Abstract
Thrombus formation leading to vaso-occlusive events is a major cause of death, and involves complex interactions between coagulation, fibrinolytic and innate immune systems. Leukocyte recruitment is a key step, mediated partly by chemotactic complement activation factors C3a and C5a. However, mechanisms mediating C3a/C5a generation during thrombosis have not been studied. In a murine venous thrombosis model, levels of thrombin–antithrombin complexes poorly correlated with C3a and C5a, excluding a central role for thrombin in C3a/C5a production. However, clot weight strongly correlated with C5a, suggesting processes triggered during thrombosis promote C5a generation. Since thrombosis elicits fibrinolysis, we hypothesized that plasmin activates C5 during thrombosis. In vitro, the catalytic efficiency of plasmin-mediated C5a generation greatly exceeded that of thrombin or factor Xa, but was similar to the recognized complement C5 convertases. Plasmin-activated C5 yielded a functional membrane attack complex (MAC). In an arterial thrombosis model, plasminogen activator administration increased C5a levels. Overall, these findings suggest plasmin bridges thrombosis and the immune response by liberating C5a and inducing MAC assembly. These new insights may lead to the development of strategies to limit thrombus formation and/or enhance resolution.
Abbreviations: MAC, membrane attack complex; VWF, von Willebrand factor; R751, arginine 751; TAT, thrombin antithrombin; IVC, inferior vena cava; VFKck, Val-Phe-Lys-chloromethylketone; PPACK, Phe-Pro-Arg-chloromethylketone; FeCl3, ferric chloride; tPA, tissue-type plasminogen activator; NETs, neutrophil extracellular traps; PAR1, protease activated receptor 1; MCP1-1, monocyte chemoattracant protein-1; IL-8, interleukin-8; FDP, fibrin degradation product
Keywords: Thrombosis, Complement, Leukocytes, Thrombin, Fibrinolysis
Highlights
-
•
Thrombin is not a major direct contributor to C5a generation during venous thrombosis in mice.
-
•
Plasmin, a protease generated in response to thrombin generation and fibrin deposition, efficiently cleaves C5 to C5a.
-
•
In an arterial thrombosis model, administration of a plasminogen activator augments C5a plasma levels.
-
•
Plasmin participates in immunothrombosis, liberating chemotactic C5a and inducing assembly of the procoagulant C5b-9.
Venous and arterial thrombosis are major causes of death and morbidity. Leukocytes are early and active participants in thrombus formation, recruited partly by complement factor C5a. We examined how C5a is generated in the setting of thrombosis. In venous thrombosis in mice, we show that thrombin, a key clot-promoting enzyme, is not a major contributor to C5a generation. Rather, plasmin, a fibrinolytic enzyme formed in response to thrombin generation and clot formation, efficiently generates C5a. The findings were validated in an arterial thrombosis model in mice. These insights may be valuable in developing therapeutic strategies to limit thrombus formation.
1. Introduction
The coagulation system and innate immunity are coordinately activated and highly integrated during venous and arterial thrombus formation and progression (von Bruhl et al., 2012, Engelmann and Massberg, 2013, Fuchs et al., 2012). Vascular endothelial activation or damage causes release of ultralarge von Willebrand factor (VWF) and P-selectin from Weibel-Palade bodies, and local activation of complement with liberation of anaphylatoxic and chemotactic factors C3a and C5a. These pathways cooperate to trigger platelet, neutrophil, and monocyte recruitment and activation (von Bruhl et al., 2012). The locally accumulated cells release proteases, reactive oxygen species, and nucleosomes, which provide a scaffold for aggregating platelets and red blood cells and further promote coagulation and fibrin formation (Fuchs et al., 2012). Several complement factors, including C3, C4, C3a, C5a and factor H are incorporated into the thrombus, where they modulate thrombus stability and the inflammatory process (Distelmaier et al., 2009, Howes et al., 2012). The fibrinolytic system and plasmin-mediated proteolysis are also intimately coupled to the axis of thrombus development and inflammation by controlling fibrin degradation, activation of matrix metalloproteinases, infiltration of monocytes/macrophages and other immune mediators, vessel wall remodeling, and ultimately thrombus resolution (Wakefield et al., 2008).
The mechanisms by which leukocytes are recruited early in thrombus formation and later during thrombus extension or resolution, are poorly understood. However, C5a, the most potent chemotactic complement activation fragment, is released following proteolytic cleavage of C5 and is considered a critical determinant of neutrophil recruitment and activation in thrombosis (Distelmaier et al., 2009, Salmon et al., 2002, Pierangeli et al., 2005). Moreover, terminal complement pathway complexes formed as C5 is activated, have multiple procoagulant properties (Langer et al., 2013, Hamilton et al., 1990). Thus, there is interest in understanding how C5a and the other major complement-derived chemotactic factor, C3a, are generated, so that novel therapeutic strategies may be designed to prevent thrombosis.
Complement activation typically proceeds via three pathways — classical, lectin and alternative — which converge to form C3 convertases that proteolyse C3 into C3b with release of C3a (Ricklin et al., 2010). As complement activation exceeds a threshold, and the density of C3b increases, the specificity of the convertase shifts from C3 to C5. The resultant C5 convertases — C3bBbC3b for the alternative pathway and C4b2aC3b for the classical/lectin pathway — efficiently cleave C5 at arginine 751 (R751), liberating C5a and generating C5b, the initiating factor for assembly of the lytic C5b-9 membrane attack complex (MAC). Although the C3/C5 convertases are well-recognized for their capacity to cleave C3 and C5, other serine proteases reportedly also exhibit convertase activity (Huber-Lang et al., 2006, Amara et al., 2010, Wiggins et al., 1981). Notably, thrombin was implicated in providing a “new pathway” to activate complement by cleaving C5 in a C3-independent manner, thereby bypassing the bona fide C5 convertases (Huber-Lang et al., 2006). However, C5 is a relatively poor substrate for thrombin cleavage at R751 (Krisinger et al., 2012), raising questions as to its importance in contributing to C5a generation during thrombus formation in vivo. We therefore explored the mechanisms by which C3a and C5a are generated using biochemical approaches and in vivo models of venous and arterial thrombosis.
