Abstract
17β-estradiol (E2) rapidly, within minutes, activates behaviors and cognition by binding to membrane estrogen receptors, activating cell signaling cascades and increasing dendritic spines. In female rodents, E2 enhances spatial memory within 2–4 hours, and spine density is increased in the CA1 area of the hippocampus within 30–60 minutes. Although chronic gonadal hormone treatments in male rats alter cognition and spines/spine synapses and acute hormone effects occur in hippocampal slices, effects of acute, in vivo hormone administration in males are unknown. Therefore, we assessed rapid effects of E2 (20 μg/kg) and testosterone (T) (750 μg/kg) on spatial memory using the object placement task and on hippocampal spine density using Golgi impregnation. Orchidectomized rats received hormones immediately after the training trial and were tested for retention 2 hours later. Vehicle-injected orchidectomized males spent equal time exploring objects in the old and new locations, but E2- or T-treated subjects spent more time exploring objects at the new location, suggesting enhanced memory. Both hormones also increased spine density in CA1, but not the dentate gyrus, by 20%–40% at 30 minutes and 2 hours after injections. This report is the first, to our knowledge, to show E2 and T enhancements of memory and spine density within such a short time frame in male rats.
Gonadal hormones exert variable effects on cognition, mostly improving, but sometimes impairing, learning and memory, depending on the specific task and neural systems involved. For hippocampal-dependent spatial memory tasks, substantial data from rodent studies indicate that ovariectomy (OVX) impairs and chronic replacement with 17β-estradiol (E2) generally enhances performance on numerous tasks (for reviews, see Refs. 1–3). Evidence for androgens modulating spatial memory in males is more limited, but orchidectomy (ORX) generally impairs and testosterone (T), and often E2, treatment enhances performance on many of the same tasks assessed in females (4–10).
It is increasingly evident that dendritic spine plasticity in the CA1 region of the hippocampus contributes to gonadal hormone-enhanced learning and memory (11, 12). Chronic treatments (days) with E2 were shown to increase hippocampal dendritic spine density 25 years ago (13), and it is now abundantly clear that dendritic spines are extremely responsive to changes in circulating estrogen. Moreover, this laboratory and others have demonstrated that OVX is associated with spatial memory decline and decreased spine density, both of which are reversed after E2 treatments (1, 14–18). In the few studies done in males, ORX decreases, and T recovers CA1 spine synapse density in rats (19, 20) and alters spine shape in mice (21). Thus, changes in spine density, number of synapses and spatial memory are associated with chronic changes in gonadal hormone levels in both male and female rodents, and these effects result from genomic changes mediated by estradiol binding to classic estrogen receptors (ERs) (1).
More recently, E2 has been demonstrated to activate behaviors within minutes. These behaviors include female rodent sexual behavior, avian male sexual displays, nutrient ingestion, social learning (22) and, relevant to this study, cognition (18, 23–25). The expression of these behaviors is dependent upon activation of different, but often interrelated areas in the brain through interaction of E2 with membrane ERs (mERs). Binding causes the activation of cell signaling cascades including ERK-dependent mammalian target of rapamycin, a key protein synthesis pathway involved in spine remodeling, the transcription factor cAMP-response element-binding protein, which mediates filopodial extension and spine formation, and the P-13 pathway involved in synapse formation (12, 18, 26–28). In female rodents, spine density is increased by 10%–35% within 30–40 minutes after treatment with E2 or E2 receptor agonists in CA1, ventromedial nucleus, arcuate nucleus, and medial prefrontal cortex (29–32). Recent studies provide evidence for a close link between signaling, dendritic spines, and memory, because hippocampal activation of ERK and mammalian target of rapamycin by E2 is necessary to enhance spatial memory consolidation in OVX mice (33) and to increase spine density (34). Furthermore, the ERα agonist, propyl pyrazole triol, enhances both object placement (OP) and CA1 spine density at 40 minutes after administration to OVX mice (32).
In this study, preliminary assessments in male rats show, for the first time, that E2 and T rapidly enhance spatial memory on the OP task (within 2 h) and increase spine density on pyramidal cells in CA1 (within 30 min). These results are discussed in relation to whether they are pharmacological in nature or are possibly related to such physiological functions as “cross talk” with nuclear receptors or mediate the effects of endocrine disrupters such as bisphenol A (BPA).
