Abstract
A variety of data suggest that estrogen action on kisspeptin (Kiss1)-containing arcuate nucleus neurons (which coexpress Kiss1, neurokinin B (the product of Tac2) and dynorphin (KNDy) neurons restrains reproductive onset and function, but roles for estrogen action in these Kiss1 neurons relative to a distinct population of rostral hypothalamic Kiss1 neurons (which does not express Tac2 or dynorphin) have not been directly tested. To test the role for estrogen receptor (ER)α in KNDy cells, we thus generated Tac2Cre and Kiss1Cre knock-in mice and bred them onto the Esr1flox background to ablate ERα specifically in Tac2-expressing cells (ERαTac2KO mice) or all Kiss1 cells (ERαKiss1KO mice), respectively. Most ERα-expressing Tac2 neurons represent KNDy cells. Arcuate nucleus Kiss1 expression was elevated in ERαTac2KO and ERαKiss1KO females independent of gonadal hormones, whereas rostral hypothalamic Kiss1 expression was normal in ERαTac2KO but decreased in ERαKiss1KO females; this suggests that ERα in rostral Kiss1 cells is crucial for control of Kiss1 expression in these cells. Both ERαKiss1KO and ERαTac2KO females displayed early vaginal opening, early and persistent vaginal cornification, increased gonadotropins, uterine hypertrophy, and other evidence of estrogen excess. Thus, deletion of ERα in Tac2 neurons suffices to drive precocious gonadal hyperstimulation, demonstrating that ERα in Tac2 neurons typically restrains pubertal onset and hypothalamic reproductive drive.
Hypothalamic-pituitary-end organ feedback systems are crucial mediators of endocrine homeostasis. In the case of the female reproductive system, gonadal estradiol acts via estrogen receptor (ER)α to mediate negative feedback during most of the reproductive cycle, as well as appropriately timed positive feedback to promote the midcycle preovulatory surge in gonadotropins (1). Because the final output for the hypothalamic control of reproductive function, GnRH neurons do not appear to express ERα, other cells that express ERα likely mediate these responses to estradiol (1).
Hypothalamic neurons that produce the neuropeptide kisspeptin (Kiss1) are one likely mediator of central estradiol feedback. Humans with mutations in KISS1 or its receptor (KISS1R) exhibit hypothalamic infertility (2, 3), and rodent studies reveal crucial roles for Kiss1 or Kiss1r in the control of puberty onset and fertility (3–7). In many species, the hypothalamus contains 2 distinct populations of Kiss1-synthesizing neurons (8–11). One set, in the arcuate nucleus (ARC) in rodents, coexpresses Kiss1, neurokinin B (NKB) (the product of Tac2), and dynorphin (KNDy) cells (12–15). The second population of hypothalamic Kiss1-containing neurons does not contain NKB or dynorphin and lies in the rostral hypothalamus (largely in the anteroventral periventricular nucleus [AVPV] region and extending into the periventricular nucleus [PeN]) in rodents. Most cells in both hypothalamic populations of Kiss1 neurons contain ERα (7, 16, 17).
Kiss1 stimulates GnRH neurons (18–20), but although both populations of Kiss1 neurons appear to synapse on GnRH neurons, estradiol regulation of the 2 Kiss1 cell types differs; estrogen suppresses Kiss1 expression in the ARC while enhancing Kiss1 expression in the AVPV (8, 21–23). Many have hypothesized that the AVPV Kiss1 neurons mediate estrogenic positive feedback, whereas KNDy neurons mediate negative feedback (17, 21, 24–27).
Deletion of ERα from all Kiss1 neurons dramatically advances puberty onset and promotes a phenotype of early-onset gonadal hyperstimulation in mice (25, 26), suggesting that estrogen action via ERα in Kiss1 neurons restrains gonadal function and pubertal onset. Selective ablation of ARC KNDy neurons in adult females blunts the increase in circulating gonadotropins that accompanies ovariectomy, but roles for these cells in development and pubertal onset have not been directly studied (28). The failure to detect normal rostral hypothalamic Kiss1-immunoreactivity (IR) in animals lacking ERα in all Kiss1 cells is consistent with a role of ERα in KNDy cells for pubertal restraint (25), but this has not been tested directly. Here, we have ablated ERα in all Kiss1 cells or specifically in Tac2 cells (leaving ERα intact in rostral hypothalamic Kiss1 cells) to distinguish roles for ERα in KNDy neurons.
Materials and Methods
Animals
All animals were bred in our colony in the Unit for Laboratory Animal Medicine at the University of Michigan in accordance with the guidelines and approval of the University Committee on the Care and Use of Animals. The Esr1flox mouse line has been previously described (29). Genotyping was by PCR to identify a loxP insertion (for primers, see Table 1). Occasional early embryonic recombination (Δ) around the loxP sites of this allele was detected using a separate forward primer together with the reverse primer used above; such animals were excluded from study.
Table 1.
Primers Used
| Genotyping | |||
|---|---|---|---|
| Cre Forward | WT Forward | Common Reverse | |
| Kiss1cre | acctggcctggtctggacac | ccaaggcagggagcttcta | gagggtgtgggcatatgagt |
| Tac2cre | acctggcctggtctggacac | cccccagctttggcatcctc | gcgaatgacagaccatctctcc |
| Forward | Reverse | Δ Forward | |
| Esr1flox | aaggctgcaaggctttcttt | gaaattcttagccacagcttc | gaatccaccagctgctgtag |
| Riboprobe Generation | |||
| Forward | Reverse | ||
| Kiss1 | aattaaccctcactaaagggagactgtgtcgccacctatgg | taatagcactcactatagggagatctagaagctccctgccttg | |
| Tac2 | aattaaccctcactaaagggagagctgtcctcagct | taatacgactcactatagggagatttgaggatgccaaagctccccctg | |
Generation of Kiss1cre and Tac2cre knock-in mice
5.6 kb of genomic DNA containing portions of exon 2 and the 3′-untranslated region (UTR) of the murine Kiss1 gene was amplified by PCR from R1 ES cells and cloned into a plasmid for insertion of a FRT-NEO-FRT-IRES-CRE cassette between the STOP codon and the polyadenylation site. Similarly, 5.2 kb of gDNA containing portions of exon 6 and the 3′-UTR of the murine Tac2 gene was amplified and cloned for insertion of the same FRT-NEO-FRT-IRES-CRE cassette. The targeting constructs were linearized using NotI and electroporated into R1 mouse embryonic stem cells at the University of Michigan Transgenic Animal Model Core. Neomycin-resistant clones were analyzed by quantitative real-time PCR (30) and further confirmed by Southern blotting using HindIII and an external 580-bp probe (Kiss1) or EcoRV and an external 560-bp probe (Tac2). Correctly targeted ES cells were injected into C57Bl/6J blastocysts to generate chimeras. Male chimeras were then bred to C57Bl/6J females, and pups were genotyped to confirm insertion of internal ribosome entry site (IRES)-cre into the appropriate locus. To remove the FRT-flanked NEO cassette, mice were bred to a line expressing Flp recombinase in the germline mouse line (Jax 012930), and excision was confirmed by PCR.
