Abstract
Background
Seasonal influenza is a major public health concern in vulnerable populations. Here we investigated the safety, tolerability, and pharmacokinetics of a broadly neutralizing monoclonal antibody (VIS410) against Influenza A in a Phase 1 clinical trial. Based on these results and preclinical data, we implemented a mathematical modeling approach to investigate whether VIS410 could be used prophylactically to lessen the burden of a seasonal influenza epidemic and to protect at-risk groups from associated complications.
Methods
Using a single-ascending dose study (n = 41) at dose levels from 2 mg/kg–50 mg/kg we evaluated the safety as well as the serum and upper respiratory pharmacokinetics of a broadly-neutralizing antibody (VIS410) against influenza A (ClinicalTrials.gov identifier NCT02045472). Our primary endpoints were safety and tolerability of VIS410 compared to placebo. We developed an epidemic microsimulation model testing the ability of VIS410 to mitigate attack rates and severe disease in at risk-populations.
Findings
VIS410 was found to be generally safe and well-tolerated at all dose levels, from 2–50 mg/kg. Overall, 27 of 41 subjects (65.9%) reported a total of 67 treatment emergent adverse events (TEAEs). TEAEs were reported by 20 of 30 subjects (66.7%) who received VIS410 and by 7 of 11 subjects (63.6%) who received placebo. 14 of 16 TEAEs related to study drug were considered mild (Grade 1) and 2 were moderate (Grade 2). Two subjects (1 subject who received 30 mg/kg VIS410 and 1 subject who received placebo) experienced serious AEs (Grade 3 or 4 TEAEs) that were not related to study drug. VIS410 exposure was approximately dose-proportional with a mean half-life of 12.9 days. Mean VIS410 Cmax levels in the upper respiratory tract were 20.0 and 25.3 μg/ml at the 30 mg/kg and 50 mg/kg doses, respectively, with corresponding serum Cmax levels of 980.5 and 1316 μg/mL. Using these pharmacokinetic data, a microsimulation model showed that median attack rate reductions ranged from 8.6% (interquartile range (IQR): 4.7%–11.0%) for 2% coverage to 22.6% (IQR: 12.7–30.0%) for 6% coverage. The overall benefits to the elderly, a vulnerable subgroup, are largest when VIS410 is distributed exclusively to elderly individuals, resulting in reductions in hospitalization rates between 11.4% (IQR: 8.2%–13.3%) for 2% coverage and 30.9% (IQR: 24.8%–35.1%) for 6% coverage among those more than 65 years of age.
Interpretation
VIS410 was generally safe and well tolerated and had good relative exposure in both serum and upper respiratory tract, supporting its use as either a single-dose therapeutic or prophylactic for influenza A. Including VIS410 prophylaxis among the public health interventions for seasonal influenza has the potential to lower attack rates and substantially reduce hospitalizations in individuals over the age of 65.
Funding
Visterra, Inc.
Keywords: Influenza, Monoclonal antibody, Epidemic modeling, Prophylaxis
Highlights
-
•
VIS410, a broadly neutralizing monoclonal antibody, neutralizes seasonal strains of influenza A.
-
•
VIS410 was found to be safe and well tolerated in a phase 1 clinical study.
-
•
VIS410 drug levels in the upper respiratory tract support treatment and prophylaxis of influenza A.
-
•
Epidemic modeling of VIS410 as a prophylactic therapy demonstrated substantial reduction of hospitalizations.
Influenza infection results in significant morbidity and mortality especially in high risk groups such as the elderly. VIS410 is a broadly neutralizing antibody engineered to bind the influenza A virus. VIS410 was shown to be safe and well tolerated in a phase 1 clinical trial in healthy adult volunteers. Measurements of drug levels of VIS410 in the upper respiratory tract demonstrated that protective levels were achieved at the site of infection. Epidemic modeling indicate that for an antibody such as VIS410 prophylactic administration to 4–6% of the population would be sufficient to substantially suppress hospitalizations related to severe influenza.
1. Introduction
Severe influenza occurs each winter especially in high-risk groups such as young children, older adults, patients with pulmonary conditions, inflammatory conditions, malignancies, and pregnant women (Newton et al., 2000, Schanzer et al., 2008). Despite available therapy with neuraminidase inhibitors, including oseltamivir, zanamivir, and peramivir; 10%–44% of hospitalized patients require intensive care and 25%–50% of these patients die. In the United States, it is estimated that as many as 400,000 patients are hospitalized with influenza each year, with as many as 50,000 deaths per year (http://www.cdc.gov/flu/about/disease/us_flu-related_deaths.h, Hamborsky et al., 2015). Furthermore, as evidenced by pandemic influenza A infections such as the 2009 “swine flu” pandemic, newly emerging influenza subtypes represent a considerable threat to global public health as they have the potential to cause significant morbidity and mortality.
The majority of the severe disease burden during seasonal influenza is experienced by individuals over the age of 65, who are susceptible to a number of complications following infection with influenza virus (Reed et al., 2015, Thompson et al., 2004). Currently available public health interventions have not significantly mitigated disease burden for the elderly. Vaccination with trivalent or tetravalent killed influenza has historically had lower measured efficacy in elderly individuals compared to adults and children (Darvishian et al., 2014, Breteler et al., 2013, Osterholm et al., 2012). Prophylaxis or early treatment with neuraminidase inhibitors is the current de facto standard of care; however, some controversy exists as to whether a direct link can be established between early oseltamivir treatment and lower hospitalization rates (Jefferson et al., 2014). Based on these shortfalls in care, there is a need to develop countermeasures to reduce or mitigate the effects of influenza in the elderly and other susceptible populations.
Recently, several broadly neutralizing antibodies against influenza have been reported, including against group 1 of influenza A (Ekiert et al., 2009), group 2 of influenza A (Ekiert et al., 2011), and against both group 1 and group 2 (Corti et al., 2011, Dreyfus et al., 2012). The benefits of broadly neutralizing antibodies are that they may enable protection of elderly individuals from influenza infection regardless of immune response and potentially provide a reliable option when considering the vaccine mismatches that occur against influenza every three to five years. Initial identification of C179, targeting the stem of influenza hemagglutinin (HA) (Okuno et al., 1993) was followed by identification of other stem-binding antibodies, including F10 (Sui et al., 2009), CR6261/CR8020 (Ekiert et al., 2009, Ekiert et al., 2011), CR9114 (Dreyfus et al., 2012), and FI6 (Corti et al., 2011), among others (Burioni et al., 2010, Kashyap et al., 2010). Using an antibody engineering approach, we developed a broadly neutralizing antibody (VIS410) that targets a unique, conserved epitope on influenza HA and binds to and neutralizes influenza A virus across group 1 and group 2 subtypes (Tharakaraman et al., 2015). In vitro, VIS410 has been shown to neutralize groups 1 and 2 influenza strains; over 40 different virus strains have been tested to date, with EC50 values ranging from 0.1 – 60 μg/mL and representing broad temporal/geographical, subtype, and epitope diversity (Tharakaraman et al., 2015, Baranovich et al., 2016). Additionally, in vivo studies in mouse models demonstrated that VIS410 administered as a prophylactic or therapeutic protects mice challenged with lethal doses of influenza A, including A/Puerto Rico/8/1934 (H1N1), A/California/04/2009 (H1N1), A/Victoria/3/1975 (H3N2), and A/Vietnam/1203/2004 (H5N1). VIS410 also demonstrated protection against newly emerging pathogenic H7N9 strains, A/Anhui/1/2013 and oseltamivir-resistant A/Shanghai/1/2013 in a lethal BALB/c mouse model (Baranovich et al., 2016). VIS410 is being developed as a single dose treatment for hospitalized patients with influenza A and is currently in phase 2 studies.