Materials and Methods
Subjects
Three cohorts of 18 8-week-old male, orchidectomized Sprague-Dawley rats (Harlan Sprague-Dawley, Inc) were used. Cohort one was used only for behavioral testing because behavioral testing alone may induce changes in dendritic spines of hippocampal neurons (17, 35). The other 2 cohorts were used for the Golgi studies. Upon arrival, subjects were double housed in clear plastic cages and maintained on a 12-hour light, 12-hour dark cycle (lights on at 7 am). Low phytoestrogen chow (Chow 2016; Harlan Teklad Global Diets) and water were available ad libitum (16). Experiments followed the NIH Guide for Care and Use of Animals and were approved by the Institutional Animal Care and Use Committee of Hunter College.
OP memory task
Two weeks after surgery and approximately 1 week after arrival, acclimation to the spatial memory task, OP, began as previously described (9, 24). The OP task is comprised of 2 trials: a sample/training trial (T1), followed by an intertrial delay interval, and a recognition/retention trial (T2). In T1, subjects explore 2 identical objects for 3 minutes in an open chamber and then are placed back in their home cage. Two hours later, they are returned to the chamber to explore the objects, one of which is moved to a new location. If rats remember the old location, then the new location is explored more than the old location because rats are exploratory and novelty seekers. Subjects that explored objects for less than 3 seconds in T1 or T2 were omitted from data analyses. Paired t tests tested differences in time spent (seconds) with objects in T2.
Acute hormone treatments
Subjects received a single sc injection at the nape of the neck of corn oil vehicle (1 mL/kg; Fisher Science Education), T (750 μg/kg), or E2 (20 μg/kg), both from Sigma-Aldrich Co, immediately after the sample trial. Posttraining treatment with E2 facilitates the acquisition/consolidation of information during T1 and enhances retention in the recognition/discrimination trial in OVX female rats (24, 25, 30, 36). The dose of E2 is the same one which enhances memory consolidation of OP in OVX females (25, 30) and results in circulating levels of 657 and 711 pg/mL at 30 minutes and 4 hours after E2 injection in OVX rats (30), a level higher than proestrus. Acute effects of T in males have not been well tested, but 2 daily sc injections of 500 μg/subject of T enhanced OP in ORX males (9) and increased CA1 spine synapse density (20). One dose of 750 μg/kg (∼225 μg/subject) is less than the chronic treatments. Rats were habituated to the sc injections via vehicle injections during habituation trials. The behavioral cohort was first tested with E2 followed 1 week later with T.
Golgi impregnation and spine counting
After acclimation to the animal facility, subjects received a single sc habituation injection of corn oil (1 mL/kg) 2 days before the experimental day. Subjects then received corn oil, E2 or T in same dose as behavior cohort and killed 30 minutes (cohort 3) or 2 hours later (cohort 2). Brains were removed and processed for Golgi impregnation using the FD Rapid GolgiStain kit (FD NeuroTechnologies, Inc) as previously described (16). Secondary basal and tertiary apical dendrites from pyramidal cells in CA1 and primary dendrites from granule cells in the dentate gyrus (DG) of the dorsal hippocampus were counted blindly using a Nikon Eclipse E400 microscope. Six cells per region/brain were included in the analysis. Neurons in both areas were chosen for analysis as follows: 1) cell bodies and dendrites were well impregnated; 2) dendrites were clearly distinguishable from adjacent cells and continuous (16). Slides were coded and spines counted under oil (100×) using a hand counter, and dendritic length measured using the Spot Advanced program, version 5.0 Windows (Diagnostic Instruments). Spine density is expressed as number of spines/10-μm dendrite. One-way ANOVAs assessed differences in spine density of each area followed by Newman-Keuls post hoc tests where appropriate.
