DK076647 and T32 ES007326.
Disclosure Summary: The authors have nothing to disclose.
For News & Views see page 1324
- BPA
- bisphenol A
- Cckar
- cholecystokinin A receptor
- E2
- 17β-estradiol
- EDC
- endocrine-disrupting chemical
- ER
- estrogen receptor
- Foxl2
- forkhead box L2
- Gnrhr
- GnRH receptor
- GPER
- G protein-coupled ER
- HPG
- hypothalamic pituitary gonadal
- Icam5
- intracellular adhesion molecule-5
- ISH
- in situ hybridization
- Nr5a1
- steroidogenic factor 1
- PND
- postnatal day
- qRT-PCR
- quantitative RT-PCR.
References
- 1. Maffini MV, Rubin BS, Sonnenschein C, Soto AM. Endocrine disruptors and reproductive health: the case of bisphenol-A. Mol Cell Endocrinol. 2006;254–255:179–186. [DOI] [PubMed] [Google Scholar]
- 2. Rochester JR. Bisphenol A and human health: a review of the literature. Reprod Toxicol. 2013;42:132–155. [DOI] [PubMed] [Google Scholar]
- 3. Hanson MA, Gluckman PD. Developmental origins of health and disease: new insights. Basic Clin Pharmacol Toxicol. 2008;102:90–93. [DOI] [PubMed] [Google Scholar]
- 4. Jacobson AG, Miyamoto DM, Ma SH. Rathke's pouch morphogenesis in the chick embryo. J Exp Zool. 1979;207:351–366. [Google Scholar]
- 5. Treier M, Rosenfeld MG. The hypothalamic-pituitary axis: co-development of two organs. Curr Opin Cell Biol. 1996;8:833–843. [DOI] [PubMed] [Google Scholar]
- 6. Carbajo-Pérez E, Watanabe YG. Cellular proliferation in the anterior pituitary of the rat during the postnatal period. Cell Tissue Res. 1990;261:333–338. [DOI] [PubMed] [Google Scholar]
- 7. Bonnefont X, Lacampagne A, Sanchez-Hormigo A, et al. Revealing the large-scale network organization of growth hormone-secreting cells. Proc Natl Acad Sci USA. 2005;102:16880–16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wen S, Ai W, Alim Z, Boehm U. Embryonic gonadotropin-releasing hormone signaling is necessary for maturation of the male reproductive axis. Proc Natl Acad Sci USA. 2010;107:16372–16377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pointis G, Latreille MT, Cedard L. Gonado-pituitary relationships in the fetal mouse at various times during sexual differentiation. J Endocrinol. 1980;86:483–488. [DOI] [PubMed] [Google Scholar]
- 10. Shelby MD. NTP-CERHR monograph on the potential human reproductive and developmental effects of bisphenol A. NTP CERHR MON. 2008;(22):v, vii–ix, 1–64 passim. [PubMed] [Google Scholar]
- 11. Kuiper GG, Lemmen JG, Carlsson B, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology. 1998;139:4252–4263. [DOI] [PubMed] [Google Scholar]
- 12. Thomas P, Dong J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: a potential novel mechanism of endocrine disruption. J Steroid Biochem Mol Biol. 2006;102:175–179. [DOI] [PubMed] [Google Scholar]
- 13. Nishihara E, Nagayama Y, Inoue S, et al. Ontogenetic changes in the expression of estrogen receptor α and β in rat pituitary gland detected by immunohistochemistry. Endocrinology. 2000;141:615–620. [DOI] [PubMed] [Google Scholar]
- 14. Ogasawara K, Nogami H, Tsuda MC, et al. Hormonal regulation of prolactin cell development in the fetal pituitary gland of the mouse. Endocrinology. 2009;150:1061–1068. [DOI] [PubMed] [Google Scholar]
- 15. Hazell GG, Yao ST, Roper JA, Prossnitz ER, O'Carroll AM, Lolait SJ. Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. J Endocrinol. 2009;202:223–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mitra SW, Hoskin E, Yudkovitz J, et al. Immunolocalization of estrogen receptor β in the mouse brain: comparison with estrogen receptor α. Endocrinology. 2003;144:2055–2067. [DOI] [PubMed] [Google Scholar]
- 17. Merchenthaler I, Lane MV, Numan S, Dellovade TL. Distribution of estrogen receptor α and β in the mouse central nervous system: in vivo autoradiographic and immunocytochemical analyses. J Comp Neurol. 2004;473:270–291. [DOI] [PubMed] [Google Scholar]