We report here the safety and pharmacokinetics of VIS410 in the serum and the upper respiratory tract, the primary target organ of infection of influenza A. Furthermore, we utilize this information to model the potential application of a broadly neutralizing antibody, such as VIS410, during an influenza outbreak to mitigate severe disease, especially for at risk-populations. We provide evidence that VIS410 is generally safe and well-tolerated in healthy subjects with protective levels of antibody achieved in the upper respiratory tract, and that it has a pharmacokinetic/pharmacodynamic (PK/PD) profile that may allow it to be used as a prophylactic during or prior to a period of high influenza activity. Taken together, these data support the development of a broadly neutralizing monoclonal antibody as a complementary strategy to existing measures for reducing the severity of seasonal influenza.
2. Methods
2.1. Production of Antibody
VIS410 was produced under current Good Manufacturing Practice (cGMP) at Gallus Biopharmaceuticals (Princeton, NJ) in a CHO cell line. After production at a 200 L scale, VIS410 was purified by protein A and ion exchange polishing steps. Testing of bulk drug substance indicated that the material was > 99% monomer, containing < 0.1 pg/mg residual DNA and < 0.1 ng/mg of host cell proteins. VIS410 material was formulated at 25 mg/mL in 40 mM Citrate–Sodium Phosphate, 150 mM NaCl, pH 6.0, containing 0.025% Tween-80.
2.2. Phase I Clinical Trial
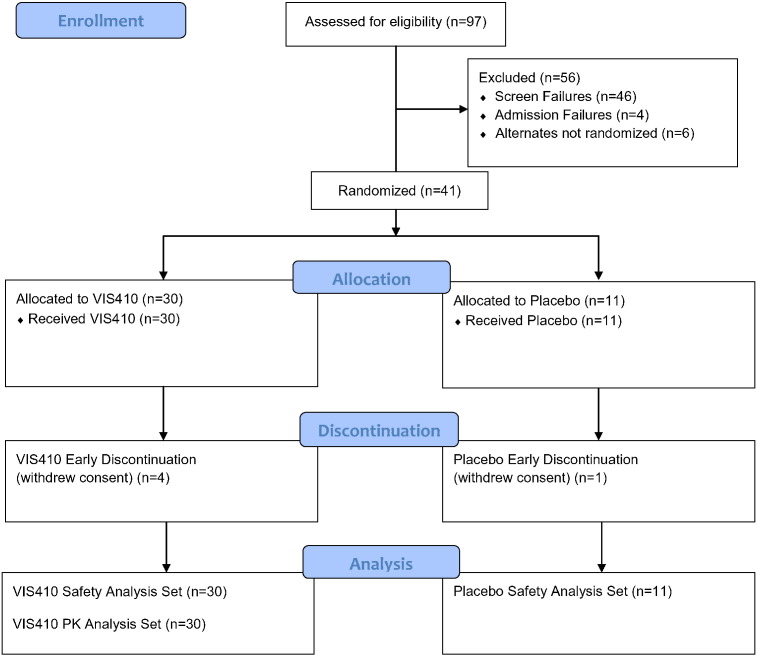
A Phase 1, double-blind, placebo-controlled, single ascending dose-escalation study was completed in healthy adult subjects (ClinicalTrials.gov identifier NCT02045472). This study was conducted according to the International Conference on Harmonization Harmonized Tripartite Guideline E6(R1): Good Clinical Practice. Institutional Research Board approval for the study was obtained in writing before the study began and all subjects signed an informed consent prior to entry in the study. The primary endpoint for the study was the safety and tolerability of VIS410 compared to placebo and the secondary endpoint was the serum pharmacokinetics of a single dose of VIS410. Eligible subjects were admitted to the clinic for dose administration and were discharged 24-h post-infusion. Overall, 30 subjects were dosed with VIS410 and 11 subjects were dosed with a placebo control infusion (Fig. 1). Nine subjects were dosed in the first cohort (Cohort 1); 6 subjects received VIS410 (2 mg/kg) and 3 subjects received placebo (sodium chloride 0.9%). Eight subjects were dosed in the subsequent cohorts (Cohorts 2 through 5) and were randomly assigned in a 6:2 ratio to receive either VIS410 or placebo. The detailed phase 1 protocol is presented in supplemental materials.
Fig. 1.
Study flow diagram outlining screening, randomization, dosing and analysis sets of subjects.
Briefly, in the first cohort (Cohort 1) the first four sentinel subjects were randomly assigned to receive either VIS410 (2 mg/kg; n = 2) or placebo (n = 2) and received study drug at least 48 h before the remaining subjects in the cohort were dosed. After the investigator had assessed that the infusions were well tolerated, the remaining subjects in the cohort were dosed concurrently (VIS410 n = 4 and placebo n = 1). In each subsequent cohort, the first 3 subjects were randomly assigned to receive either VIS410 (n = 2) or placebo (n = 1) and received study drug at least 48 h before the remaining subjects in the cohort were dosed. After the investigator had assessed that the infusions were well tolerated, the remaining members of the cohort (VIS410 n = 4 and placebo n = 1) were dosed concurrently. Dose escalation to the next dosing level occurred after the Safety Monitoring Committee (SMC) comprised of the investigator, an independent medical monitor, and the sponsor reviewed the safety data through Day 7 after the infusion.
Assessment of safety by the SMC was determined from vital sign measurements; physical examinations; hematology, chemistry, and urinalysis laboratory testing; 12-lead triplicate electrocardiograms (ECGs); use of concomitant medications; and review of adverse events (AEs). Blood samples for pharmacokinetic (PK) analysis and for assessment of antidrug antibodies (ADA) to VIS410 were obtained before and after the infusion during the 120-day study period (Days 1, 2, 3, 7, 14 ± 1, 28 ± 3, 56 ± 7, and 120 ± 7). Nasopharyngeal (NP) swabs, to assess upper respiratory VIS410 concentrations, were collected before and after the infusion from subjects in the 15, 30, and 50 mg/kg cohorts (Days 1, 3 and 7).
2.3. Pharmacokinetic and Antidrug Antibody Assays
Blood samples were collected at the time points indicated in Table S6. Serum was aliquoted and stored at − 20 °C to − 80 °C. All samples were tested for IgG antibody concentrations using an enzyme-linked immunosorbent assay performed by Charles River Laboratories (Senneville, QC). Nasopharyngeal swabs (one from each nostril) for the analysis of the local concentration of VIS410 were taken using COPAN flocked swabs from subjects in the 15, 30, and 50 mg/kg cohorts at the time points indicated in Table S6. The swabs from each nostril were combined in 1 transport tube containing 3 mL COPAN Universal Transport Medium and stored at − 70 °C. Samples were tested for VIS410 by an immunoassay performed by Charles River Laboratories. The samples for ADA analysis were collected in serum separator tubes. Serum was aliquoted and stored at − 20 °C to − 80 °C. The enzyme-linked immunosorbent assay was performed by Charles River Laboratories. Descriptive statistics were used to summarize data between groups. Statistical comparisons of the frequency of adverse events for placebo versus VIS410 receiving subjects used Fisher's exact test. All statistical analyses were conducted using SAS® software Version 9.3 (SAS Institute, Inc., Cary, NC), and the PK analysis was conducted using Phoenix WinNonlin® Version 6.2.1 (Pharsight Corporation, St Louis, MO).
2.4. Individual-Based Population Model
An individual-based microsimulation was developed based on a previously developed model (Boni et al., 2013), and is similar in structure and design to an array of microsimulation models that have been developed over the past decade (Ferguson et al., 2005, Germann et al., 2006, Longini et al., 2005). We used standard techniques in individual-based microsimulation methods to test the population-level effects of deploying VIS410 during a typical winter influenza A epidemic and to perform sensitivity analyses on key unknown parameters.
