Abstract
Germ cell transport across the seminiferous epithelium during spermatogenesis requires the intricate coordination of cell junctions, signaling proteins, and both actin- and microtubule (MT)-based cytoskeletons. Although the involvement of cytoskeletons in germ cell transport has been suggested, the precise mechanism(s) remains elusive. Based on growing evidence that actin and MT interactions underlie fundamental cellular processes, such as cell motility, it is unlikely that actin- and MT-based cytoskeletons work independently to regulate germ cell transport in the testis. Using rats treated with adjudin, a potential male contraceptive that disrupts spermatid adhesion and transport in the testis, as a study model, we show herein that actin- and MT-based cytoskeletons are both necessary for transport of spermatids and residual bodies/phagosomes across the seminiferous epithelium in adult rat testes. Analysis of intratubular expression of F-actin and tubulin revealed disruption of both actin and MT networks, concomitant with misdirected spermatids and phagosomes in rats treated with adjudin. Actin regulatory proteins, epidermal growth factor receptor pathway substrate 8 and actin-related protein 3, were mislocalized and down-regulated at the actin-rich anchoring junction between germ and Sertoli cells (apical ectoplasmic specialization) after adjudin treatment. Nonreceptor tyrosine kinase p-FAK-Tyr407, known to regulate F-actin nucleation via actin-related protein 3, was also mislocalized and down-regulated at the apical ectoplasmic specialization, corroborating the observation of actin cytoskeleton disruption. Additionally, spatiotemporal expression of MT regulatory protein end-binding protein 1, shown to be involved in MT-actin cross talk herein, was also disrupted after adjudin treatment. In summary, spermatid/phagosome transport across the epithelium during spermatogenesis requires the coordination between actin- and MT-based cytoskeletons.
Studies have conclusively demonstrated that disruption of the anchoring device at the Sertoli cell-spermatid interface, known as the apical ectoplasmic specialization (ES), induces premature release of spermatids from the seminiferous epithelium. Treatment of rodents with toxicants (eg, carbendazim, colchicine) (1, 2), T implants (which leads to a considerable reduction in intratesticular androgen as well as suppression of LH release from the pituitary gland) (3, 4), or small interfering RNA duplexes to knock down actin bundling proteins (such as ezrin [5], palladin [6] and plastin [7] in the rat testis) can cause premature spermatid release in non-stage VIII tubules, mimicking spermiation, which only occurs at stage VIII of the epithelial cycle in the rat. Furthermore, in these studies elongated spermatids were also found trapped inside the epithelium in some tubules well beyond stage VIII. The reason for this entrapment of elongated spermatids is not known because it is expected that if the apical ES at the Sertoli-spermatid (steps 8–19 spermatids) interface is disrupted, then all spermatids should be depleted from the epithelium.
In this context, it is noted that the apical ES is a testis-specific, actin-rich anchoring junction typified by the presence of tightly packed actin microfilament bundles that are sandwiched in between cisternae of endoplasmic reticulum and the Sertoli cell plasma membrane at the cortical zone. This intercellular junction is first detected in step 8 spermatids during spermiogenesis and persists until step 19, which is the final stage of spermatid maturation. Spermatids are transformed into mature spermatazoa prior to their release at spermiation, which takes place at stage VIII of the epithelial cycle (for reviews, see references 8–11). The location of the apical ES varies at the adluminal compartment within the seminiferous epithelium because it depends on the progression of spermatid transformation. Nonetheless, it was speculated that exposure of rodents to toxicants led to a disruption of the actin- and/or microtubule (MT)-based cytoskeleton, which in turn impeded spermatid transport, possibly involving nonreceptor protein kinases (for reviews, see references 12–19).
In vitro studies using human and/or rodent Sertoli cells have shed light on these earlier observations regarding the entrapment of spermatids inside the epithelium when other elongating/elongated spermatids near the adluminal edge were depleted from the epithelium. For instance, treatment of human and/or rat Sertoli cells with toxicants (eg, cadmium, bisphenol A, perfluorooctanesulfonate) was found to cause truncation and the misorganization of F-actin in those cells. These toxicants disrupted the spatiotemporal expression of actin binding proteins, in particular, actin barbed-end capping and bundling protein, epidermal growth factor receptor pathway substrate 8 (Eps8), actin cross-linking and bundling protein, palladin, and branched actin polymerization protein, actin-related protein 3 (Arp3), at the Sertoli cell cortical zone (20–22). However, it remains unclear how a loss of plasticity of actin microfilaments at the Sertoli cell cortical zone due to a failure in actin filament conversion between their bundled and unbundled/branched configuration caused by disrupted spatiotemporal expression of Eps8 and the Arp2/3 complex would cause entrapment of spermatids deep inside the epithelium.
Studies have shown that the MT-based cytoskeleton that provides the track for the transport of spermatids and cell organelles (eg, residual bodies, phagosomes) in the epithelium during spermatogenesis is likely working in concert with the actin-based cytoskeleton (for reviews, see references 13, 23, and 24). We thus hypothesize that toxicants may disrupt the track function conferred by either the polarized MTs or actin microfilaments or both, causing the failure of spermatid and residual body/phagosome transport across the epithelium. Residual bodies are the cytoplasmic remnants released from spermatids during spermiation at early stage VIII of the epithelial cycle in the adluminal compartment near the tubule lumen, but they are soon engulfed by the Sertoli cell, transforming into phagosomes (phagocytic vesicles) while being transported down to the base of the seminiferous epithelium at late stage VIII to be processed and degraded via the lysosomal degradation pathway (25–27). Another possibility is that the apical ES function in spermatids that are embedded inside the epithelium, unlike spermatids, which reside near the tubule, is not compromised and/or disrupted so that these spermatids remain tightly anchored onto the Sertoli cell in the epithelium. Similar to toxicant treatments in rodents, the potential male contraceptive drug, adjudin, led to failure in spermatid adhesion and transport attributed to defects in apical ES function (for a review, see reference 28). Furthermore, tubules that displayed extensive exfoliation of spermatids after adjudin treatment were also found to have spermatids entrapped deep inside the epithelium (for a review, see reference 28). We thus performed a detailed analysis using adjudin-treated rats as a model to understand the intriguing interactions between MT- and actin-based cytoskeletons that led to premature exfoliation of germ cells while causing entrapment of spermatids inside the epithelium. These unexpected findings illustrate a novel mechanism that regulates the transport of spermatids and residual bodies/phagosomes during spermatogenesis and identify some of the crucial players involved.
Materials and Methods
Animals and antibodies
The use of animals for experiments in this study was approved by the Rockefeller University Institutional Animal Care and Use Committee (protocol numbers 12506 and 15780-H). Adult male Sprague Dawley rats (250–300 g body weight [b.w.]) were purchased from Charles River Laboratories. Antibodies used in this study are listed in Table 1.
Table 1.
Antibodies Used for Various Experiments in This Report
| Antibody | Source and Nature of Antigen | Vendor and Catalog Number | Host Species and Antibody Nature | Working Dilution and Application |
|---|---|---|---|---|
| Arp3 | Recombinant human Arp3 protein | Sigma-Aldrich, A5979 | Mouse monoclonal | 1:200 IF |
| EB1 | Amino acids 239-268 from the N-terminus, which is located near the C-terminus of EB1 of human origin | Santa Cruz Biotechnology, sc-374474 | Mouse monoclonal | 1:200 IHC |
| EB1 | Amino acids 133-202 of peptide fragment from the N-terminus, which is located near the C-terminus of EB1 of human origin | Santa Cruz Biotechnology, sc-15347 | Rabbit polyclonal | 1:300 IF |
| Eps8 | Amino acids 628-821 from the N-terminus of mouse Eps8 | BD Biosciences, 610143 | Mouse monoclonal | 1:100 IF |
| p-FAK-Tyr407 | A synthetic phosphopeptide from human FAK containing tyrosine 407 | ThermoFisher Scientific, 44-650G | Rabbit polyclonal | 1:100 IF |
| β1-Integrin | Against peptide fragment at N-terminus of β-integrin | Santa Cruz Biotechnology, sc-6622 | Goat polyclonal | 1:100 IF |
| Nectin-3 | Against a peptide fragment near the C-terminus of human nectin-3 | Santa Cruz Biotechnology, sc-14806 | Goat polyclonal | 1:100 IF |
| α-Tubulin | Full-length native (purified) protein of chicken α-tubulin, but this monoclonal antibody recognized epitope of amino acid residues 426-450 from the N-terminus | Abcam, ab7291 | Mouse monoclonal | 1:500 IHC |
Abbreviations: IF, immunofluorescence microscopy; IHC, immunohistochemistry.
Treatment of rats with adjudin, 1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide
Adult male rats (∼250–300 g b.w.) were treated with a single dose of adjudin (50 mg/kg b.w.) by oral gavage. Adjudin was prepared as a suspension in 0.05% methylcellulose (0.05 g methylcellulose in 100 mL Milli-Q water) with a final concentration of 20 mg/mL adjudin by oral gavage using a Cadence Science feeding needle (16-gauge/3-inch needle, with a 3-mm ball at the tip) as described (29). After the administration of the drug, the rats were euthanized by CO2 asphyxiation using slow (20%–30%/min) displacement of chamber air with compressed CO2 at specified time points. Testes were removed immediately and then either snap frozen in liquid nitrogen or fixed with Bouin's fixative (Polysciences, Inc). Testes fixed by Bouin's fixative were embedded in paraffin. Each time point had at least three to four rats, including controls. Samples from control and treatment groups were processed simultaneously for hematoxylin and eosin staining (H&E), immunohistochemistry (IHC), or immunofluorescence to eliminate interexperimental variations.
Hematoxylin and eosin staining
Testes embedded in paraffin were mounted on slides and stained with H&E. Paraffin wax was dissolved and sections were rehydrated by passing the sections through xylene, decreasing strengths of ethanol (100% to 0%), and then Milli-Q water. After rehydration, sections were stained with Mayer's hematoxylin, washed with tap water, and then stained with eosin (Richard-Allan Scientific). Thereafter, sections were dehydrated with increasing strengths of ethanol (70%–100%) and xylene and mounted in Poly-Mount (Polysciences).