2. Materials and Methods
2.1. Materials
Human complement C3a and C5a were measured using Quidel MicroVue C3a Plus or C5a ELISA kits (Cedarlane Laboratories, Burlington, Ontario). Murine thrombin–antithrombin (TAT) levels were measured using Enzygnost TAT micro ELISA (Siemens, Munich, Germany). Human complement proteins C3 and C5 were obtained from Complement Technology, Inc. (Tyler, TX) and human hemostatic enzymes (plasmin, factor Xa and thrombin) were from Haematologic Technologies, Inc. (Essex Junction, VT).
2.2. ELISAs to Measure C3a and C5a
Murine complement C5a levels were determined using the mouse complement component C5a duoset and accompanying standard from R&D systems (catalog #DY2150; Minneapolis, MN). An ELISA for murine complement C3a was established using a murine C3a standard and antibodies from BD Biosciences (Mississauga, Canada). The capture rat monoclonal anti-mouse C3a antibody (catalog # 55820, clone: I87-1162) was coated overnight onto 96-well plates in 100 μL of PBS at a concentration of 2 μg/mL. Wells were washed × 3 with wash buffer (R&D catalog #WA126) followed by blocking for 2 h with 300 μl of reagent diluent (R&D systems catalog #DY995). Plasma samples in duplicate were diluted 1/50 and 1/125 in sample diluent to a final volume of 100 μl and incubated for 2 h. After 3 washes, 100 μl of the biotinylated detection monoclonal rat anti-mouse C3a antibody (clone I87-419, catalog #55821) 0.5 μg/mL in reagent diluent was incubated for 1 h. Wells were washed × 4 and incubated for 20 min at room temperature with 100 μl of streptavidin-biotinylated horseradish peroxidase (1:3000), followed by 2 washes, and development with the substrate solution containing o-phenylenediamine using a plate reader set to 450 nm. A standard curve was generated with purified murine C3a (catalog # 558618). The sensitivity range of the assay was 0.1 nM to 2.5 nM. Intra-assay and inter-assay precision was 8–10%.
2.3. Animal Models
All experiments with animals were approved by the University of North Carolina at Chapel Hill Institutional Animal Care and Use Committees. The mice (C57Bl/6) were male and between 6 and 8 weeks of age. The number of animals for each model was determined based on previous work that showed a broad range of TAT levels in the respective models (Machlus et al., 2011a, Machlus et al., 2011b). On each day of the experiments, animals were randomly assigned to receive the stated treatment or to be used for baseline measurements. Quantification of biomarkers was performed in a blinded fashion wherein an experimenter, different from the one who performed the procedures on the animals, carried out the ELISAs on coded samples that were only de-coded after results had been generated.
2.4. Murine model of Venous Thrombosis
The inferior vena cava (IVC) stasis model was performed as previously described (Aleman et al., 2013). Briefly, mice were anesthetized with 1·5–2% isoflurane in oxygen and human prothrombin (to 300%, final, mouse plus human prothrombin) or vehicle was infused via tail vein injection. Prothrombin was infused to give a broader range of thrombin generation and clot weight. Following sterile laparotomy, the intestines were exteriorized, the IVC was dissected bluntly, and side branches were ligated with 8–0 prolene suture and lumbar branches closed by cautery. The IVC was separated from the aorta by blunt dissection and completely ligated with 8–0 prolene suture. After replacing the intestines, the muscle layer was closed with 5–0 vicryl suture and skin closed with 8–0 prolene suture and skin glue. Mice recovered with analgesia (buprenorphine, 0·05 mg/kg subcutaneous). After 12 h, blood was drawn from the IVC above the ligation site into 3.2% sodium citrate and processed to platelet-poor plasma by centrifugation at 5000 × g for 10 min. Thrombi were collected and weighed. Plasmas were stored at − 80 °C for analysis of TAT, C3a and C5a levels by a person that was blinded to the treatment group. Two samples showing hemolysis were excluded.
2.5. In Vitro Generation of C3a or C5a by Hemostatic Enzymes
The relative efficiency, time course, and rate of complement cleavage by plasmin, factor Xa or thrombin were determined using a series of assays. Briefly, complement C3 (20 μM) or C5 (2 μM) was incubated with 100 nM plasmin or 250 nM factor Xa or thrombin. Reactions were quenched at various time points and C3a or C5a levels were quantified by ELISA. To determine the relative efficiency of cleavage, 2 μM of C5 was incubated with 100 nM of plasmin, factor Xa or thrombin at 37 °C. After 10 min the reaction was quenched with the appropriate chloromethylketone (Val-Phe-Lys-chloromethylketone (VFKck) for plasmin and Phe-Pro-Arg-chloromethylketone (PPAck) for factor Xa and thrombin). In similar experiments, aliquots of the reaction mixture were sub-sampled into chloromethylketones at various time points to determine the time courses of C3 and C5 cleavages by plasmin, factor Xa and thrombin. The kinetics of C5a generation were determined by incubating 0–3 μM of C5 with 100 nM of plasmin at 37 °C. Reactions were quenched after 1 min and C5a levels were quantified by ELISA. Similar experiments substituting factor Xa or thrombin for plasmin were conducted, but the amount of C5a generated in these assays over 30 min (for factor Xa) or 1 h (for thrombin) was below the limit of detection for the assay.
C5a generation occurring during clot formation and degradation was assessed in vitro by incubating physiological concentrations of fibrinogen (9 μM), plasminogen (2 μM), antiplasmin (1 μM) and C5 (400 nM) at 37 °C. High (1 μM) or low (10 nM) concentrations of thrombin were added to induce clot formation and 10 nM of tPA was used to induce plasminogen activation and clot lysis. The tPA concentration chosen resulted in complete clot lysis in 30 min. At the end of the 30-minute incubation period, enzymes were quenched with PPAck and VFKck, the sample centrifuged and supernatant or sample stored at − 80 °C for quantification of C5a levels. Background C5a signal when PPAck/VFKck was added at t = 0 were subtracted.
