Abstract
Background
Geraniol is a plant-derived phytochemical possessing anti-cancer action. The anti-carcinogenic effect of geraniol was investigated in the diethylnitrosamine (DEN)-induced hepatocarcinogenic rat model.
Methods
Male Wistar rats were intraperitoneally injected with 300 μL of phosphate-buffered saline (PBS) (G1; n = 4) or DEN (100 mg/kg body weight) dissolved in PBS (G2; n = 8) every 2 weeks on experimental weeks 2, 4 and 6. The rats were treated with a low concentration (0.07%) of geraniol (G3; n = 9) and high concentration (0.35%) of geraniol (G4; n = 7) for 12 weeks. To evaluate the effects of geraniol on the DEN-induced hepatocarcinogenesis, we compared the relative liver weight, serum aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP) levels and expression levels of proliferating cell nuclear antigen (PCNA) and glutathione S transferase-P (GST-P) by immunohistochemical analyses among each group.
Results
Relative liver weight was significantly higher in G2 than in G1 (P < 0.01). Both serum AST and ALT levels were significantly higher in G2 than in G3 and in G4 (P < 0.05). Serum ALP levels did not show a significant difference among each group. Percentages of both PCNA- and GST-P- positive area were significantly decreased in G3 and in G4 compared to in G2 (P < 0.001, respectively), suggesting anti-hepatocarcinogenic effects of geraniol.
Conclusion
Geraniol is a promising compound useful for suppression of hepatocellular carcinoma. The mechanisms of action are required to be clarified in the future intensive study.
Keywords: diethylnitrosamine, geraniol, hepatocellular carcinoma
Hepatocellular carcinoma (HCC) is one of the most common malignancies in Japan, responsible for over thirty thousand deaths per year.1 Diagnostic imaging for HCC in its early stage has progressed including contrast-enhanced ultrasound and gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid (Gd-EOB) MRI. Therapeutic options for HCC including radiofrequency ablation (RFA), transcatheter arterial chemoembolization (TACE), surgical resection and molecular-targeted therapy (Sorafenib), have also increased. Despite these advances in diagnosis and treatment, the prognosis of patients with HCC remains unsatisfactory.2 In order to improve the outcomes of patients with HCC, novel chemopreventive and therapeutic compounds are urgently required.
Geraniol is a natural acyclic monoterpene contained in the essential oils of many aromatic plants such as geranium and lemon.3 It is widely used as an aromatic component in many cosmetic products. In addition to its aromatic properties, geraniol has been reported to demonstrate anti-tumor activity in a variety of cancer cells including lung,4 pancreatic,5 colon6 and liver.7 The mechanisms of anti-cancer effects of geraniol include inhibition of angiogenesis3 and the mevalonate pathway,4 modulation of the Ras-extracellular signal-regulated kinase (ERK) signaling pathway8 and inhibition of NF-κB,9 then leading to the modulation of cellular proliferation and apoptosis. However, little is known about geraniol inhibiting hepatocarcinogenesis in vivo.
Diethylnitrosamine (DEN) is an established hepatocarcinogen in rats.10 DEN has been reported to induce hepatocarcinogenesis by alteration of the DNA structure, formation of alkyl DNA adducts and induction of chromosomal aberrations and micronuclei in the liver.11 In the present study, we evaluated anti-hepatocarcinogenic effects of geraniol in DEN-induced hepatocarninogenic rats.
MATERIALS AND METHODS
Chemicals
Geraniol and PBS were purchased from Cosmo Bio (Tokyo, Japan). DEN was purchased from Sigma Aldrich (St. Louis, MO). Pentobarbital was obtained from Dainippon Sumitomo Pharma (Osaka, Japan). Antibodies against proliferating cell nuclear antigen (PCNA) and glutathione S‑transferase (GST)-P were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and Assay Designs (Ann Arbor, MI), respectively.
Animals, treatments and tissue collection
Male Wistar rats weighing 200 g or less were purchased from Japan SLC. (Hamamatsu, Japan). The rats were randomized, divided into four groups and were housed two per cage with rice husks for bedding in an air‑ventilated room under a 12‑h light/dark cycle. In the animal room, the temperature (22 ˚C) and humidity (55%) were kept constant. The animals were allowed free access to food and tap water ad libitum during the experiment. All animals received humane care and protocols were approved by the Animal Ethics Committee of Tottori University (approval number 13-Y-35).
