Abstract
Background
Prostate cancer is the most common cancer in men, and radical prostatectomy (RP) often results in erectile dysfunction (ED) and a substantially reduced quality of life. The efficacy of current interventions, principal treatment with PDE-5 inhibitors, is not satisfactory and this condition presents an unmet medical need. Preclinical studies using adipose-derived stem cells to treat ED have shown promising results. Herein, we report the results of a human phase 1 trial with autologous adipose-derived regenerative cells (ADRCs) freshly isolated after a liposuction.
Methods
Seventeen men suffering from post RP ED, with no recovery using conventional therapy, were enrolled in a prospective phase 1 open-label and single-arm study. All subjects had RP performed 5–18 months before enrolment, and were followed for 6 months after intracavernosal transplantation. ADRCs were analyzed for the presence of stem cell surface markers, viability and ability to differentiate. Primary endpoint was the safety and tolerance of the cell therapy while the secondary outcome was improvement of erectile function. Any adverse events were reported and erectile function was assessed by IIEF-5 scores. The study is registered with ClinicalTrials.gov, NCT02240823.
Findings
Intracavernous injection of ADRCs was well-tolerated and only minor events related to the liposuction and cell injections were reported at the one-month evaluation, but none at later time points. Overall during the study period, 8 of 17 men recovered their erectile function and were able to accomplish sexual intercourse. Post-hoc stratification according to urinary continence status was performed. Accordingly, for continent men (median IIEFinclusion = 7 (95% CI 5–12), 8 out of 11 men recovered erectile function (IIEF6months = 17 (6–23)), corresponding to a mean difference of 0.57 (0.38–0.85; p = 0.0069), versus inclusion. In contrast, incontinent men did not regain erectile function (median IIEF1/3/6 months = 5 (95% CI 5–6); mean difference 1 (95% CI 0.85–1.18), p > 0.9999).
Interpretation
In this phase I trial a single intracavernosal injection of freshly isolated autologous ADRCs was a safe procedure. A potential efficacy is suggested by a significant improvement in IIEF-5 scores and erectile function. We suggest that ADRCs represent a promising interventional therapy of ED following prostatectomy.
Funding
Danish Medical Research Council, Odense University Hospital and the Danish Cancer Society.
Abbreviations: RP, radical prostatectomy; ED, erectile dysfunction; PDE-5, phosphodiesterase-5; ADRC, adipose-derived regenerative cells; SVF, stromal vascular fraction; IIEF-5, international index of erectile function-5; EHS, erection hardness score; ICIQ-UI SF, incontinence questionnaire – urinary incontinence – short form questionnaire; BMI, body mass index; CFU-F, fibroblastoid colony forming units; NSAID, nonsteroidal antiinflammatory drug; LUTS, lower urinary tract symptoms
Keywords: Adipose-derived regenerative cells, Adipose-derived stromal vascular fraction, Adipose-derived stem cells, Cell therapy, Erectile dysfunction, Clinical trial
Highlights
-
•
In this phase I study a single intracavernous injection of adipose-derived regenerative cells was a safe and well-tolerated procedure
-
•
Seventeen men suffering from erectile dysfuncion after radical prostatectomy were enrolled
-
•
The adipose-derived regenerative cells were used directly after a abdominal liposuction
-
•
8/11 continent men regained the ability to have sexual intercourse, suggesting potential efficacy of ADRC injections
1. Introduction
The promising potential of stem cell therapy for various diseases has been subject to much basic research and has attracted significant clinical interest. In clinical practice, however, such interventions remain largely experimental outside of bone marrow transplantation and autologous stem cell transplantation as related to chemotherapy (Dohner et al., 2015). Clinical implementation of stem cell treatment for erectile dysfunction (ED) represents a plausible candidate for such an approach. It has been reported that mesenchymal stem cells from bone marrow or adipose tissue can correct ED in animal models (Gimble et al., 2012, Lin et al., 2012). Prostate cancer is the most common male cancer affecting 17% of all men (Chung and Gillman, 2014), of which approximately 25% receive a prostatectomy. Due to penile nerve injury, up to 86% of patients experience ED (Salonia et al., 2012, Tal et al., 2009, Weyne and Albersen, 2014) following prostatectomy. ED is defined as the consistent or recurrent inability to attain or maintain an erection sufficient for satisfactory sexual performance (JAMA, 1993, Montorsi et al., 2010). ED following prostatectomy is an important medical condition that substantially decreases quality of life of the afflicted men and their sexual partners (Litwin et al., 1998). Besides prostatectomy, ED risk factors include widespread diseases such as hypertension and obesity, but also medications such as β-blockers and anti-depressants, as well as major life-style factors like smoking and alcohol use cause ED (Shabsigh et al., 2005). Moreover, age is a risk factor; approximately one third of men in their forties report ED symptoms, while more than half of men over 60 years suffer from ED (Lewis et al., 2010). Although the prevalence and impact of ED remain substantial, current penile rehabilitation therapy following prostatectomy mainly consists of treatment with PDE-5 inhibitors or injection therapy, which have an unimpressive clinical efficacy around 27% or lower (Chung and Gillman, 2014, Weyne and Albersen, 2014, Weyne et al., 2015). This condition therefore presents a significant unmet medical need.