2.6. In Vitro Generation of C5b,6 by Hemostatic Enzymes
Complement factors C5 (400 nM) and C6 (500 nM) were incubated with 100 nM of plasmin or 250 nM of factor Xa or thrombin. Parallel reactions were quenched over time with an appropriate chloromethylketone. The amount of functional C5b,6 generated was subsequently quantified using a chicken erythrocyte hemolytic assay (Wat et al., 2014). Since plasmin readily cleaves C5, we also incubated 500 nM of C5b,6 with 100 nM of plasmin to determine if C5b,6 activity diminishes over time. In these experiments, plasmin was quenched with VFKck.
2.7. Ferric Chloride Model of Arterial Thrombosis
Mice were anesthetized with 1·5–2% isoflurane in oxygen. Ferric chloride injury to the carotid artery was performed as described (Aleman et al., 2013). Briefly, the right common carotid artery was exposed, dried, and treated with ferric chloride (7·5 or 10% on 0·5 × 1·0-mm filter paper) for 2 min. The artery was washed with warm saline and blood flow was continuously monitored by Doppler ultrasonic flow probe (Indus Instruments). The time to vessel occlusion was defined as the time between FeCl3 administration and lack of flow for 60 s. Blood was sampled into citrate from the IVC 5 min after stable vessel occlusion (defined as continuous occlusion for 1 min) or after 40 min if no occlusion occurred.
2.8. Thrombolysis Model
Thrombolysis was assessed in mice subjected to FeCl3 carotid artery thrombosis. After 5 consecutive minutes of vessel occlusion, mice were infused with Tenecteplase (5 mg/kg, generous gift of Genentech, CA) through a saphenous vein intravenous catheter constructed of pulled PE-10 tubing (Braintree Scientific, Braintree, MA) with a 3·0-mil (0·076-mm diameter) cleaning wire (Hamilton Company, Reno NV) placed into the lumen as a stylet, as described (Machlus et al., 2011a), while continuously monitoring carotid blood flow. Blood was sampled from the IVC 5 min after the return of blood flow or 30 min after Tenecteplase infusion if the clot did not lyse.
2.9. Statistics
The relationships between prothrombotic and complement activation markers were assessed using Spearman rank correlation. C3a and C5a levels were compared using t-tests or the Wilcoxon rank sum test (Tenecteplase-treated versus untreated mice). P < 0·05 was considered statistically significant.
3. Results
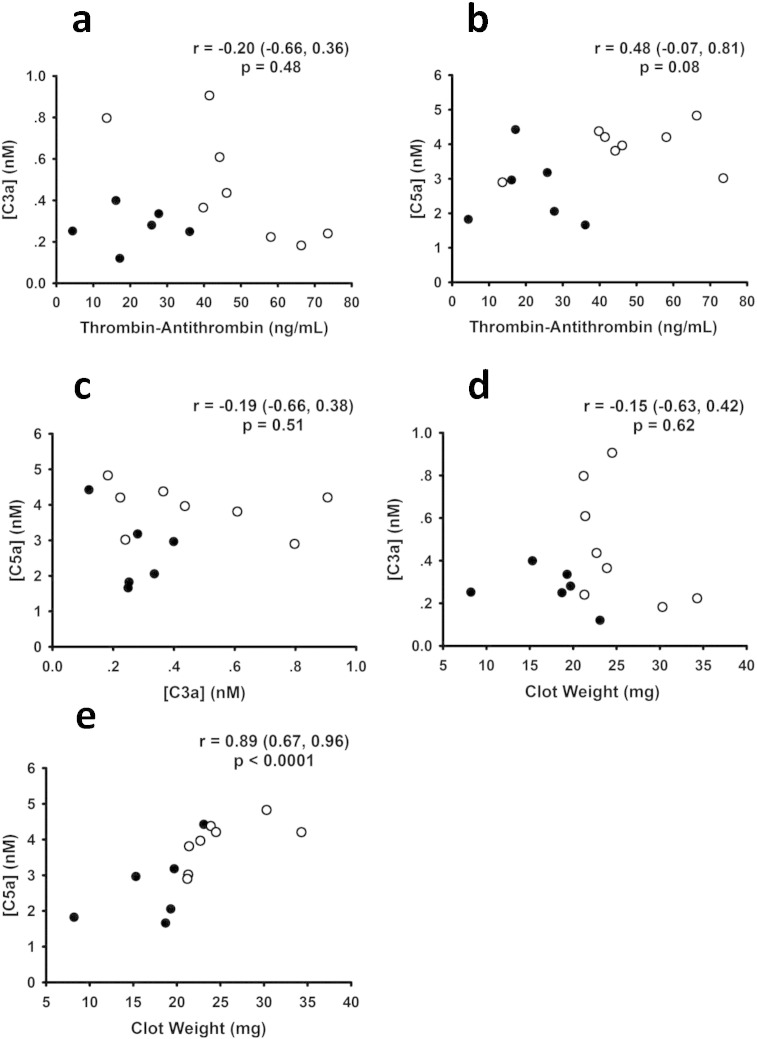
We studied the role of thrombin in complement activation using an in vivo murine model of ligation (stasis)-induced IVC thrombosis (von Bruhl et al., 2012, Aleman et al., 2013). Plasma levels of activation markers of coagulation and complement were measured by ELISA. Baseline TAT levels were 3·8 ± 4·0 ng/mL (n = 5), as previously reported (Aleman et al., 2013). 24 h after IVC ligation, plasma TAT levels rose to 21·2 ± 11 ng/mL (mean ± SD, n = 6). Prothrombin was infused in some mice to give a broader range of thrombin generation and clot weight (Aleman et al., 2013) (Fig. 1 — open circles — mice infused with prothrombin; solid circles — mice infused with vehicle). When prothrombin was infused just prior to IVC ligation (Aleman et al., 2013), TAT levels measured at 24 h were significantly higher (47·9 ± 19 ng/mL, n = 8, p < 0·009). As expected, clot weights directly correlated with TAT levels (r = 0·66, p < 0·01). Plasma levels of C5a and C3a in venous thrombosis were elevated as compared to baseline (unchallenged) levels (C5a = 0·43 ± 0·15 nM; C3a < 0.1 nM; n = 6). Interestingly, circulating levels of complement activation markers C3a and C5a correlated poorly with TAT levels (Fig. 1a, b), suggesting that thrombin does not directly activate complement in this experimental model of venous thrombosis. Notably, C5a also correlated poorly with C3a (Fig. 1c), suggesting that C5a was generated to a large extent via C3/C5 convertase-independent pathways. Furthermore, there was no relationship between clot weight and C3a levels (Fig. 1d). There was, however, a strong direct correlation between clot weight and C5a levels (Fig. 1e). Taken together, these findings show that processes triggered during venous thrombosis are associated with C5a generation, but suggest that thrombin is not the major activator of C5 under these conditions.