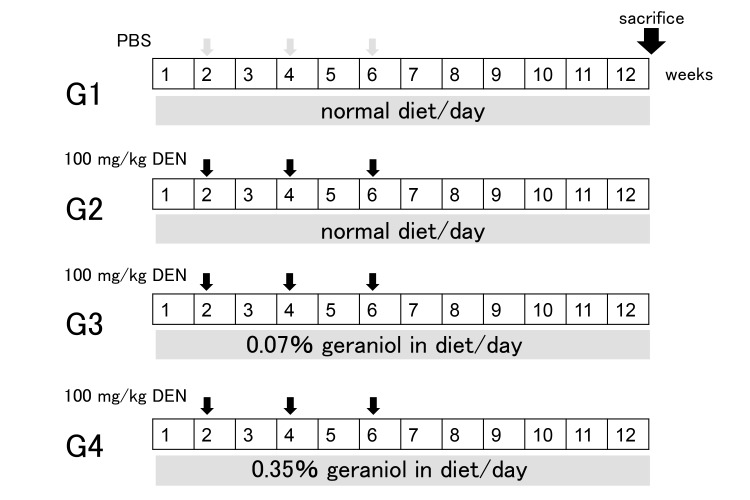
As a control group, animals in group 1 (G1) were intraperitoneally injected with 300 μL of PBS (n = 4) every 2 weeks on experimental weeks 2, 4 and 6. In order to generate a hepatocarcinogenic model, animals in groups 2 (G2, n = 8), 3 (G3, n = 9) and 4 (G4, n = 7) were intraperitoneally injected with DEN (100 mg/kg body weight) dissolved in PBS every 2 weeks on experimental weeks 2, 4 and 6. In order to examine the anti-cancer effects of geraniol on the liver, the rats in group 3 and 4 were fed with 0.07% and 0.35% geraniol in liquid form, respectively during the experimental period (Fig. 1).
Fig. 1.
Experimental schedules.
Animals in group 1 (G1) were intraperitoneally injected with 300 μL of PBS (n =4) once every 2 weeks on experimental weeks 2, 4 and 6. Animals in groups 2 (G2, n = 8), 3 (G3, n = 9) and 4 (G4, n = 7) were intraperitoneally injected with DEN (100 mg/kg body weight) dissolved in PBS once every 2 weeks on experimental weeks 2, 4 and 6. The animals in group 3 and 4 were treated with 0.07% and 0.35% geraniol in liquid form, respectively, during 12 weeks. DEN, diethylnitrosamine; PBS, phosphate-buffered saline.
One week subsequent to the final treatments, the animals were sacrificed under anesthesia using pentobarbital. Blood samples were collected via cardiac puncture and serum samples were stored at –30 ˚C until analysis. Immediately following the excision of the livers, the livers were weighed and portions of the samples were stored in 10% neutral buffered formalin for histological examination.
Measurement of serum transaminase and alkaline phosphatase levels
Serum aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP) levels were measured at SRL. (Tokyo, Japan).
Histology and immunohistochemistry
The rat liver tissues embedded in paraffin were stained with hematoxylin and eosin. For immunohistochemistry with the PCNA and GST-P antibodies, Histofine Simple Stain Rat MAX PO was employed (Nichirei Biosciences, Tokyo, Japan). Briefly, after routine dewaxing with xylene and hydration through a graded ethanol series, the sections were incubated with 1.5% hydrogen peroxide solution for 15 min at room temperature to quench endogenous peroxidase activity. The sections were washed in PBS, blocked with 1.5% serum solution and incubated with primary antibodies overnight at 4 °C. After rinsing with PBS, the sections were incubated with biotinylated secondary antibody for 30 min at room temperature and horse HRP-conjugated ABC solution (Vector Laboratories, Burlingame, CA) was applied for 30 min at room temperature. Peroxidase activity was developed with DAB solution (Vector Laboratories). Counterstaining was performed with hematoxylin. The PCNA labeling indices were represented as the percentage of positively stained nuclei by counting 1000 cells in a field at x200 magnification. The GST-P-positive area was measured on images captured by a Charge Coupled Device (CCD) camera on a Windows computer.
Statistical analysis
Values are expressed as mean ± SD. Means were compared using the Student t-test. Statistical significance was inferred at P < 0.05.