Immunohistochemistry
Paraffin sections were rehydrated as described in H&E. After rehydration, antigen retrieval was performed by heating sections in 10 mM citrate buffer (pH 6.0) in a microwave for 7 minutes (Table 1). Sections were allowed to cool and then washed in Tris-buffered saline (TBS; 50 mM Tris-HCl, at pH 7.6, at 22°C, containing 150 mM NaCl). Endogenous peroxidase activity was blocked by submerging sections in 3% H2O2 in TBS (vol/vol) for 10 minutes. Sections were then blocked with either 10% normal goat or horse serum in TBS for 30 minutes. After blocking, sections were incubated with primary antibody (Table 1) at 4°C overnight. Thereafter, sections were incubated with secondary antibody (biotinylated horse anti-mouse or biotinylated goat anti-rabbit IgG, 1:300 to 1:400 dilution in TBS; Vector Laboratories) for 1 hour at room temperature, washed with TBS, and then incubated with horseradish peroxidase-streptavidin (Invitrogen) (1:250 dilution in TBS). Sections were washed with TBS and then incubated with 3,3′-diaminobenzidine (Sigma) solution. Following the 3,3′-diaminobenzidine incubation, sections were washed with Milli-Q water and then stained with hematoxylin and rinsed with tap water. Sections were then dehydrated with increasing strengths of ethanol (70%–100%) and xylene. After dehydration steps, sections were mounted with Poly-Mount. Images were captured using an Olympus BX61 microscope with a DP71 12.5 MPx digital camera (Olympus America) and MicroSuite FIVE software package (version 1224; Olympus Soft Imaging System Corp). Images were converted to TIFF formats, and layouts were performed in Adobe Creative Suite (version 6.0).
Immunofluorescence staining and filamentous (F)-actin staining
Testes snap frozen in liquid nitrogen were cut in a cryostat (−20°C) at 7 μm thickness and mounted onto positively charged slides. Sections were then fixed in 3.7% paraformaldehyde (in PBS [wt/vol]) for 10 minutes, washed in PBS (10 mM NaH2PO4, pH 7.4, at 22°C, containing 0.15M NaCl), and permeabilized with 0.1% Triton X-100 (Sigma) in PBS for 10 minutes. After permeabilization, sections were washed in PBS, blocked with 4% BSA for 30 minutes, and then incubated with primary antibody (Table 1) diluted in PBS overnight at 4°C. Thereafter, slides were washed in PBS and then incubated with secondary antibody (goat or rabbit IgG-Alexa Fluor 555; mouse or rabbit IgG-Alexa Fluor 488), Alexa Fluor 488 (green fluorescence)- or rhodamine (red fluorescence)-phalloidin conjugate diluted in PBS for staining of F-actin for 30 minutes at room temperature (ThermoFisher Scientific). Slides were washed and then mounted with ProLong Gold antifade mountant with 4′,6-diamidino-2-phenylindole (Molecular Probes) for visualization of cell nuclei. Fluorescence images were captured using a Nikon Eclipse 90i fluorescence microscope and Nikon NIS Elements 3.2 imaging software package (Nikon Instruments Inc).
Results
Adjudin treatment that induces spermatid exfoliation also disrupts F-actin organization across the entire seminiferous epithelium
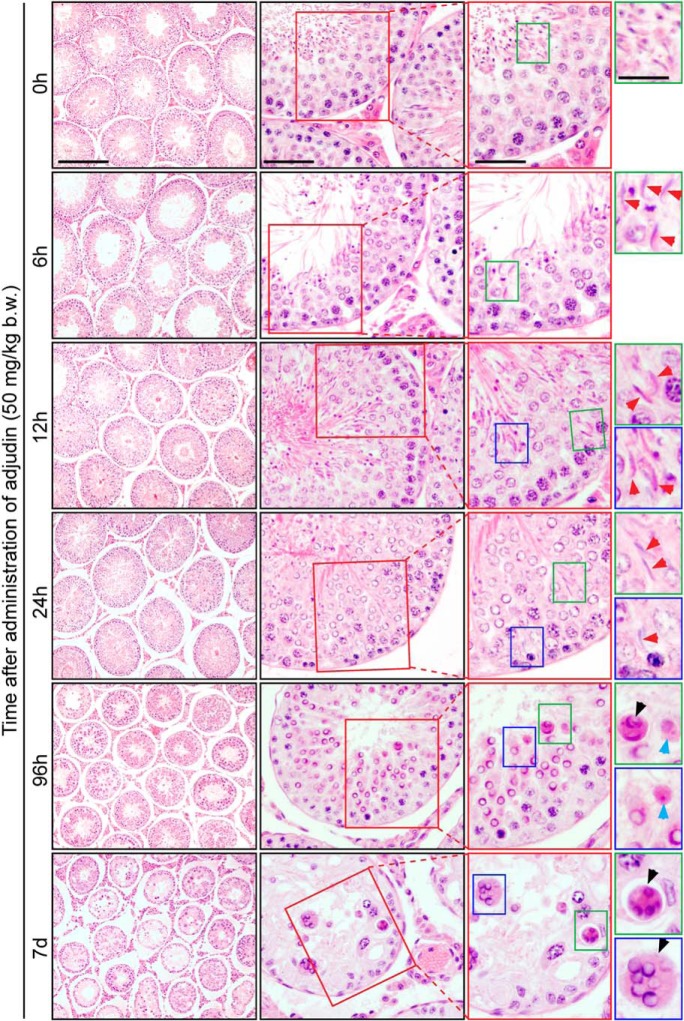
After a single dose of adjudin at 50 mg/kg b.w. administered to adult rats by oral gavage (n = 4 rats per time point including normal control rats at 0 h), spermatid exfoliation was detected as early as 6 hours, in which spermatids at stages V-VIII that resided near the tubule lumen underwent premature release as noted in 6, 12, and 24 hours, analogous to spermiation (Figure 1), consistent with earlier reports (30, 31). However, elongating/elongated spermatids residing deep inside the seminiferous epithelium remain embedded in the epithelium (see red arrowheads in Figure 1), failing to be transported to the tubule lumen to undergo spermiation. Furthermore, residual bodies/phagosomes were found near the tubule lumen in an apparently stage IX tubule (blue arrowheads in Figure 1) frequently seen in adjudin treated rats, which should have been transported to the basal compartment near the tunica propria (27, 32). Furthermore, some giant cells containing multinucleated round spermatids (black arrowheads in Figure 1), which would be processed to become phagosomes as seen in rodents exposed to other environmental toxicants, were also found near the tubule lumen (for reviews, see references 33 and 34).
Figure 1.
Defects in spermatid and phagosome transport were detected in conjunction with premature release of spermatids and other changes after the treatment of adult rats with adjudin. H&E staining of testis cross-sections of a rat treated with adjudin for 0 hour, 6 hours, 12 hours, 24 hours, 96 hours, or 7 days. Colored boxes (red, green, and blue) are magnified images to highlight the progressive changes in the premature release of spermatids, analogous to spermiation, defects in the transport of spermatids and residual bodies/phagosomes, or appearance of multi-nucleated germ cells, due to adjudin-mediated apical ES disruption and other disruptive changes in the testis. Red boxes highlight tubules undergoing either spermiation (0 h) or premature spermatid release (6 h to 7 d). Green and blue boxes identify location of spermatids in the seminiferous epithelium at 0–24 hours; red arrowheads in 6- to 24-hour tubules point out spermatids trapped in the epithelium. By 96 hours and later, spermatids are no longer present in the seminiferous tubules; green and blue boxes at these time points highlight other defects in transport of phagosomes/residual bodies (blue arrowhead) and/or formation of multi-nucleated round spermatids (black arrowheads). Data shown were representative findings of one experiment, but two additional experiments of three rats per time point yielded similar results. Scale bar in left, middle, and right column, 360 μm, 80 μm, and 60 μm, respectively; scale bar in inset, 40 μm.
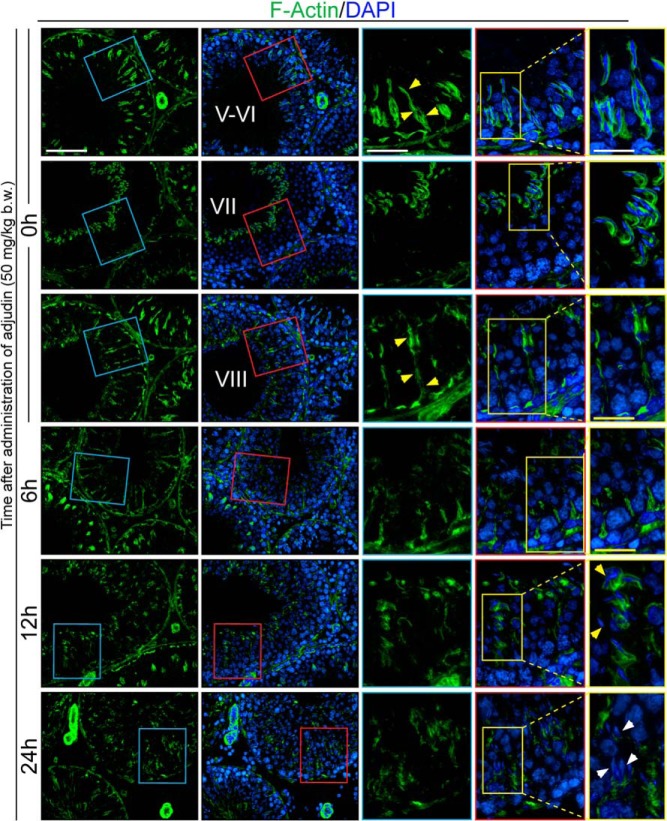
We next examined whether this failure of spermatid release for those spermatids residing inside the epithelium was related to changes in F-actin organization because spermatids are anchored onto the epithelium via the apical ES, largely conferred by an extensive network of actin microfilament bundles (for reviews, see references 8, 10, and 15). In normal testes at 0 hour, polarized actin microfilaments appeared as distinctive linear stalks spanning the seminiferous epithelium were noted in virtually all stages of the epithelial cycle (Figure 2). In stages V-VI and VII, in addition to its localization at the basal ES/BTB adjacent to the basement membrane, F-actin closely associated with elongated spermatids at the apical ES (yellow box) being transported toward the luminal edge of the epithelium, preceding stage VIII when the release of sperm at spermiation occurred.