Mouse model validation
Kiss1cre/+ and Tac2cre/+ males were bred to homozygous cre-inducible eYFP reporter females (Jax 006148) to generate Kiss1eYFP and Tac2eYFP animals, respectively. Dual-label immunohistochemistry/in situ hybridization (IHC/ISH) was performed as previously described (31) on brains from adult female Kiss1eYFP and Tac2eYFP mice. Enhanced yellow fluorescent protein (eYFP) was detected using a chicken anti-green fluorescent protein primary antibody (1:5000; Abcam).
Perfusion and IHC
Blood was drawn from the heart of female mice (age 21 d or 9–10 wk) after administration of an overdose of sodium pentobarbital. They were then perfused transcardially with PBS (pH 7.4) followed by 10% formalin. Blood was allowed to clot for 30 minutes at room temperature and then centrifuged for 15 minutes at 2000g, and the serum was removed and stored at −20°C. The brain was removed and postfixed in 10% formalin for 2–4 hours and then dehydrated in 30% sucrose in PBS until the time of sectioning. Brains were cut in 30-μm coronal sections on a sliding microtome, collected in 4 representative series, and stored at −20°C in cryoprotectant (32).
For Kiss1 immunostaining, free-floating brain sections were thoroughly washed with PBS to remove the cryoprotectant, incubated in 1% H2O2 in PBS, blocked in 3% normal donkey serum/3% Triton X-100 in PBS and then incubated with primary antibody (rabbit anti-Kiss1, 1:2000; Millipore) overnight. On the following day, sections were washed again with PBS and then incubated in biotinylated donkey-antirabbit (1:200; Jackson ImmunoResearch) for 2 hours, followed by avidin-biotin-complex labeling (Vectastain Elite kit; Vector Laboratories). Signals were developed with diaminobenzidine resulting in a brown precipitate.
For eYFP and ERα immunostaining, sections were blocked as described above and then incubated in primary antibodies (chicken anti-green fluorescent protein, 1:5000 [Abcam]; rabbit anti-ERα, 1:1000 [Santa Cruz Biotechnology, Inc]) overnight. Brain sections were washed and then incubated with Alexa Fluor-conjugated secondary antibodies (goat antichicken 488 and donkey antirabbit 568, 1:250; Invitrogen). Sections were mounted onto gelatin-coated slides and coverslipped with ProLong Antifade mounting medium (Invitrogen).
Longitudinal study
To ablate ERα from Kiss1- and Tac2-neurons, we crossed each Cre/+ line (Tac2cre/+ and Kiss1cre/+) to Esr1flox/flox mice, and then crossed the resultant Cre/+;Esr1flox/+ males to Esr1flox/+ females. The F2 litters contained Cre/+;Esr1flox/flox (ERαKiss1KO and ERαTac2KO, respectively) mice, along with Esr1flox/flox and Cre/+ control mice for study. When appropriate, Cre/+ and Esr1flox/flox mice were combined into one larger control group for comparison with the appropriate Cre/+;Esr1flox/flox animals.
Females were checked daily for vaginal opening beginning at postnatal day 10. Mice were individually housed beginning at postnatal day 21; body weight was measured weekly. After vaginal opening, estrous cycle phase was determined daily based on vaginal cytology. At 8 weeks of age, body composition was determined by a Minispec LF90II (Bruker Optics) analyzer. Tissue was harvested from 9-week-old mice (some controls in diestrus and some in estrus, based on vaginal cytology). Using a mouse brain matrix, the ARC and a triangle of tissue containing the AVPV/PeN plus preoptic area were dissected and individually snap frozen on dry ice. Brain tissue was stored at −80° for later RNA extraction. Ovaries and uterus were removed, weighed, and fixed overnight in Z-fix (Anatech) and then transferred to 70% ethanol for storage. Ovaries were sectioned at 4 μm on a cryostat and stained with hematoxylin and eosin by the University of Michigan Comprehensive Cancer Center Tissue Core. The number of follicles or corpora lutea was counted by an individual blind to the genotype.
RNA was extracted from frozen microdissected brain tissue using TRIzol (Invitrogen) and then converted to cDNA using the iScript cDNA Synthesis kit (Bio-Rad). Gene expression was analyzed in triplicate by quantitative RT-PCR using Gapdh (endogenous control; VIC) and Kiss1 (FAM) TaqMan assays (Applied Biosystems). Relative mRNA expression values were calculated by the 2−ΔΔCt method.
Ovariectomy
Nine-week-old female mice (ERαKiss1KO, ERαTac2KO and their littermate controls) were ovariectomized (OVX) under isoflurane anesthesia. Blood was collected in a heparinized capillary tube from the tail vein before ovariectomy. Seven days after ovariectomy, all mice were killed by anesthetic overdose, and hypothalamic regions were microdissected as described above.
Microscopy and image analysis
Microscopic images were obtained using an Olympus BX-51 microscope with a DP30BW camera (Olympus) or a Nikon Eclipse 90i microscope with a DS-Fi1 color camera (Nikon) (for color images [ovaries histology, dual IHC/ISH]). Images from fluorescent labeling experiments were pseudocolored and merged using Adobe Photoshop.
Hormone analysis
Serum or plasma was analyzed by the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core (estradiol) or the University of Michigan Chemistry Core (LH and FSH). Estradiol concentrations were determined by ELISA (Calbiotech) (intraassay coefficient of variation (CV) 6.3%; interassay CV 8.1%), whereas LH and FSH concentrations were assessed using the Millipore MILLIPLEX MAP rat pituitary panel; all were run in a single assay (intraassay CV 9.4% [FSH] and 14.1% [LH]).
Statistics
Data were analyzed and graphs were generated using either OriginPro 8 or GraphPad Prism software. Data were normally distributed. Student's t test was used when only 2 groups were compared. ANOVA with Bonferroni post hoc analysis was used when comparing 3 or more groups. Differences were deemed significant if P < .05. Data are presented as mean ± SEM.
Results
Generation and validation of Tac2 and Kiss1 cell-specific mouse lines
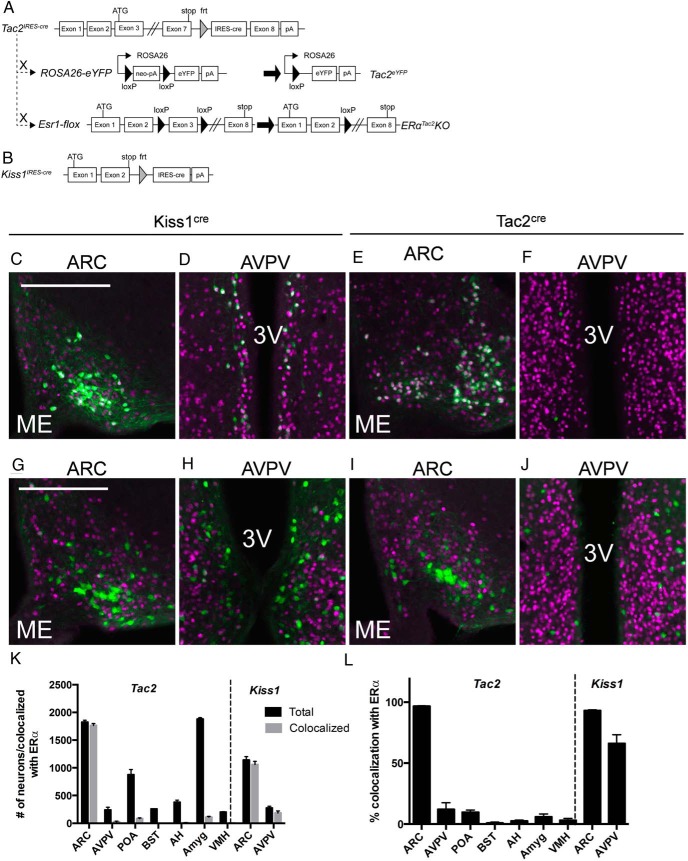
Reasoning that neurons coexpressing NKB and ERα likely specify KNDy cells, we set out to generate tools to ablate the gene encoding ERα (Esr1) in Tac2- or all Kiss1-expressing cells. We thus generated knock-in mouse lines in which we inserted an IRES plus the coding sequence of cre recombinase in the 3′-UTR of the terminal exons of the Tac2 and Kiss1 loci (Tac2cre and Kiss1cre mice, respectively) to mediate the expression of cre from the mRNA encoding the relevant neuropeptide (Figure 1, A and B).