Briefly, the model simulates an age-structured population of one million individuals living in a city with 1,000 pre-defined neighborhoods or locations. Daily work commutes, random within-city travel, household structure, pre-existing immunity, and age-based social contacts are included in the model. Influenza infection and potential hospitalization are modeled by randomly infecting individuals by location or household, in proportion to the current level of infections and contacts in that location or household. Infection, seasonality, contact structure, hospitalization, and the clinical course and epidemiology of influenza in the model were validated using characteristics of past influenza epidemics of influenza A (see supplemental materials).
In the microsimulation, VIS410 was deployed as a population-wide prophylaxis strategy. A small percentage of individuals received VIS410 prophylaxis (between 0% and 6%) in the early stages of the epidemic, and two modes of distribution were included: randomly to all individuals or randomly to only elderly individuals (> 65 years old). We varied the distribution time between eight weeks prior to the epidemic peak and the date of the modeled epidemic peak. VIS410 levels in individuals were modeled using an exponential decay function, with a half-life of 13 days. VIS410 was modeled to be administered at a level that was 8-fold over a minimally protective dose of approximately 1–2 mg/kg based on preclinical estimates, corresponding to over 3 half-lives of protection. Levels of VIS410 over the protective threshold confer a 90% reduction in the probability of being infected, with the protection decreasing exponentially as a function of VIS410 levels below the minimally protective threshold. Levels of VIS410 below 0.1-fold of the protective threshold were considered to be non-protective. This behavior is shown in Supplemental Figure S1.
2.5. Role of the Funding Source
The funder of the study oversaw trial management, data collection, statistical analyses, model design, interpretation, and the writing and review of the report. The corresponding author had full access to all data in the study, model code, and simulation results, and had final responsibility for the decision to submit the publication.
3. Results
VIS410 is an engineered human IgG1 antibody that targets a unique, conserved conformational epitope on the stem of Influenza A virus HA protein (Tharakaraman et al., 2015). Previous studies have identified that VIS410 has broad reactivity against both group 1 and group 2 influenza A strains and is effective against seasonal H1 and H3 influenza viruses as well as H7N9 virus (Baranovich et al., 2016).
A Phase 1, placebo-controlled, single ascending dose study of VIS410 in healthy volunteers was initiated at a single site in North America. Five cohorts were dosed with levels ranging from 2 to 50 mg/kg (Table 1). A total of 41 subjects were enrolled in the phase 1 study. Overall, 36 subjects (87.8%) completed the study and 5 subjects (12.2%) discontinued early. All 41 subjects (100.0%) who received study drug (VIS410 or placebo) were included in the safety analysis set. All 30 subjects (100.0%) who received a dose of VIS410 and had at least one evaluable PK parameter were included in the PK analysis set. Five subjects (12.2%) withdrew consent (4 of 30 subjects [13.3%] who received VIS410 and 1 of 11 subjects [9.1%] who received placebo). For 1 subject (Subject 402; 30 mg/kg VIS410), the investigator was unblinded to the subject's treatment due to serious adverse events (SAEs) of leukopenia and herpes simplex esophagitis. This SAE was ultimately found to be unrelated to the study drug as a primary herpes simplex virus type 1 infection was confirmed based on analysis of pre and post event serology.
Table 1.
Summary of subject disposition.
| VIS410 |
||||||||
|---|---|---|---|---|---|---|---|---|
| 2 mg/kg (n = 6) | 5 mg/kg (n = 6) | 15 mg/kg (n = 6) | 30 mg/kg (n = 6) | 50 mg/kg (n = 6) | Total (N = 30) | Placebo (N = 11) | Overall (N = 41) | |
| Total number of subjects, no. (%) | ||||||||
| Completed | 6 (100) | 4 (66.7) | 5 (83.3) | 5 (83.3) | 6 (100) | 26 (86.7) | 10 (90.9) | 36 (87.8) |
| Discontinued | 0 | 2 (33.3) | 1 (16.7) | 1 (16.7) | 0 | 4 (13.3) | 1 (9.1) | 5 (12.2) |
| Primary reason for discontinuation, no. (%) | ||||||||
| Subject withdrew consent | 0 | 2 (33.3) | 1 (16.7) | 1 (16.7) | 0 | 4 (13.3) | 1 (9.1) | 5 (12.2) |
| Study population, no. (%) | ||||||||
| Safety analysis seta | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 30 (100) | 11 (100) | 41 (100) |
| Pharmacokinetic analysis setb | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 30 (100) | 0 | 30 (73.2) |
Note: Percentages were based on the number of subjects within each group and overall.
The safety analysis set included all subjects who received a dose of VIS410 or placebo.
The pharmacokinetic analysis set included all subjects who received a dose of VIS410 and had at least 1 evaluable pharmacokinetic parameter.
3.1. Safety Results
Overall, 27 of 41 subjects (65.9%) reported a total of 67 treatment emergent adverse events (TEAEs). TEAEs were reported by 20 of 30 subjects (66.7%) who received VIS410 and by 7 of 11 subjects (63.6%) who received placebo. 18 of 41 subjects (43.9%) overall (16 of 30 subjects [53.3%] who received VIS410 and 2 of 11 subjects [18.2%] who received placebo; p > 0.05) experienced TEAEs related to study drug. 14 of 16 TEAEs related to study drug were considered mild (Grade 1) and 2 were moderate (Grade 2).
Overall, the highest percentage of subjects that reported TEAEs were classified as nervous system disorders (11 subjects; 26.8%) followed by gastrointestinal (GI) disorders and infections and infestations (10 subjects; 24.4% each). The percentage of subjects reporting nervous system disorders was similar following administration of VIS410 (7 of 30 subjects; 23.3%) compared with placebo (4 of 11 subjects; 36.4%) and did not reach statistical significance; no notable differences were observed across VIS410 dose levels. Gastrointestinal disorders were reported by subjects who received VIS410 only (10 of 30 subjects; 33.3% for VIS410 receiving subjects, compared to 0% for placebo, p < 0.05). The percentage of subjects reporting GI disorders was highest in the 50 mg/kg VIS410 cohort (5 of 6 subjects; 83.3%). The percentage of subjects reporting infections and infestations was similar following administration of VIS410 (6 of 30 subjects; 20.0%) compared with placebo (4 of 11 subjects; 36.4%); no notable differences were observed across VIS410 dose levels (See Supplementary Tables S7 and S8).
Subjects who developed clinically significant upper respiratory infections had viral testing of their nasopharyngeal swabs by the site investigator for the duration of the study (Day 120). None were found to have influenza although the 30 mg/kg and 50 mg/kg cohorts were dosed from December 2014 to January 2015 where, based on state reported epidemiology, there were high relative incidences of influenza A and other respiratory virus infections (http://www.health.state.mn.us/divs/idepc/diseases/flu/stats/2014summary.pdf). A summary table of infections and infestations lists the relevant infections and dose groups (See Supplementary Table S10). The most frequently reported TEAEs overall were diarrhea reported by 10 of 41 subjects (24.4%) and headache reported by 8 of 41 subjects (19.5%). The most frequently reported drug-related TEAE overall was diarrhea (9 of 41 subjects; 22.0%) that generally occurred following infusion and spontaneously resolved within 24 h. Most GI TEAEs were mild (Grade 1) with the exception of 2 subjects in the 50 mg/kg dose that had moderate (Grade 2) grading of their symptoms.