Figure 2.
Adjudin perturbs F-actin organization in the seminiferous epithelium that induces defects in spermatid/phagosome transport as well as disruption of apical ES that leads to spermatid exfoliation. Cross-sections of frozen testes were stained to visualize F-actin (green fluorescence) after treatment of rats with adjudin; F-actin distribution at 0 hour throughout the seminiferous tubules was representative of normal rat testes. Stages V-VI, VII, and VIII depicted at 0 hour show typical localization of F-actin in the seminiferous epithelium and serves as a reference to compare changes in F-actin localization due to adjudin. Yellow boxes and arrows at 0 hour highlight the changes in F-actin association with spermatids as they are transported to the luminal edge by stage VIII. Yellow boxes at 6, 12, and 24 hours focus on the gradual changes in the F-actin distribution after treatment with adjudin. Yellow arrowheads at 12 hours and white arrowheads at 24 hours point to misoriented and trapped spermatids in the epithelium, respectively. Scale bar (white) in first, third, and fifth columns, 100 μm, 60 μm, and 40 μm, respectively; scale bar (yellow) in fifth column, 50 μm. DAPI, 4′,6-diamidino-2-phenylindole.
Unlike stages V-VI tubules, F-actin at stage VII no longer organized into long bundles that spanned the entire length of the epithelium because spermatid transport was virtually completed at this late stage VII tubule shown here. In the stage VIII tubule, F-actin association with elongated spermatids (step 19 spermatids) considerably subsided through apical ES degeneration to facilitate the release of fully developed spermatids (ie, spermatozoa) into the lumen at spermiation; however, available F-actin then reorganized into long bundles that span the length of the epithelium (yellow box), which presumably served as the tracks for other spermatogenesis-related events such as the transport of phagosomes and the recycling of apical ES components. Furthermore, spermatids being transported across the epithelium were enveloped in these stalks (annotated by yellow arrowheads in Figure 2 in stage V-VI tubules at 0 hour); polarized actin microfilaments are known to provide the means for the transport of spermatids and organelles (eg, phagosomes) by serving as the vehicles (for reviews, see references 8, 35, and 36). However, at late stage VII when the transport of elongated spermatids across the apical compartment was completed, the actin-based stalks considerably diminished but then reappeared at stage VIII (Figure 2). The stalks most likely reappear at this stage to carry out the transport of phagosomes derived from residual bodies, the excess cytoplasm of spermatids, near the tubule lumen back to the basal compartment for lysosomal degradation.
After the adjudin treatment, by 6, 12, and 24 hours, the organization of F-actin in the epithelium was grossly affected because the prominent F-actin stalks apparently being used to support spermatid and phagosome transport were no longer detected across the epithelium (Figure 2). At 6 hours, changes in F-actin distribution were mild vs control testes at 0 hour but began to display signs of abnormality. For instance, like a normal stage VIII tubule undergoing spermiation, F-actin association with spermatids at the apical ES considerably diminished, whereas F-actin tracks were still detected after 6 hours of adjudin treatment; however, as evidenced by the weaker F-actin staining, some of the tracks had become truncated, no longer stretching across the epithelium (yellow box) as seen in the seminiferous epithelium of control tubules at 0 hour (Figure 2). By 12 and 24 hours after treatment with adjudin, F-actin microfilaments were considerably truncated throughout the epithelium. Spermatids that were entrapped in the epithelium at 12 hours (yellow box) still associated with F-actin, but the distribution was abnormal because actin microfilaments no longer tightly covered the spermatid heads but diffusely localized and spermatids displayed defects in polarity (annotated by yellow arrowheads) in which their heads no longer pointed toward the basement membrane but deviated by at least 90º. By 24 hours, F-actin association with remaining spermatids (yellow box) was considerably diminished and virtually no F-actin was detected surrounding spermatids that were entrapped in the epithelium (white arrowheads), F-actin in the epithelium was disorganized, and no long actin bundles stretching across the epithelium were detected (Figure 2).
Adjudin treatment disrupts apical ES function in elongated spermatids residing near the tubule lumen and those embedded in the epithelium
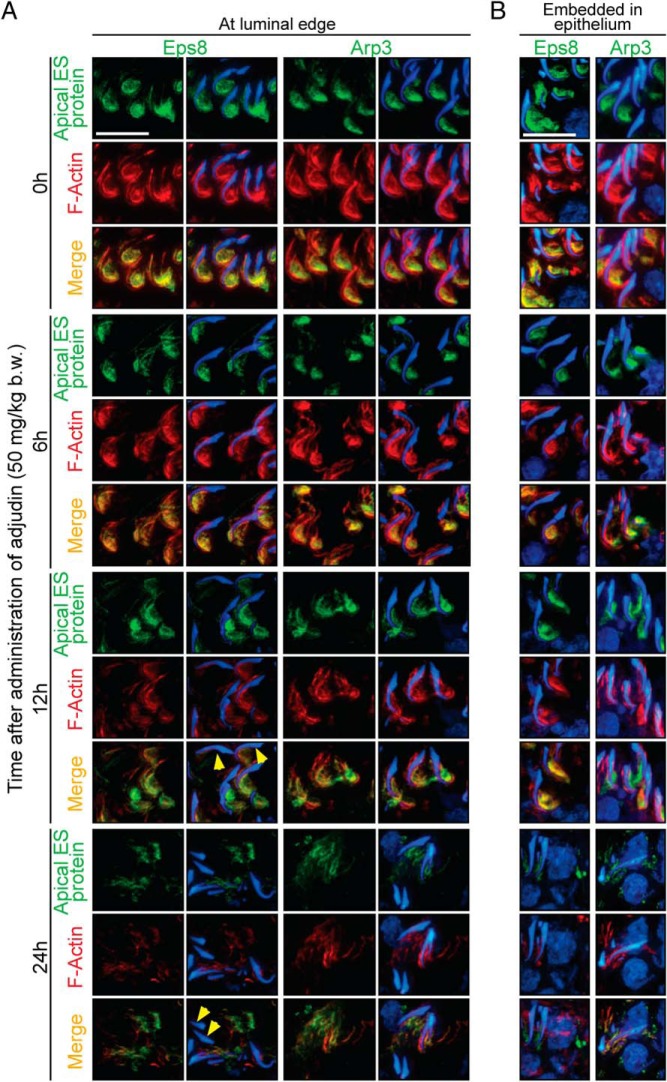
In normal rat testes (0 hour), apical ES integrity is conferred by F-actin, which is dependent on the proper spatiotemporal expression of actin barbed-end capping and bundling protein, Eps8 (bundles actin microfilaments), and also branched actin polymerization protein, Arp3 (modulates actin microfilament branching/unbundling). These proteins reorganize actin, conferring cell plasticity at the apical ES. The dynamic nature of F-actin at the apical ES in turn supports spermatid adhesion and/or endocytic vesicle-mediated protein trafficking events such as protein endocytosis and recycling (for reviews, see references 10, 37, and 38) for spermatids residing near the tubule lumen (Figure 3A) or spermatids embedded in the seminiferous epithelium (Figure 3B). However, after the adjudin treatment, considerable changes in F-actin organization, mediated by changes in the spatial expression of Eps8 and Arp3, were noted (Figure 3, A and B).
Figure 3.
Adjudin perturbs F-actin organization at the apical ES of spermatids residing at (or near) the luminal edge but also those embedded inside the seminiferous epithelium that fail to undergo exfoliation. Changes in spatiotemporal expression of actin microfilament-organizing proteins, Eps8 and Arp3, effectively disrupted apical ES function at the luminal edge (A) and deep inside the apical compartment (B) at the indicated time points after treatment with adjudin; changes are shown by colocalization of Eps8 (green fluorescence) or Arp3 (green fluorescence) and F-actin (red fluorescence). A, Spatiotemporal expression of Eps8 and Arp3 vs F-actin distribution at stage VII/early VIII apical ES in the normal rat (0 h) was compared with apical ES of spermatids undergoing premature spermiation after 6, 12, and 24 hours of adjudin treatment. Scale bar, 25 μm. B, Spatiotemporal expression of Eps8 or Arp3 vs F-actin at stage VI apical ES in the normal rat (0 h) were compared with apical ES of spermatids embedded inside the epithelium after 6, 12, and 24 hours of adjudin treatment, as indicated by the presence of spermatocytes in these micrographs. Cell nuclei were visualized by 4',6-diamidino-2-phenylindole. Scale bar, 25 μm.
At stage VII/early stage VIII of the seminiferous epithelial cycle in normal rat testes at 0 hour, Eps8 (green fluorescence) or Arp3 (green fluorescence) colocalized with F-actin (red fluorescence) at the apical ES; these proteins and F-actin were highly expressed and/or localized at the concave (ventral) side of elongated spermatid heads to support the following: 1) endocytic vesicle-mediated protein trafficking (eg, endocytosis and recycling of apical ES proteins) that took place at this site, and 2) spermatid adhesion (Figure 3A). By 6 hours, a mild but notable down-regulation of Eps8 and Arp3 was detected; the restricted spatial expression of Eps8 and Arp3 that conferred F-actin organization displayed signs of defects in which their expression was considerably reduced in comparison with the control (0 h) (Figure 3A). In addition, F-actin no longer surrounded spermatids as extensively as noted in control testes (0 h). By 12 hours, changes in the distribution of F-actin and the two regulatory proteins were more pronounced; in addition to considerable reduction in expression, Eps8 and Arp3 were no longer restrictively expressed at the concave side of spermatid heads but throughout most of the spermatid head. Furthermore, some elongated spermatids displayed defects in polarity, in which their heads no longer pointed toward the basement membrane, deviating by at least 90° from the intended orientation (yellow arrowheads in Figure 3A). Apical ES changes found near the luminal edge (Figure 3A) were similar to those found at the apical ES of elongated spermatids embedded inside the epithelium (Figure 3B) during adjudin-induced spermatid exfoliation. These findings thus support the notion that adjudin exerts its disruptive effects on the apical ES across the epithelium, regardless of the relative location of elongating/elongated spermatids.