Figure 1.
Generation of mice to study Kiss1- and Tac2-expressing neurons and coexpression of ERα and eYFP in Tac2eYFP and Kiss1eYFP female mice. Tac2cre mice carry an IRES-cre cassette in the 3′-UTR of the endogenous murine Tac2 gene (A). Kiss1cre mice carry the same IRES-cre cassette in the 3′-UTR of the murine Kiss1 gene (B). Both cre lines were crossed to ROSA26-eYFP and Esr1-flox animals to generate fluorescent reporter and neuron-specific ERα deletion strains (shown for Tac2cre). eYFP- and ERα-IR (pseudocolored green and purple, respectively) in the ARC (C) and the AVPV (D) of a Kiss1eYFP mouse and in the ARC (E) and AVPV (F) of a Tac2eYFP mouse. eYFP- and ERα-IR (pseudocolored green and purple, respectively) in the ARC (G) and the AVPV (H) of a Kiss1eYFP/ERαKiss1KO mouse and in the ARC (I) and AVPV (J) of a Tac2eYFP/ERαTac2KO mouse. Neurons expressing both eYFP and ERα appear white. 3V, third ventricle; ME, median eminence. K, Counts of Tac2 and Kiss1 neurons in Tac2eYFP and Kiss1eYFP female mice in the indicated brain regions (black bars) and numbers of Tac2 and Kiss1 neurons colocalized with ERα (gray bars). L, Data from K plotted to show % Tac2 and Kiss1 neurons colocalizing with ERα in each region. Mean ± SEM is plotted (from a 1:4 series through the brains of n = 3 animals per genotype).
We crossed Tac2cre and Kiss1cre mice onto the cre-activated ROSA26-eYFP reporter mouse line, generating Tac2eYFP and Kiss1eYFP animals to reveal sites of cre expression (Figure 1, A and B). To examine the overlap of YFP expression with Kiss1- and Tac2-expression within the brain in these 2 novel mouse lines, we performed dual-label IHC/ISH on hypothalamic sections from Kiss1eYFP and Tac2eYFP female mice (Supplemental Figure 1). In the ARC of Tac2eYFP females, most (96%) eYFP-IR colocalized with Tac2 mRNA and Kiss1 mRNA (97%). Although we observed Kiss1 hybridization adjacent to the third ventricle in the AVPV of Tac2eYFP females, we found few eYFP cells in that area (and no colocalization), consistent with the absence of Tac2 in AVPV/PeN Kiss1 neurons. Similarly, Kiss1 probe and eYFP-IR colocalized in Kiss1eYFP sections, confirming the coexpression of cre and Kiss1 in these mice.
We also examined the colocalization of ERα with eYPF in Tac2eYFP and Kiss1eYFP mice. In Kiss1eYFP mice, nearly all (93 ± 11%; n = 3) of ARC eYFP cells express ERα and approximately 2/3 (66 ± 7%; n = 3) of AVPV eYFP cells express ERα (Figure 1, C and D). In Tac2eYFP female mice, nearly all (96 ± 1%; n = 3) ARC eYFP cells express ERα, but we detected no eYFP/ERα cells in the AVPV/PeN (Figure 1, E, F, and K). Although we found occasional eYFP/ERα cells elsewhere in the brain of Tac2eYFP mice, they did not cluster in any particular region and were few in number (Figure 1K). Thus, ARC Tac2/ERα cells, which approximate KNDy cells, represent the vast majority (∼90%) of central nervous system Tac2/eYFP+ERα cells.
Cre-mediated excision of Esr1flox by Kisscre ablated ERα from Kiss1 cells in both the ARC and AVPV/PeN, as revealed by the lack of ERα-IR in eYFP neurons in Kiss1eYFP/ERαflox/flox females (0% of ARC and 6% of AVPV/PeN eYFP cells contained ERα-IR; n = 2) (Figure 1, F–I). Excision of Esr1flox by Tac2cre similarly ablated ERα from Tac2 cells in the ARC of a Tac2eYFP/ERαflox/flox mouse (4% of ARC eYFP cells contained ERα-IR; n = 2) (Figure 1, K and L).
Consistent with previous reports, we detected eYFP cells in the ovary of Kiss1eYFP (Supplemental Figure 2). We also detected evidence of Tac2 expression in the pituitary and ovary of Tac2 reporter mice. Although our IHC analysis detected no ERα in these cells in WT animals (data not shown), it remains possible that Kiss1cre and Tac2cre ablate functional ERα from some or all of the cells that express Kiss1 or Tac2 outside of the brain (others report Kiss1 expression in pancreatic islets, adipose, and other tissues, as well) (33, 34), in addition to deleting ERα from cells in the ARC and/or AVPV/PeN. Although our analysis (Supplemental Figure 1) and other reports suggest that virtually all ARC Tac2 cells coexpress Kiss1 (12, 13), it is also theoretically possible that some non-KNDy cells are affected in the brain of Tac2eYFP/ERαflox/flox animals, although AVPV/PeN Kiss1 neurons should be unaffected.