RESULTS
Relative liver weight and serum liver enzyme levels
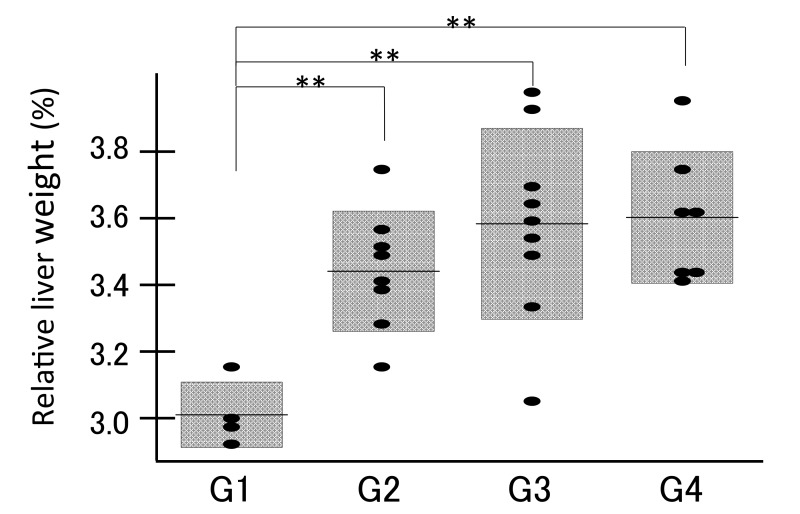
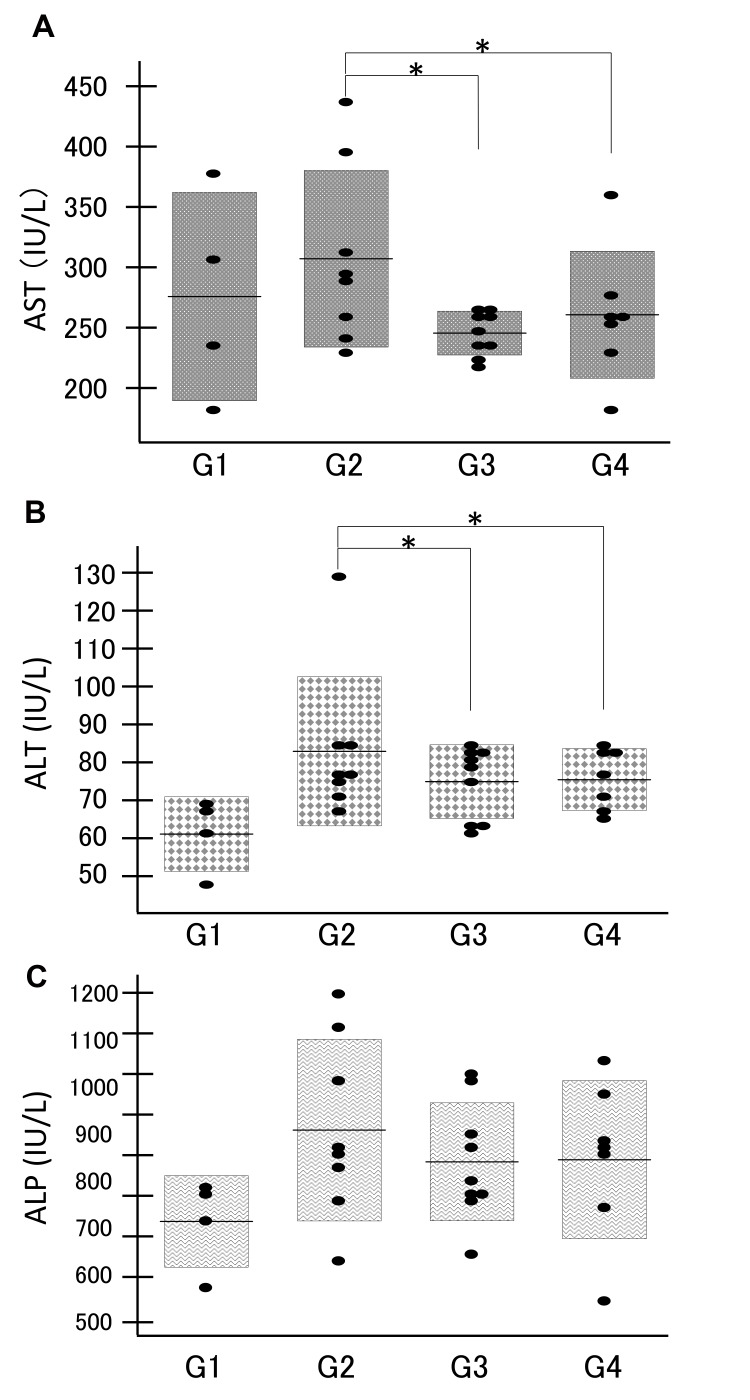
The relative liver weight (liver weight/body weight) was significantly higher in the DEN groups (G2, G3 and G4) than in the control group (G1) (Fig. 2, P <0.01), presumably due to the development of liver tumors. Among the DEN groups G2, G3 and G4, the relative liver weight did not show a significant difference. Both serum transaminases (AST and ALT) were significantly higher in the DEN administration group G2 than in the geraniol treatment groups G3 and G4 (P < 0.05, Figs. 3A and B). Serum ALP levels did not show significant differences among the DEN groups G2, G3 and G4 (Fig. 3C). These data suggest that both low (0.07%) and high (0.35%) concentrations of geraniol attenuated the liver injury and hepatic tumor formation.