At the cellular level, ED is thought to be caused by neuro-vascular or hormonal dysfunction resulting in impaired vasodilatation of penile arteries (Salonia et al., 2012, Weyne and Albersen, 2014). When the natural nocturnal erection is lost, the penis tissue enters a chronic hypoxic state leading to vascular dysfunction (Mulhall et al., 2010). Adipose-derived regenerative cells (ADRCs, also referred to as stromal vascular fraction, SVF) are able to differentiate into vascular cells and neurons in vitro (Gimble et al., 2013, Lin et al., 2008, Zuk, 2010), and a large body of preclinical work shows a surprisingly good effect of ADRC injection into the corpora cavernosa (Lin et al., 2012). One human study reported improved erectile function using umbilical cord blood cells in 7 diabetic ED patients (Bahk et al., 2010). This latter study demonstrates proof of concept for the use of cell therapy in clinical ED treatment, even if the etiology of ED in diabetics may reflect less nerve injury as compared to patients after radical prostatectomy (RP) (Schauer et al., 2015). The safety of applying freshly isolated autologous ADRCs for non-homologous use in intracavernous injections has not previously been explored.
We here report safety and preliminary efficacy outcomes from a phase 1 clinical trial using autologous ADRCs for the treatment of ED in 17 men after radical prostatectomy.
2. Methods
2.1. Study Design and Eligibility Criteria
Study population (Fig. 1): Seventeen patients with ED after RP (3 open, 14 robot-assisted laparoscopic prostatectomies) were enrolled between May 2014 and September 2015 in this prospective, open-label, single-arm and single-center study. All subjects had RP performed at Odense University Hospital, Denmark, 5–18 months prior to enrolment. Inclusion criteria were: age > 18 years. Clinical follow-up was required to show organ-confined prostate cancer without metastasis. Patients had to be sexually active before RP and expressing a wish to remain sexually active. Appropriate pharmacological intervention (PDE-5 or PGE1 analog) must have been tried and deemed insufficient to allow for inclusion. The participants were suggested to continue medication throughout the study period if they felt the slightest effect. Patients with no initial effect were encouraged to retry pharmacological treatment, to see if they had changed responder-status. Patients were told to continue other regular medications and pelvic physiotherapy during the trial.
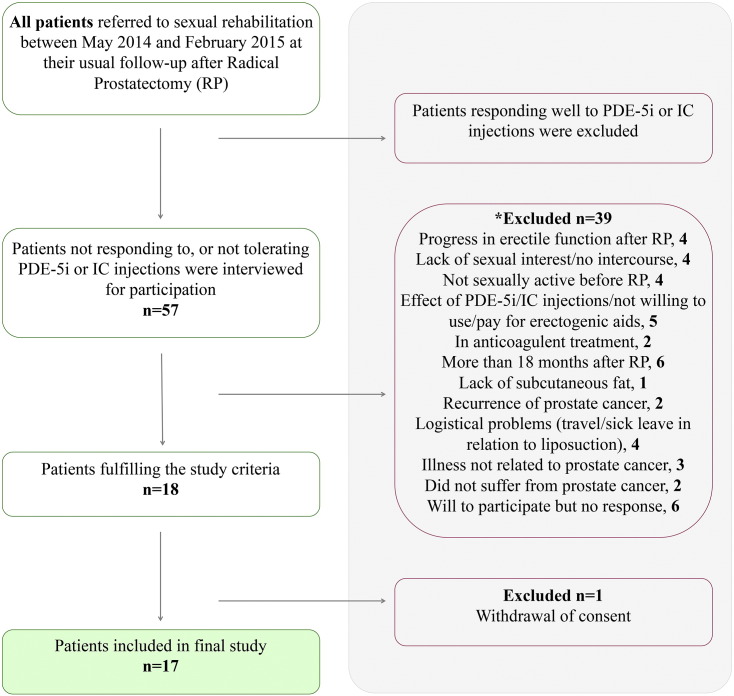
Fig. 1.
Study overview. *Some patients were excluded based on several criteria. (IC:Intracavernous; RP:Radical Prostatectomy).
Exclusion criteria: treatment with anticoagulants; insufficient subcutaneous fat; lack of sexual interest. We used the following outcome measurements. As described in (Olsen et al., 2015), the International Index of Erectile Function-5 (IIEF-5) and Erection Hardness Score (EHS) were used to assess erectile function before RP at study inclusion and 1, 3 and 6 months after ADRC treatment. Information on urinary incontinence was assessed by International Consultation on Incontinence Questionnaire – Urinary Incontinence – Short form questionnaire (ICIQ-UI SF). Adverse events were recorded at each visit by inspection of the injection site and posing the patients an open question, “Did you experience any discomfort related to the operation since the last visit?”, followed by specific questions regarding pain, bleeding and swelling at the liposuction site as well as at the penis area.
2.2. Approvals
The study was approved by the Danish National Ethics Committee (no. 37054), The Danish Health and Medicines Authority (EUDRA-CT number 2013–004220-11) and the Danish Data Protection Agency (2008–58-0035). The study was performed in accordance with the Declaration of Helsinki and ICH-GCP guidelines and monitored by the GCP Unit at Odense University Hospital. All patients gave written informed consent before participation. This study is registered at ClinicalTrials.gov (NCT02240823, Phase 1 Study. Can Fat Derived Stem Cells (SVF) be used in the Treatment of Erectile Dysfunction After Prostatectomy).