Fig. 1.
Coagulation and complement activation in venous thrombosis. Following stasis-induced thrombosis of the inferior vena cava (see Materials and Methods section), levels of thrombin-antithrombin complexes (TAT), C3a, and C5a were measured by ELISA. C3a (a) and C5a (b) levels correlated poorly with TAT levels. C3a and C5a levels also poorly correlated (c), implying the existence of C3-independent pathways to generate C5a. Clot weight poorly correlated with C3a (d), but strongly correlated with C5a (e). Each dot represents a separate mouse, n = 14. Solid circles are untreated mice (infused with vehicle); open circles are mice that were infused with prothrombin to increase thrombin generation (see Materials and Methods section). Correlation coefficients (r) with 95% confidence levels and p-values are indicated on each panel.
To determine the potential mechanisms of C5a generation in vivo, we used in vitro assays in purified systems to compare C5a generation following cleavage of C5 by thrombin, factor Xa or plasmin. Plasmin was considered a likely candidate of complement activation in the setting of a fibrin clot because, 1) plasmin is known to cleave C5 to yield chemotactically-active C5a in vitro (Amara et al., 2010), and 2) fibrin is an essential cofactor for tissue-type plasminogen activator (tPA)-mediated plasmin generation (Horrevoets et al., 1997).
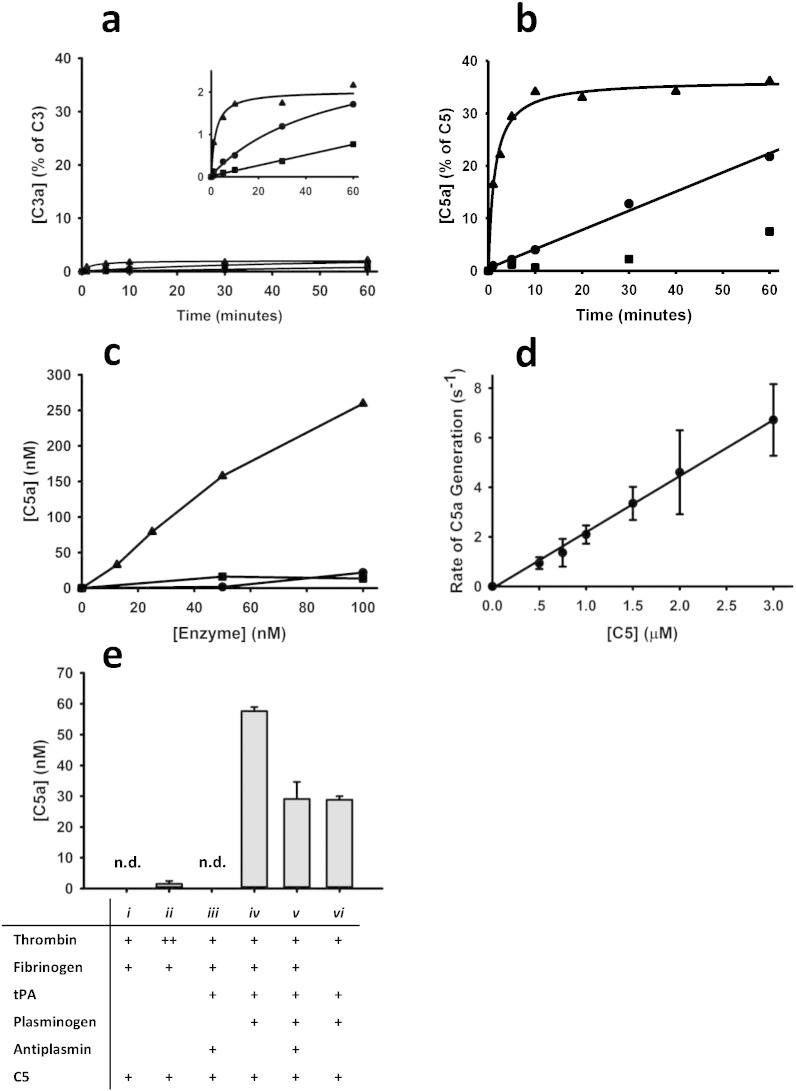
Incubation of C3 or C5 with thrombin, factor Xa or plasmin revealed that plasmin is much more effective than thrombin or factor Xa in cleaving C3 and C5 to generate C3a and C5a, respectively (Fig. 2a, b). Plasmin more readily cleaved C5 than C3, with ~ 30% of C5 (~ 700 nM) being converted to C5a, and only ~ 2% of C3 (~ 450 nM) cleaved to form intact C3a. The low turnover of C3 by plasmin, thrombin and fXa precluded further in vitro interrogation. The incomplete cleavage of C5 to C5a under the conditions employed may reflect competitive inhibition by an abundance of cleavage products (molecular weight > 30–70 kDa) that were detected following SDS-PAGE (not shown and (Barthel et al., 2012)). We compared the efficiency of C5 cleavage by various concentrations (0–100 nM) of each of the three enzymes (Fig. 2c). During a 10-minute incubation period, plasmin generated substantially more C5a than factor Xa or thrombin.
Fig. 2.