Fig. 2.
The relative liver weight compared among the groups (G1, G2, G3 and G4) demonstrated that the relative liver weight of the DEN groups (G2, G3 and G4) was significantly higher than that of the control group (G1). G1; PBS, G2; DEN, G3; DEN+0.07% geraniol, G4; DEN+0.35% geraniol. **P < 0.01. DEN, diethylnitrosamine; PBS, phosphate-buffered saline.
Fig. 3.
Serum AST (A), ALT (B) and ALP (C) levels compared among the groups (G1, G2, G3 and G4) demonstrated that serum transaminase levels were significantly higher in the DEN administration group G2 than in the geraniol treatment groups G3 and G4 (P < 0.05) and that serum ALP levels did not show any significant differences among the DEN groups G2, G3 and G4. *P < 0.05. ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; DEN, diethylnitrosamine.
Macroscopic and histological examinations
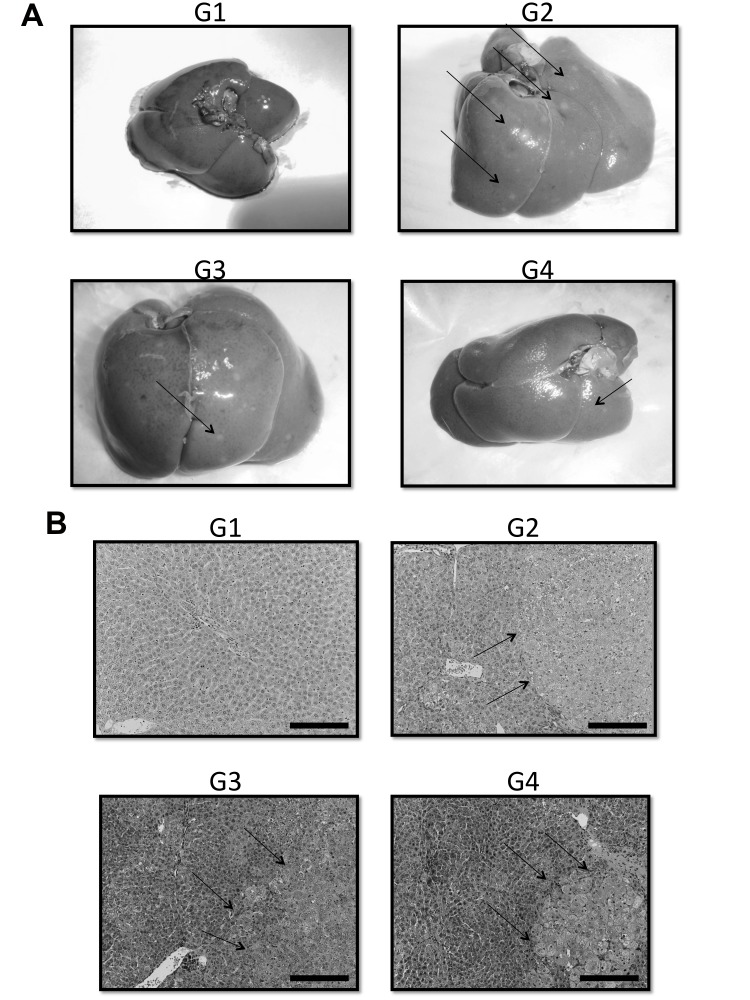
Macroscopic and microscopic features of the liver were evaluated. As expected, in the liver of control rats (G1), no tumors were observed (Figs. 4A and B). Multiple white tumor formation was macroscopically observed in the DEN groups G2, G3 and G4 (Fig. 4A, arrows).