2.3. Procedures
2.3.1. Adipose Tissue Collection, ADRC Preparation, − Delivery and Characterization
Briefly, adipose tissue collection was conducted during general anesthesia, and harvesting was performed with water-jet-assisted liposuction. Following immediate isolation of ADRCs, using an automated processing Celution® 800/CRS system (Cytori Therapeutics, San Diego, California, USA), these were injected into corpus cavernosum. ADRCs were characterized by cell count, viability and subpopulation. For details, refer to supplementary material.
2.4. Statistical Analysis
This was an exploratory pilot study and no sample size estimates were calculated. We planned to include 30 patients with an interim efficacy analysis upon reaching 15 evaluable patients. In case of a clinically relevant positive signal at the interim analysis, the inclusion of patients would be discontinued at this point. Data are presented as medians and interquartile ranges (IQR) with exception of ADRC characteristics, which are mean (SD). IIEF outcomes were analyzed by repeated measurement two-way ANOVA with Sidak's post-hoc test for multiple comparisons, following log transformation of data. Effect sizes are presented as geometric mean differences with 95% confidence intervals. EHS outcomes were analyzed by Friedman's test for multiple non-parametric comparisons with Dunn's post-hoc test for multiple comparisons. ICIQ-UI SF outcomes were analyzed by Wilcoxon's matched pairs signed rank test. Associations between ADRCs and volume of liposuction and donor age were analyzed by Pearson's product–moment correlation. ADRC phenotypes between groups were analyzed by the Mann–Whitney U test.
All statistical analyses were performed using Prism 6 (GraphPad Software, La Jolla, CA, USA).
2.5. Role of the Funding Source
The study sponsors had no role in the study design, data collection, data analysis, data interpretation or writing of the report. All authors had full access to all data. The corresponding author had final responsibility for the decision to submit for publication.
3. Results
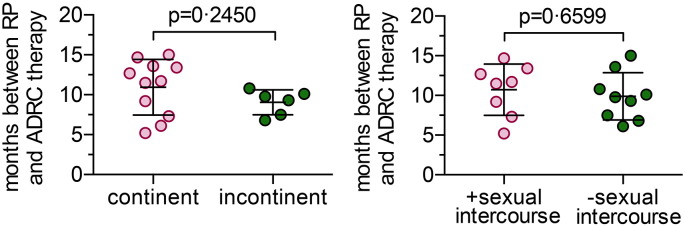
We included 17 men (Fig. 1) aged between 46 and 69 years (63 (9), median (IQR)) years with ED after prostatectomy to intracavernosal injection with their own ADRCs freshly isolated after a liposuction. All patients had high IIEF-5 scores (23 (3), median (IQR)) and EHS scores (4 (1), median (IQR)), and reported an active sex life before RP. Eleven men were continent while 6 were incontinent at the time of inclusion, but baseline characteristics including age, BMI, smoking, alcohol intake, physical activity, degree of co-morbidities and medication were similar for the two groups (Table 1). Time between RP and ADRC treatment 10.1 (5.7) (median (IQR) months with no difference (p = 0.2450, Mann–Whitney test) between continent and incontinent men is shown in Supplemental Fig. S1.
Table 1.
Baseline characteristics.
| All patients (n = 17) |
Continent (n = 11) |
Incontinent (n = 6) |
|
|---|---|---|---|
| Age, years | 63.0 (9.0) | 63.0 (10.0) | 63.0 (5.8) |
| Body mass index, kg/m2 | 30.3 (5.0) | 28.7 (5.1) | 30.7 (2.4) |
| Hypertensive | 6 | 5 | 1 |
| Preoperative PSA, ng/ml | 7.3 (5.4) | 8.2 (5.1) | 6.9 (3.2) |
| Surgical approach | |||
| Robotic prostatectomy | 14 | 7 | 7 |
| Open retropubic prostatectomy | 3 | 3 | 0 |
| Nerve-sparing approach | |||
| Nerve-sparing RP | 5 | 4 | 1 |
| Unilateral nerve-sparing RP | 3 | 3 | 0 |
| None nerve-sparing RP | 9 | 4 | 5 |
| Pathologic stage | |||
| pT2c | 15 | 10 | 5 |
| pT3b | 2 | 1 | 1 |
| Pathologic Gleason score | |||
| Gleason 3 + 3 | 2 | 2 | 0 |
| Gleason 3 + 4 | 9 | 7 | 2 |
| Gleason 4 + 3 | 5 | 2 | 3 |
| Gleason 4 + 5 | 1 | 0 | 1 |
| Severe LUTS1 prior to diagnosis of prostate cancer. | 5 | 0 | 5 |
Data are median (IQR).1Severe LUTS defined as > 15 DANPSS points. DANPSS, Danish Validated Questionnaire on LUTS and LUTS-bother. PSA, Prostate Specific Antigen. RP, Radical Prostatectomy.
Supplemental Fig. S1.
The time between prostatectomy and ADRC intervention was similar in all groups. The time interval in months between radical prostatectomy and ADRC injection was tested (Mann–Whitney test) in patients grouped according to their (A) urinary continence status, or (B) ability to have sexual intercourse after 6 months. In both cases, no significant difference was observed.