Results
Acute estradiol and T treatments enhance OP
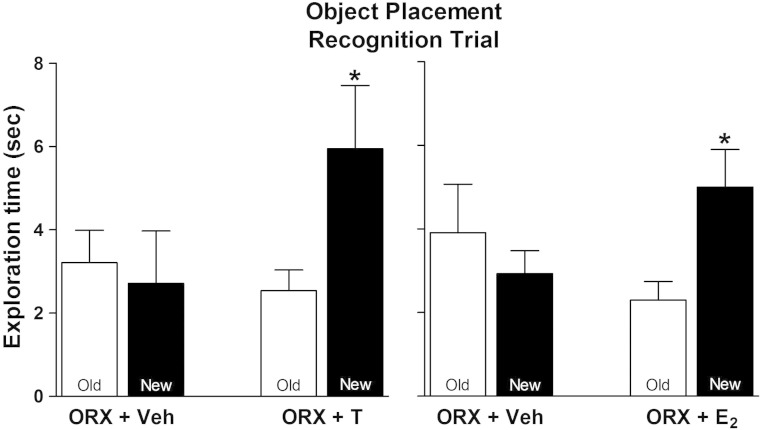
Figure 1 shows results of OP testing using a posttraining treatment paradigm: subjects explored identical objects in the training trial, were injected with hormones immediately after the training trial, and were then tested 2 hours later for exploration of objects in the old and new locations in the retention trial. Vehicle-injected ORX males spent the same amount of time exploring objects in the old and new locations (Figure 1A), suggesting poor memory for the old location. When E2 was given immediately after the training trial, the subjects spent more time exploring objects at the new than the old location (∼2 vs 5 s; P < .05), suggesting better memory for the old vs the new location. A similar pattern was seen after T treatment (Figure 1B): vehicle-treated ORX males spent the same amount of time exploring at the old and new locations, whereas T-treated males spent more time exploring the object at the new location (∼2 vs 6 s; P < .05).
Figure 1.
Effects of acute T and E2 treatment on OP. Time spent exploring objects at old and new location is shown for vehicle- and E2-treated (left panel) and vehicle- and T-treated (right panel) subjects (n = 6 and 7 for vehicle- and hormone-treated groups, respectively). Entries are mean ± SEM. *, P < .05, by paired t test.
Acute estradiol and T treatments increase spine density in CA1
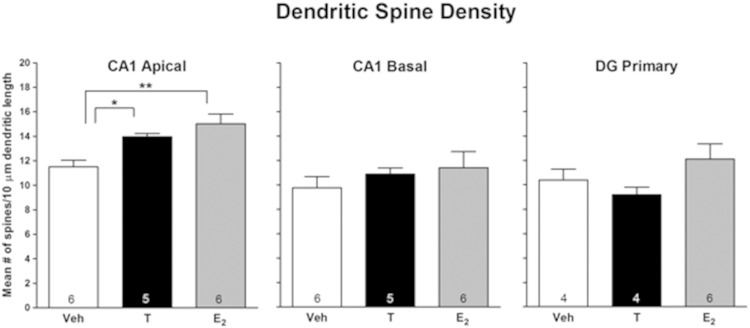
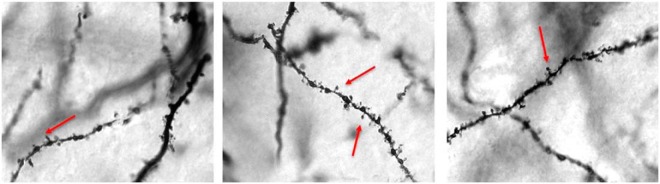
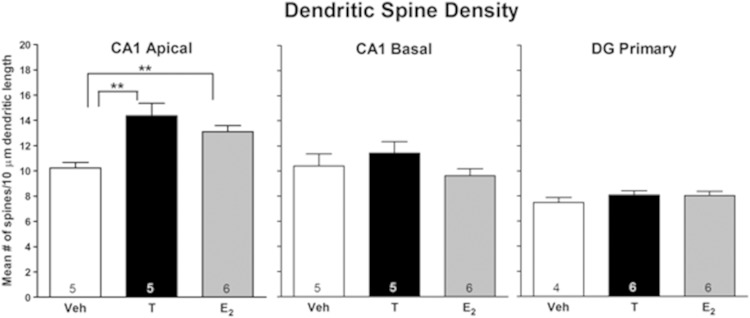
Hormonal effects on spine density in the hippocampus were determined for CA1 pyramidal cells and DG granule cells. Measurements were first made 2 hours after injection of the hormones, the time when subjects in the behavioral cohort received retention testing (see Figure 1). Both hormones increased spine density in apical dendrites of pyramidal cells in CA1 (P < .003) (Figure 2). E2 treatment increased spine density by 31%, whereas T increased spine density by 21%. No treatment effects were noted in basal dendrites of CA1 neurons or in granule cells in the DG. The next experiment examined hormonal effects 30 minutes after hormone treatment, the time when memory would be consolidating in the behavioral cohort. Figure 3 shows representative photomicrographs of Golgi-impregnated tertiary apical dendrites from CA1 30 minutes after oil, T, and E2. Similar to effects at 2 hours, spine density was increased in apical (P < .003) but not in basal dendrites in CA1, and no effect was seen in the DG (Figure 4). E2 treatment increased spine density by 28%, whereas T increased spine density by 40%. Thus, spine density was increased on the apical branch of CA1 cells by both hormones during the time of both memory consolidation and retention testing.