- 18. Wang W, Hafner KS, Flaws JA. In utero bisphenol A exposure disrupts germ cell nest breakdown and reduces fertility with age in the mouse. Toxicol Appl Pharmacol. 2014;276:157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Losa-Ward SM, Todd KL, McCaffrey KA, Tsutsui K, Patisaul HB. Disrupted organization of RFamide pathways in the hypothalamus is associated with advanced puberty in female rats neonatally exposed to bisphenol A. Biol Reprod. 2012;87:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xi W, Lee CK, Yeung WS, et al. Effect of perinatal and postnatal bisphenol A exposure to the regulatory circuits at the hypothalamus-pituitary-gonadal axis of CD-1 mice. Reprod Toxicol. 2011;31:409–417. [DOI] [PubMed] [Google Scholar]
- 21. Monje L, Varayoud J, Muñoz-de-Toro M, Luque EH, Ramos JG. Exposure of neonatal female rats to bisphenol A disrupts hypothalamic LHRH pre-mRNA processing and estrogen receptor α expression in nuclei controlling estrous cyclicity. Reprod Toxicol. 2010;30:625–634. [DOI] [PubMed] [Google Scholar]
- 22. Fernández M, Bianchi M, Lux-Lantos V, Libertun C. Neonatal exposure to bisphenol a alters reproductive parameters and gonadotropin releasing hormone signaling in female rats. Environ Health Perspect. 2009;117:757–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–536. [DOI] [PubMed] [Google Scholar]
- 24. Patisaul HB, Fortino AE, Polston EK. Neonatal genistein or bisphenol-A exposure alters sexual differentiation of the AVPV. Neurotoxicol Teratol. 2006;28:111–118. [DOI] [PubMed] [Google Scholar]
- 25. Rubin BS, Lenkowski JR, Schaeberle CM, Vandenberg LN, Ronsheim PM, Soto AM. Evidence of altered brain sexual differentiation in mice exposed perinatally to low, environmentally relevant levels of bisphenol A. Endocrinology. 2006;147:3681–3691. [DOI] [PubMed] [Google Scholar]
- 26. Cao J, Mickens JA, McCaffrey KA, Leyrer SM, Patisaul HB. Neonatal Bisphenol A exposure alters sexually dimorphic gene expression in the postnatal rat hypothalamus. Neurotoxicology. 2012;33:23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kundakovic M, Gudsnuk K, Franks B, et al. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc Natl Acad Sci USA. 2013;110:9956–9961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brannick KE, Craig ZR, Himes AD, et al. Prenatal exposure to low doses of bisphenol A increases pituitary proliferation and gonadotroph number in female mice offspring at birth. Biol Reprod. 2012;87:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nishida Y, Yoshioka M, St-Amand J. Sexually dimorphic gene expression in the hypothalamus, pituitary gland, and cortex. Genomics. 2005;85:679–687. [DOI] [PubMed] [Google Scholar]
- 30. Poling MC, Kauffman AS. Sexually dimorphic testosterone secretion in prenatal and neonatal mice is independent of kisspeptin-Kiss1r and GnRH signaling. Endocrinology. 2012;153:782–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tian L, Stefanidakis M, Ning L, et al. Activation of NMDA receptors promotes dendritic spine development through MMP-mediated ICAM-5 cleavage. J Cell Biol. 2007;178:687–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tian L, Nyman H, Kilgannon P, et al. Intercellular adhesion molecule-5 induces dendritic outgrowth by homophilic adhesion. J Cell Biol. 2000;150:243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Matsuno H, Okabe S, Mishina M, Yanagida T, Mori K, Yoshihara Y. Telencephalin slows spine maturation. J Neurosci. 2006;26:1776–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nyman-Huttunen H, Tian L, Ning L, Gahmberg CG. α-Actinin-dependent cytoskeletal anchorage is important for ICAM-5-mediated neuritic outgrowth. J Cell Sci. 2006;119:3057–3066. [DOI] [PubMed] [Google Scholar]
- 35. Nantie LB, Himes AD, Getz DR, Raetzman LT. Notch signaling in postnatal pituitary expansion: proliferation, progenitors, and cell specification. Mol Endocrinol. 2014;28:731–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Goldberg LB, Aujla PK, Raetzman LT. Persistent expression of activated Notch inhibits corticotrope and melanotrope differentiation and results in dysfunction of the HPA axis. Dev Biol. 2011;358:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yoshihara Y, Oka S, Nemoto Y, et al. An ICAM-related neuronal glycoprotein, telencephalin, with brain segment-specific expression. Neuron. 1994;12:541–553. [DOI] [PubMed] [Google Scholar]