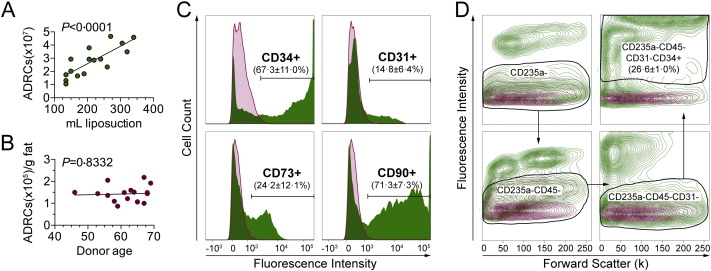
The characteristics of ADRCs isolated by the automated Celution® system (Table 2) were comparable to those previously reported for other ADRCs/SVF (Domenis et al., 2015) including yield (ADRCs/g fat tissue, 1.4 × 105, 3.7 × 104; mean, SD), cell size (10.7, 0.2; mean, SD), viability (84.7, 3.0; mean, SD) and, percentage of fibroblastoid colony forming units (%CFU-F, 1.5, 0.8; mean, SD). The number of ADRCs obtained per patient was strongly correlated to the amount of lipoaspirate (Fig. 2A), while we observed no relationship between age and yield per gram fat tissue (Fig. 2B). A large proportion of the freshly isolated ADRCs expressed the surface markers CD34 (67.3, 11.0%; mean, SD) and CD90 (71.3, 7.4%; mean, SD), whereas CD31 and CD73 each defined smaller subpopulations (14.8%, 6.4% and 24.2%, 12.1%, respectively; mean, SD) (Fig. 2C). The fraction of stromal stem cells as defined phenotypically by the markers CD235a-CD45-CD31-CD34 +, encompassed approximately 26% of the parent ADRCs (Fig. 2D), which is similar to what has been shown by others (Domenis et al., 2015, Aronowitz and Ellenhorn, 2013).
Table 2.
Characterization of the freshly isolated ADRCs.*
| Parameter | All (n = 17) | Continent (n = 11) | Incontinent (n = 6) | |
|---|---|---|---|---|
| ADRCs/mL liposuction | 1.3 × 105 (3.3 × 104) | 1.3 × 105 (3.8 × 104) | 1.2 × 105 (1.9 × 104) | |
| ADRCs/g fat tissue* | 1.4 × 105 (3.7 × 104) | 1.5 × 105 (4.3 × 104) | 1.3 × 105 (2.1 × 104) | |
| Injected ADRCs | 2.2 × 107 (9.0 × 106) | 2.1 × 107 (8.3 × 106) | 2.3 × 107 (1.1 × 107) | |
| Cell size (μm) | 10.7 (0.2) | 10.7 (0.2) | 10.6 (0.2) | |
| Viability (%) | 84.7 (3.0) | 85.3 (2.5) | 83.8 (3.8) | |
| CFU-F (%) | 1.5 (0.8) | 1.4 (0.8) | 1.6 (0.7) | |
| Cell surface marker expression | CD34 (%) | 67.3 (11.0) | 67.8 (12.2) | 660.3 (9.3) |
| CD31 (%) | 14.8 (6.4) | 16.2 (6.2) | 12.1 (6.5) | |
| CD73 (%) | 24.2 (12.1) | 25.1 (13.0) | 22.5 (11.2) | |
| CD90 (%) | 71.3 (7.4) | 73.0 (5.7) | 68.3 (9.6) | |
1 mL = 0.9 g fat tissue. Data are mean (SD). Differences between groups were analyzed using Mann–Whitney test.
Fig. 2.
Characterics of the isolated ADRCs. (A) The total yield of nucleated ADRCs per isolation was positively correlated to the input volume of liposuction. (B) ADRC yield per gram fat tissue was independent of donor age. (C) Representative histograms showing expression levels of CD34, CD31, CD73 and CD90 (green) in single-stained ADRCs as analyzed by flow cytometry. The appropriate isotype control is depicted as overlay (purple) in each case. Population percentages are given as mean ± SD, n = 17. (D) The population of CD235a-CD45-CD31-CD34 + ADRCs was identified in multi-stained samples by flow cytometry using a stepwise gating strategy as indicated by the arrows. The number of cells is expressed as a percentage of the entire single-cell ADRC population (mean ± SD, n = 3). In each density plot (from one representative patient), the corresponding isotype control is shown as overlay (purple). P-values represent Pearson correlation coefficients in A and B.
The men received between 8.4–37.2 million ADRCs immediately after cell isolation and were discharged from hospital the same day. No serious adverse events were reported. Five patients reported minor events related to the liposuction and ADRC injection at the one-month evaluation time point. Two men had experienced transient redness and swelling at the injection sites. One had a scrotal and penile hematoma that resolved within 14 days. This patient had taken large doses of NSAID medicine for back pain in the days before the treatment. Finally, 2 patients reported abdominal pain and tenderness for 2–6 days after the liposuction. All events resolved without intervention and at the 3- and 6-month evaluations, no patients reported any side- or adverse events.