Hemostatic enzymes generate C5a. a, b, c. In a purified system, plasmin (100 nM) (▴), factor Xa (250 nM) (•) or thrombin (250 nM) (■) was incubated with C3 or C5 for varying periods of time and C3a (a) or C5a (b) was measured by ELISA. Plasmin efficiently generated C3a (a) (inset is higher magnification to show relative initial rates of C3a generation) and C5a (b) within 1 min, whereas factor Xa or thrombin took much longer to generate appreciable amounts of C3a or C5a. c. C5 (2 μM) was incubated for 10 min with varying concentrations of each of the three enzymes, after which C5a levels were measured by ELISA. Plasmin was significantly more efficient at cleaving C5 than factor Xa or thrombin. d. The rate of C5a generation by plasmin increased linearly as the concentration of C5 increased. The slope of the line implies that plasmin cleaves C5, generating C5a with a catalytic efficiency of 2.3 ± 0.6 × 104 M− 1 s− 1 (Distelmaier et al., 2009). e. Thrombin (10 nM, 1 μM; i, ii, respectively) or tPA (10 nM) (iii) did not generate significant levels of C5a from C5. Addition of plasminogen (2 μM) to the system enabled plasmin generation and was associated with C5a generation in the absence (iv) or presence (v) of 1 μM of antiplasmin. Fibrinogen (9 μM) (vi) is not essential for C5a generation when tPA, plasminogen and C5 are present, but it enhances C5a generation in the context of tPA induced plasminogen activation (v). The data presented in a, b are a single representative experiment and the data for all other experiments (c–e) represent means ± SD for 3 replicates. n.d. = not detectable.
In kinetic assays, the rate of C5a generation by plasmin increased linearly as the concentration of C5 increased (Fig. 2d). The catalytic efficiency, inferred from the slope of the plot, was 2·3 ± 0·6 × 104 M− 1 s− 1 (Distelmaier et al., 2009). This is similar to the published rate of C5 cleavage by the bona fide alternative pathway C5 convertase and the soluble monomeric classical/lectin pathway C3/C5 convertase (Rawal and Pangburn, 1998, Rawal and Pangburn, 2001). This rate of C5 cleavage by plasmin is therefore consistent with the premise that plasmin has a physiologically relevant role in generation of C5a.
Since plasmin is rarely, if ever, free in circulation, we next tested whether plasmin is capable of generating C5a in the presence of physiological concentrations of antiplasmin and fibrinogen, that when converted to fibrin, binds plasminogen and plasmin with high affinity. We first confirmed that even at very high concentrations, thrombin generates almost undetectable amounts of C5a (Fig. 2e, conditions i, ii). In the absence of plasminogen, tPA was incapable of cleaving C5 to yield C5a (Fig. 2e, condition iii). When C5 was incubated with tPA and plasminogen in the presence of fibrin(ogen) (Fig. 2e, condition v), readily detectable amounts of C5a were generated, and this occurred even in the presence of physiological concentrations of antiplasmin (condition iv). Absence of fibrinogen, a cofactor for tPA-mediated conversion of plasminogen to plasmin, resulted in the generation of measurable, but less, C5a than with fibrinogen (condition v versus vi). Overall, the data confirm that in the presence of physiological concentrations of hemostatic proteins, C5a can be generated in a plasmin-dependent manner.
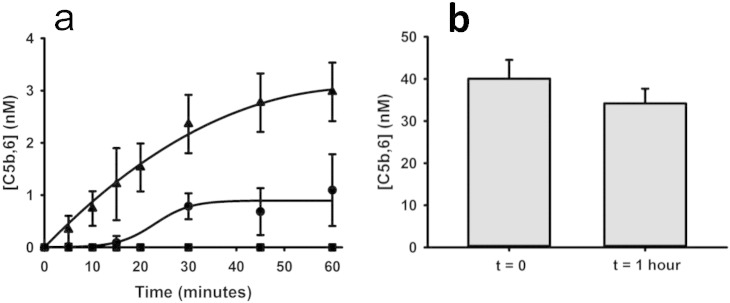
Convertase-mediated release of C5a from C5 occurs in parallel with generation of C5b that is required for MAC formation. Using a terminal pathway hemolytic assay (Wat et al., 2014), we showed that in the presence of excess C6, cleavage of C5 by factor Xa or plasmin yielded a C5b,6 complex that could assemble with C7, C8 and C9 to form a fully functional lytic MAC. The efficiency of C5b,6 generation by plasmin, factor Xa and thrombin mirrored that observed for C5a (Fig. 3a). Over 60 min, 100 nM of plasmin generated 3·0 ± 0.6 nM C5b,6, whereas even 250 nM of factor Xa (which is ~ 2-fold higher than the plasma concentration of factor X) generated only 1·1 ± 0·7 nM of C5b,6. Consistent with its inefficient cleavage of C5, thrombin (250 nM) did not generate any detectable C5b,6. Thus, although plasmin can cleave C5 at several sites (Barthel et al., 2012), exposure of C5b,6 to plasmin for 1 h did not appreciably decrease the functionality of the MAC (Fig. 3b).
Fig. 3.
Hemostatic enzymes generate C5b,6. a. Cleavage of C5 by plasmin (▴), factor Xa (•), or thrombin (■) in the presence of excess C6, C7, C8 and C9, resulted in the generation of functional C5b,6, measured by a terminal pathway erythrocyte hemolytic assay. Thrombin did not generate any measurable C5b,6 in this experimental system. b. The functional integrity of C5b,6, measured by the terminal pathway hemolytic assay, did not decay appreciably over a 1-hour period when incubated with plasmin. The data presented represent means ± SD for 3 replicates.