Analysis of Kiss1 expression in ERαKiss1KO and ERαTac2KO females
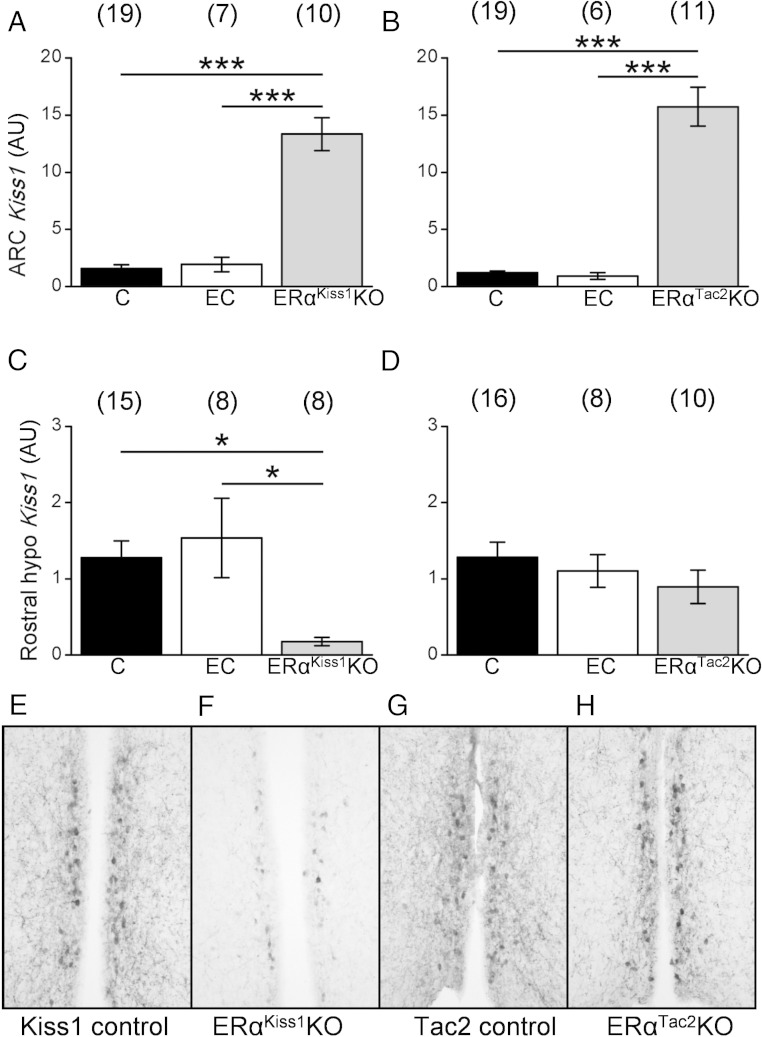
In wild-type animals, estrogen promotes AVPV Kiss1 expression while inhibiting ARC Kiss1 expression (Smith et al [21] and Frazao et al [26]). To examine the potential role for ERα in specific cell types for the control of Kiss1 expression in these areas in our mouse models, we extracted RNA from microdissected tissue (ARC or the AVPV/PeN-containing rostral hypothalamus) from ERαKiss1KO and ERαTac2KO females to determine relative Kiss1 mRNA levels (Figure 2). Deletion of ERα from Kiss1 and Tac2 neurons increased ARC Kiss1 expression in ERαKiss1KO and ERαTac2KO females, consistent with a cell-autonomous role for ERα in the suppression of Kiss1 expression in KNDy cells (Figure 2, A and B). Deletion of ERα from all Kiss1 cells in ERαKiss1KO females decreased Kiss1 in the rostral hypothalamus, whereas deletion of ERα solely from KNDy neurons in ERαTac2KO females did not alter Kiss1 mRNA in the rostral hypothalamus (Figure 2, C and D). Similarly, AVPV Kiss1-IR was diminished in adult ERαKiss1KO females (Figure 2, E and F), but not ERαTac2KO females (Figure 2, G and H), consistent with the observed changes in rostral hypothalamic gene expression. This could reflect a decrease in the total number of AVPV Kiss1 cells and/or decreased intensity of staining and soma detection due to a decrease in Kiss1 content in each AVPV Kiss1 neuron. Thus, ERα in Tac2 neurons suppresses ARC Kiss1 expression, whereas ERα in non-Tac2 cells independently controls rostral hypothalamic Kiss1 mRNA expression.
Figure 2.
Kiss1 expression in intact ERαKiss1KO and ERαTac2KO females. Relative expression of Kiss1 in the ARC (A and B) and rostral hypothalamus (C and D) of intact female ERαKiss1KO (left panels) and ERαTac2KO (right panels) mice. Controls were collected in diestrus/metestrus (control) or estrus (estrous control). One-way ANOVA, Bonferroni post hoc: *, P < .05; ***, P < .001. Numbers in parentheses above each bar denote the n for each measurement. Representative Kiss1-IR (from ≥3 animals of each genotype) in the AVPV/PeN of intact adult females of the indicated genotype (E–H).
Estradiol feedback regulation
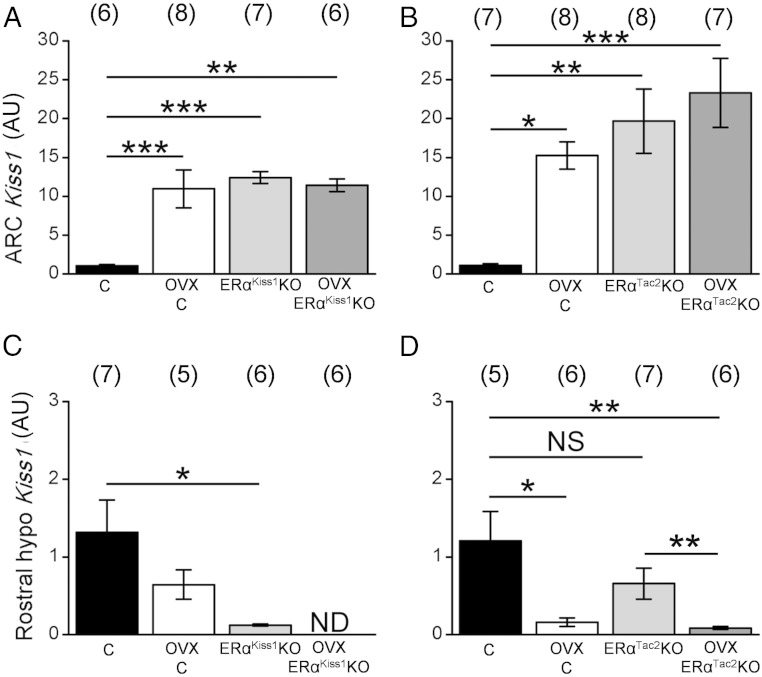
To assess gonadal feedback on Kiss1 expression in ERαKiss1KO and ERαTac2KO mice, we also examined ARC and rostral hypothalamic Kiss1 mRNA after ovariectomy (Figure 3). ARC Kiss1 expression in control animals rose dramatically as a result of ovariectomy, to levels that were indistinguishable from those of intact or OVX ERαKiss1KO and ERαTac2KO females. In contrast, ARC Kiss1 expression was not changed by ovariectomy in the ERαKiss1KO or ERαTac2KO females (Figure 3, A and B). Thus, ERα in KNDy neurons suppresses Kiss1 expression by these cells in response to gonadal signals.
Figure 3.
Kiss1 expression in intact and OVX ERαKiss1KO and ERαTac2KO females. Relative expression of Kiss1 in the ARC (A and B) or rostral hypothalamus (C and D) of intact and OVX control and ERαKiss1KO (left panels) or ERαTac2KO (right panels) adult females. Numbers in parentheses above each bar denote the n for each measurement. Two-way ANOVA, Bonferroni post hoc: *, P < .05; **, P < .01; ***, P < .001. NS, not significant; ND, not detected.
Although ovariectomy tended to decrease rostral hypothalamic Kiss1 expression in control animals, as reported previously (21), this difference was only significant in the control littermates of ERαTac2KO females (Figure 3, C and D). As above, rostral hypothalamic Kiss1 expression was diminished in intact ERαKiss1KO but not ERαTac2KO females. Ovariectomy decreased rostral hypothalamic Kiss1 expression not only in ERαTac2KO females but also further reduced the already low rostral hypothalamic Kiss1 expression in ERαKiss1KO mice (Figure 3C).