There were no subjects with SAEs that were related to study drug. Approximately 3 weeks following infusion, a subject that received 30 mg/kg of VIS410 developed a primary HSV-1 infection with associated esophagitis and transient leukopenia. Based on serological data, the HSV-1 infection was confirmed to be primary and given that the clinical sequelae of HSV-1 were consistent with the clinical manifestations, the SAE was considered to be unrelated to the study drug by the site investigator. This subject resolved their leukopenia spontaneously and resolved their esophagitis following treatment with valacyclovir administered by their treating physician. No subjects discontinued from the study due to a TEAE, and all TEAEs resolved by the end of the study. Overall, mean clinical laboratory results, vital sign measurements, and ECG values observed after dosing were similar to baseline levels. Mean changes from baseline were also similar across VIS410 dose levels, and no apparent treatment- or dose-related trends were observed.
3.2. Pharmacokinetic Results
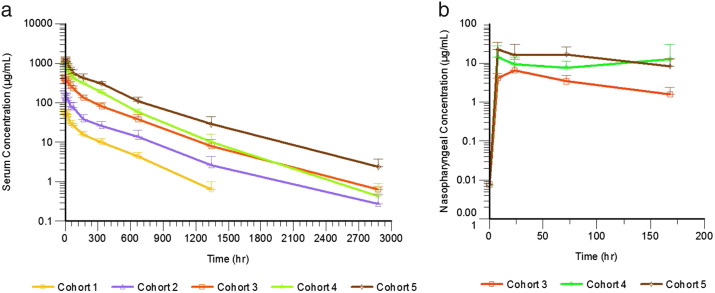
Mean AUC0–t, AUC0–∞, and Cmax for VIS410 increased approximately proportional to dose (Fig. 2 and Table 2). Across the dose cohorts, mean t1/2 values of VIS410 ranged between 250.72 and 376.38 h and median Tmax values ranged between 1.92 and 3.50 h. The mean clearance values of VIS410 ranged from 11.41 to 14.07 mL/h and mean volume of distribution values ranged from 4914.4 to 6189.8 mL, across all of the doses tested. We observed a dose proportional increase in the Cmax for VIS410, which ranged from 58.6 μg/mL at a 2 mg/kg dose to 1316 μg/mL at a 50 mg/kg dose. Additionally, we examined PK samples for the presence of anti-drug antibodies (ADA). Of note, no preexisting ADAs were detected before administration of VIS410. We found that four subjects (three at 5 mg/ml and one at 15 mg/ml dose levels) developed low titer ADA, 120 days after administration of VIS410. Notably, the presence of ADA did not alter drug PK as exclusion of ADA-positive subject data from the analysis did not substantially affect calculated PK parameters, including half-life and drug exposure.
Fig. 2.
Pharmacokinetic Profiles of VIS410 in (a) Serum and (b) Nasopharyngeal Samples. Mean concentrations along with the corresponding standard deviation at each time point were plotted on a logarithmic scale for each dose level. Cohort dose levels are as follows: Cohort 1: 2 mg/kg; Cohort 2: 5 mg/kg; Cohort 3: 15 mg/kg; Cohort 4: 30 mg/kg; and Cohort 5: 50 mg/kg.
Table 2.
Mean (CV) serum pharmacokinetic parameters of VIS410.
| Parameter (unit) | VIS410 |
||||
|---|---|---|---|---|---|
| 2 mg/kg (N = 6) | 5 mg/kg (N = 6) | 15 mg/kg (N = 6) | 30 mg/kg (N = 6) | 50 mg/kg (N = 6) | |
| AUC0–t (hr·μg/mL) | 10,828 (11) | 28,026 (50) | 90,332 (33) | 163,914 (41) | 322,070 (16) |
| AUC0–∞ (hr·μg/mL) | 11,074 (12) |
36,086 (25) | 100,410 (20) | 190,921 (10) | 323,451 (16) |
| Cmax (μg/mL) | 58.6 (16·8) | 180.5 (29.6) |
446.1 (13.6) |
980.5 (16.7) |
1316.0 (14.3) |
| Tmax (hr)a | 3.00 (1.92, 3.00) |
3.50 (1.92, 4.00) |
3.00 (1.92, 3.08) |
2.46 (1.92, 4.00) |
1.92 (1.92, 3.00) |
| t1/2 (hr)b | 250.7 (11.6) |
293.1 (38.5) |
341.2 (17.1) |
288.6 (15.3) |
376.4 (16.0) |
| CL (mL/h) | 14.1 (10.4) |
12.6 (28.6) |
12.9 (40.8) |
11.7 (19.7) |
11.4 (6.4) |
| Vd (mL) | 5089 (16) |
4914 (21) |
6027 (22) |
4779 (15) |
6190 (17) |
Abbreviations: AUC, area under the curve; Cmax, maximal concentration of VIS410; Tmax, time at which maximal concentration is achieved; t1/2, half-life; CL, clearance; Vd, volume of distribution; CV, coefficient of variation; hr., hours; Kel, terminal elimination rate constant.
Note: Kel-associated pharmacokinetic parameters for Subject 202 (5 mg/kg VIS410) and Subject 306 (15 mg/kg VIS410) were set to missing due to > 20% extrapolation of AUC0–∞.
For Tmax, the median (minimum, maximum) values are presented.
VIS410 serum half-life (12.9 days), was calculated by averaging the mean t1/2 of all cohorts.
Nasopharyngeal (NP) swabs were collected for the 15, 30, and 50 mg/kg VIS410 treatment groups but not for the 2 and 5 mg/kg groups. Following a single IV infusion of VIS410 over 120 min, across the dose cohorts tested, NP VIS410 concentrations appeared to increase with each increasing dose level, in a similar manner to serum Cmax levels (Table 3 and Fig. 2). Mean NP VIS410 concentrations reached peak levels within 24 h after dosing for all the dose cohorts tested and remained measurable throughout the collection period. Mean NP VIS410 concentrations for the ADA-negative subset were comparable to the PK analysis set demonstrating that the ADA status did not influence VIS410 NP concentrations.
Table 3.
VIS410 nasopharyngeal pharmacokinetic Cmax statistics.
| Cohort | Dose (mg/kg) | n | Mean Cmax ± SD (μg/mL) |
|---|---|---|---|
| 3 | 15 | 6 | 7.6 ± 5.2 |
| 4 | 30 | 6 | 20.0 ± 16.3 |
| 5 | 50 | 6 | 25.3 ± 10.4 |
Cmax — maximum observed nasal concentration.