- 38. Peretz J, Craig ZR, Flaws JA. Bisphenol A inhibits follicle growth and induces atresia in cultured mouse antral follicles independently of the genomic estrogenic pathway. Biol Reprod. 2012;87:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Peretz J, Gupta RK, Singh J, Hernández-Ochoa I, Flaws JA. Bisphenol A impairs follicle growth, inhibits steroidogenesis, and downregulates rate-limiting enzymes in the estradiol biosynthesis pathway. Toxicol Sci. 2011;119:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Döhler KD, Wuttke W. Changes with age in levels of serum gonadotropins, prolactin and gonadal steroids in prepubertal male and female rats. Endocrinology. 1975;97:898–907. [DOI] [PubMed] [Google Scholar]
- 41. Kim HJ, Gieske MC, Hudgins S, et al. Estrogen receptor α-induced cholecystokinin type A receptor expression in the female mouse pituitary. J Endocrinol. 2007;195:393–405. [DOI] [PubMed] [Google Scholar]
- 42. Wakeling AE. Similarities and distinctions in the mode of action of different classes of antioestrogens. Endocr Relat Cancer. 2000;7:17–28. [DOI] [PubMed] [Google Scholar]
- 43. Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. [DOI] [PubMed] [Google Scholar]
- 44. Shupnik MA, Gharib SD, Chin WW. Estrogen suppresses rat gonadotropin gene transcription in vivo. Endocrinology. 1988;122:1842–1846. [DOI] [PubMed] [Google Scholar]
- 45. Dihl F, Begeot M, Loevenhruck C, Dubois MP, Dubois PM. Ontogeny of gonadotropic and thyrotropic cells in fetal mouse anterior pituitary. Comparison between two species C57 BL6 and Balb/C. Anat Embryol (Berl). 1988;178:21–27. [DOI] [PubMed] [Google Scholar]
- 46. Chen HT. Sexual dimorphism of pituitary gonadotropes during postnatal development in the rat. Mol Cell Endocrinol. 1988;57:33–39. [DOI] [PubMed] [Google Scholar]
- 47. Ishikawa M, Murai E, Hashiguchi Y, Iguchi T, Sato T. Effects of diethylstilbestrol on luteinizing hormone-producing cells in the mouse anterior pituitary. Exp Biol Med (Maywood). 2014;239:311–319. [DOI] [PubMed] [Google Scholar]
- 48. DonCarlos LL, McAbee M, Ramer-Quinn DS, Stancik DM. Estrogen receptor mRNA levels in the preoptic area of neonatal rats are responsive to hormone manipulation. Brain Res Dev Brain Res. 1995;84:253–260. [DOI] [PubMed] [Google Scholar]
- 49. Kurian JR, Olesen KM, Auger AP. Sex differences in epigenetic regulation of the estrogen receptor-α promoter within the developing preoptic area. Endocrinology. 2010;151:2297–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tsai HW, Grant PA, Rissman EF. Sex differences in histone modifications in the neonatal mouse brain. Epigenetics. 2009;4:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schwarz JM, Nugent BM, McCarthy MM. Developmental and hormone-induced epigenetic changes to estrogen and progesterone receptor genes in brain are dynamic across the life span. Endocrinology. 2010;151:4871–4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nugent BM, Wright CL, Shetty AC, et al. Brain feminization requires active repression of masculinization via DNA methylation. Nat Neurosci. 2015;18:690–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Steinmetz R, Brown NG, Allen DL, Bigsby RM, Ben-Jonathan N. The environmental estrogen bisphenol A stimulates prolactin release in vitro and in vivo. Endocrinology. 1997;138:1780–1786. [DOI] [PubMed] [Google Scholar]
- 54. Benson DL, Yoshihara Y, Mori K. Polarized distribution and cell type-specific localization of telencephalin, an intercellular adhesion molecule. J Neurosci Res. 1998;52:43–53. [DOI] [PubMed] [Google Scholar]