Fig. 4.
Representative macroscopic (A) and microscopic (B) features of the liver in the four groups (G1, G2, G3 and G4) demonstrated that no tumors were observed in the liver of control rats (G1) and that multiple white tumor formation was macroscopically observed in the DEN groups G2, G3 and G4. In the histological analysis, the white nodules induced by DEN were hyperplastic nodules (hematoxylin and eosin staining, Bars express 100 µm). Arrows indicate the representative nodules. DEN, diethylnitrosamine.
In the histological analysis, the white nodules induced by DEN were hyperplastic nodules (Fig. 4B).
Expression levels of PCNA and GST-P
PCNA is an essential regulator of the cell cycle and its expression is a useful tool for the study of cell proliferation.12 Among the glutathione S-transferases (GSTs), a family of detoxification enzymes catalyzing the conjugation of glutathione with a large number of carcinogens, placental GST (GST-P) is specifically expressed during rat hepatocarcinogenesis.13
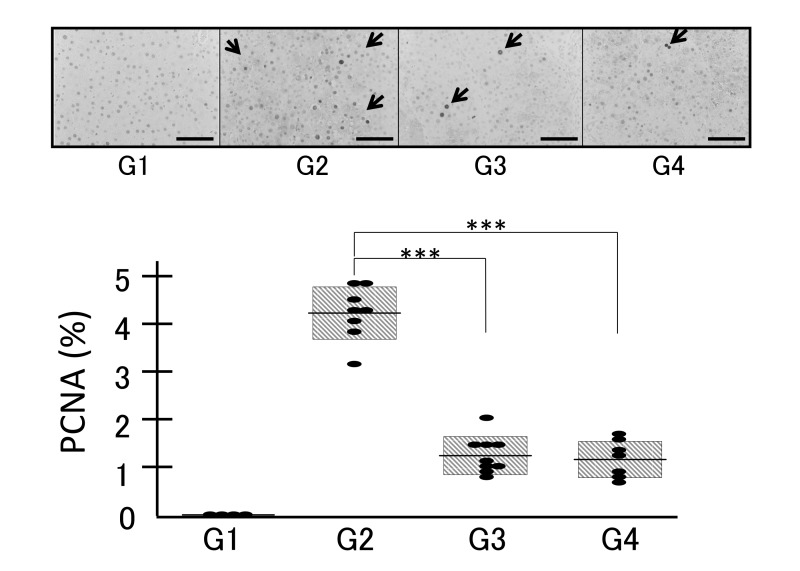
In order to confirm the anti-tumor effects of geraniol, we evaluated the expression levels of PCNA and GST-P by immunohistochemical analyses. Immunostaining with the PCNA (Fig. 5) and GST-P (Fig. 6) antibodies revealed that no positive cells or areas were observed in the control livers (Figs. 5 and 6, G1). Administration of DEN induced the nuclear expression of PCNA (Fig. 5, G2). Treatment with both low (0.07%) and high (0.35%) concentrations of geraniol significantly attenuated the percentages of PCNA-positive cells in G3 and G4 compared to G2 (Fig. 5, P < 0.001).
Fig. 5.
Expression levels of PCNA examined by immunohistochemical analysis in the four groups (G1, G2, G3 and G4) demonstrated that no positive cells were observed in the control livers and that administration of DEN induced the nuclear expression of PCNA (G2), which was significantly attenuated by the treatment with both low (0.07%, G3) and high (0.35%, G4) concentrations of geraniol. ***P < 0.001. Bars express 200 µm. Arrows indicate representative PCNA-positive nuclei. DEN, diethylnitrosamine.PCNA, proliferating cell nuclear antigen.