3.3. Modeling of Population-Level Benefits
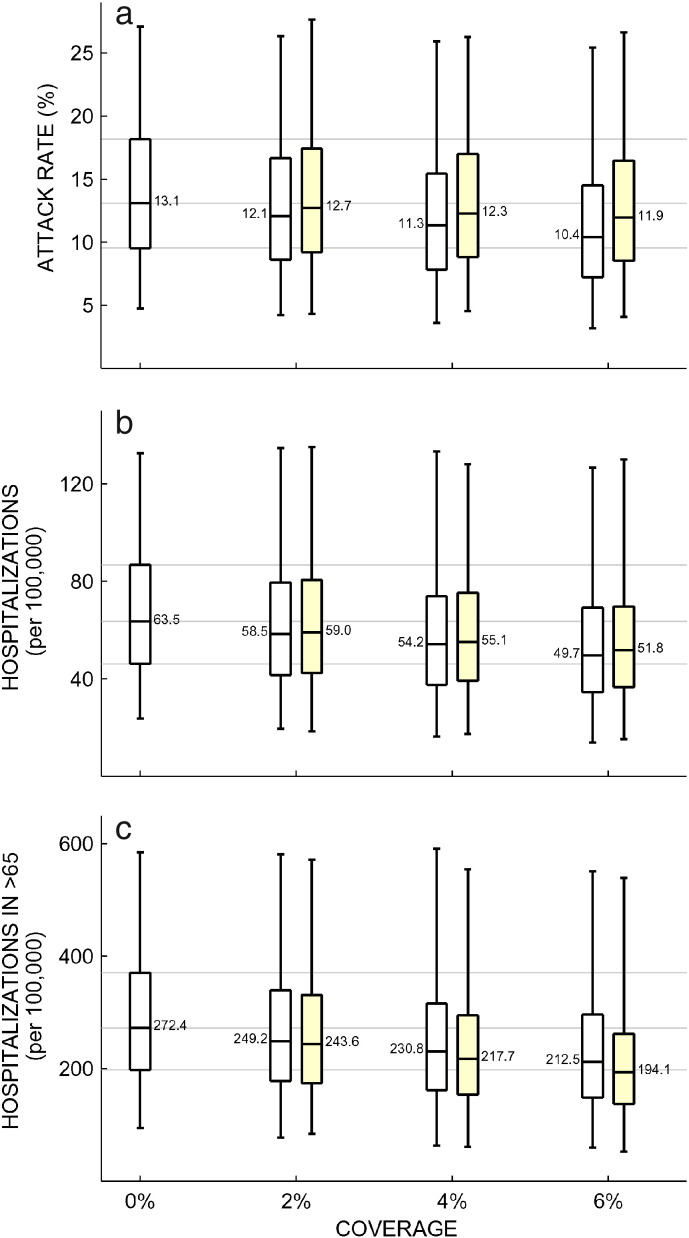
Using the measured half-life and biodistribution information as well as information on protective levels in animals, we sought to model if a population-level prophylaxis strategy with VIS410 would be able to reduce influenza burden during a single influenza A season. The microsimulation results indicated that prophylaxis of even a small percentage of the population can have a substantial impact on the outcome of the epidemic as measured by the reduction in both attack rates and hospitalization rates for the elderly. Simulations were carried out for a range of temperate-zone influenza epidemic scenarios corresponding to attack rates between 4.8% and 27%. Attack rates and hospitalization rates for the 3 coverage levels explored in this study are shown in Table 4. For a prophylactic dose of 8-fold over a protective threshold of 1–2 mg/kg, as estimated from preclinical prophylactic experiments, and with administration initiated 0–8 weeks prior to the epidemic peak, median reductions in attack rates from 50 simulations were 8.6% (IQR: 4.7%–11.0%) for 2% coverage, 16.1% (IQR: 8.1%–20.9%) for 4% coverage, and 22.6% (IQR: 12.7%–30.0%) for 6% coverage (Fig. 3a). The associated reductions in hospitalization of the elderly were 8.8% (IQR: 4.9%–11.6%), 16.5% (IQR: 8.8%–21.9%) and 22.9% (IQR: 13.0%–30.6%), respectively, for the three coverage scenarios (Fig. 3c).
Table 4.
Results of microsimulation measurements.
| Coverage |
||||||
|---|---|---|---|---|---|---|
| Metric | Age | Admin | Untreated (IQR) | 2% (IQR) | 4% (IQR) | 6% (IQR) |
| Attack rate (%) | 0–5 | All | 9.1 (6.7–12.7) | 8.4 (6.0–11.7) | 7.8 (5.4–10.8) | 7.2 (5.0–10.1) |
| Elderly | 8.9 (6.4–12.3) | 8.6 (6.2–12.0) | 8.4 (6.0–11.7) | |||
| 6–15 | All | 15.6 (11.6–21.8) | 14.5 (10.4–20.1) | 13.6 (9.4–18.6) | 12.6 (8.7–17.5) | |
| Elderly | 15.3 (11.2–21.1) | 14.9 (10.7–20.7) | 14.7 (10.5–20.2) | |||
| 16–25 | All | 13.1 (9.5–18.3) | 12.1 (8.6–16.8) | 11.3 (7.8–15.5) | 10.4 (7.2–14.6) | |
| Elderly | 12.8 (9.2–17.6) | 12.4 (8.9–17.3) | 12.2 (8.6–16.9) | |||
| 26–34 | All | 11.4 (8.2–15.9) | 10.5 (7.4–14.6) | 9.8 (6.7–13.5) | 9.0 (6.2–12.7) | |
| Elderly | 11.1 (8.0–15.4) | 10.8 (7.7–15.0) | 10.5 (7.5–14.6) | |||
| 35–49 | All | 18.6 (13.5–25.5) | 17.1 (12.3–23.5) | 16.0 (11.1–21.8) | 14.8 (10.3–20.5) | |
| Elderly | 18.1 (13.1–24.7) | 17.5 (12.6–24.2) | 17.2 (12.3–23.6) | |||
| 50–64 | All | 12.3 (9.0–17.1) | 11.4 (8.2–15.7) | 10.7 (7.4–14.5) | 9.8 (6.8–13.6) | |
| Elderly | 12.1 (8.7–16.5) | 11.7 (8.4–16.1) | 11.4 (8.1–15.6) | |||
| > 65 | All | 7.2 (5.2–10.0) | 6.6 (4.7–9.1) | 6.2 (4.3–8.4) | 5.7 (4.0–7.8) | |
| Elderly | 6.5 (4.7–8.8) | 5.8 (4.1–7.8) | 5.2 (3.7–6.9) | |||
| All ages | All | 13.1 (9.5–18.2) | 12.1 (8.6–16.7) | 11.3 (7.8–15.4) | 10.4 (7.2–14.5) | |
| Elderly | 12.7 (9.2–17.4) | 12.3 (8.8–17.0) | 11.9 (8.5–16.5) | |||
| Hospitalization rate (per 100 K) | 0–5 | All | 72.1 (52.1–102.8) | 67.5 (46.0–95.1) | 62.9 (42.9–87.4) | 58.3 (38.3–81.3) |
| Elderly | 70.6 (49.1–98.2) | 70.6 (47.5–96.6) | 67.5 (47.5–95.1) | |||
| 6–15 | All | 5.3 (2.6–7.9) | 4.4 (2.6–7.0) | 4.4 (2.6–6.2) | 4.4 (2.6–6.2) | |
| Elderly | 5.3 (3.5–7.0) | 4.4 (2.6–7.0) | 4.4 (2.6–7.0) | |||
| 16–25 | All | 27.0 (19.0–37.2) | 24.8 (16.8–35.0) | 22.6 (15.3–32.1) | 21.2 (13.9–29.9) | |
| Elderly | 26.3 (18.2–36.5) | 25.5 (17.5–35.7) | 24.8 (16.8–35.0) | |||
| 26–34 | All | 23.5 (16.1–31.6) | 21.3 (14.4–29.3) | 19.5 (13.2–27.6) | 17.8 (12.1–25.3) | |
| Elderly | 22.4 (15.5–31.0) | 21.8 (14.9–30.4) | 21.3 (14.4–29.3) | |||
| 35–49 | All | 31.5 (22.3–43.1) | 29.1 (20.3–39.7) | 27.1 (18.5–37.3) | 24.7 (16.9–34.9) | |
| Elderly | 31.0 (21.8–42.1) | 30.0 (20.8–41.2) | 29.5 (20.3–40.2) | |||
| 50–64 | All | 55.0 (38.7–74.7) | 51.1 (35.4–69.6) | 47.2 (32.0–64.6) | 43.2 (29.8–60.1) | |
| Elderly | 53.3 (37.6–73.0) | 51.7 (36.5–70.7) | 50.5 (35.4–69.1) | |||
| > 65 | All | 272.4 (198.3–370.6) | 249.2 (178.1–339.5) | 230.8 (161.3–315.5) | 212.5 (148.6–296.3) | |
| Elderly | 243.6 (174.1–331.5) | 217.7 (154.2–294.7) | 194.1 (137.4–262.0) | |||
| All ages | All | 63.5 (46.1–86.7) | 58.5 (41.4–79.4) | 54.2 (37.5–74.0) | 49.7 (34.5–69.1) | |
| Elderly | 59.0 (42.3–80.6) | 55.1 (39.1–75.3) | 51.8 (36.6–69.7) | |||
Fig. 3.