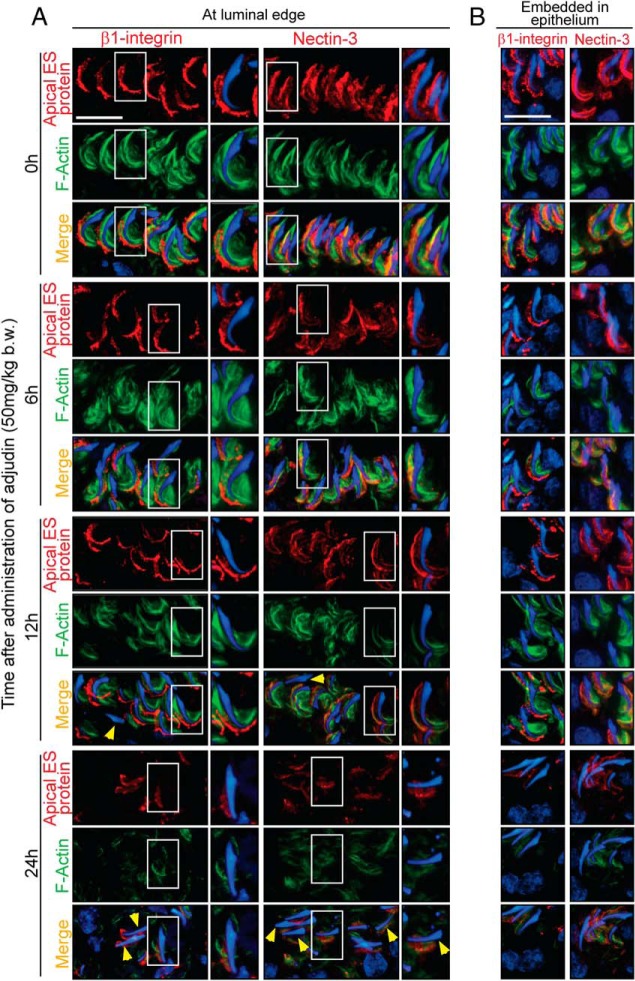
We next examined whether the apical ES adhesion function was grossly affected after adjudin treatment for spermatids residing near the tubule lumen vs those embedded in the epithelium. In normal rat testes at 0 hour, β1-integrin (a Sertoli cell specific integral membrane protein at the apical ES) and nectin-3 (a spermatid specific integral membrane protein at the apical ES) that use F-actin for support and attachment were localized predominantly to the convex side of spermatid heads in spermatids residing near the tubule lumen (Figure 4A) or embedded in the epithelium (Figure 4B). After the adjudin treatment, time-dependent changes in their localization were detected. By 6 hours, the expression of β1-integrin and nectin-3 at the apical ES were mildly but notably down-regulated (Figure 4A); and by 12 and 24 hours, considerable down-regulation and mislocalization were detected because β1-integrin and nectin-3 no longer tightly attached onto the convex side of spermatid heads but were diffusing away; and in some spermatids that displayed defects in polarity (yellow arrowheads), β1-integrin or nectin-3 was either absent or considerably down-regulated (Figure 4A). Similar changes were noted for spermatids embedded inside the epithelium (Figure 4B). Collectively these findings illustrate that the apical ES was similarly compromised for spermatids residing near the tubule lumen and those embedded inside the epithelium after the adjudin treatment. Yet, unlike elongated spermatids near the lumen, elongated spermatids embedded in the epithelium failed to undergo spermiation.
Figure 4.
Apical ES function of spermatids located near the luminal edge and those embedded deep inside the epithelium was disrupted after adjudin treatment. Spatiotemporal expression of apical ES integral membrane proteins was examined to confirm changes in F-actin organization that were mediated through disrupted expression of Eps8 and Arp3 at the apical ES. Integral membrane proteins: β1-integrin (a Sertoli cell specific apical ES protein) and nectin-3 (a spermatid specific apical ES protein) vs F-actin were examined at the luminal edge of the apical compartment (A) and within the seminiferous epithelium (B) (as evidenced by the presence of spermatocytes) after treatment with adjudin. At 0 hour, both β1-integrin (red fluorescence) and nectin-3 (red fluorescence) were predominantly expressed on the convex (dorsal) side of the elongated spermatid heads, colocalizing with F-actin (green fluorescence) at the site to confer spermatid adhesion, wherein F-actin was also expressed at the convex side of spermatid heads in this stage VII tubule. Cell nuclei were visualized by 4′,6-diamidino-2-phenylindole. Scale bar, 25 μm (A and B).
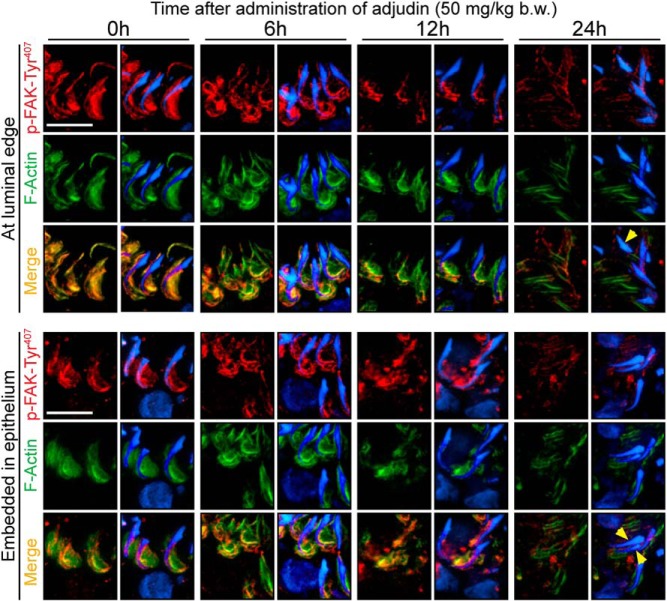
Adjudin treatment perturbs the spatiotemporal expression of an F-actin-regulatory protein, p-FAK-Tyr407, at the apical ES
Studies have shown that p-FAK-Tyr407 is a regulator of apical ES and is spatiotemporally expressed at that site (for a review, see reference 61). It is predominantly localized to the concave side of spermatid head, as seen in stage VII tubule spermatids of the normal rat testis at 0 hour (red fluorescence in Figure 5); spermatids located near both the tubule lumen and embedded inside the epithelium (Figure 5) expressed p-FAK-Tyr407, perhaps used to confer proper F-actin (green fluorescence) organization to maintain apical ES integrity. However, after the adjudin treatment, the spatial localization of p-FAK-Tyr407 was gradually disrupted over time. For instance, by 6 hours, p-FAK-Tyr407 no longer tightly associated with the concave side of spermatid heads but instead diffused away and covered much of the tip of spermatid heads including the convex side. By 12 hours, p-FAK-Tyr407 was no longer intensely localized at the concave side of the spermatid head, and by 24 hours its expression was considerably down-regulated and diffusely localized in the epithelium, similar to F-actin (Figure 5). In spermatids that had defects in polarity (yellow arrowheads), virtually no p-FAK-Tyr407 expression was detected (Figure 5). These changes were similar for elongated spermatids residing near the tubule lumen and those embedded in the epithelium (Figure 5), illustrating that disruption of apical ES integrity was due to defects in F-actin organization at both sites.
Figure 5.
Disruption of F-actin organization at the Sertoli-spermatid interface is mediated through changes in the restricted spatiotemporal expression of the apical ES regulator p-FAK-Tyr407. p-FAK-Tyr407 (red fluorescence), a nonreceptor protein tyrosine kinase known to regulate ES dynamics via its effects on Arp-mediated actin nucleation, was highly expressed at the spermatid head, predominantly at the concave (ventral) side, and colocalized with F-actin (green fluorescence) in this stage VII tubule from control testes at 0 hour, both in spermatids near the luminal edge (upper panel) and also those embedded inside the seminiferous epithelium (lower panel). Following adjudin treatment, localization and/or expression of p-FAK-Tyr407 at the apical ES was either compromised or down-regulated in elongated spermatids found near the tubule lumen (upper panel) or embedded inside the epithelium (lower panel). Cell nuclei were visualized by 4′,6-diamidino-2-phenylindole. Scale bar, 25 μm in both panels.