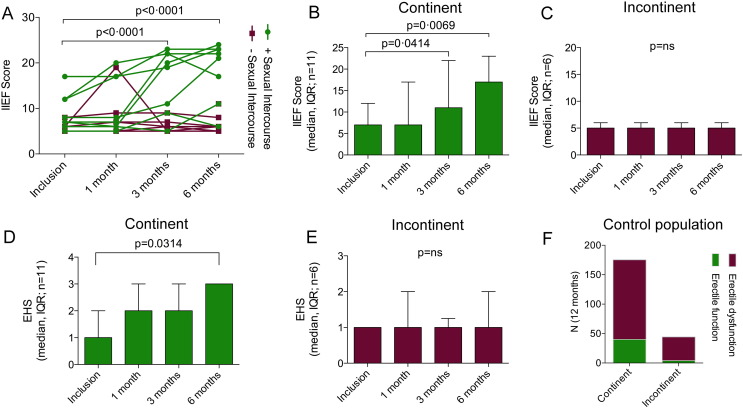
During the study period, eight out of 17 participants recovered erectile function after ADRC injection with the ability to complete intercourse (Fig. 3A). Post-hoc stratification according to urine continence at inclusion revealed an apparent strong conditional association to the regain of function. Preliminary efficacy was solely demonstrated in the patients that were continent at inclusion (Fig. 3). In the continent group, the IIEF-5 score was unchanged 7 (Shabsigh et al., 2005) (median (IQR) (mean 0.92 (95% CI 0.78–1.1), p = 0.3859, RM one-way ANOVA with Sidak's multiple comparisons test) 1 month after the treatment, but significantly increased to 11 (Gimble et al., 2013) (0.66, (0.44–0.98), p = 0.0414) after 3 months and to 17 (Gimble et al., 2013) (0.57 (0.38–0.85), p = 0.0069) after 6 months (Fig. 3B). The EHS scores supported these data although with slight differences. The EHS data were significantly increased at 6 months (3(1) (p = 0.0314, Friedman's with Dunn's multiple comparisons test) as compared to inclusion (1(1)), while no change was observed after 1 month and 3 months (Fig. 3D). In the group of incontinent men, the IIEF-5 scores were similar after 1, 3 and 6 months and did not differ from the score at the time of inclusion (5 (1)(median (IQR) (mean 1 (95% CI 0·85–1.18), p > 0.9999, RM one-way ANOVA with Sidak's multiple comparisons test), Fig. 3C). Likewise, EHS data for this group were unchanged throughout the study period (fig. 3E). There were no differences in ADRC characteristics between the groups of continent versus incontinent men (table 2). To estimate the rate of spontaneous reversion of ED post RP in our department, we reviewed the medical records for 2010–2013. These included records of 165 prostatectomized men with information about their sexual status and urine continence. There were 135 urine continent men and 30 incontinent men. In these groups, 40 and 4 men, respectively, reported having adequate sexual function, and were able to complete intercourse with or without medication 6 and 12 months after operation (Fig. 3F).
Fig. 3.
Effect of ADRC therapy. (A) IIEF scores for each patient at inclusion, 1, 3 and 6 months after a single intracavernous bolus of autologous ADRCs. Patients that regained their ability to have sexual intercourse (green closed circles) had significantly better IIEF scores after 3 and 6 months, but not after 1 month (Two-Way ANOVA following log transformation). (B, C) Patients were stratified according to their urinary incontinence status. (B) In the group of continent men (n = 11), significantly improved IIEF scores were observed after 3 and 6 months compared to their inclusion scores (RM one-way ANOVA with Sidak's multiple comparisons test following log transformation). (C) No changes in IIEF scores were observed in the group of incontinent men (n = 6) during the study period (RM one-way ANOVA with Sidak's multiple comparisons test, following log transformation). Similarly, the EHS scores were significantly improved after 6 months in the continent (D) but not in the incontinent group (E) (Friedman's test with Dunn's multiple comparisons test). (F) Spontaneous reversion of ED post RP in our department was established reviewing 165 medical records. In groups of 135 urine continent men and 30 incontinent men, 40 and 4 men, respectively, showed spontaneous reversion of ED.
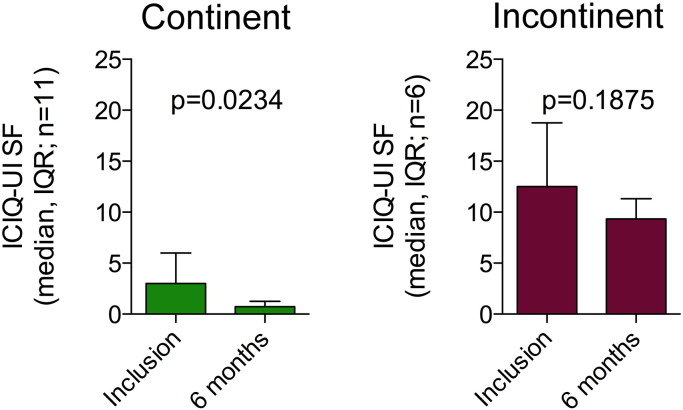
Additionally, urinary incontinence scores (ICIQ-UI SF) were significantly lower 6 months after ADRC injection in continent men 0 (Dohner et al., 2015) (median (IQR))(p = 0.0234, Wilcoxon matched pairs signed rank test) as compared to inclusion 3 (Tal et al., 2009) and a trend (8 (9) versus 12.5 (10.25)(p = 0.1875) was seen in incontinent men as well (Supplemental Fig. S2), suggesting that ADRC treatment may also have a positive effect on incontinence per se.
Supplemental Fig. S2.
Urinary continence was improved. Improved urinary continence scores with a reduction in ICIQ-UI SF score 6 months post-transplantation was significant for continent men, and a trend was revealed for incontinent men as well (Wilcoxon matched pairs signed rank test).