Body weight and composition in ERαKiss1KO and ERαTac2KO females
The body mass of ERαKiss1KO females was not significantly altered compared with littermate controls at early ages, but at 8 weeks of age, ERαKiss1KO females were slightly heavier than control littermates (Supplemental Figure 3). A similar effect was observed in ERαTac2KO females, which were slightly heavier than their littermate controls starting at 5 weeks of age (Supplemental Figure 3). Body composition analysis of 8-week-old females revealed that both ERαKiss1KO and ERαflox/flox mice had lower body fat percentage than Kiss1IRES−cre/+ mice and also that ERαKiss1KO females have increased fluid percentage compared with both littermate control genotypes (Supplemental Figure 3). The same effect was observed in ERαTac2KO mice and their littermate controls; Tac2IRES−cre/+ mice have significantly higher fat percentage compared with both ERαflox/flox and ERαTac2KO animals and we found increased fluid percentage in ERαTac2KO females compared with both littermate control genotypes (Supplemental Figure 3).
Reproductive function in ERαKiss1KO and ERαTac2KO females
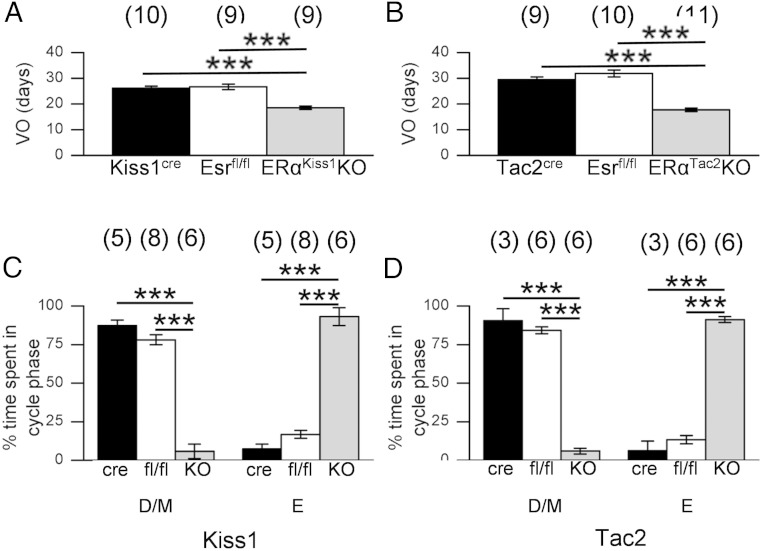
Vaginal opening is an external signal of puberty onset in rodents; administration of Kiss1 accelerates this and other markers of pubertal onset (20). Vaginal opening occurred in ERαKiss1KO and ERαTac2KO mice significantly earlier than their littermate controls (Figure 4, A and B). Vaginal cytology revealed early cornification of the vaginal epithelium in ERαKiss1KO and ERαTac2KO females, most of which displayed estrogenized (cornified) cytology when first checked at 21 days of age; in contrast, controls exhibited first estrus between 35 and 40 days of age (data not shown). Furthermore, ERαKiss1KO and ERαTac2KO mice spent significantly less time in diestrus or metestrus and significantly more time in estrus than their littermate controls (Figure 4, C and D).
Figure 4.
Advanced vaginal opening and persistent cornification in ERαKiss1KO and ERαTac2KO females. Average age at vaginal opening of littermate controls and ERαKiss1KO females (A) and ERαTac2KO females (B). Numbers in parentheses above each bar denote the n for each measurement. Representative vaginal cytology over time for control and ERαKiss1KO females (C) and control and ERαTac2KO females (D). Percentage of time spent in diestrus/metestrus and estrus (based on vaginal cytology) from the time of vaginal opening until 8 weeks of age for mice of the indicated genotypes. Numbers in parentheses above each bar denote the n for each measurement. One-way ANOVA, Bonferroni post hoc: ***, P < .001. VO, vaginal opening; C, cornified epithelial cells; N, nucleated epithelial cells; L, leukocytes; D/M, diestrus/metestrus; E, estrus.
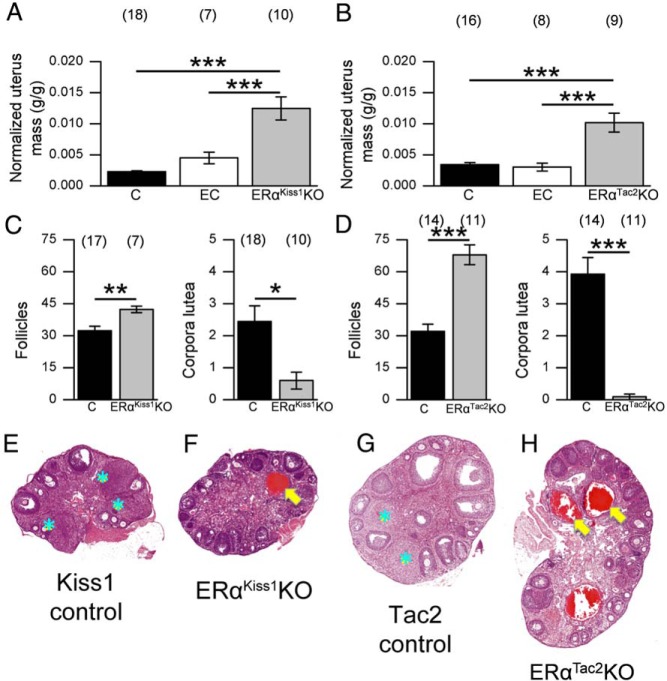
In addition to their precocious and persistent estrus, both ERαKiss1KO and ERαTac2KO females exhibit additional abnormalities in their reproductive organs, including enlarged, fluid-filled uteri (Figure 5, A and B). Furthermore, we observed an increase in the total number of follicles, including hemorrhagic cysts, within the ovaries of ERαKiss1KO and ERαTac2KO females, coupled with a reduced number of corpora lutea (Figure 5, C–H). Thus, ablation of ERα from either Kiss1- or Tac2-expressing neurons results in early vaginal opening and estrogenization, along with ovarian and uterine histology consistent with hyperstimulation of the hypothalamic-pituitary-gonadal axis.
Figure 5.
Ovarian and uterine phenotypes of adult ERαKiss1KO and ERαTac2KO females. Uterine mass (normalized to body mass [g/g]) of 9-week-old control (C) (Kiss1IRES−Cre/+ and ERαflox/flox females dissected in diestrus/metestrus), estrous control (EC) (littermate controls dissected in estrus), and ERαKiss1KO females (A) (n = 7–18). Uterine mass of control, estrous control, and ERαTac2KO females (B) (n = 8–16). Number of follicles and corpora lutea per 2 ovary sections per animal from control and ERαKiss1KO (C) or ERαTac2KO (D) females. Numbers in parentheses above each bar denote the n (number of animals) for each measurement. One-way ANOVA (A and B), Bonferroni post hoc: ***, P < .001. Student's t test (C and D): *, P < .05; **, P < .01; ***, P < .001. Representative hematoxylin and eosin (H&E)-stained ovary sections from littermate control (E) and ERαKiss1KO females (F) and littermate control (G) and ERαTac2KO females (H). Blue stars denote corpora lutea, whereas yellow arrows indicate hemorrhagic cysts. All images were taken at the same magnification (×4) and were scaled identically.