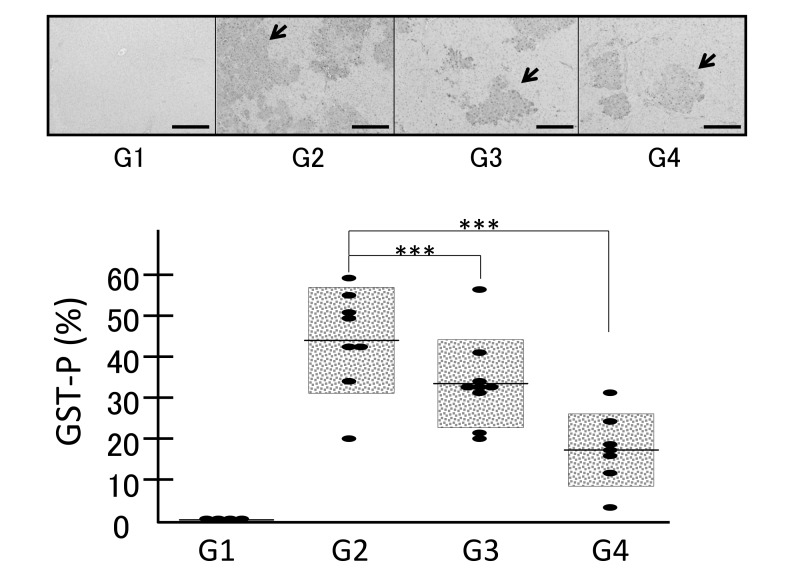
Fig. 6.
Expression levels of GST-P examined by immunohistochemical analysis in the four groups (G1, G2, G3 and G4) demonstrated that no positive area was observed in the control livers and that administration of DEN induced GST-P-positive area (G2), which was significantly inhibited by the treatment with both low (0.07%, G3) and high (0.35%, G4) concentrations of geraniol, revealing no significant difference between G3 and G4. ***P < 0.001. Bars express 200 µm. Arrows indicate representative GST-P-positive area. DEN, diethylnitrosamine; GST-P, glutathione S transferase-P.
Immunostaining with the GST-P antibody revealed mostly similar results as PCNA. Administration of DEN induced the appearance of a GST-P-positive area (Fig. 6, G2). The GST-P-positive area was significantly decreased in G3 and G4 compared to G2 (Fig. 6, P < 0.001), although there was no significant difference between G3 and G4. These results indicate that geraniol could be a potent compound useful for the prevention of HCC.
DISCUSSION
HCC is the third-leading cause of death from cancer and the fifth most prevalent malignancy worldwide.14, 15 Since most HCC is complicated with diseased liver including chronic hepatitis and liver cirrhosis secondary to infection with hepatitis B virus (HBV), hepatitis C virus (HCV) and alcohol, regular surveillance of these patients who are at high risk of HCC by serum tumor markers and imaging has been conducted in Japan.16 However, the prognosis of patients with HCC remains poor because HCC is often diagnosed at advanced stages due to the inefficiency of ultrasonography instrument operators, dropout among the patients targeted by the screening program17 and the presence of a number of undiagnosed carriers of HBV and HCV18 which are difficult to include in the screening program. Thus, novel preventive and therapeutic approaches for these patients are urgently required.
Geraniol is an isoprenic derivative contained in the essential oils of many aromatic plants such as geranium and lemon.3 Beside its cosmetic use, geraniol has been demonstrated to have some bioactive properties including anti-inflammation,19 anti-angiogenesis3 and anti-tumor effects.5 Anti-tumor effects of geraniol have been demonstrated on a variety of cells including lung,4 pancreatic,5 colon6 and liver.7 In the liver, anti-tumor effects of geraniol have been demonstrated in culture cells7, 20 and rats.21–23
Thus geraniol is a promising candidate for the prevention and therapy of HCC. However, the number of studies investigating the anti-tumor effects of geraniol on hepatocarcinogenesis is quite limited. Therefore, we sought to further examine the anti-hepatocarcinogenic effects of geraniol employing the DEN-induced hepatocarcinogenic rat model which we have previously established.24–26 In the present study, we have demonstrated that oral treatment by geraniol of the DEN-induced hepatocarcinogenic rats significantly decreased serum transaminase levels and tumor formation of the liver based on the immunohistochemical analyses by the PCNA and GST-P antibodies. We determined the concentrations of geraniol based on a previous report.22 However, appropriate concentrations of geraniol in humans need to be evaluated in future studies.