Loss of apical ES integrity alone is not sufficient to induce elongated spermatid release—the presence of MT-based tracks is necessary to support the transport and release of spermatids
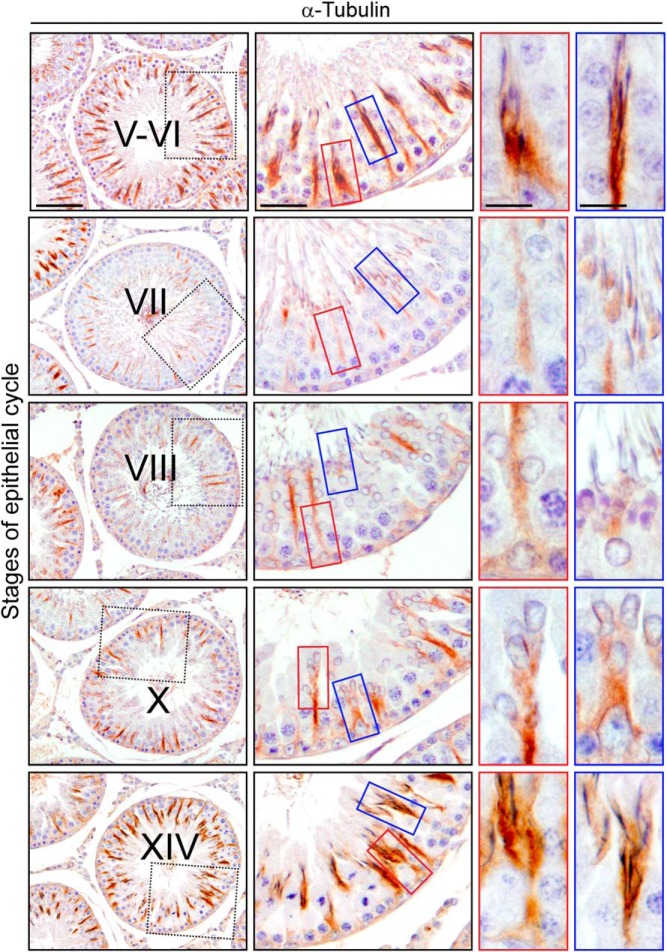
We next investigated what causes the failure of elongated spermatids embedded in the epithelium to be released from the epithelium after apical ES disruption using the adjudin model. We hypothesized that this might be due to disrupted MT function that impeded the provision of tracks for proper transport of spermatids (and residual bodies/phagosomes) after the adjudin treatment. In normal rat testes, α-tubulin, the integrated constructive component of MTs, assumes a spoke-like pattern in the cross sections of seminiferous tubules. The spokes are the tracks presumably used for transport of the elongating/elongated spermatids across the seminiferous epithelium in virtually all stages of the epithelial cycle (Figure 6). Spermatids being transported across the epithelium were tightly associated with tubulin as seen by the staining for α-tubulin in the normal rat testes (Figure 6), supporting the concept that spermatids are transported along MT tracks. Whereas α-tubulin was present in all stages, its expression pattern throughout the epithelium was stage specific. For instance, in stage V-VI, spermatids were being transported from the basal compartment to the apical compartment of the epithelium in preparation for spermiation at the luminal edge. It should be noted that α-tubulin expression gradually subsided when spermatid transport was completed in late stage VII (MT tracks diminished), and was localized predominantly at the concave side of spermatid heads (blue box in Figure 6). By late stage VIII when spermiation began to take place, α-tubulin expression at the concave side of spermatids heads was considerably diminished, but its expression in the epithelium began to reemerge as the MT tracks were reassembled (blue and red boxes in Figure 6). The reappearance of these tracks at this time is likely to support the transport of phagosomes and endocytosed apical ES proteins for recycling.
Figure 6.
Expression of α-tubulin in the seminiferous epithelium of normal adult rat testes. Cross-sections of normal adult rat testes were stained to visualize expression of α-tubulin, the building block of MTs, in select stages (V-VI, VII, VIII, X, and XIV) of the seminiferous epithelial cycle by IHC. The red and blue boxes highlight intense staining of α-tubulin surrounding the spermatids in transit along MT tracks. By stage VII, when elongated spermatids approach the luminal edge, α-tubulin is no longer detected around entire spermatids and is predominantly localized at the concave side of spermatid heads (blue box) instead. The overall intensity of α-tubulin staining throughout the stage VII tubule is less in comparison with the earlier stages (V-VI) and subsequent stages (X-XIV), and considerably fewer long bundles of microtubules are found at this stage. In stages after spermiation (ie, X and XIV), α-tubulin was intensely expressed along the Sertoli cell stalks, surrounding developing spermatids (red and blue boxes). Scale bar in first, second, third, and fourth column, 200 μm, 70 μm, 30 μm and 30 μm, respectively.
However, after adjudin treatment spermatids located at or near the tubule lumen began to be depleted from the epithelium by 6 hours (Figure 7) due to the loss of apical ES integrity (Figures 2–4), resulting from disruption of the underlying F-actin network (Figures 2 and 3) likely contributed by changes in the spatiotemporal expression of the p-FAK-Tyr407 (Figure 5). Interestingly, some spermatids at this time point remained trapped inside the epithelium (red box in Figure 7). By 12 hours, in an apparently stage VII tubule, elongated spermatids were also found to be entrapped inside the epithelium, whereas other spermatids that were located near the tubule lumen had undergone spermiation prematurely (red box). Interestingly by 24 hours, the time by which apical ES integrity and F-actin organization were compromised and disrupted (Figures 2–4), some elongated step 19 spermatids were still found embedded in the epithelium (green arrowheads), even in stage X tubules, which should contain only step 10 spermatids (red arrowheads in Figure 7). By 96 hours MT arrangement was considerably disorganized and did not assume the spoke-like pattern of normal seminiferous tubules (0 h); MT bundles were no longer polarized as evidenced by scattered and dispersed α-tubulin staining. As shown in Figure 7, the failed transport of elongated spermatids to the tubule lumen at the appropriate stages of the epithelial cycle appeared to be associated with the lack of MT tracks across the epithelium. Even when some short track-like structures were found, they no longer reached the tubule lumen as found in control testes at 0 hour (Figure 7) to support the release of elongated spermatids into the tubule lumen.
Figure 7.
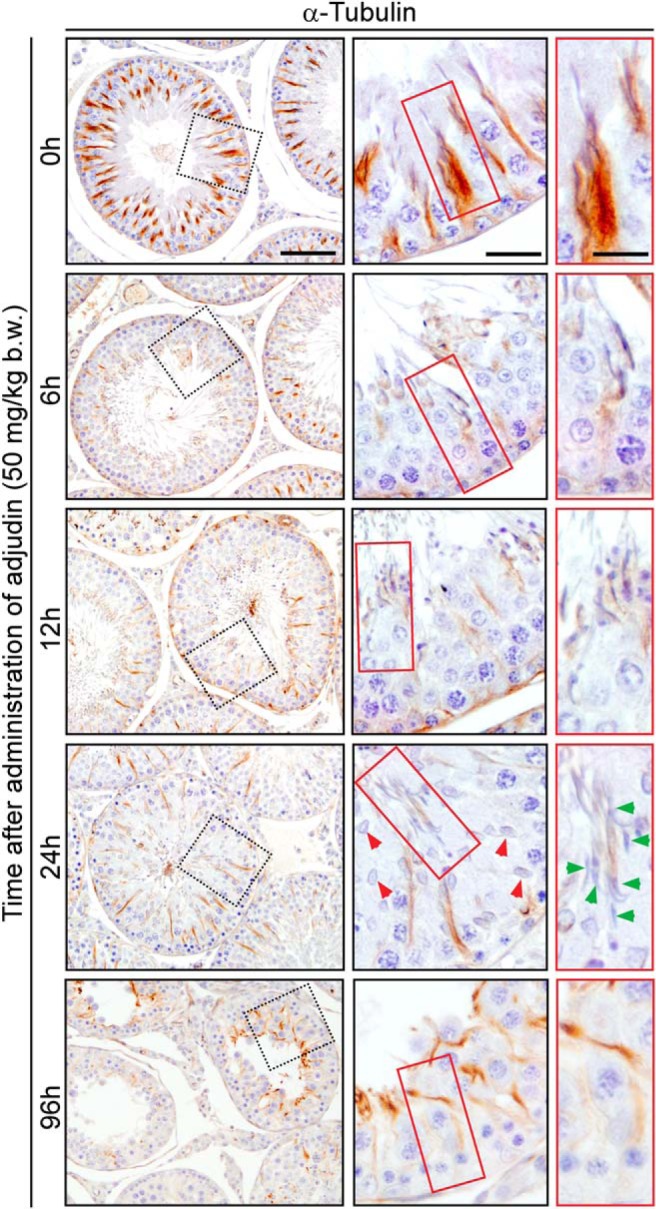
Adjudin induces disruption of transport tracks conferred by MTs in the seminiferous epithelium. Cross-sections of rat testes were stained to visualize changes in expression of α-tubulin/MTs after rats were treated with adjudin. Evidence of MT track disruption was visible by as early as 6 hours after adjudin treatment. In normal testes, spermatids, phagosomes, and other organelles (eg, endosomes) used the tracks conferred by MTs for transport across the epithelium as noted at 0 hour. The boxed areas in red at each time point were enlarged and shown on the right panel to provide more structural details, illustrating the MT-based tracks along the path in which elongating/elongated spermatids were being transported. Red and green arrowheads denote step 10 and trapped step 19 spermatids, respectively, in the epithelium. In addition to disruption of MT tracks, adjudin also led to a reduction in seminiferous tubule diameter, by 96 hours, decreasing approximately 30%–40%. Scale bar in first, second, and third column, 200 μm, 70 μm, and 30 μm, respectively.