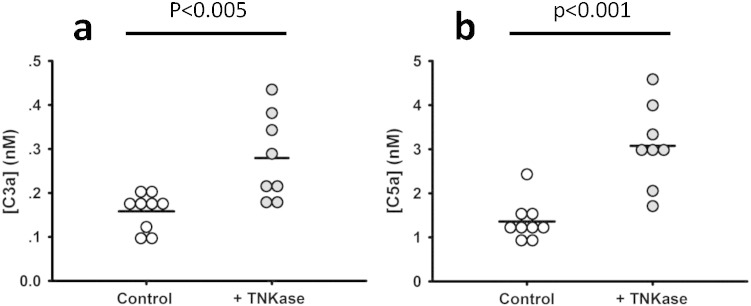
In view of these in vitro findings, we tested the association between thrombin generation and complement activation in a second, independent thrombosis/thrombolysis model, and used this model to determine whether C5 is activated by thrombolytic pathways in vivo. Using wild-type mice with stable carotid artery thrombi induced by ferric chloride, we first showed that 5 min after occlusion, TAT levels correlated poorly with systemic levels of C3a and C5a (r = − 0·36, p = 0·39 for C3a; r = − 0·05, p = 0·91 for C5a; n = 8), consistent with our observations in the venous thrombosis model. To determine the impact of plasminogen activation in this setting, we intravenously administered the tPA analog Tenecteplase (n = 8) into mice that had been challenged with arterial occlusion and measured C3a and C5a 5 min after restoration of blood flow, or 30 min after infusion if blood flow was not restored (Fig. 4a, b). Of these, 3 mice did not re-perfuse and 5 mice did re-perfuse. Regardless of outcome, all mice that received Tenecteplase were included in the analysis. As compared to ferric chloride-challenged mice that were not infused with Tenecteplase (n = 9), Tenecteplase caused a significant (~ 2-fold) increase in C3a and C5a levels. Notably, this increase is in line with published studies in a small group of patients treated with recombinant tPA following acute myocardial infarction (Bennett et al., 1987). Our finding that C5 could not be cleaved by recombinant tPA (Fig. 2d, iii) makes it highly unlikely that the elevated C5a levels were attributable to Tenecteplase-mediated generation of C5. We further excluded this possibility by showing in vitro that exposure of C5 to high concentrations of tPA (up to 200 nM) for 10 and 30 min, did not yield measurable amounts of C5a (data not shown). Taken together, these data are consistent with a direct effect of plasmin on complement activation during thrombosis.
Fig. 4.
Effects of plasminogen activator-mediated thrombolysis on C3a and C5a levels. a, b. Stable carotid artery thrombosis was induced in wild-type mice using the ferric chloride model (see Materials and Methods section), after which the plasminogen activator Tenecteplase was administered intravenously as noted. C3a (a) and C5a (b) levels were significantly increased by Tenecteplase infusion. Each dot represents a separate mouse (n = 9 controls, n = 8 infused with Tenecteplase).
4. Discussion
Thrombus formation leading to pathological vaso-occlusive events (e.g. acute coronary syndrome, stroke, deep vein thrombosis and pulmonary embolus) is a major cause of death worldwide (Mozaffarian et al., 2015, Raskob et al., 2014). Initiation, propagation, and resolution of a thrombus rely on the recruitment of platelets and inflammatory cells, and this is mediated partly by local release of the complement activation factor C5a. C5a is a potent anaphylatoxic peptide, inducing a range of pro-inflammatory and pro-thrombotic effects via its cognate G-protein-coupled receptors, C5aR and C5L2. C5a activates platelets, leukocytes and endothelial cells, upregulates expression of adhesion molecules, induces secretion of pro-inflammatory and procoagulant cytokines, promotes tissue factor expression by neutrophils and release of tissue factor-containing microparticles, induces the formation of neutrophil extracellular traps (NETs), and amplifies complement activation through positive feedback loops (Oikonomopoulou et al., 2012). Given the current findings, we propose a model in which plasmin, via liberation of C5a, contributes to leukocyte trafficking during thrombus formation, propagation and/or resolution (Fig. 5). The precise local contribution of C5a (and C3a) to thrombus formation and resolution, is difficult to ascertain, particularly since clearance of these peptides is short and likely dynamically changes in this setting. Nonetheless, with generation of C5a, terminal complement pathway complexes form which also regulate coagulation. C5b-7 induces tissue factor expression by monocytic cells (Langer et al., 2013), while the MAC induces VWF and P-selectin secretion, platelet microparticle release, and endothelial cell and platelet membrane changes that favor prothrombinase assembly and thrombin generation (Hamilton et al., 1990, Wiedmer et al., 1986, Sims et al., 1988). Since C5 activation is associated with many disease states, including acute lung injury, arthritis, sepsis (Huber-Lang et al., 2006, Kessel et al., 2014, Yan and Gao, 2012), and thrombosis (Distelmaier et al., 2009, Cheung et al., 1994), the present studies suggest that interventions at the level of plasmin may have broad clinical utility.
Fig. 5.
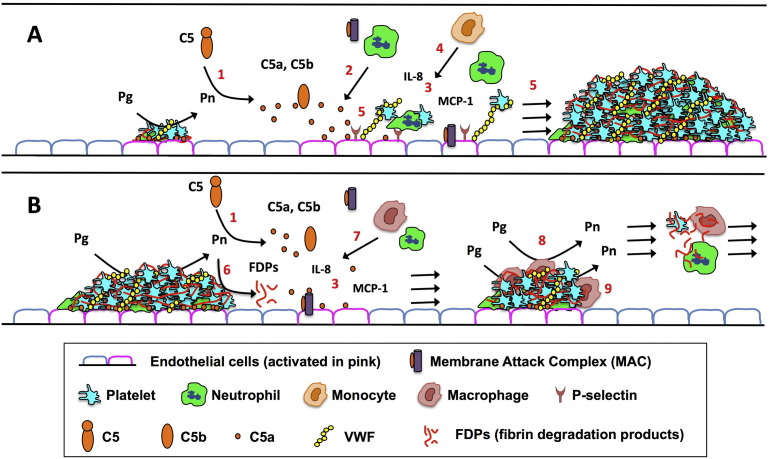
Proposed contributions of plasmin-mediated C5 activation in thrombosis. During a. thrombus formation, progression, and b. resolution, fibrin deposition promotes plasmin generation, which activates complement. The traditional complement activation pathways likely also participate (not shown). The effects of plasmin-mediated complement activation on thrombus growth and resolution depend on the timing and localization of C5a generation, and assembly of the membrane attack complex (MAC). a. Thrombus formation: Following endothelial cell activation/damage, C5 (and C3) is activated, generating C5a and C5b (1), the latter of which binds to C6–C9 to form the membrane-damaging, procoagulant MAC. C5a is a potent chemoattractant for platelets and neutrophils (2) and activates cells to express monocyte chemoattractant protein-1 (MCP-1) and interleukin (IL)-8 (3). These pathways cooperate to recruit and activate platelets, monocytes and neutrophils (4), with release of reactive oxygen species, proteases and nucleosomes, all of which enhance thrombus formation (5). b. Thrombus resolution: As plasmin degrades fibrin into fibrin degradation products (FDPs) (6), it also generates C5a and C5b (1). C5a induces the release of factors (e.g. MCP-1, IL-8) (3) that recruit macrophages and neutrophils (7), which promote clot resolution by augmenting plasmin generation (8), fibrin degradation, and phagocytic clearance of clot-associated debris (9).