Microsimulation results of VIS410 prophylactic use. Changes in attack rate (a), overall hospitalization rate (b), and hospitalization rate in individuals older than 65 years of age (c) as a function of the population-level prophylaxis coverage. The boxplots aggregate outcomes over time of administration and transmission setting, as these epidemiological variables might in some cases be difficult to predict. The boxplots for 0% coverage summarize 750 individual simulations, while the boxplots for 2% to 6% coverage summarize 3750 simulations each. The yellow boxplots show results for VIS410 administration to the elderly only, while the white boxplots show general population VIS410 administration. The whiskers show the full range of outcomes, and the median value is shown next to the median line of each boxplot. With one exception, all pairwise comparisons between different coverage levels, when keeping the group administration method fixed (“all” or “elderly only”), show a statistically significant difference by the Mann–Whitney test (p = 0·002); the one exception is in panel A when comparing no coverage to 2% coverage and distribution to the elderly only (significant at p = 0·05). Note that in panel B, when comparing elderly versus general population distribution, the Mann–Whitney p-values are p = 0·21 (2% coverage), p = 0·05 (4% coverage), and p = 0·005 (6% coverage).
In addition to investigating coverage levels, we assessed whether administration of VIS410 prophylaxis to the elderly would be an improvement over general population administration. In the microsimulations, general-population administration results in larger reductions in attack rate than administration to the elderly alone, partially because of the nature of social contacts by which individuals are more likely to associate with those in their same age group. However, prophylaxis of elderly populations was associated with a larger reduction in elderly hospitalizations than distribution to the population at large. The median reductions in > 65 years old hospitalizations were 11.4% (IQR: 8.2%–13.3%) for 2% coverage, 21.6% (IQR: 17.4%–24.9%) for 4% coverage, and 30.9% (IQR: 24.8%–35.1%) for 6% coverage when VIS410 was administered to the elderly only. Hospitalization rate in the elderly is an important outcome measure as this age group makes up the majority of influenza hospitalizations and is particularly vulnerable to severe outcomes. Hospitalization rates across all age groups differed by a small amount (± 4%) when comparing general-population prophylaxis to prophylaxis of the elderly only (Fig. 3b). The impact of VIS410 prophylaxis on seasonal influenza epidemics shown in Fig. 3 represents an aggregation across a number of simulation variables (including severity of the epidemic, and date of administration of VIS410 relative to the date of peak activity). When the analysis is restricted to a single epidemic scenario, the effect of VIS410 on the severity of the epidemic is much more pronounced (Supplemental Figure S5).
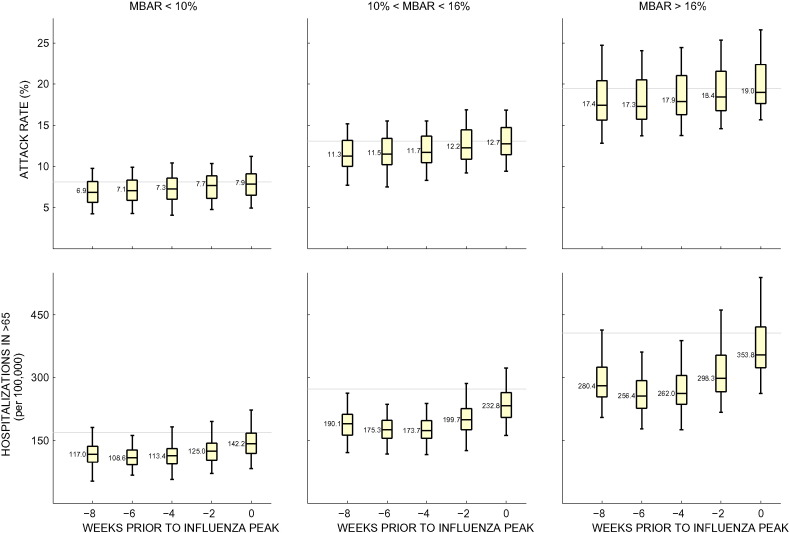
An additional critical parameter that had a large influence on attack rates and hospitalizations was the timing of VIS410 deployment (Fig. 4). For an influenza season of moderate intensity, in our model, administration of VIS410 four to eight weeks prior to peak prevalence resulted in a reduction of hospitalizations of 34.3% (IQR: 31.9%–36.6%) for 6% coverage, but the impact on hospitalizations was more marginal when administered zero to two weeks prior to the peak, with the reduction of hospitalizations at 13.9% (IQR: 12.1%–15.4%). The absolute case reduction of a prophylaxis strategy is very sensitive to the individual protective period assumed for an administered dose of VIS410, which is longer than 40 days in our model (Supplemental Figure S1). If prophylaxis is distributed too late, the majority of individuals will have already been infected, but if given too early, the prophylactic effects of VIS410 administration would wane before the major part of the epidemic wave passes through the population. Administration just prior to the peak is not optimal for population prophylaxis. At this period, approximately 30–40% of the season's infections have already occurred, and the opportunity is lost to protect individuals who become infected during the early and slow phase of the epidemic.
Fig. 4.
Results of VIS410 prophylactic use stratified by date of administration and transmission setting. Median baseline attack rate (MBAR) is used to separate the simulations into those that have low (< 10%), medium (10%–16%), or high (> 16%) median attack rates when coverage is zero. In these simulations, VIS410 was administered to the elderly only and coverage was set to 6%. Each boxplot corresponds to 250 simulations. The whiskers show the full range of outcomes, and the median value is shown next to the median line of each boxplot. The gray line denotes the baseline median for each scenario when coverage is zero.
4. Discussion
Influenza A remains a major public health threat based on seasonal infections and the potential for pandemic infection. Broadly neutralizing monoclonal antibodies such as VIS410 represent a potentially important class of therapies. While vaccination remains the mainstay of public health prophylactic strategies, we investigated whether passive prophylaxis could provide a reasonable supplemental strategy, especially for at-risk populations such as the elderly and immunocompromised, who have suboptimal responses to seasonal vaccination. Given the current state-of-the-art in the large-scale production of antibodies, it should be possible to rapidly ensure availability of an adequate supply of monoclonal antibody at a reasonable cost (Kelley, 2007) during an influenza season compared to production of a vaccine that must incorporate novel strains (generally > 6 months).
We are initially developing VIS410 for the treatment of hospitalized patients with influenza A for the following reasons. (Newton et al., 2000) There are no approved treatments for hospitalized influenza patients, representing a large unmet need. (Schanzer et al., 2008) Administration of polyclonal antisera has demonstrated the ability to reduce morbidity and mortality in this population (Hung et al., 2013). (http://www.cdc.gov/flu/about/disease/us_flu-related_deaths.htm, n.d.) The data from VIS410 in several preclinical models have demonstrated the ability to rapidly reduce viral titers by greater than one log10 and reduce ARDS in lethal models of H7N9 (Baranovich et al., 2016). Pre-clinical data in the ferret suggest that VIS410 can also prevent aerosol transmission of influenza (H1N1) despite its short half-life in this animal model (Lakdawala et al., 2013). As demonstrated in this Phase 1 study, the relatively safe profile of an antibody therapy enables dosing at high levels through bolus administration that can potentially enable a more rapid drop in viral loads compared to many small molecule therapies.