Figure 2.
Acute effects of T and E2 on hippocampal spine density 2 hours later. Entries are mean number of spines/10 μm. Number inside bar is n/group. *, P < .05; **, P < .01 by Newman-Keuls test.
Figure 3.
Representative photomicrographs of Golgi-impregnated CA1 pyramidal cells. Tertiary apical dendrites in CA1 are shown 30 minutes after oil (left), T (middle), or estradiol (right) treatment. 100× under oil. Arrows denote spines.
Figure 4.
Acute effects of T and E2 on hippocampal spine density 30 minutes later. Entries are mean number of spines/10 μm. Number inside bar is n/group. **, P < .01; by Newman-Keuls test.
Discussion
The current study provides novel findings showing that ORX male rats respond to acute administration of E2 or T with rapid enhancements in memory consolidation and increases in CA1 apical dendritic spine density. E2 effects occur at the same dose, time course and in the same hippocampal location, CA1, as in female rats (25, 30–32). Acute effects of T on memory or spines have not previously been investigated in vivo in either sex. However, Meyer et al (37) demonstrated that prepubertal ORX blocks normal increases in CA1 spines, an effect reversed by chronic T administration. In addition, Leranth et al (19, 20) found changes in spine synapse density in apical dendrites in CA1 of male rats after chronic ORX and T, but not estradiol, replacement, and Li et al (21) reported that chronic ORX induced aberrant morphologies of spines, which were reversed after chronic T replacement in CA1 of thy1-GFP transgenic male mice. Our results demonstrate that E2 and T effects on spine density in adult males also occur rapidly.
Although acute changes in spines and signaling molecules have not been investigated in males in vivo, effects in vitro are similar to females. Addition of E2 to hippocampal slices from male rats causes an induction of long-term potentiation (LTP), activation of several downstream signaling pathways including ERK and phosphatidylinositide 3-kinase and a 30% increase in spine density in CA1 neurons in 30 minutes (38). Kramár et al (39) investigated the acute facilitatory effects of estrogen on glutamatergic transmission and LTP and showed that brief infusion of E2 into adult male hippocampal slices triggers actin polymerization via signaling cascades that enhance LTP and spinogenesis. These changes were suggested by the authors to underlie estrogen's rapid promotion of learning and memory which is consistent with the currently reported enhancements in memory consolidation. Similar mechanisms for T promotion of memory have been shown recently, wherein 30% increases in CA1 spine density within 2 hours after T or dihydrotestosterone were present in male rat slices (40) and inhibition of specific signaling pathways including ERK, MAPK, protein kinase A, and protein kinase C and a block of all effects by an androgen receptor antagonist indicated that enhanced spinogenesis was mediated by synaptic/extranuclear androgen receptors, which rapidly drive kinase networks. Thus, in vivo and in vitro evidence in females and in vitro evidence in males supports that either E2 or T may rapidly enhance memory in both sexes through interactions with mERs, enhancements in cell signaling and increases in spines.