From studies with mouse models (Huber-Lang et al., 2006, Hoth et al., 2014, Khan et al., 2013, Auger et al., 2012, Zecher et al., 2014, Borkowska et al., 2014), several groups have concluded that thrombin is the major coagulation enzyme that generates C5a under pathologic conditions. This role for thrombin was supported by observations that thrombin generates C5 in vitro (Huber-Lang et al., 2006), and inhibition of thrombin dampens severity of disease and reduces C5a levels in murine models of disease (Huber-Lang et al., 2006, Hoth et al., 2014, Khan et al., 2013, Auger et al., 2012, Zecher et al., 2014, Borkowska et al., 2014). How do we reconcile these findings with the fact that the residues flanking the R751 C5 convertase cleavage site necessary to generate C5a lack similarity to thrombin cleavage sites in all other classic thrombin substrates (e.g., protein C, PAR1, fibrinogen, factor V, factor VIII) (Krisinger et al., 2012), and that thrombin is an inefficient cutter of C5 at that site (Krisinger et al., 2012)?
That thrombin participates in C5a generation during coagulation is not challenged by the present findings. However, this reaction likely does not occur via direct C5 cleavage. Indeed, in our experiments, with thrombin concentrations that more closely approximate the dynamics of thrombin generation in plasma and blood (Brummel et al., 2002, Dielis et al., 2008), C5a could not be measured. Moreover, the C5T product that is generated by thrombin-mediated cleavage of C5 at the thrombin-sensitive R947 site (Krisinger et al., 2012), does not exhibit C5a-like chemotactic/migration properties in vitro (data not shown). The present studies are consistent with the concept that thrombin contributes to C5a generation, but indirectly via plasmin-mediated events. Thrombin is fundamentally important for the initiation of fibrinolysis since it generates fibrin, an important cofactor for tPA-mediated plasmin generation. Thrombin further amplifies plasmin generation by inducing endothelial secretion of tPA and expression of urokinase-type plasminogen activator (van den Eijnden-Schrauwen et al., 1995). Given the relative kinetics of thrombin versus plasmin in generating C5a, it is unlikely that thrombin, alone, generates C5a. Rather, our data support the premise that, in combination with C5 convertase, plasmin (and/or downstream proteolytic effectors of plasmin) is the major mediator, and that the amount of thrombin only affects C5a levels insofar as thrombin affects the kinetics of fibrinolysis/plasmin generation. The caveat to this premise is that very high thrombin concentrations that may transiently accumulate during thrombus formation (Brummel-Ziedins et al., 2005) could cleave C5 at R751 to generate C5a (Krisinger et al., 2012). Interestingly, reports of a role for thrombin in generating C5a come from studies with mice lacking C3 (Huber-Lang et al., 2006, Khan et al., 2013, Auger et al., 2012, Borkowska et al., 2014). These mice, for unexplained reasons, have elevated levels of prothrombin (Huber-Lang et al., 2006) which can result in increased generation of thrombin following activation of coagulation (Aleman et al., 2013, Kyrle et al., 1998). It is reasonable to consider that the high thrombin levels reached in C3-deficient mice might contribute to C5a generation. However, with excess thrombin and fibrin deposition, plasmin generation would also be markedly increased, and plasmin would be significantly favored over thrombin in generating C5a from C5. Indeed, this apparent paradox of heightened plasmin generation with a larger thrombus is in line with our observation that IVC clot weights strongly correlated with C5a levels. By interfering with deposition of fibrin, a critical cofactor for plasmin generation, thrombin inhibition would reduce C5a generation. Overall, thrombin is important in C5a generation, but indirectly, via enhanced production of plasmin. These findings are consistent with, and indeed, extend previous in vitro studies in which high concentrations of plasmin cleaved C5 (Amara et al., 2010). Attempts to measure murine levels of plasma plasmin–antiplasmin complexes were confounded by a lack of reliable assays. However, in vivo gain-of-function studies using pharmacologic-intervention (Tenecteplase infusion), allow us to conclude that plasmin plays a physiologically relevant role in the generation of C5a.
Although plasmin generation is increased acutely and to a lesser extent, chronically, in patients with thrombotic disorders (Wada et al., 1989), the role of plasmin in complement-mediated events during thrombus formation, propagation, and resolution is unknown. Our finding that plasmin drives C5a generation in mouse models of thrombosis exposes plasmin and/or its downstream effectors as potential therapeutic targets for limiting production of procoagulant and pro-inflammatory effectors. These insights are likely to be applicable to thrombi in arteries and veins, in spite of the differences with regard to etiology of these presentations. Indeed, and most notably, both presentations involve activation of inflammatory pathways and liberation of fibrinolytic activity in response to the thrombus (Engelmann and Massberg, 2013, Wolberg et al., 2012). The impact of any intervention would likely be dependent on its timing in relation to thrombus formation and resolution (Fig. 5). Thus, early dampening of C5a and MAC assembly may thwart leukocyte accumulation and thrombus initiation and propagation. Later interventions may hinder resolution of the clot, but may also reduce long-term inflammatory sequelae of thrombosis, such as occurs in post-thrombotic syndrome. Intuitively, administering agents to suppress fibrinolysis during a thrombotic event at any stage may be unappealing, and thus an optimal intervention strategy/agent might preserve fibrinolysis while restricting C5 activation. Direct blocking of C5 activation is highly efficacious in preventing complement-mediated thrombosis in atypical hemolytic uremic syndrome and paroxysmal nocturnal hemoglobinuria (Wong and Kavanagh, 2015). Similar successes were not, however, observed for acute myocardial infarction (APEX AMI Investigators et al., 2007), which might be due to the fact that therapy was initiated early at presentation. The variable responses in these reports underline the need for further study, using models that represent different vascular disorders.