In this study, we sought to investigate whether VIS410 could be a useful therapy and/or prophylactic countermeasure. To this end, we demonstrate here that VIS410 is generally safe and well tolerated, even at the relatively high dose levels of 30 mg/kg and 50 mg/kg. The most common AE related to study drug was loose stool or diarrhea (10 of 40 subjects; 24.4%). Most subjects had minor and transient loose stool that resolved spontaneously. Two of the six subjects at the highest dose of 50 mg/ml had moderate diarrhea with associated nausea and vomiting that resolved within 6 h. None of the subjects with diarrhea had any clinically significant issues such as hypotension and there were no associated laboratory abnormalities. The time of onset and transient nature of these AEs suggest that they may be related to an infusion reaction and options such as slowing infusion or pretreatment can be explored in future development to further mitigate these AEs. There is precedence for infusion reaction related GI events as previously observed in both IVIG therapy and licensed monoclonal antibodies such as infliximab and appears to be related to mast cell activation that correlates to the period around the Cmax phase of the initial infusion (Gammagard, 2014, Kang and Saif, 2007, Remicade, 2011). Serum PK was approximately dose proportional, and nasal PK of the target organ (nasopharyngeal/upper respiratory tract) demonstrated a partitioning compared to serum of 1:53. ADA was observed at very low levels in subjects treated with VIS410 (4 of 30). The presence of ADA in response to treatment with IgG1 monoclonal antibodies such as VIS410 is not unique and has been observed in marketed human monoclonal antibodies such as adalimumab (Bartelds et al., 2011). If ADAs were to have a clinically-relevant impact on efficacy, a change in the PK of VIS410 would be expected as a result of ADA appearance. However, this was not observed in any of the subjects with ADA. While this was a small study in healthy volunteers, and ADAs will continue to be monitored thru the development program, these observations would suggest that an impact of ADAs on acute treatment or a one-time prophylaxis of influenza is unlikely. However, it may be likely that the 13% (4 of 30) of subjects who produced ADAs, upon re-exposure to VIS410, would elicit a similar immune response and produce ADAs again. Because the time-course to elicit the ADA response upon re-exposure and clinical significance of this hypothetical concern is unknown, it would be difficult to speculate on the potential impact that re-administration may have for VIS410 therapy either as a treatment or prophylactic modality.
Given the phase 1 trial results, we sought to address a complementary question whether VIS410 could be successfully deployed in the event of an epidemic outbreak to improve public health outcomes. We predicted that given VIS410's half-life, its distribution to the primary site of influenza A infection (nasopharynx) and its potency, that limited, directed use of the agent would reduce the total burden of disease. We note that universal prophylaxis is unlikely to be practical or feasible. To test the hypothesis of the ability of VIS410 to reduce influenza disease burden, we developed a micro-simulation of seasonal influenza that is in good general agreement with observed attack rates. In multiple scenarios, administration of a broadly neutralizing antibody like VIS410 at an estimated dose of 8–16 mg/kg to the at-risk elderly, for example in nursing homes and within the hospital, prior to the peak of an influenza outbreak reduces the frequency of serious influenza. We find that this effect can be achieved even with administration of VIS410 at a relatively low coverage (between 2% and 6%), having a measurable impact on mitigating hospitalization events in an influenza A outbreak.
Sensitivity analysis of the models indicates that timing of administration may be a crucial component of the decision-making process for the deployment of VIS410 as a prophylaxis. Our analysis suggests that between four to eight weeks prior to an epidemic peak is the optimal timing for deployment, and this is also dependent on the dose given which determines the length of an individual's protective period. As recently developed climate-based models have made influenza peak forecasting possible with a four to six week lead time (Shaman et al., 2013, Shaman and Karspeck, 2012), it may in fact be possible to have accurate enough influenza prediction to begin the early roll-out of a prophylaxis. One of the key challenges may be determining whether an influenza season will be short or long, and the forecasting exercises would need to be re-run with this exact scenario in mind: timed deployment of a population-level prophylactic whose aim is to stem transmission and reduce hospitalizations in the elderly.
Desirable outcomes for influenza public health interventions include reductions in attack rates and hospitalizations across all age groups. For hospitalization reductions in particular, it is usually not possible to prioritize one age group over another, and for this reason there is a long unresolved question in influenza about the age-targeting of public health interventions — should high-contact or high-vulnerability individuals be targeted for intervention? Targeting high-contact individuals may have a larger impact on mitigating the epidemic as a whole, including larger attack-rate reductions in high-vulnerability individuals (Fig. 3a). On the other hand, targeting high-vulnerability individuals has a more direct and measurable impact on the individuals that receive prophylaxis (Fig. 3c), and it may make it easier to argue for higher coverage levels if it can be clearly seen that protection is highly efficacious on an individual level.
The general indirect benefits seen in this population modeling exercise are seen in all population-level analyses of public health interventions for infectious disease. It is important to remember that the precise outcomes from the population exercise come with many caveats, including the geographic, demographic, and contact structure of the population in question; individual variation in the protective period; interaction between VIS410 therapy and acquisition/loss of influenza-specific immunity; and the sometimes unpredictable shape of influenza epidemics. For long-term effects of VIS410 as a public health strategy, the relationship with immunity will need to be better understood. One promising piece of preliminary information is that VIS410 targets a non-immunodominant epitope. As such, in animals, there is no measureable difference in the strength of the native immunological response between infected, untreated animals, and infected VIS410-treated animals. In both cases, re-challenge with the same virus results in no infection due to a memory response. For short-term effects (single epidemic season), it is likely that the general prophylaxis principles described in this analysis will be robust to different characteristics of temperate-zone influenza epidemics, but individual cities or states will need to perform analyses and make decisions that are specific to their populations and their past experience with influenza. Finally, an additional consideration is that VIS410 targets only influenza A. In the context of a complete prophylactic strategy employing a monoclonal antibody or antibodies, coverage of influenza B is also anticipated to be important.
In summary, based on the results presented here, we find that the safety and pharmacokinetic profile of VIS410 possesses potential for not only treating influenza A on an individual level but also as a public health strategy to mitigate the effects of seasonal or pandemic influenza based on its ability to reduce the overall burden of disease when strategically administered in a vulnerable population. The findings from this study warrant and require verification through well-controlled clinical trials.
4.1. Contributors
AMW, MFB, ZS, and JMT wrote the manuscript. JMT oversaw the Phase I clinical trials. CAH and JMT designed the phase 1 study and interpreted phase 1 data, and SB implemented the phase 1 study. SES, KJS and PFS performed analysis for serum and nasal PK data. AMW, MFB, KV, and ZS designed, analyzed, and interpreted the epidemic modeling study. MY and AMW wrote the epidemic modeling code with input from MFB.
4.2. Declaration of Interests
AMW, KJS, KV, SES, SB, CAH, ZS, and JMT are employees of Visterra. MY was previously an employee of Visterra. MFB was funded by Visterra, Inc. for this work. PFS is a consultant to Visterra.
Acknowledgments
The authors would like to acknowledge Greg Babcock for work on the discovery and pre-clinical evaluation of VIS410 and Drs. Jolene Berg and Paul Fredlund for their oversight and monitoring of the trial. MFB is a Wellcome Trust/Royal Society Sir Henry Dale Fellow.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2016.02.021.
Appendix A. Supplementary Data
Supplementary material.