Gonadotropins and gonadal hormones
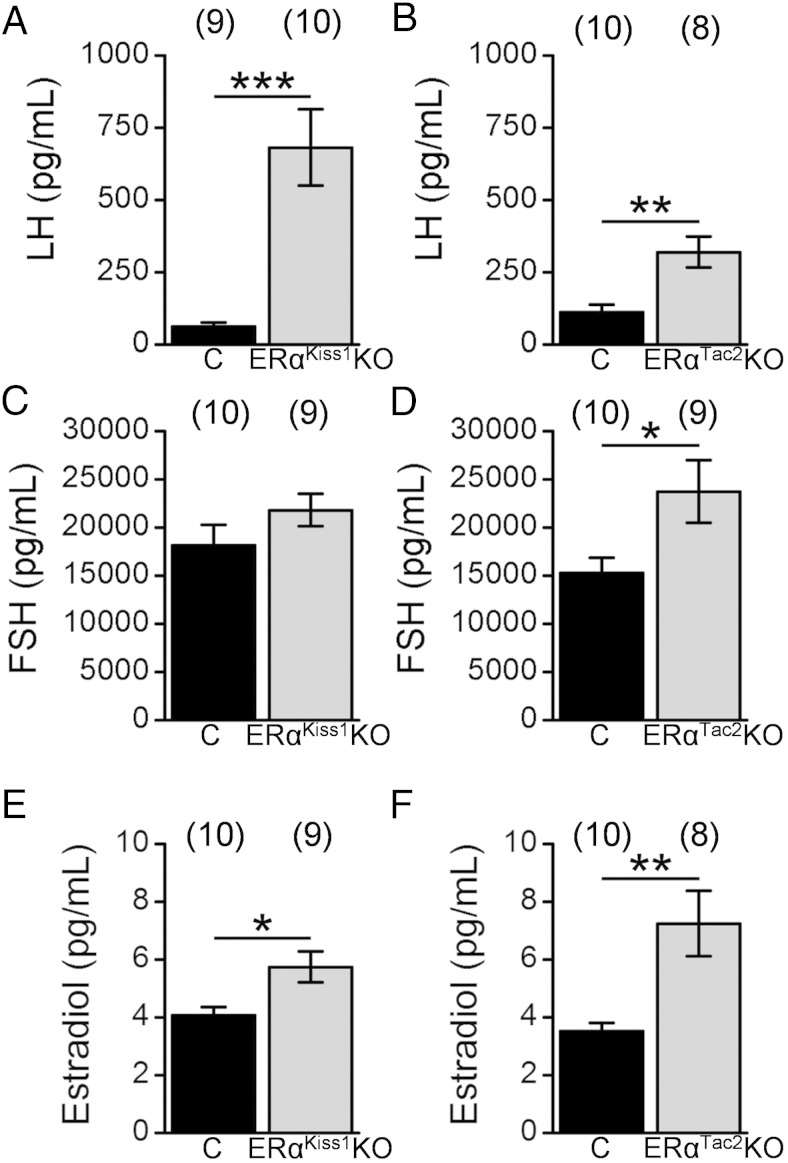
To assess whether deletion of ERα from Kiss1 or Tac2 cells altered gonadotropin and/or estradiol levels consistent with the phenotype of the ERαKiss1KO and ERαTac2KO females, we analyzed serum from juvenile (3 wk old) (Figure 6) and adult (9 wk old) (Figure 7) animals. Serum LH concentrations were elevated in juvenile ERαKiss1KO females compared with littermate controls (Figure 6A), although FSH was not affected (Figure 6C). Not surprisingly, given their precocious vaginal opening, juvenile ERαKiss1KO females also had significantly higher estradiol levels than their littermate controls (Figure 6E). We observed a similar effect on serum LH and estradiol in the ERαTac2KO females (Figure 6B). The ERαTac2KO females displayed elevated FSH concentrations (Figure 6D), however.
Figure 6.
Gonadotropin and estradiol levels of intact juvenile ERαKiss1KO and ERαTac2KO females. Serum LH (A and B), FSH (C and D), and estradiol (E and F) in control (C) and ERαKiss1KO (left panels) or ERαTac2KO females (right panels). Numbers in parentheses above each bar denote the n for each measurement. Student's t test: *, P < .05; **, P < .01; ***, P < .001.
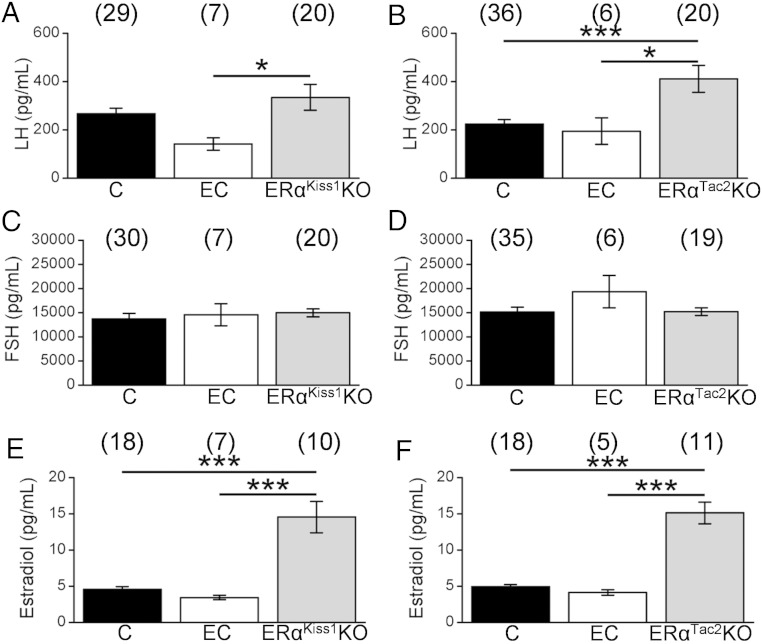
Figure 7.
Gonadotropin and estradiol levels in intact adult ERαKiss1KO and ERαTac2KO females. Serum LH (A and B), FSH (C and D), and estradiol (E and F) levels of 9-week-old diestrous control (C), estrous control (EC), and ERαKiss1KO (left panels) or ERαTac2KO females (right panels). Numbers in parentheses above each bar denote the n for each measurement. One-way ANOVA, Bonferroni post hoc: *, P < .05; ***, P < .001.
Because ERαKiss1KO and ERαTac2KO females showed persistent vaginal cornification, we assayed serum from a group of littermate controls in estrus, as well as diestrus, alongside the adult ERαKiss1KO and ERαTac2KO animals. Adult female ERαKiss1KO animals displayed elevated LH concentrations compared with estrous, but not diestrous, controls, whereas LH concentrations in adult ERαTac2KO females were elevated compared with both cycle stages in control mice (Figure 7, A and B). FSH levels were similar among all genotypes in the adult females (Figure 7, C and D). Both ERαKiss1KO and ERαTac2KO adult females displayed estradiol levels that were approximately 3 times higher than controls (Figure 7, E and F).
Discussion
Important aspects of estradiol feedback upon reproductive neuroendocrine activity has been postulated to be mediated by ERα-expressing Kiss1 neurons of the AVPV and ARC, but the roles of the Kiss1 neurons in these individual regions has not been empirically distinguished. To test directly roles for ERα in Tac2 (including KNDy) neurons, while maintaining endogenous ERα in AVPV/PeN Kiss1 neurons, we genetically ablated ERα in all Kiss1-expressing cells or in Tac2 cells. The essentially complete colocalization of cre reporter expression and mRNA from the relevant neuropeptide is as expected based upon the expression of cre from the Tac2 and Kiss1 mRNAs in these knock-in mice, and based upon the previous findings of others with similar cre lines and for the colocalization of Tac2 and Kiss1 in the ARC (12, 13, 25, 26, 35). Because KNDy neurons coexpress the product of the Tac2 gene (NKB), as well as Kiss1, we used our novel Tac2cre mouse line to ablate ERα in KNDy neurons, but not AVPV/PeN Kiss1 cells. Because ARC Tac2/ERα cells, which approximate KNDy cells, represent the vast majority (∼90%) of central nervous system Tac2/eYFP+ERα cells; our results therefore likely reflect effects of ERα deletion in ARC Tac2 cells.