4. Discussion
In this clinical trial autologous non-expanded stem cells have been used for the treatment of ED following radical prostatectomy. In recent years, much attention has been given to stem cell-based therapies in general, and specifically in the field of urology where there has been a great deal of hype in relation to treatment of erectile dysfunction (ED) (Khera et al., 2015). Until now, there has been only one other published clinical trial reporting a beneficial effect of non-autologous umbilical cord cell transplantation in 3/7 men with type 2 DM (Bahk et al., 2010). Earlier this year, this lack of substantial evidence from clinical trials led to a commentary in which Khera et al. raised their concerns regarding stem cell treatment of ED patients outside an approved protocol (Khera et al., 2015).
In this study, a single intracavernous injection of freshly isolated autologous ADRCs was a feasible, safe and well-tolerated approach for treating ED following radical prostatectomy. We did not observe any serious adverse events within a 6-month time frame, which – while clearly not sufficient for a long-term safety evaluation – nevertheless is reassuring. The safety from clinical trials encompassing more than 1000 patients receiving bone marrow or adipose tissue-derived mesenchymal stem/stromal cells, does not suggest that serious adverse events is a clinically important issue (Casiraghi et al., 2013). Importantly, post-hoc stratification demonstrated that the majority of continent men with ED after RP recovered erectile function within 3 months, and that this effect persisted for 6 months. This potential efficacy of the ADRC therapy was promisingly high, reaching 8/11 (73%). In contrast, such effect was absent in the group of incontinent men. These different outcomes are unlikely to result from underlying differences in the amount of injected stem cells or in their phenotypes, since all analyzed parameters were statistically equal between groups. However, the incontinent men may represent individuals where damage to the neurovascular bundle has resulted in partial disruption of urethral rhabdosphincter innervation as reported recently (Reeves et al., 2015). Also, it should be noted that these patients had lower inclusion IIEF5 and EHS scores compared to the continent group and that 5 of the 6 incontinent men were initially referred for prostate evaluation due to irritable lower urinary tract symptoms before the RP. This suggests that the incontinent men, have a more advanced neurovascular degeneration altogether which agrees with previous reports showing a difference in spontaneous recovery between continent and incontinent patients (Gandaglia et al., 2012). Finally, there is likely to be a psychological element, since incontinent men may feel less sexually attractive because of the incontinence. We believe the observation that incontinent men have no effect of ADRC therapy for ED is important for future studies and clinical decisions regarding which patient groups should be offered the therapy. ADRC therapy seems to partly alleviate the patient's urinary incontinence symptoms as measured by the ICIQ-UI SF scores (Supplemental Fig. S2). While the clinical significance thereof is unclear, these data are promising for treatment of incontinence per se.
Our results hold promise for the outcome of other ongoing clinical trials with stem cells for ED therapy, especially one trial in RP patients using allogeneic cultured stem cells derived from bone marrow (NCT01983709), and for tests of mesenchymal stem cells in a wide range of human diseases. For evaluation of the clinical impact, it will be important to characterize the quality, stability and potency of different types and preparations of mesenchymal stem cells. For instance, it is not known if cultured ADRCs or ADRCs from another person, other mesenchymal stem cells or even induced pluripotent stem (iPS) cells would work as well as the freshly isolated stem cells used herein. If cultured non-autologous ADRCs or iPS cells work equally well both from a safety and efficacy point of view, this would have a large impact for the field since cells from stem cell banks could then be used. We investigated ED after RP, which is considered a severe form of ED with nerve injury; however, it is not known if stem cell therapy would work in patients with ED due to diabetes, cardiovascular disease, lower urinary tract diseases or simply age. Our results as well as results from the Korean study in diabetic men (Bahk et al., 2010) suggest that stem cell therapy may be effective for different types of ED.
Our study has several limitations. This pilot study was unblinded without a control group. We cannot discern if the positive effect is a result of the urologist interviews or the patient's own expectations to stem cell therapy or if in fact the stem cells themselves by differentiation have helped regenerate the erectile function (Gimble et al., 2013, Zuk, 2010) or if a paracrine mechanism is responsible (Fandel et al., 2012, Kimbrel et al., 2014). In general, reported degrees of ED after RP vary greatly (14%–86%) depending on risk stratification and patient selection, the experience of the surgeon, type of operation and the measure and definition of ED (Weyne and Albersen, 2014). The result that 8/11 (73%) of the continent men recovered erectile function after ADRC therapy is promising, although further placebo-controlled trials are needed to differentiate possible stem cell effects from spontaneous regeneration. Regarding spontaneous regeneration, background data from review of 165 medical journals from our urology department showed that only 40/135 (29.6%) continent men and 4/30 (13.3%) incontinent men recovered their erectile function 6 months and 1 year after RP using conventional therapy. In a previous RCT study of much milder forms of ED from our group (Olsen et al., 2015), only 7/57 (12.3%) men in a placebo control group recovered erectile function (IIEF scores above 10) after 6 months. Likewise, others have analyzed all 11 reported, randomized, controlled trials to enclose a penile rehabilitation of 20–25% following RP (Schauer et al., 2015). In the present paper, we report that a much higher number of the stem cell-treated RP-induced ED men with urinary continence had high IIEF scores after 6 months. Finally, the nine months' IIEF score data from 423 men with ED after RP in the REACTT placebo-controlled study showed relatively low recovery of erectile function after RP; 25.5% had high IIEF scores in the “5 mg tadalafil (Cialis®) 3 times a day group” versus 14.2% with high IIEF scores in the placebo group (Montorsi et al., 2014).