In the current study, we did not explore the mechanisms of antitumor action by geraniol. Although information available is limited, induction of apoptosis was involved in the anti-proliferative effects of geraniol in rats.21, 22 In general, the proposed mechanisms by which miscellaneous plant-derived chemicals exhibit anti-tumor effects on the liver include cell cycle arrest, induction of apoptosis and inhibition of angiogenesis.27 It is conceivable that geraniol, a plant-derived compound, also modulated these cellular mechanisms. It is essential to investigate the modulation of signaling pathways which are relevant to the cell cycle, apoptosis and angiogenesis in DEN-administered rats after treating them with geraniol in future studies.
The authors declare no conflict of interest.
REFERENCES
- 1. Akita T, Ohisa M, Kimura Y, Fujimoto M, Miyakawa Y, Tanaka J. Validation and limitation of age-period-cohort model in simulating mortality due to hepatocellular carcinoma from 1940 to 2010 in Japan. Hepatol Res. 2014; 44: 713-9. . [DOI] [PubMed] [Google Scholar]
- 2. Kanda T, Ogasawara S, Chiba T, Haga Y, Omata M, Yokosuka O. Current management of patients with hepatocellular carcinoma. World J Hepatol. 2015; 7: 1913-20. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wittig C, Scheuer C, Parakenings J, Menger MD, Laschke MW. Geraniol suppresses angiogenesis by downregulating vascular endothelial growth factor (VEGF)/VEGFR-2 signaling. PLoS One. 2015; 10: e0131946. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Galle M, Crespo R, Kladniew BR, Villegas SM, Polo M, de Bravo MG. Suppression by geraniol of the growth of A549 human lung adenocarcinoma cells and inhibition of the mevalonate pathway in culture and in vivo: potential use in cancer chemotherapy. Nutr Cancer. 2014; 66: 888-95. . [DOI] [PubMed] [Google Scholar]
- 5. Jin X, Sun J, Miao X, Liu G, Zhong D. Inhibitory effect of geraniol in combination with gemcitabine on proliferation of BXPC-3 human pancreatic cancer cells. J Int Med Res. 2013; 41: 993-1001. . [DOI] [PubMed] [Google Scholar]
- 6. Carnesecchi S, Bras-Gonçalves R, Bradaia A, Zeisel M, Gossé F, Poupon MF, et al. Geraniol, a component of plant essential oils, modulates DNA synthesis and potentiates 5-fluorouracil efficacy on human colon tumor xenografts. Cancer Lett. 2004; 215: 53-9. . [DOI] [PubMed] [Google Scholar]
- 7. Crespo R, Montero Villegas S, Abba MC, de Bravo MG, Polo MP. Transcriptional and posttranscriptional inhibition of HMGCR and PC biosynthesis by geraniol in 2 Hep-G2 cell proliferation linked pathways. Biochem Cell Biol. 2013; 91: 131-9. . [DOI] [PubMed] [Google Scholar]
- 8. Chaudhary SC, Siddiqui MS, Athar M, Alam MS. Geraniol inhibits murine skin tumorigenesis by modulating COX-2 expression, Ras-ERK1/2 signaling pathway and apoptosis. J Appl Toxicol. 2013; 33: 828-37. . [DOI] [PubMed] [Google Scholar]
- 9. Ahmad ST, Arjumand W, Seth A, Nafees S, Rashid S, Ali N, et al. Preclinical renal cancer chemopreventive efficacy of geraniol by modulation of multiple molecular pathways. Toxicology. 2011; 290: 69-81. . [DOI] [PubMed] [Google Scholar]
- 10. Hussein UK, Mahmoud HM, Farrag AG, Bishayee A. Chemoprevention of diethylnitrosamine-initiated and phenobarbital-promoted hepatocarcinogenesis in rats by sulfated polysaccharides and aqueous extract of ulva lactuca. Integr Cancer Ther. 2015; 14: 525-45. . [DOI] [PubMed] [Google Scholar]
- 11. Verna L, Whysner J, Williams GM. N-nitrosodiethylamine mechanistic data and risk assessment: bioactivation, DNA-adduct formation, mutagenicity, and tumor initiation. Pharmacol Ther. 1996; 71: 57-81. . [DOI] [PubMed] [Google Scholar]