Although our IHC analysis detected no ERα in the pituitary and ovarian cells that exhibit Kiss1cre- and Tac2cre-mediated recombination in animals containing wild-type Esr1 (data not shown), it remains possible that Kiss1cre and Tac2cre ablate functional ERα from some or all of the cells that express Kiss1 or Tac2 outside of the brain (others report Kiss1 expression in pancreatic islets, adipose, and other tissues, as well) (33, 34), in addition to deleting ERα from cells in the ARC and/or AVPV/PeN. Although our analysis (Supplemental Figure 1) and other reports suggest that virtually all ARC Tac2 cells coexpress Kiss1 (12, 13), it is also theoretically possible that some non-KNDy cells are affected in the brain of Tac2eYFP/ERαflox/flox animals, although AVPV/PeN Kiss1 neurons should be unaffected.
The ablation of ERα from all Kiss1 cells in our hands resulted in early vaginal opening and cornification of vaginal epithelial cells, and a phenotype of ovarian hyperstimulation characterized by elevated circulating gonadotropins and estradiol, fluid-filled uteri, increased follicle development with hemorrhagic cysts, and few corpora lutea, consistent with previous findings (25, 26).
Our finding of a similar phenotype upon the deletion of ERα from Tac2 neurons thus refines our understanding of the locus of central estradiol negative feedback on puberty onset and gonadal restraint. The similarities between the phenotypes presented by ERαKiss1KO and ERαTac2KO females suggest that the precocious estrogenization and ovarian hyperstimulation results from the loss of ERα from the ARC KNDy neurons, because this is common to both knockout lines.
Body weight was increased in both ERαKiss1KO and ERαTac2KO females due to increased fluid content; this likely reflects the increase in fluid in their uteri, as noted above. In contrast, there was no effect of ablation of ERα from Kiss1 or Tac2 cells on adiposity (although the presence of the Esr1Flox allele decreased adiposity compared with cre only controls).
Others have shown that the ablation of ARC KNDy cells in adult animals blunts the increase in LH secretion that follows ovariectomy (28). The present data suggest that the deletion of ERα from Tac2-expressing neurons sets in motion a series of changes that likely begins with an increase in Kiss1 expression and culminates in increased gonadotropin drive. That is, during normal prepubertal development, estradiol restrains a positive signal from KNDy neurons, rather than promoting an inhibitory KNDy-derived signal. The negligible to modest elevations in LH, FSH, and estradiol concentrations in adult ERαKiss1KO and ERαTac2KO females relative to the elevations observed in younger animals also suggests the potential for compensatory circuit reorganization with age in these animals.
Circulating estradiol controls Kiss1 mRNA expression differently in the 2 hypothalamic Kiss1 cell types, suppressing Kiss1 in ARC cells and supporting Kiss1 in rostral hypothalamic neurons (21, 26). Ablation of ERα from all Kiss1 neurons or specifically from KNDy neurons promotes ARC Kiss1 gene expression, mimicking the levels found in OVX animals. These findings are consistent with a crucial role for estrogen/ERα in KNDy cells in the repression of Kiss1 in these cells. Although deletion of ERα from KNDy neurons in ERαTac2KO mice did not alter AVPV Kiss1 expression, deletion of ERα from all Kiss1 cells decreased Kiss1 expression in this area, demonstrating that estrogen acts via ERα in AVPV/PeN Kiss1 cells to support Kiss1 expression in these cells. Similarly, AVPV Kiss1-IR is diminished in adult ERαKiss1KO females, but not ERαTac2KO females, consistent with the observed changes in rostral hypothalamic gene expression. Thus, ERα in Tac2 neurons suppresses ARC Kiss1 expression, whereas ERα in non-Tac2 cells independently controls rostral hypothalamic Kiss1 mRNA expression. This could reflect a decrease in the total number of AVPV Kiss1 cells and/or decreased intensity of staining and soma detection due to a decrease in Kiss1 content in each AVPV Kiss1 neuron. This is consistent with previous findings suggesting that early deletion of ERα in Kiss1 neurons interferes with the normal development (and Kiss1 expression) of these cells (25).
Because the deletion of ERα alters Kiss1 expression oppositely in ARC vs AVPV Kiss1 cells, ERα may recruit different cofactors to the Kiss1 promoter region in each cell type, or ERα may act via distinct second order transcriptional regulators in each population of Kiss1 cells. Indeed, estrogen may increase Kiss1 expression by a unique epigenetically regulated enhancer in AVPV/PeN Kiss1 cells (36).
Because ovariectomy further suppressed already low Kiss1 expression in the rostral hypothalamus of ERαKiss1KO females, gonadal hormones contribute to AVPV Kiss1 expression not only via ERα in AVPV Kiss1 neurons, but also via mechanisms independent of ERα in these cells. Thus, in contrast to Tac2 neurons in the ARC, where the control of Kiss1 expression absolutely requires ERα, a second gonadal signal (distinct from estrogen/ERα) independently controls the expression of Kiss1 by AVPV neurons. Additionally, the finding that estrogen decreases LH concentrations in ERαKiss1KO females demonstrates the existence of other (non-Kiss1) loci of estrogenic negative feedback in adults (37). Indeed, we observed similar effects in ERαKiss1KO and ERαTac2KO females (Supplemental Figure 4). Although estrogenic feedback on the pituitary is likely to participate, other sites in the ARC or elsewhere may also play a role (38).
Our study was not designed to specifically test the postulate that the non-KNDy Kiss1 neurons of the rostral hypothalamus mediate estrogen positive feedback to the reproductive axis. Furthermore, there may be developmental changes in the circuitry of Kiss1 cells and/or other neurons that control reproductive function in our knockout animals; indeed, others have suggested that early deletion of ERα from AVPV Kiss1 cells impairs their development and expression of Kiss1 (25).
Overall, our findings demonstrate that ERα in Tac2 neurons normally restrains pubertal onset and hypothalamic reproductive drive, because deletion of ERα from these cells does not alter AVPV/PeN ERα or Kiss1 expression, but promotes precocious onset of puberty and subsequent gonadal hyperfunction. The ARC KNDy neurons thus constitute a critical element of the sensitivity to gonadal steroids that regulate puberty onset and sexual maturation.
Acknowledgments
We thank David Olson, Michael Lehman, Lique Coolen, and members of the Myers lab for helpful discussions.
This work was supported by the Animal Phenotyping Core and the Clinical Core of the Michigan Diabetes Research Center (National Institutes of Health [NIH] Grant P30 DK020572); the American Diabetes Association; the American Heart Association; the Marilyn H. Vincent Foundation (M.G.M.); and NIH Grants DK057768 (to M.G.M.), HD041469 (to S.M.M.), and HD069702 (to C.F.E.). The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/NIH (SCCPIR) Grant U54-HD28934.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ARC
- arcuate nucleus
- AVPV
- anteroventral periventricular nucleus
- CV
- coefficient of variation
- ER
- estrogen receptor
- eYFP
- enhanced yellow fluorescent protein
- IHC/ISH
- immunohistochemistry/in situ hybridization
- IR
- immunoreactivity
- IRES
- internal ribosome entry site
- Kiss1
- kisspeptin
- KNDy
- Kiss1, NKB, and dynorphin
- NKB
- neurokinin B
- OVX
- ovariectomized
- PeN
- periventricular nucleus
- UTR
- untranslated region.