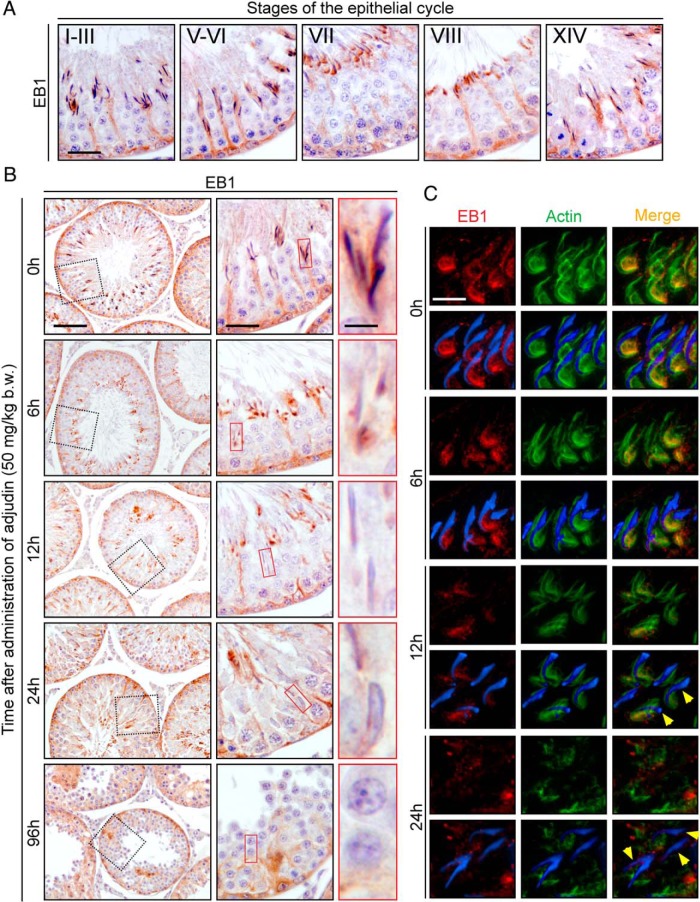
Loss of transport tracks conferred by MT after adjudin treatment is mediated by impaired localization of a plus-end tracking protein (+TIP), end-binding protein 1 (EB1)
We next examined the molecular mechanism by which the disruption of the MT-based track-like structures in the epithelium after adjudin treatment occurred. EB1 was shown to be an important regulator of MT integrity in the testis because its knockdown was found to perturb the organization of MTs in Sertoli cells (39). As noted in normal rat testes at 0 hour, EB1 also assumed a track-like configuration in the epithelium during the epithelial cycle and tightly associated with elongating/elongated spermatids being transported across the epithelium (Figure 8A), consistent with a recent report (39). After the adjudin treatment, as soon as 6 hours when apical ES integrity was compromised and spermatids at or near the tubule lumen were depleting from the epithelium, EB1 expression was considerably down-regulated; and by 12 and 24 hours, α-tubulin was almost undetectable and the track-like structures conferred by MT virtually vanished in the epithelium (Figure 8B). This thus impeded spermatid transport. More importantly, EB1 is known to regulate both actin microfilaments and MT dynamics in Sertoli cells and it also colocalized with actin microfilaments at the ES in Sertoli cells (39). We next examined changes in EB1 spatial expression at the apical ES in relation to F-actin at the apical ES after the adjudin treatment (Figure 8C). In normal testes at 0 hour, EB1 was localized prominently at the concave side of spermatid heads, colocalized with F-actin, in stage VII tubules (Figure 8C), consistent with data shown in Figure 8A. However, by 6 hours after adjudin treatment, the expression of EB1 in stage VII tubules considerably diminished (Figure 8C). In addition to considerable down-regulation, EB1 was also diffusing away from the concave side of spermatid heads by 12 and 24 hours, and EB1 was virtually undetectable in most spermatids that had defects in polarity (see yellow arrowheads in Figure 8C). These findings support the notion that the adjudin-induced MT disruption was mediated by changes in the spatial expression and/or localization of EB1 that impeded the organization of track-like structures for proper transport of elongated spermatids. Figure 9 summarizes the concept that MT tracks and actin microfilaments support the transport of spermatids; it also illustrates that adjudin-mediated disruption of these ultrastructures would induce premature release of spermatids into the tubule lumen while causing entrapment of elongated spermatids in the epithelium.
Figure 8.
Adjudin induced MT-based track disruption in the seminiferous epithelium is mediated by changes in the spatiotemporal expression of +TIP EB1. A, Expression of EB1 in normal rat testis visualized by IHC. EB1 expression was similar to α-tubulin in the seminiferous epithelium (see Figure 6). EB1, a MT-stabilizing protein, was tightly associated with spermatids across the epithelium, ensuring the integrity of the tracks for spermatid and other organelle transport. Similar to α-tubulin, the expression of EB1 was mildly diminished at late stage VII when elongated spermatids had been transported to the luminal edge, but its expression across the seminiferous epithelium was up-regulated again by stage VIII, presumably to aid in the transport of phagosomes to the basal compartment for their eventual degradation. Scale bar, 80 μm. B, Changes in EB1 expression after treatment with adjudin are similar to that of α-tubulin-based MTs shown in Figure 7. Within the epithelium, EB1 expression was closely associated with elongating spermatids as they were being transported from the basal to apical compartment as shown in normal testes at 0 hour. After treatment with adjudin, association of EB1 with spermatids was considerably altered. At 6 hours, changes in EB1 localization began to emerge. While spermatids near the tubule lumen were undergoing spermiation prematurely (red box), some spermatids were still embedded inside the epithelium, even though apical ES function and the underlying F-actin network in these spermatids had been disrupted (see Figures 2–4). More importantly, spermatids trapped inside the epithelium had considerably weakened EB1 immunostaining compared with normal control tubules. By 96 hours, the epithelium was virtually devoid of elongated spermatids. Scale bar in first, second, and third column, 200 μm, 80 μm, and 20 μm, respectively. C, Colocalization of EB1 (red fluorescence) and F-actin (green fluorescence) after adjudin treatment. Both EB1 and F-actin expression at the apical ES diminished significantly after adjudin treatment. At 0 hour, EB1 was predominantly localized at the concave side of spermatid heads and colocalized with F-actin, at the apical ES in normal testes. By 6–12 hours, EB1 expression at the apical ES was considerably diminished. After 24 hours, both EB1 and F-actin expression was no longer restricted at the apical ES but diffusely localized concurrent with misorientation of many spermatids, displaying defects in polarity (yellow arrowheads). Cell nuclei were visualized by 4′,6-diamidino-2-phenylindole. Scale bar, 25 μm.
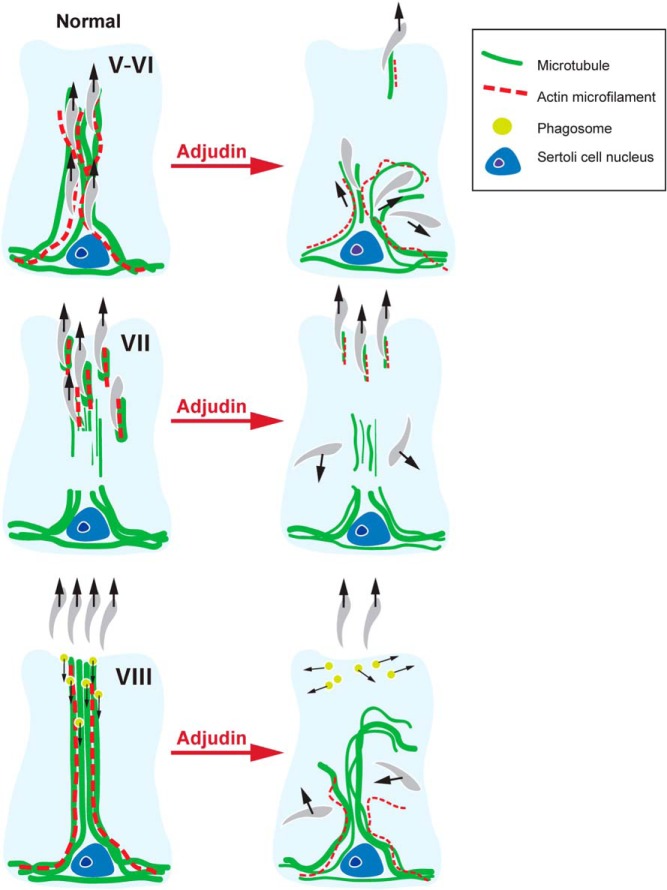
Figure 9.
Schematic diagram illustrating gradual disruption of MT- and actin-based cytoskeletons in the Sertoli cell after treatment with adjudin that leads to premature spermatid release at the luminal edge but also entrapment of spermatids inside the epithelium. The proposed model is based on findings in this report. Stages V-VI (upper panel), VII (middle panel), and VIII (lower panel) of the epithelial cycle in the rat are depicted before and after treatment with adjudin. In a normal stage V-VI, spermatids are transported up through the epithelium, prior to spermiation at stage VIII along MT tracks (green) in conjunction with actin microfilaments (red) in the Sertoli cell. At stage VII, spermatids are in transit toward the tubule lumen. The columnar structure of MT bundles, serving as the tracks, begin to diminish during this stage because the spermatids have been transported to their final destination. Although the presence of actin and tubulin subside along the tracks, they are still tightly associated with the spermatids, especially at the concave side of the apical ES prior to spermiation. At stage VIII, the apical ES undergoes degeneration, which allows spermatids to be released into the tubule lumen at spermiation. Although tubulin and actin, which constitute the MT- and actin-based cytoskeletons at the apical ES, are absent during spermiation, they begin to reassemble to facilitate the transport of phagosomes, which are residual bodies or multi-nucleated giant cells phagocytosed by Sertoli cells, found near the apical lumen. The track-like structures reappear at late stage VIII to transport these phagosomes to the base of the epithelium so that they can be digested by the Sertoli cell via the lysosome-based degradation pathway. As shown in this diagram, MT- and actin-based cytoskeletons are disrupted after treatment with adjudin. A stage V-VI tubule affected by adjudin undergoes premature spermiation and MT tracks collapse, which leads to the entrapment of spermatids within the epithelium. A stage VII tubule is similarly affected by adjudin, causing premature spermatid release into the lumen and entrapment of spermatids in the epithelium because the MT tracks are further diminished due to the effects of the drug. In a normal stage VIII tubule, spermatids are not present within the epithelium because they should already be transported to the edge of tubule lumen in preparation for spermiation. However, a stage VIII tubule affected by adjudin may contain some spermatids trapped within the epithelium due to the gradual disruption of the MT tracks before it develops into a stage IX tubule.
Discussion
The aim of this study was to examine the mechanism by which germ cells and residual bodies/phagosomes are transported within the seminiferous epithelium of the rat testes during the epithelial cycle. We chose this model because adjudin is a known cell adhesion disruptor by perturbing apical ES function, which causes defects in spermatid transport, such as premature release of spermatids, leading to infertility in adult male rats. Moreover, although adjudin induces infertility, its effects are highly reversible at a dose of 50 mg/kg b.w. (for a review, see reference 40), which was used in this study, making it a suitable model to investigate cellular events of spermatogenesis, in particular spermatid adhesion and transport. More importantly, similar to other toxicant models (eg, carbendazim, 1,2-hexanediol) (for reviews, see references 14, 17, and 41), many elongating/elongated spermatids were found to be entrapped inside the epithelium long after premature spermiation had occurred, even though adjudin effectively induces premature release of elongating/elongated spermatids from the epithelium (for a review, see reference 28).