Hojo et al (41) showed increased spines in the DG 2 hours after E2 application to hippocampal slices from males, an effect suppressed by blocking ERK. In contrast, no spine changes were present in the DG after acute, in vivo E2 or T administration to males (current study) or acute (9, 34) or chronic in vivo E2 to females (11, 13). Differences in hormonal effects found in vivo vs in vitro may result from loss of afferent subcortical inputs, which have been shown to alter the synaptic density responses to E2 in females (42) and T in males in CA1 after gonadectomy (43). Thus, it is important to assess effects of hormone signaling in vivo in males. At least one recent study suggests the presence of sex differences in E2 effects on signaling to the endocannabinoid system (44). In addition, the basis for the sex difference in responses of spines on CA1 dendrites to acute E2 needs exploring; the apical dendritic tree is affected in males, whereas the basal tree was affected in females (current study, 30–32). Whether some species differences may exist should also be investigated because application of E2 to hippocampal slices from mice maintained LTP and spine synapses in females but not in males (38, 39), and chronic E2 administration to African green monkeys increased DG spine synapses (47). Finally, the hippocampus actively converts T to E2 (2); thus, the possibility that aromatization of T may mediate the T effects needs evaluation. The short time frame of spine induction (30 min) and the demonstration of T-dependent signaling in CA1 (38–40) argues against this possibility.
The physiological relevance of rapid effects of gonadal hormones in males, as well as in females, is currently unclear. The dose of estradiol given here, 20 μg/kg, results in levels of E2 in OVX rats that are higher than at proestrus (30). Nonetheless, it can be speculated that activation of hormonal signaling mechanisms may facilitate genomic responses, and thus, nuclear cross talk may serve as a “priming” mechanism for long-term changes associated with classic receptor mechanisms (see Ref. 2 for further discussion). Inhibition of mERs may underlie some deleterious effects of endocrine disrupters like BPA. When BPA is given to gonadally intact male rats immediately after an OP trial, BPA impairs retention 2 hours later and is associated with 10% decreases in spine density in CA1 apical and basal dendrites (48). In females, BPA also rapidly blocks E2 -enhanced OP and increased CA1 spine density when E2 is given after training (30). Additionally, BPA rapidly, within 40 minutes, completely abolishes estradiol-induced hippocampal synaptogenesis (49). Thus, in both males and females, BPA may bind to E2 or T membrane receptors and rapidly impair memory and spino-/synaptogenesis. Overall, further investigations are necessary to understand whether or not there are possible role(s) in neuroendocrine function for these rapid, novel gonadal hormones effects in both males and females.
Acknowledgments
This work was supported by National Institutes of Health Grants R25-GM-60665 (Research Initiative for Scientific Enhancement) and RR003037 from National Center for Research Resources.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BPA
- bisphenol A
- DG
- dentate gyrus
- E2
- 17β-estradiol
- ER
- estrogen receptor
- mER
- membrane ER
- LTP
- long-term potentiation
- OP
- object placement
- ORX
- orchidectomized
- OVX
- ovariectomy
- T
- testosterone
- T1
- sample/training trial
- T2
- recognition/retention trial.
References
- 1. Luine VN. Estradiol and cognition function: past, present and future. Horm Behav. 2014;66:602–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Frick KM. Molecular mechanisms underlying the memory-enhancing effects of estradiol. Horm Behav. 2015;74:4–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tuscher JJ, Fortress AM, Kim J, Frick KM. Regulation of object recognition and object placement by ovarian sex steroid hormones. Behav Brain Res. 2015;285:140–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Frick KM, Kim J, Tuscher JJ, Fortress AM. Sex steroid hormones matter for learning and memory: estrogenic regulation of hippocampal function in male and female rodents. Learn Mem. 2015;22:472–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Daniel JM, Winsauer PJ, Moerschbaecher JM. Castration in rats impairs performance during acquisition of a working memory task and exacerbates deficits in working memory produced by scopolamine and mecamylamine. Psychopharmacology. 2003;170:294–300. [DOI] [PubMed] [Google Scholar]