References
- 1. Wintermantel TM, Elzer J, Herbison AE, Fritzemeier KH, Schutz G. Genetic dissection of estrogen receptor signaling in vivo. Ernst Schering Found Symp Proc. 2006;(1):25–44. [DOI] [PubMed] [Google Scholar]
- 2. Topaloglu AK, Tello JA, Kotan LD, et al. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N Engl J Med. 2012;366(7):629–635. [DOI] [PubMed] [Google Scholar]
- 3. Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614–1627. [DOI] [PubMed] [Google Scholar]
- 4. d'Anglemont de Tassigny X, Fagg LA, et al. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci USA. 2007;104(25):10714–10719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dungan HM, Gottsch ML, Zeng H, et al. The role of kisspeptin-GPR54 signaling in the tonic regulation and surge release of gonadotropin-releasing hormone/luteinizing hormone. J Neurosci. 2007;27(44):12088–12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lapatto R, Pallais JC, Zhang D, et al. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology. 2007;148(10):4927–4936. [DOI] [PubMed] [Google Scholar]
- 7. Clarkson J, d'Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J Neurosci. 2008;28(35):8691–8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clarkson J, d'Anglemont de Tassigny X, Colledge WH, Caraty A, Herbison AE. Distribution of kisspeptin neurones in the adult female mouse brain. J Neuroendocrinol. 2009;21(8):673–682. [DOI] [PubMed] [Google Scholar]
- 9. Foradori CD, Amstalden M, Goodman RL, Lehman MN. Colocalisation of dynorphin a and neurokinin B immunoreactivity in the arcuate nucleus and median eminence of the sheep. J Neuroendocrinol. 2006;18(7):534–541. [DOI] [PubMed] [Google Scholar]
- 10. Gottsch ML, Navarro VM, Zhao Z, et al. Regulation of Kiss1 and dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. J Neurosci. 2009;29(29):9390–9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roa J, Vigo E, Castellano JM, et al. Hypothalamic expression of KiSS-1 system and gonadotropin-releasing effects of kisspeptin in different reproductive states of the female rat. Endocrinology. 2006;147(6):2864–2878. [DOI] [PubMed] [Google Scholar]
- 12. Goodman RL, Lehman MN, Smith JT, et al. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148(12):5752–5760. [DOI] [PubMed] [Google Scholar]
- 13. Kauffman AS, Navarro VM, Kim J, Clifton DK, Steiner RA. Sex differences in the regulation of Kiss1/NKB neurons in juvenile mice: implications for the timing of puberty. Am J Physiol Endocrinol Metab. 2009;297(5):E1212–E1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29(38):11859–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. True C, Kirigiti M, Ciofi P, Grove KL, Smith MS. Characterisation of arcuate nucleus kisspeptin/neurokinin B neuronal projections and regulation during lactation in the rat. J Neuroendocrinol. 2011;23(1):52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roa J, Castellano JM, Navarro VM, Handelsman DJ, Pinilla L, Tena-Sempere M. Kisspeptins and the control of gonadotropin secretion in male and female rodents. Peptides. 2009;30(1):57–66. [DOI] [PubMed] [Google Scholar]
- 17. Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006;26(25):6687–6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Han SK, Gottsch ML, Lee KJ, et al. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25(49):11349–11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gottsch ML, Cunningham MJ, Smith JT, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145(9):4073–4077. [DOI] [PubMed] [Google Scholar]
- 20. Navarro VM, Fernandez-Fernandez R, Castellano JM, et al. Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J Physiol. 2004;561(pt 2):379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146(9):3686–3692. [DOI] [PubMed] [Google Scholar]
- 22. Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology. 2008;149(4):1979–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shibata M, Friedman RL, Ramaswamy S, Plant TM. Evidence that down regulation of hypothalamic KiSS-1 expression is involved in the negative feedback action of testosterone to regulate luteinising hormone secretion in the adult male rhesus monkey (Macaca mulatta). J Neuroendocrinol. 2007;19(6):432–438. [DOI] [PubMed] [Google Scholar]
- 24. Clarkson J, Boon WC, Simpson ER, Herbison AE. Postnatal development of an estradiol-kisspeptin positive feedback mechanism implicated in puberty onset. Endocrinology. 2009;150(7):3214–3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mayer C, Acosta-Martinez M, Dubois SL, et al. Timing and completion of puberty in female mice depend on estrogen receptor α-signaling in kisspeptin neurons. Proc Natl Acad Sci USA. 2010;107(52):22693–22698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Frazao R, Dungan Lemko HM, et al. Estradiol modulates Kiss1 neuronal response to ghrelin. Am J Physiol Endocrinol Metab. 2014;306(6):E606–E614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30(6):713–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, et al. Arcuate kisspeptin/neurokinin B/dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology. 2012;153(6):2800–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Feng Y, Manka D, Wagner KU, Khan SA. Estrogen receptor-α expression in the mammary epithelium is required for ductal and alveolar morphogenesis in mice. Proc Natl Acad Sci USA. 2007;104(37):14718–14723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Soliman GA, Ishida-Takahashi R, Gong Y, et al. A simple qPCR-based method to detect correct insertion of homologous targeting vectors in murine ES cells. Transgenic Res. 2007;16(5):665–670. [DOI] [PubMed] [Google Scholar]
- 31. Lam DD, Leinninger GM, Louis GW, et al. Leptin does not directly affect CNS serotonin neurons to influence appetite. Cell Metab. 2011;13(5):584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Münzberg H, Jobst EE, Bates SH, et al. Appropriate inhibition of orexigenic hypothalamic arcuate nucleus neurons independently of leptin receptor/STAT3 signaling. J Neurosci. 2007;27(1):69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Castellano JM, Gaytan M, Roa J, et al. Expression of KiSS-1 in rat ovary: putative local regulator of ovulation? Endocrinology. 2006;147(10):4852–4862. [DOI] [PubMed] [Google Scholar]
- 34. Hauge-Evans AC, Richardson CC, Milne HM, Christie MR, Persaud SJ, Jones PM. A role for kisspeptin in islet function. Diabetologia. 2006;49(9):2131–2135. [DOI] [PubMed] [Google Scholar]
- 35. Gottsch ML, Popa SM, Lawhorn JK, et al. Molecular properties of Kiss1 neurons in the arcuate nucleus of the mouse. Endocrinology. 2011;152(11):4298–4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lomniczi A, Loche A, Castellano JM, et al. Epigenetic control of female puberty. Nat Neurosci. 2013;16(3):281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dubois SL, Acosta-Martínez M, DeJoseph MR, et al. Positive, but not negative feedback actions of estradiol in adult female mice require estrogen receptor α in kisspeptin neurons. Endocrinology. 2015;156(3):1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yeo SH, Herbison AE. Estrogen-negative feedback and estrous cyclicity are critically dependent upon estrogen receptor-α expression in the arcuate nucleus of adult female mice. Endocrinology. 2014;155(8):2986–2995. [DOI] [PubMed] [Google Scholar]