- 12. Alenzi FQ, El-Nashar EM, Al-Ghamdi SS, Abbas MY, Hamad AM, El-Saeed OM, et al. Investigation of Bcl-2 and PCNA in hepatocellular carcinoma: Relation to chronic HCV. J Egypt Natl Canc Inst. 2010; 22: 87-94. . [PubMed] [Google Scholar]
- 13. Sakai M, Muramatsu M. Regulation of glutathione transferase P: a tumor marker of hepatocarcinogenesis. Biochem Biophys Res Commun. 2007; 357: 575-8. . [DOI] [PubMed] [Google Scholar]
- 14. El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007; 132: 2557-76. . [DOI] [PubMed] [Google Scholar]
- 15. Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006; 6: 674-87. . [DOI] [PubMed] [Google Scholar]
- 16. Makuuchi M, Kokudo N, Arii S, Futagawa S, Kaneko S, Kawasaki S, et al. Development of evidence-based clinical guidelines for the diagnosis and treatment of hepatocellular carcinoma in Japan. Hepatol Res. 2008; 38: 37-51. . [DOI] [PubMed] [Google Scholar]
- 17. Okano J, Sawada S, Imamoto R, Abe R, Fujise Y, Murawaki Y. Challenges of diagnosing primary hepatocellular carcinoma. J Tottori med Ass. 2014; 42: 10-6. Japanese. [Google Scholar]
- 18. Tanaka J, Koyama T, Mizui M, Uchida S, Katayama K, Matsuo J, et al. Total numbers of undiagnosed carriers of hepatitis C and B viruses in Japan estimated by age- and area-specific prevalence on the national scale. Intervirology. 2011; 54: 185-95. . [DOI] [PubMed] [Google Scholar]
- 19. Khan AQ, Khan R, Qamar W, Lateef A, Rehman MU, Tahir M, et al. Geraniol attenuates 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced oxidative stress and inflammation in mouse skin: possible role of p38 MAP Kinase and NF-κB. Exp Mol Pathol. 2013; 94: 419-29. . [DOI] [PubMed] [Google Scholar]
- 20. Polo MP, Crespo R, de Bravo MG. Geraniol and simvastatin show a synergistic effect on a human hepatocarcinoma cell line. Cell Biochem Funct. 2011; 29: 452-8. . [DOI] [PubMed] [Google Scholar]
- 21. Cardozo MT, de Conti A, Ong TP, Scolastici C, Purgatto E, Horst MA, et al. Chemopreventive effects of β-ionone and geraniol during rat hepatocarcinogenesis promotion: distinct actions on cell proliferation, apoptosis, HMGCoA reductase, and RhoA. J Nutr Biochem. 2011; 22: 130-5. . [DOI] [PubMed] [Google Scholar]
- 22. Ong TP, Heidor R, de Conti A, Dagli ML, Moreno FS. Farnesol and geraniol chemopreventive activities during the initial phases of hepatocarcinogenesis involve similar actions on cell proliferation and DNA damage, but distinct actions on apoptosis, plasma cholesterol and HMGCoA reductase. Carcinogenesis. 2006; 27: 1194-203. . [DOI] [PubMed] [Google Scholar]
- 23. Yu SG, Hildebrandt LA, Elson CE. Geraniol, an inhibitor of mevalonate biosynthesis, suppresses the growth of hepatomas and melanomas transplanted to rats and mice. J Nutr. 1995; 125: 2763-7. . [DOI] [PubMed] [Google Scholar]
- 24. Imamoto R, Okano J, Sawada S, Fujise Y, Abe R, Murawaki Y. Null anticarcinogenic effect of silymarin on diethylnitrosamine-induced hepatocarcinogenesis in rats. Exp Ther Med. 2014; 7: 31-8. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abe R, Okano J, Imamoto R, Fujise Y, Murawaki Y. Sequential analysis of diethylnitrosamine-induced hepatocarcinogenesis in rats. Exp Ther Med. 2012; 3: 371-8. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fujise Y, Okano J, Nagahara T, Abe R, Imamoto R, Murawaki Y. Preventive effect of caffeine and curcumin on hepato-carcinogenesis in diethylnitrosamine-induced rats. Int J Oncol. 2012; 40: 1779-88. . [DOI] [PubMed] [Google Scholar]
- 27. Okano J, Fujise Y, Abe R, Imamoto R, Murawaki Y. Chemoprevention against hepatocellular carcinoma. Clin J Gastroenterol. 2011; 4: 185-97. . [DOI] [PubMed] [Google Scholar]