Collectively our findings provide compelling evidence, using the adjudin model, that the apical ES and the underlying actin- and MT-based cytoskeletons are indeed disrupted, affecting spermatids positioned near the tubule lumen and those within the seminiferous epithelium. However, the striking observation is that even though the actin-based adhesion proteins at the apical ES, namely β1-integrin (42–45) and nectin-3 (46), were mislocalized and/or down-regulated, spermatids remained embedded inside the epithelium. These findings thus suggest that a loss of actin-based cytoskeletal function concomitant with apical ES disruption alone is not sufficient to induce spermatid depletion from the epithelium. Rather, we noted that in addition to the loss of actin cytoskeleton function, the disruption of the MT cytoskeleton also led to the phenotype observed, namely disruption of spermatid transport. The track-like structures assembled across the seminiferous epithelium conferred by MTs (for reviews, see references 13, 23, and 24) were grossly disrupted after the adjudin treatment and considerably down-regulated and totally absent in most tubules examined, suggesting that the presence of MT-based tracks are necessary to support spermatid transport across the epithelium in the absence of functional apical ES so that spermatids can be released into the tubule lumen. Furthermore, the lack of MT-based tracks also impedes the transport of residual bodies/phagosomes across the epithelium because the residual bodies/phagosomes remained near the tubule lumen in stage VIII–IX tubules after the adjudin treatment, when they should have been transported to the basal compartment for degradation (27, 32). Collectively these findings suggest that the apical ES and the underlying actin-based cytoskeleton require the presence of the MT-conferred tracks to support the release of spermatids into the tubule lumen at spermiation and the transport of residual bodies/phagosomes near the tubule lumen into the base of the epithelium, thus illustrating the requirement of tight coordination between the actin- and the MT-based cytoskeletons to support cell transport events, as summarized in Figure 9.
Recent studies on various cellular transport events have revealed that tight coordination between actin- and MT-based cytoskeletons is required (for reviews, see references 47 and 48); proteins such as Tau (49), formin mDia (50), WASP homolog-associated protein with actin, membranes and microtubules (51), TipAct (52), and G proteins (for a review, see reference 53) are some of these players that coorganize actin and MT networks. We chose to investigate EB1 (a plus-end tracking protein [+TIP]) because it has been previously demonstrated that it plays a crucial role in cross talk between actin- and MT-based cytoskeletons of Sertoli cells in the rat testes (for a review, see references 24, 54, and 55). In addition, a recent study reported that +TIP protein TipAct works in concert with EB3 (related to EB1) to coordinate actin- and MT-based cytoskeleton dynamics (52). As such, future studies are needed to explore the involvement of other +TIP proteins in coordinating the actin- and MT-based cytoskeletons to support spermatid and/or residual body/phagosome transport during the epithelial cycle of spermatogenesis.
We have illustrated that EB1 was involved in the timely transport of spermatids and residual bodies/phagosomes when apical ES and actin- and MT-based cytoskeletons were disrupted due to adjudin treatment because its expression and/or localization along the track-like structures in the epithelium was grossly perturbed. These observations in vivo using the adjudin model are also consistent with data from an in vitro study in which EB1 was silenced in Sertoli cells by RNA interference. In that study, EB1 knockdown was shown to induce a disruption in both actin- and MT-based cytoskeletons: actin microfilaments became defragmented and MTs no longer extended to the Sertoli cell cortex and instead wrapped around the nucleus (39). The notion that EB1 is involved in coordinating actin- and MT-based cytoskeleton-mediated cellular processes is also supported by studies in motile mammalian cells, such as oesteoclasts, macrophages, and dendritic cells, to facilitate their movement on extracellular matrix mediated by actin-rich podosomes (56) (for reviews, see references 57–59). In podosomes, EB1 appears to exert its regulatory function to coorganize actin- and MT-based networks via cortactin and Src (56). We have shown that p-FAK-Tyr407, a known ES regulator in the testis (for reviews, see references 60 and 61) and a partner of c-Src (for a review, see reference 62), is also a likely partner that works in concert with EB1 to induce coordination between actin- and MT-based networks to regulate spermatid and residual body/phagosome transport. Although the detailed molecular mechanism(s) by which the two cytoskeletal networks are coordinated require additional studies, we have provided a useful model (eg, Figure 9) and likely candidates (eg, EB1, p-FAK-Tyr407) to design functional studies in the future.
In this context, it is of interest to note that MTs serving as the tracks for transport are located inside the Sertoli cell, while elongating/elongated spermatids are found outside the Sertoli cell. However, spermatids tightly adhere onto the MT- (and possibly actin-) based tracks in the Sertoli cell through the apical ES, creating a cellular microenvironment in which spermatids and Sertoli cells are only separated by their apposing plasma membranes. Thus, MTs that are located near the Sertoli cell plasma membrane adjacent to the actin microfilament bundles at the apical ES can work in concert to provide the track-like structures to support spermatid transport through a mechanism involving actin- and MT-specific motor proteins (see Figure 9). On the other hand, because elongating/elongated spermatids are required to be transported up but also down the seminiferous epithelium during the epithelial cycle, it is possible that there are two types of polarized MT-conferred tracks to support the two contrasting spermatid transports. However, MT-binding and/or regulatory proteins, such as MAP/microtubule affinity-regulating kinases and end-binding proteins, can also serve as the molecular switches to regulate different transport events that occur in opposite directions along a common MT track, analogous to traffic switches that modulate a railroad track for an incoming vs outgoing train without the necessity of building two parallel tracks. These possibilities will be carefully evaluated in future studies.
Acknowledgments
We thank Dr Katarzyna Chojnacka for her helpful advice on the experiments pertinent to morphological analysis. We also thank Dr Dolores Mruk for her critical discussion throughout the course of this study.
Author contributions included the following: E.I.T. and C.Y.C. designed the research, performed the research and experiments pertinent to the use of live animals, contributed new reagents/analytic tools, and analyzed the raw data. E.I.T., W.M.L., and C.Y.C. critically evaluated the data. E.I.T. and C.Y.C. prepared the figures and wrote the paper. All authors reviewed and approved the manuscript.
This work was supported by Grant R01 HD056034 from the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development (to C.Y.C.) and Grant U54 HD029990 from Project 5 (to C.Y.C.); National Natural Science Foundation of China/Research Grants Council of Hong Kong (NSFC/RGC) Joint Research Scheme (N_HKU 717/12 to W.M.L.), General Research Fund from RGC (771513 to W.M.L.), and CRCG Seed Funding, University of Hong Kong (to W.M.L.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Arp3
- actin-related protein 3
- b.w.
- body weight
- EB1
- end-binding protein 1
- Eps8
- epidermal growth factor receptor pathway substrate 8
- ES
- ectoplasmic specialization
- F-actin
- filamentous actin
- H&E
- hematoxylin and eosin staining
- IHC
- immunohistochemistry
- MT
- microtubule
- TBS
- Tris-buffered saline
- +TIP
- plus-end tracking protein.
References
- 1. Nakai M, Miller MG, Carnes K, Hess RA. Stage-specific effects of the fungicide carbendazim on Sertoli cell microtubules in rat testis. Tissue Cell. 2002;34:73–80. [DOI] [PubMed] [Google Scholar]
- 2. Allard EK, Johnson KJ, Boekelheide K. Colchicine disrupts the cytoskeleton of rat testis seminiferous epithelium in a stage-dependent manner. Biol Reprod. 1993;48:143–153. [DOI] [PubMed] [Google Scholar]
- 3. O'Donnell L, McLachlan R, Wreford N, de Kretser D, Robertson D. Testosterone withdrawal promotes stage-specific detachment of round spermatids from the rat seminiferous epithelium. Biol Reprod. 1996;55:895–901. [DOI] [PubMed] [Google Scholar]
- 4. O'Donnell L, Stanton P, Bartles J, Robertson D. Sertoli cell ectoplasmic specializations in the seminiferous epithelium of the testosterone-suppressed adult rat. Biol Reprod. 2000;63:99–108. [DOI] [PubMed] [Google Scholar]
- 5. Gungor-Ordueri NE, Tang EI, Celik-Ozenci C, Cheng CY. Ezrin is an actin binding protein that regulates Sertoli cell and spermatid adhesion during spermatogenesis. Endocrinology. 2014;155:3981–3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Qian X, Mruk DD, Wong EWP, Lie PPY, Cheng CY. Palladin is a regulator of actin filament bundles at the ectoplasmic specialization in the rat testis. Endocrinology. 2013;154:1907–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li N, Mruk DD, Wong CK, Lee WM, Han D, Cheng CY. Actin-bundling protein plastin 3 is a regulator of ectoplasmic specialization dynamics during spermatogenesis in the rat testis. FASEB J. 2015;29:3788–3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vogl AW, Vaid KS, Guttman JA. The Sertoli cell cytoskeleton. Adv Exp Med Biol. 2008;636:186–211. [DOI] [PubMed] [Google Scholar]
- 9. Wong EWP, Mruk DD, Cheng CY. Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochim Biophys Acta. 2008;1778:692–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nat Rev Endocrinol. 2010;6:380–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004;25:747–806. [DOI] [PubMed] [Google Scholar]