References
- Baranovich T., Jones J.C., Russier M. The hemagglutinin stem-binding monoclonal antibody VIS410 controls influenza virus-induced acute respiratory distress syndrome. Antimicrob. Agents Chemother. 2016 doi: 10.1128/AAC.02457-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartelds G.M., Krieckaert C.M., Nurmohamed M.T. Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA. 2011;305(14):1460–1468. doi: 10.1001/jama.2011.406. [DOI] [PubMed] [Google Scholar]
- Boni M.F., Nguyen T.D., de Jong M.D., van Doorn H.R. Virulence attenuation during an influenza A/H5N1 pandemic. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2013;368(1614):20120207. doi: 10.1098/rstb.2012.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breteler J.K., Tam J.S., Jit M., Ket J.C., De Boer M.R. Efficacy and effectiveness of seasonal and pandemic A (H1N1) 2009 influenza vaccines in low and middle income countries: a systematic review and meta-analysis. Vaccine. 2013;31(45):5168–5177. doi: 10.1016/j.vaccine.2013.08.056. [DOI] [PubMed] [Google Scholar]
- Burioni R., Canducci F., Mancini N. Monoclonal antibodies isolated from human B cells neutralize a broad range of H1 subtype influenza A viruses including swine-origin influenza virus (S-OIV) Virology. 2010;399(1):144–152. doi: 10.1016/j.virol.2009.12.014. [DOI] [PubMed] [Google Scholar]
- Corti D., Voss J., Gamblin S.J. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science. 2011;333(6044):850–856. doi: 10.1126/science.1205669. [DOI] [PubMed] [Google Scholar]
- Darvishian M., Bijlsma M.J., Hak E., van den Heuvel E.R. Effectiveness of seasonal influenza vaccine in community-dwelling elderly people: a meta-analysis of test-negative design case–control studies. Lancet Infect. Dis. 2014;14(12):1228–1239. doi: 10.1016/S1473-3099(14)70960-0. [DOI] [PubMed] [Google Scholar]
- Dreyfus C., Laursen N.S., Kwaks T. Highly conserved protective epitopes on influenza B viruses. Science. 2012;337(6100):1343–1348. doi: 10.1126/science.1222908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekiert D.C., Bhabha G., Elsliger M.A. Antibody recognition of a highly conserved influenza virus epitope. Science. 2009;324(5924):246–251. doi: 10.1126/science.1171491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekiert D.C., Friesen R.H., Bhabha G. A highly conserved neutralizing epitope on group 2 influenza A viruses. Science. 2011;333(6044):843–850. doi: 10.1126/science.1204839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson N.M., Cummings D.A., Cauchemez S. Strategies for containing an emerging influenza pandemic in Southeast Asia. Nature. 2005;437(7056):209–214. doi: 10.1038/nature04017. [DOI] [PubMed] [Google Scholar]
- Gammagard Liquid [Package Insert]. Westlake Village, CA USA; Baxter; 2014.
- Germann T.C., Kadau K., Longini I.M., Jr., Macken C.A. Mitigation strategies for pandemic influenza in the United States. Proc. Natl. Acad. Sci. U. S. A. 2006;103(15):5935–5940. doi: 10.1073/pnas.0601266103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamborsky J., Kroger A., Wolfe S., editors. Epidemiology and Prevention of Vaccine-Preventable Diseases. 13th ed. Public Health Foundation; Washington, D.C.: 2015. [Google Scholar]
- Centers for Disease Control U. http://www.cdc.gov/flu/about/disease/us_flu-related_deaths.htm.
- Hung I.F., To KK, Lee C.K. Hyperimmune IV immunoglobulin treatment: a multicenter double-blind randomized controlled trial for patients with severe 2009 influenza A(H1N1) infection. Chest. 2013;144(2):464–473. doi: 10.1378/chest.12-2907. [DOI] [PubMed] [Google Scholar]
- Jefferson T., Jones M., Doshi P., Spencer E.A., Onakpoya I., Heneghan C.J. Oseltamivir for influenza in adults and children: systematic review of clinical study reports and summary of regulatory comments. BMJ. 2014;348:g2545. doi: 10.1136/bmj.g2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S.P., Saif M.W. Infusion-related and hypersensitivity reactions of monoclonal antibodies used to treat colorectal cancer - identification, prevention, and management. J. Support. Oncol. 2007;5(9):451–457. [PubMed] [Google Scholar]
- Kashyap A.K., Steel J., Rubrum A. Protection from the 2009 H1N1 pandemic influenza by an antibody from combinatorial survivor-based libraries. PLoS Pathog. 2010;6(7) doi: 10.1371/journal.ppat.1000990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley B. Very large scale monoclonal antibody purification: the case for conventional unit operations. Biotechnol. Prog. 2007;23(5):995–1008. doi: 10.1021/bp070117s. [DOI] [PubMed] [Google Scholar]
- Lakdawala S., Baz M., E. L. Options for the Control of Influenza VIII. International Society for Influenza and Other Respiratory Virus Diseases; Cape Town, South Africa: 2013. Therapy or prophylaxis with an HA-stem antibody (VIS410) limits respiratory droplet transmission of influenza viruses in the ferret model. [Google Scholar]
- Longini I.M., Jr., Nizam A., Xu S. Containing pandemic influenza at the source. Science. 2005;309(5737):1083–1087. doi: 10.1126/science.1115717. [DOI] [PubMed] [Google Scholar]
- Newton D.W., Treanor J.J., Menegus M.A. Clinical and laboratory diagnosis of influenza virus infections. Am. J. Manag. Care. 2000;6(5 Suppl):S265–S275. [PubMed] [Google Scholar]
- Okuno Y., Isegawa Y., Sasao F., Ueda S. A common neutralizing epitope conserved between the hemagglutinins of influenza A virus H1 and H2 strains. J. Virol. 1993;67(5):2552–2558. doi: 10.1128/jvi.67.5.2552-2558.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterholm M.T., Kelley N.S., Sommer A., Belongia E.A. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect. Dis. 2012;12(1):36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- Reed C., Chaves S.S., Daily Kirley P. Estimating influenza disease burden from population-based surveillance data in the United States. PLoS One. 2015;10(3) doi: 10.1371/journal.pone.0118369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remicade [Package Insert]. Horsham, PA USA: Janssen Biotech; Revised 2011.
- Schanzer D.L., Langley J.M., Tam T.W. Co-morbidities associated with influenza-attributed mortality, 1994–2000, Canada. Vaccine. 2008;26(36):4697–4703. doi: 10.1016/j.vaccine.2008.06.087. [DOI] [PubMed] [Google Scholar]
- Shaman J., Karspeck A. Forecasting seasonal outbreaks of influenza. Proc. Natl. Acad. Sci. U. S. A. 2012;109(50):20425–20430. doi: 10.1073/pnas.1208772109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaman J., Karspeck A., Yang W., Tamerius J., Lipsitch M. Real-time influenza forecasts during the 2012–2013 season. Nat. Commun. 2013;4:2837. doi: 10.1038/ncomms3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui J., Hwang W.C., Perez S. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat. Struct. Mol. Biol. 2009;16(3):265–273. doi: 10.1038/nsmb.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharakaraman K., Subramanian V., Viswanathan K. A broadly neutralizing human monoclonal antibody is effective against H7N9. Proc. Natl. Acad. Sci. U. S. A. 2015 doi: 10.1073/pnas.1502374112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson W.W., Shay D.K., Weintraub E. Influenza-associated hospitalizations in the United States. JAMA. 2004;292(11):1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- Weekly Influenza & Respiratory Illness Activity Report. Summary of the 2014-15 Influenza Season. MINNESOTA DEPARTMENT OF HEALTH. http://www.health.state.mn.us/divs/idepc/diseases/flu/stats/2014summary.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.