We did not include objective measurement for the recovery of erectile function, like measurements of penile hemodynamics or nerve impulse speed. We used EHS and IIEF scores that are generated on the basis of patient questionnaires and thus could be biased by several factors including interviewer effects and differences in the patients' understanding of the questions. In order to mitigate interviewer bias, we used the same interviewer throughout the study, and interviewed the patients at different time points using the same questions. A recent study shows correlation between objective measurements and EHS (Matsuda et al., 2014).
In conclusion, our findings suggest that autologous, freshly isolated ADRCs are safe to use and possess potential efficacy in the treatment of ED after RP. These findings need confirmation in an adequately powered, randomized and placebo-controlled trial.
The following are the supplementary data related to this article.
Supplementary material
5. Contributors
MKH and CHJ contributed equally to this work. Literature search: MKH, CHJ, SPS; Conception: SPS; Design: SPS, LL, MKH, CHJ, PD; Recruitment: MKH, LL; Surgical procedures: JAS, NT, MKH; Cell processing and laboratory procedures: CHJ; Data Collection: MKH, CHJ; Data analysis and interpretation: SPS, DCA, CHJ, MKH, PD; Production of figures and tables: SPS, CHJ, DCA, MKH. Manuscript writing: SPS, DCA, CHJ, MKH, PD. All authors have contributed to review and revision and approved the final manuscript version.
Conflicts of Interest
SPS has asked the Danish Health Authorities for permission to offer the treatment to the public in a private hospital. All other authors declare no conflicts of interest.
Interpretation
Our pilot data suggest that a single intracavernous injection of freshly isolated autologous ADRCs is a safe, and potentially efficient treatment of ED following radical prostatectomy. These results suggest that ADSCs can restore normal erectile function and such therapy may become a significant in the treatment of ED. Our results hold promise for the outcome of other ongoing clinical trials with stem cells for ED therapy, especially one trial in RP patients using allogeneic, cultured stem cells derived from bone marrow (NCT01983709), and for tests of mesenchymal stem cells in a wide range of human diseases.
Acknowledgment
This study was funded by Odense University Hospital (11/31936), The Danish Centre for Regenerative Medicine (14/50427) and the Danish Cancer Society. We thank Lars Melholt Rasmussen, prof. MD, Odense University Hospital and Bruce Conklin, MD, PhD, The Gladstone Inst. USCF, San Francisco, USA for critical reading of the manuscript.
References
- Aronowitz J.A., Ellenhorn J.D. Adipose stromal vascular fraction isolation: a head-to-head comparison of four commercial cell separation systems. Plast. Reconstr. Surg. 2013;132(6)):932e–939e. doi: 10.1097/PRS.0b013e3182a80652. [DOI] [PubMed] [Google Scholar]
- Bahk J.Y., Jung J.H., Han H., Min S.K., Lee Y.S. Treatment of diabetic impotence with umbilical cord blood stem cell intracavernosal transplant: preliminary report of 7 cases. Exp. Clin Transplant. 2010;8(2):150–160. [PubMed] [Google Scholar]
- Casiraghi F., Remuzzi G., Abbate M., Perico N. Multipotent mesenchymal stromal cell therapy and risk of malignancies. Stem Cell Rev. 2013;9(1):65–79. doi: 10.1007/s12015-011-9345-4. [DOI] [PubMed] [Google Scholar]
- Chung E., Gillman M. Prostate cancer survivorship: a review of erectile dysfunction and penile rehabilitation after prostate cancer therapy. Med. J. Aust. 2014;200(10):582–585. doi: 10.5694/mja13.11028. [DOI] [PubMed] [Google Scholar]
- Dohner H., Weisdorf D.J., Bloomfield C.D. Acute myeloid leukemia. N. Engl. J. Med. 2015;373(12):1136–1152. doi: 10.1056/NEJMra1406184. [DOI] [PubMed] [Google Scholar]
- Domenis R., Lazzaro L., Calabrese S. Adipose tissue derived stem cells: in vitro and in vivo analysis of a standard and three commercially available cell-assisted lipotransfer techniques. Stem Cell Res. Ther. 2015;6:2. doi: 10.1186/scrt536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fandel T.M., Albersen M., Lin G. Recruitment of intracavernously injected adipose-derived stem cells to the major pelvic ganglion improves erectile function in a rat model of cavernous nerve injury. Eur. Urol. 2012;61(1):201–210. doi: 10.1016/j.eururo.2011.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandaglia G., Suardi N., Gallina A. Preoperative erectile function represents a significant predictor of postoperative urinary continence recovery in patients treated with bilateral nerve sparing radical prostatectomy. J. Urol. 2012;187(2):569–574. doi: 10.1016/j.juro.2011.10.034. [DOI] [PubMed] [Google Scholar]