- 12. O'Donnell L, Nicholls PK, O'Bryan MK, McLachlan RI, Stanton PG. Spermiation: the process of sperm release. Spermatogenesis. 2011;1:14–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O'Donnell L, O'Bryan MK. Microtubules and spermatogenesis. Semin Cell Dev Biol. 2014;30:45–54. [DOI] [PubMed] [Google Scholar]
- 14. O'Donnell L. Mechanisms of spermiogenesis and spermiation and how they are disturbed. Spermatogenesis. 2014;4:e979623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Qian X, Mruk DD, Cheng YH, et al. Actin binding proteins, spermatid transport and spermiation. Semin Cell Dev Biol. 2014;30:75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wan HT, Mruk DD, Wong CKC, Cheng CY. The apical ES-BTB-BM functional axis is an emerging target for toxicant-induced infertility. Trends Mol Med. 2013;19:396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boekelheide K, Neely MD, Sioussat TM. The Sertoli cell cytoskeleton: a target for toxicant-induced germ cell loss. Toxicol Appl Pharmacol. 1989;101:373–389. [DOI] [PubMed] [Google Scholar]
- 18. Boekelheide K, Fleming SL, Allio T, et al. 2,5-Hexanedione-induced testicular injury. Annu Rev Pharmacol Toxciol. 2003;43:125–147. [DOI] [PubMed] [Google Scholar]
- 19. Xiao X, Mruk DD, Wong CKC, Cheng CY. Germ cell transport across the seminiferous epithelium during spermatogenesis. Physiology. 2014;29:286–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xiao X, Mruk DD, Tang EI, et al. Environmental toxicants perturb human Sertoli cell adhesive function via changes in F-actin organization medicated by actin regulatory proteins. Hum Reprod. 2014;29:1279–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wan HT, Mruk DD, Wong CKC, Cheng CY. Perfluorooctanesulfonate (PFOS) perturbs male rat Sertoli cell blood-testis barrier function by affecting F-actin organization via p-FAK-Tyr407—an in vitro study. Endocrinology. 2014;155:249–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Siu ER, Wong EWP, Mruk DD, Sze KL, Porto CS, Cheng CY. An occludin-focal adhesion kinase protein complex at the blood-testis barrier: a study using the cadmium model. Endocrinology. 2009;150:3336–3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tang EI, Mruk DD, Cheng CY. MAP/microtubule affinity-regulating kinases, microtubule dynamics, and spermatogenesis. J Endocrinol. 2013;217:R13–R23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tang EI, Mruk DD, Lee WM, Cheng CY. Cell-cell interactions, cell polarity, and the blood-testis barrier. In: Ebnet K, ed. Cell Polarity. 1st ed Geneva: Springer International Publishing; 2015:303–326. [Google Scholar]
- 25. Charron M, Wright WW. Proteases and protease inhibitors. In: Skinner MK, Griswold MD, eds. Sertoli Cell Biology. New York, Elsevier Science; 2005:121–152. [Google Scholar]
- 26. Morales C, Clermont Y, Nadler NJ. Cyclic endocytic activity and kinetics of lysosomes in Sertoli cells of the rat: a morphometric analysis. Biol Reprod. 1986;34:207–218. [DOI] [PubMed] [Google Scholar]
- 27. Clermont Y, Morales C, Hermo L. Endocytic activities of Sertoli cells in the rat. Ann NY Acad Sci. 1987;513:1–15. [DOI] [PubMed] [Google Scholar]
- 28. Cheng CY. Toxicants target cell junctions in the testis—insights from the indazole-carboxylic acid model. Spermatogenesis. 2014;4:e981485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cheng CY, Silvestrini B, Grima J, et al. Two new male contraceptives exert their effects by depleting germ cells prematurely from the testis. Biol Reprod. 2001;65:449–461. [DOI] [PubMed] [Google Scholar]
- 30. Chen YM, Lee NPY, Mruk DD, Lee WM, Cheng CY. Fer kinase/Fer T and adherens junction dynamics in the testis: an in vitro and in vivo study. Biol Reprod. 2003;69:656–672. [DOI] [PubMed] [Google Scholar]
- 31. Grima J, Silvestrini B, Cheng CY. Reversible inhibition of spermatogenesis in rats using a new male contraceptive, 1-(2,4-dichlorobenzyl)-indazole-3-carbohydrazide. Biol Reprod. 2001;64:1500–1508. [DOI] [PubMed] [Google Scholar]
- 32. Cheng CY, Mruk DD. Biochemistry of Sertoli cell/germ cell junctions, germ cell transport, and spermiation in the seminiferous epithelium. In: Griswold M, ed. Sertoli Cell Biology. 2nd ed Amsterdam: Elsevier; 2014:333–383. [Google Scholar]
- 33. Boekelheide K, Johnson KJ, Richburg JH. Sertoli cell toxicants. In: Skinner MK, Griswold MD, eds. Sertoli Cell Biology. New York: Elsevier Science; 2005:345–382. [Google Scholar]
- 34. Li L, Heindel J. Sertoli cell toxicants. In: Korach K, ed. Reproductive and Developmental Toxicology. New York: Marcel Dekker; 1998. [Google Scholar]
- 35. Vogl A, Pfeiffer D, Mulholland D, Kimel G, Guttman J. Unique and multifunctional adhesion junctions in the testis: ectoplasmic specializations. Arch Histol Cytol. 2000;63:1–15. [DOI] [PubMed] [Google Scholar]
- 36. Lee NPY, Cheng CY. Ectoplasmic specialization, a testis-specific cell-cell actin-based adherens junction type: is this a potential target for male contraceptive development. Hum Reprod Update. 2004;10:349–369. [DOI] [PubMed] [Google Scholar]
- 37. Vogl AW, Du M, Wang XY, Young JS. Novel clathrin/actin-based endocytic machinery associated with junction turnover in the seminiferous epithelium. Semin Cell Dev Biol. 2014;30:55–64. [DOI] [PubMed] [Google Scholar]
- 38. Su WH, Mruk DD, Cheng CY. Regulation of actin dynamics and protein trafficking during spermatogenesis—insights into a complex process. Crit Rev Biochem Mol Biol. 2013;48:153–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tang EI, Mok KW, Lee WM, Cheng CY. EB1 regulates tubulin and actin cytoskeletal networks at the Sertoli cell blood-testis barrier in male rats—an in vitro study. Endocrinology. 2015;156:680–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cheng CY, Mruk DD, Silvestrini B, et al. AF-2364 [1-(2,4-dichlorobenzyl)-1H-indazole-3-carbohydrazide] is a potential male contraceptive: A review of recent data. Contraception. 2005;72:251–261. [DOI] [PubMed] [Google Scholar]
- 41. Johnson KJ. Testicular histopathology associated with disruption of the Sertoli cell cytoskeleton. Spermatogenesis. 2014;4:e979106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Palombi F, Salanova M, Tarone G, Farini D, Stefanini M. Distribution of β1 integrin subunit in rat seminiferous epithelium. Biol Reprod. 1992;47:1173–1182. [DOI] [PubMed] [Google Scholar]
- 43. Salanova M, Stefanini M, De Curtis I, Palombi F. Integrin receptor α6β1 is localized at specific sites of cell-to-cell contact in rat seminiferous epithelium. Biol Reprod. 1995;52:79–87. [DOI] [PubMed] [Google Scholar]
- 44. Siu MKY, Wong CH, Xia W, Mruk DD, Lee WM, Cheng CY. The β1-integrin-p-FAK-p130Cas-DOCK180-RhoA-vinculin is a novel regulatory protein complex at the apical ectoplasmic specialization in adult rat testes. Spermatogenesis. 2011;1:73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Siu MKY, Cheng CY. Interactions of proteases, protease inhibitors, and the β1 integrin/laminin γ3 protein complex in the regulation of ectoplasmic specialization dynamics in the rat testis. Biol Reprod. 2004;70:945–964. [DOI] [PubMed] [Google Scholar]
- 46. Ozaki-Kuroda K, Nakanishi H, Ohta H, et al. Nectin couples cell-cell adhesion and the actin scaffold at heterotypic testicular junctions. Curr Biol. 2002;12:1145–1150. [DOI] [PubMed] [Google Scholar]
- 47. Poulter NS, Thomas SG. Cytoskeletal regulation of platelet formation: coordination of F-actin and microtubules. Int J Biochem Cell Biol. 2015;66:69–74. [DOI] [PubMed] [Google Scholar]
- 48. Burianek LE, Soderling SH. Under lock and key: Spatiotemporal regulation of WASP family proteins coordinates separate dynamic cellular processes. Semin Cell Dev Biol. 2013;24:258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Elie A, Prezel E, Guerin C, et al. Tau co-organizes dynamic microtubule and actin networks. Sci Rep. 2015;5:9964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pan J, Lordier L, Meyran D, et al. The formin DIAPH1 (mDia1) regulates megakaryocyte proplatelet formation by remodeling the actin and microtubule cytoskeletons. Blood. 2014;124:3967–3977. [DOI] [PubMed] [Google Scholar]
- 51. Shen QT, Hsiue PP, Sindelar CV, Welch MD, Campellone KG, Wang HW. Structural insights into WHAMM-mediated cytoskeletal coordination during membrane remodeling. J Cell Biol. 2012;199:111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Preciado Lopez M, Huber F, Grigoriev I, et al. Actin-microtubule coordination at growing microtubule ends. Nat Commun. 2014;5:4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schappi JM, Krbanjevic A, Rasenick MM. Tubulin, actin and heterotrimeric G proteins: coordination of signaling and structure. Biochim Biophys Acta. 2014;1838:674–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sayas CL, Avila J. Regulation of EB1/3 proteins by classical MAPs in neurons. Bioarchitecture. 2014;4:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jiang K, Akhmanova A. Microtubule tip-interacting proteins: a view from both ends. Curr Opin Cell Biol. 2011;23:94–101. [DOI] [PubMed] [Google Scholar]
- 56. Biosse Duplan M, Zalli D, Stephens S, et al. Microtubule dynamic instability controls podosome patterning in osteoclasts through EB1, cortactin, and Src. Mol Cell Biol. 2014;34:16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Linder S, Kopp P. Podosomes at a glance. J Cell Sci. 2006;118:2079–2082. [DOI] [PubMed] [Google Scholar]
- 58. Schachtner H, Calaminus SD, Thomas SG, Machesky LM. Podosomes in adhesion, migration, mechanosensing and matrix remodeling. Cytoskeleton (Hoboken). 2013;70:572–589. [DOI] [PubMed] [Google Scholar]
- 59. Georgess D, Machuca-Gayet I, Blangy A, Jurdic P. Podosome organization drives osteoclast-mediated bone resorption. Cell Adh Migr. 2014;8(3):191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cheng CY, Mruk DD. Regulation of blood-testis barrier dynamics by focal adhesion kinase (FAK). An unexpected turn of events. Cell Cycle. 2009;8:3493–3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Li SY, Mruk DD, Cheng CY. Focal adhesion kinase is a regulator of F-actin dynamics: new insights from studies in the testis. Spermatogenesis. 2013;3:e25385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Xiao X, Mruk DD, Cheng FL, Cheng CY. c-Src and c-Yes are two unlikely partners of spermatogenesis and their roles in blood-testis barrier dynamics. Adv Exp Med Biol. 2012;763:295–317. [DOI] [PMC free article] [PubMed] [Google Scholar]