- 55. Mitsui S, Saito M, Hayashi K, Mori K, Yoshihara Y. A novel phenylalanine-based targeting signal directs telencephalin to neuronal dendrites. J Neurosci. 2005;25:1122–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kelly EA, Tremblay ME, Gahmberg CG, Tian L, Majewska AK. Subcellular localization of intercellular adhesion molecule-5 (telencephalin) in the visual cortex is not developmentally regulated in the absence of matrix metalloproteinase-9. J Comp Neurol. 2014;522:676–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mori K, Fujita SC, Watanabe Y, Obata K, Hayaishi O. Telencephalon-specific antigen identified by monoclonal antibody. Proc Natl Acad Sci USA. 1987;84:3921–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Imamura K, Mori K, Oka S, Watanabe Y. Variations by layers and developmental changes in expression of telencephalin in the visual cortex of cat. Neurosci Lett. 1990;119:118–121. [DOI] [PubMed] [Google Scholar]
- 59. Taniguchi Y, Yasutaka S, Kominami R, Shinohara H. Proliferation and differentiation of rat anterior pituitary cells. Anat Embryol (Berl). 2002;206:1–11. [DOI] [PubMed] [Google Scholar]
- 60. Okada H, Tokunaga T, Liu X, Takayanagi S, Matsushima A, Shimohigashi Y. Direct evidence revealing structural elements essential for the high binding ability of bisphenol A to human estrogen-related receptor-γ. Environ Health Perspect. 2008;116:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sohoni P, Sumpter JP. Several environmental oestrogens are also anti-androgens. J Endocrinol. 1998;158:327–339. [DOI] [PubMed] [Google Scholar]
- 62. Krüger T, Long M, Bonefeld-Jørgensen EC. Plastic components affect the activation of the aryl hydrocarbon and the androgen receptor. Toxicology. 2008;246:112–123. [DOI] [PubMed] [Google Scholar]
- 63. Moriyama K, Tagami T, Akamizu T, et al. Thyroid hormone action is disrupted by bisphenol A as an antagonist. J Clin Endocrinol Metab. 2002;87:5185–5190. [DOI] [PubMed] [Google Scholar]
- 64. Furutani Y, Kawasaki M, Matsuno H, Mitsui S, Mori K, Yoshihara Y. Vitronectin induces phosphorylation of ezrin/radixin/moesin actin-binding proteins through binding to its novel neuronal receptor telencephalin. J Biol Chem. 2012;287:39041–39049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ning L, Tian L, Smirnov S, et al. Interactions between ICAM-5 and β1 integrins regulate neuronal synapse formation. J Cell Sci. 2013;126:77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tsukita S, Yonemura S. Cortical actin organization: lessons from ERM (ezrin/radixin/moesin) proteins. J Biol Chem. 1999;274:34507–34510. [DOI] [PubMed] [Google Scholar]
- 67. Furutani Y, Matsuno H, Kawasaki M, Sasaki T, Mori K, Yoshihara Y. Interaction between telencephalin and ERM family proteins mediates dendritic filopodia formation. J Neurosci. 2007;27:8866–8876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Le Tissier PR, Hodson DJ, Lafont C, Fontanaud P, Schaeffer M, Mollard P. Anterior pituitary cell networks. Front Neuroendocrinol. 2012;33:252–266. [DOI] [PubMed] [Google Scholar]
- 69. Budry L, Lafont C, El Yandouzi T, Chauvet N, Conéjero G, Drouin J, Mollard P. Related pituitary cell lineages develop into interdigitated 3D cell networks. Proc Natl Acad Sci USA. 2011;108:12515–12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Navratil AM, Knoll JG, Whitesell JD, Tobet SA, Clay CM. Neuroendocrine plasticity in the anterior pituitary: gonadotropin-releasing hormone-mediated movement in vitro and in vivo. Endocrinology. 2007;148:1736–1744. [DOI] [PubMed] [Google Scholar]
- 71. Sanchez-Cardenas C, Fontanaud P, He Z, et al. Pituitary growth hormone network responses are sexually dimorphic and regulated by gonadal steroids in adulthood. Proc Natl Acad Sci USA. 2010;107:21878–21883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Schaeffer M, Hodson DJ, Meunier AC, et al. Influence of estrogens on GH-cell network dynamics in females: a live in situ imaging approach. Endocrinology. 2011;152:4789–4799. [DOI] [PubMed] [Google Scholar]
- 73. Alim Z, Hartshorn C, Mai O, et al. Gonadotrope plasticity at cellular and population levels. Endocrinology. 2012;153:4729–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]