- 6. Gibbs R. Testosterone and estradiol produce different effects on cognitive performance in male rats. Horm Behav. 2005;48:268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sandstrom NJ, Kim JH, Wasserman MA. Testosterone modulates performance on a spatial working memory task in male rats. Horm Behav. 2006;50:18–26. [DOI] [PubMed] [Google Scholar]
- 8. Aubele T, Kaufman R, Montalmant F, Kritzer M. Effects of gonadectomy and hormone replacement on a spontaneous novel object recognition task in adult male rats. Horm Behav. 2008;54:244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Luine V. Recognition memory tasks in neuroendocrine research. Behav Brain Res. 2015;285:158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hawley WR, Grissom EM, Martin RC, Halmos MB, Bart CL, Dohanich GP. Testosterone modulates spatial recognition memory in male rats. Horm Behav. 2013;63:559–565. [DOI] [PubMed] [Google Scholar]
- 11. Frankfurt M, Luine V. The evolving role of dendritic spines and memory: interaction(s) with estradiol. Horm Behav. 2015;74:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arevalo MA, Azcoitia I, Gonzalez-Burgos I, Garcia-Segura LM. Signaling mechanisms regulating synaptic plasticity and memory by estradiol. Horm Behav. 2015;74:19–27. [DOI] [PubMed] [Google Scholar]
- 13. Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10:1286–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li C, Brake WG, Romeo RD, et al. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc Natl Acad Sci USA. 2004;101:2185–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wallace M, Luine V, Arellanos A, Frankfurt M. Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex. Brain Res. 2006;1126:176–182. [DOI] [PubMed] [Google Scholar]
- 16. Luine V, Attalla S, Mohan G, Costa A, Frankfurt M. Dietary phytoestrogens enhance spatial memory and spine density in the hippocampus and prefrontal cortex of ovariectomized rats. Brain Res. 2006;1126:183–187. [DOI] [PubMed] [Google Scholar]
- 17. Beltrán-Campos V, Prado-Alcalá RA, León-Jacinto U, et al. Increase of mushroom spine density in CA1 apical dendrites produced by water maze training is prevented by ovariectomy. Brain Res. 2011;1369:119–130. [DOI] [PubMed] [Google Scholar]
- 18. Luine VN, Frankfurt M. Estrogens facilitate memory processing through membrane mediated mechanisms and alterations in spine density. Front Neuroendocrinol. 2012;33:388–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leranth C, Szigeti-Buck K, Maclusky NJ, Hajszan T. Bisphenol A prevents the synaptogenic response to testosterone in the brain of adult male rats. Endocrinology. 2008;149:988–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leranth C, Petnehazy O, MacLusky N. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J Neurosci. 2003;23:1588–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li M, Masugi-Tokita M, Takanami K, Yamada S, Kawata M. Testosterone has sublayer-specific effects on dendritic spine maturation mediated by BDNF and PSD-95 in pyramidal neurons in the hippocampus CA1 area. Brain Res. 2012;1484:76–84. [DOI] [PubMed] [Google Scholar]
- 22. Luine V, Frankfurt M. Introduction to the special issue estradiol and cognition: molecules to mind. Horm Behav. 2015;74:1–3. [DOI] [PubMed] [Google Scholar]
- 23. Luine VN. Estradiol: mediator of memories, spine density and cognitive resilience to stress in female rodents [published online ahead of print August 1, 2015]. J Steroid Biochem Mol Biol. doi: 10.1016/j.jsbmb.2015.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. [DOI] [PubMed] [Google Scholar]
- 25. Inagaki T, Gautreaux C, Luine V. Acute estrogen treatment facilitates recognition memory consolidation and alters monoamine levels in memory-related brain areas. Horm Behav. 2010;58:415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Frick KM, Fernandez SM, Harburger LL. A new approach to understanding the molecular mechanisms through which estrogens affect cognition. Biochim Biophys Acta. 2010;1800:1045–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Almey A, Cannell E, Bertram K, Filardo E, Milner TA, Brake WG. Medial prefrontal cortical estradiol rapidly alters memory system bias in female rats: ultrastructural analysis reveals membrane-associated estrogen receptors as potential mediators. Endocrinology. 2014;155:4422–4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McEwen BS, Akama KT, Spencer-Segal JL, Milner TA, Waters EM. Estrogen effects on the brain: actions beyond the hypothalamus via novel mechanisms. Behav Neurosci. 