- Gimble J.M., Bunnell B.A., Frazier T. Adipose-Derived Stromal/Stem Cells: A Primer. Organogenesis. 2013;9(1):3–10. doi: 10.4161/org.24279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimble J.M., Bunnell B.A., Guilak F. Human adipose-derived cells: an update on the transition to clinical translation. Regen. Med. 2012;7(2):225–235. doi: 10.2217/rme.11.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH Consensus Conference. Impotence NIH Consensus Development Panel on Impotence. JAMA. 1993;270(1):83–90. [PubMed] [Google Scholar]
- Khera M., Albersen M., Mulhall J.P. Mesenchymal stem cell therapy for the treatment of erectile dysfunction. J. sex. Med. 2015;12(5):1105–1106. doi: 10.1111/jsm.12871. [DOI] [PubMed] [Google Scholar]
- Kimbrel E.A., Kouris N.A., Yavanian G.J. Mesenchymal stem cell population derived from human pluripotent stem cells displays potent immunomodulatory and therapeutic properties. Stem Cells Dev. 2014;23(14):1611–1624. doi: 10.1089/scd.2013.0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R.W., Fugl-Meyer K.S., Corona G. Definitions/Epidemiology/Risk Factors for Sexual Dysfunction. J. Sex. Med. 2010;7(4 Pt 2):1598–1607. doi: 10.1111/j.1743-6109.2010.01778.x. [DOI] [PubMed] [Google Scholar]
- Lin C.S., Xin Z.C., Deng C.H., Ning H., Lin G., Lue T.F. Recent advances in andrology-related stem cell research. Asian. J. Androl. 2008;10(2):171–175. doi: 10.1111/j.1745-7262.2008.00389.x. [DOI] [PubMed] [Google Scholar]
- Lin C.S., Xin Z.C., Wang Z. Stem Cell Therapy for Erectile Dysfunction: A Critical Review. Stem Cells Dev. 2012;21(3):343–351. doi: 10.1089/scd.2011.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwin M.S., Nied R.J., Dhanani N. Health-Related Quality of Life in men with Erectile Dysfunction. J. Gen. Intern. Med. 1998;13(3):159–166. doi: 10.1046/j.1525-1497.1998.00050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda Y., Hisasue S., Kumamoto Y. Correlation between erection hardness score and nocturnal penile tumescence measurement. J. Sex. Med. 2014;11(9):2272–2276. doi: 10.1111/jsm.12617. [DOI] [PubMed] [Google Scholar]
- Montorsi F., Adaikan G., Becher E. Summary of the recommendations on sexual dysfunctions in men. J. Sex. Med. 2010;7(11):3572–3588. doi: 10.1111/j.1743-6109.2010.02062.x. [DOI] [PubMed] [Google Scholar]
- Montorsi F., Brock G., Stolzenburg J.U. Effects of tadalafil treatment on erectile function recovery following bilateral nerve-sparing radical prostatectomy: a randomised placebo-controlled study (REACTT) Eur. Urol. 2014;65(3):587–596. doi: 10.1016/j.eururo.2013.09.051. [DOI] [PubMed] [Google Scholar]
- Mulhall J.P., Bella A.J., Briganti A., McCullough A., Brock G. Erectile function rehabilitation in the radical prostatectomy patient. J. Sex. Med. 2010;7(4 Pt 2):1687–1698. doi: 10.1111/j.1743-6109.2010.01804.x. [DOI] [PubMed] [Google Scholar]
- Olsen A.B., Persiani M., Boie S., Hanna M., Lund L. Can low-intensity extracorporeal shockwave therapy improve erectile dysfunction? A prospective, randomized, double-blind, placebo-controlled study. Scand.J. Urol. 2015;49(4):329–333. doi: 10.3109/21681805.2014.984326. [DOI] [PubMed] [Google Scholar]
- Reeves F., Everaerts W., Murphy D.G. Stimulation of the Neurovascular Bundle Results in Rhabdosphincter Contraction in a Proportion of Men Undergoing Radical Prostatectomy. Urology. 2015 doi: 10.1016/j.urology.2015.09.016. [DOI] [PubMed] [Google Scholar]
- Salonia A., Burnett A.L., Graefen M. Prevention and management of postprostatectomy sexual dysfunctions. Part 1: choosing the right patient at the right time for the right surgery. Eur. Urol. 2012;62(2):261–272. doi: 10.1016/j.eururo.2012.04.046. [DOI] [PubMed] [Google Scholar]
- Schauer I., Keller E., Muller A., Madersbacher S. Have rates of erectile dysfunction improved within the past 17 years after radical prostatectomy? A systematic analysis of the control arms of prospective randomized trials on penile rehabilitation. Andrology. 2015;3(4):661–665. doi: 10.1111/andr.12060. [DOI] [PubMed] [Google Scholar]
- Shabsigh R., Perelman M.A., Lockhart D.C., Lue T.F., Broderick G.A. Health issues of men: prevalence and correlates of erectile dysfunction. J. Urol. 2005;174(2):662–667. doi: 10.1097/01.ju.0000165389.73148.d1. [DOI] [PubMed] [Google Scholar]
- Tal R., Alphs H.H., Krebs P., Nelson C.J., Mulhall J.P. Erectile function recovery rate after radical prostatectomy: a meta-analysis. J. Sex. Med. 2009;6(9):2538–2546. doi: 10.1111/j.1743-6109.2009.01351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyne E., Albersen M. Decade in review-sexual dysfunction: post-RP erectile dysfunction-therapies for the next decade. Nat. Rev. Urol. 2014;11(11):616–618. doi: 10.1038/nrurol.2014.274. [DOI] [PubMed] [Google Scholar]
- Weyne E., Castiglione F., Van der Aa F., Bivalacqua T.J., Albersen M. Landmarks in erectile function recovery after radical prostatectomy. Nat. Rev. Urol. 2015;12(5):289–297. doi: 10.1038/nrurol.2015.72. [DOI] [PubMed] [Google Scholar]
- Zuk P.A. The adipose-derived stem cell: looking back and looking ahead. Mol. Biol. Cell. 2010;21(11):1783–1787. doi: 10.1091/mbc.E09-07-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material