2012;126:4–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Christensen AP, Dewing P, Micevych P. Membrane-initiated estradiol signaling induces spinogenesis required for female sexual receptivity. J Neurosci. 2011;30:17583–17589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Inagaki T, Frankfurt M, Luine V. Estrogen-induced memory enhancements are blocked by acute Bisphenol A in adult female rats: role of dendritic spines. Endocrinology. 2012;153:3357–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Phan A, Gabor CS, Favaro KJ, et al. Low doses of 17β-estradiol rapidly improve learning and increase hippocampal dendritic spines. Neuropsychopharmacology. 2012;37:2299–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Phan A, Lancaster KE, Armstrong JN, MacLusky NJ, Choleris E. Rapid effects of estrogen receptor α and β selective agonists on learning and dendritic spines in female mice. Endocrinology. 2011;152:1492–1502. [DOI] [PubMed] [Google Scholar]
- 33. Fortress AM, Fan L, Orr PT, Zhao Z, Frick KM. Estradiol-induced object recognition memory consolidation is dependent on activation of mTOR signaling in the dorsal hippocampus. Learn Mem. 2013;20:147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tuscher JJ, Luine V, Frankfurt M, Frick KM. Estradiol-mediated spine changes in the dorsal hippocampus and medial prefrontal cortex of ovariectomized female mice depend on ERK and mTOR activation in the dorsal hippocampus. J Neurosci. 2016;36:1483–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leuner B, Falduto J, Shors T. Associative memory formation increases the observation of dendritic spines in the hippocampus. J Neurosci. 2003;26:2595–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Packard M. Posttraining estrogen and memory modulation. Horm Behav. 1998;34:126–139. [DOI] [PubMed] [Google Scholar]
- 37. Meyer G, Ferres-Torres R, Mas M. The effects of puberty and castration on hippocampal dendritic spines of mice. A Golgi study. Brain Res. 1978;155:108–112. [DOI] [PubMed] [Google Scholar]
- 38. Hasegawa Y, Hojo Y, Kojima H, et al. Estradiol rapidly modulates synaptic plasticity of hippocampal neurons: involvement of kinase networks. Brain Res. 2015;1621:147–161. [DOI] [PubMed] [Google Scholar]
- 39. Kramár EA, Babayan AH, Gall CM, Lynch G. Estrogen promotes learning-related plasticity by modifying the synaptic cytoskeleton. Neuroscience. 2013;239:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hatanaka Y, Hojo Y, Mukai H, et al. Rapid increase of spines by dihydrotestosterone and testosterone in hippocampal neurons: dependence on synaptic androgen receptor and kinase networks. Brain Res. 2015;1621:121–132. [DOI] [PubMed] [Google Scholar]
- 41. Hojo Y, Munetomo A, Mukai H, et al. Estradiol rapidly modulates spinogenesis in hippocampal dentate gyrus: involvement of kinase networks. Horm Behav. 2015;74:149–156. [DOI] [PubMed] [Google Scholar]
- 42. Leranth C, Shanabrough M, Horvath TL. Hormonal regulation of hippocampal spine synapse density involves subcortical mediation. Neuroscience. 2000;101:349–356. [DOI] [PubMed] [Google Scholar]
- 43. Kovacs E.-G, MacLusky NJ, Leranth C. Effects of testosterone on hippocampal CA1 spine synaptic density in the male rat are inhibited by fimbria/fornix transection. Neuroscience. 2003;122:807–810. [DOI] [PubMed] [Google Scholar]
- 44. Tabatadze N, Huang G, May RM, Jain A, Woolley CS. Sex Differences in molecular signaling at inhibitory synapses in the hippocampus. J Neurosci. 2015;35(32):11252–11265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vierk R, Bayer J, Freitag S, et al. Structure-function-behavior relationship in estrogen-induced synaptic plasticity. Horm Behav. 2015;74:139–148. [DOI] [PubMed] [Google Scholar]
- 46. Fester L, Rune GM. Sexual neurosteroids and synaptic plasticity in the hippocampus. Brain Res. 2015;1621:162–169. [DOI] [PubMed] [Google Scholar]
- 47. Leranth C, Hajszan T, Szigeti-Buck K, Bober J, MacLusky NJ. Bisphenol A prevents the synaptogenic response to estradiol in hippocampus and prefrontal cortex of ovariectomized nonhuman primates. Proc Natl Acad Sci USA. 2008;105:14187–14191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Eilam-Stock T, Serrano P, Frankfurt M, Luine V. Bisphenol-A impairs memory and reduces dendritic spine density in adult male rats. Behav Neurosci. 2012;126:175–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. MacLusky NJ, Hajszan T, Leranth C. The environmental estrogen bisphenol A inhibits estradiol-induced hippocampal synaptogenesis. Environ Health Perspect. 2005;113:675–679. [DOI] [PMC free article] [PubMed] [Google Scholar]