Contributors
JHF, ASW and EMC designed experiments, analyzed data and wrote the manuscript and managed the project. JHF, MMA, BLW, AMO, VL and MH performed experiments. KAF analyzed data and edited the manuscript.
Declaration of Interests
We declare no competing interests.
Ethical Research Conduct
All aspects of the work covered in this manuscript were conducted with the ethical approval of all relevant bodies and these are acknowledged within the manuscript.
Role of the Funding Sources
None of the funders had any input into the design of the experiments, the collection, analysis or interpretation of the data, the writing of the manuscript, or the decision to submit it for publication.
Acknowledgments
JHF was supported by a Banting Fellowship. EMC is supported by operating grants from the Canadian Institutes of Health Research (CIHR), the Natural Sciences and Engineering Research Council of Canada (NSERC), and the Canada Foundations for Innovation (CFI). He holds a CSL Behring Research Chair and a Tier 1 Canada Research Chair in Endothelial Cell Biology and is an Adjunct Scientist with the Canadian Blood Services. ASW was supported by funding from the National Institutes of Health (R01HL094740 and R56HL094740). BLW was supported by a training grant to the University of North Carolina (T32HL069768) and MMA was supported by NIH grant F31HL112608. KAF was supported by the Queen's University Terry Fox Foundation Training Program in Transdisciplinary Cancer Research in partnership with the CIHR. Partial funding was provided by Biogen. We thank Drs. Amit Nathwani and Jay L. Degen for critically reviewing the manuscript.
References
- Aleman M.M., Walton B.L., Byrnes J.R. Elevated prothrombin promotes venous, but not arterial, thrombosis in mice. Arterioscler. Thromb. Vasc. Biol. 2013;33(8):1829–1836. doi: 10.1161/ATVBAHA.113.301607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara U., Flierl M.A., Rittirsch D. Molecular intercommunication between the complement and coagulation systems. J. Immunol. 2010;185(9):5628–5636. doi: 10.4049/jimmunol.0903678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APEX AMI Investigators, Armstrong P.W., Granger C.B. Pexelizumab for acute ST-elevation myocardial infarction in patients undergoing primary percutaneous coronary intervention: a randomized controlled trial. JAMA. 2007;297(1):43–51. doi: 10.1001/jama.297.1.43. [DOI] [PubMed] [Google Scholar]
- Auger J.L., Haasken S., Binstadt B.A. Autoantibody-mediated arthritis in the absence of C3 and activating Fcgamma receptors: C5 is activated by the coagulation cascade. Arthritis Res. Ther. 2012;14(6):R269. doi: 10.1186/ar4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthel D., Schindler S., Zipfel P.F. Plasminogen is a complement inhibitor. J. Biol. Chem. 2012;287(22):18831–18842. doi: 10.1074/jbc.M111.323287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett W.R., Yawn D.H., Migliore P.J. Activation of the complement system by recombinant tissue plasminogen activator. J. Am. Coll. Cardiol. 1987;10(3):627–632. doi: 10.1016/s0735-1097(87)80206-1. [DOI] [PubMed] [Google Scholar]
- Borkowska S., Suszynska M., Mierzejewska K. Novel evidence that crosstalk between the complement, coagulation, and fibrinolysis proteolytic cascades is involved in mobilization of hematopoietic stem/progenitor cells (HSPCs) Leukemia. 2014 doi: 10.1038/leu.2014.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummel K.E., Paradis S.G., Butenas S., Mann K.G. Thrombin functions during tissue factor-induced blood coagulation. Blood. 2002;100(1):148–152. doi: 10.1182/blood.v100.1.148. [DOI] [PubMed] [Google Scholar]
- Brummel-Ziedins K.E., Vossen C.Y., Butenas S., Mann K.G., Rosendaal F.R. Thrombin generation profiles in deep venous thrombosis. J. Thromb. Haemost. 2005;3(11):2497–2505. doi: 10.1111/j.1538-7836.2005.01584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung A.K., Faezi-Jenkin B., Leypoldt J.K. Effect of thrombosis on complement activation and neutrophil degranulation during in vitro hemodialysis. J. Am. Soc. Nephrol. 1994;5(1):110–115. doi: 10.1681/ASN.V51110. [DOI] [PubMed] [Google Scholar]
- Dielis A.W., Castoldi E., Spronk H.M. Coagulation factors and the protein C system as determinants of thrombin generation in a normal population. J. Thromb. Haemost. 2008;6(1):125–131. doi: 10.1111/j.1538-7836.2007.02824.x. [DOI] [PubMed] [Google Scholar]
- Distelmaier K., Adlbrecht C., Jakowitsch J. Local complement activation triggers neutrophil recruitment to the site of thrombus formation in acute myocardial infarction. Thromb. Haemost. 2009;102(3):564–572. doi: 10.1160/TH09-02-0103. [DOI] [PubMed] [Google Scholar]
- Engelmann B., Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 2013;13(1):34–45. doi: 10.1038/nri3345. [DOI] [PubMed] [Google Scholar]
- Fuchs T.A., Brill A., Wagner D.D. Neutrophil extracellular trap (NET) impact on deep vein thrombosis. Arterioscler. Thromb. Vasc. Biol. 2012;32(8):1777–1783. doi: 10.1161/ATVBAHA.111.242859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton K.K., Hattori R., Esmon C.T., Sims P.J. Complement proteins C5b-9 induce vesiculation of the endothelial plasma membrane and expose catalytic surface for assembly of the prothrombinase enzyme complex. J. Biol. Chem. 1990;265(7):3809–3814. [PubMed] [Google Scholar]
- Horrevoets A.J., Pannekoek H., Nesheim M.E. A steady-state template model that describes the kinetics of fibrin-stimulated [Glu1]- and [Lys78]plasminogen activation by native tissue-type plasminogen activator and variants that lack either the finger or kringle-2 domain. J. Biol. Chem. 1997;272(4):2183–2191. doi: 10.1074/jbc.272.4.2183. [DOI] [PubMed] [Google Scholar]
- Hoth J.J., Wells J.D., Jones S.E., Yoza B.K., McCall C.E. Complement mediates a primed inflammatory response after traumatic lung injury. J. Trauma Acute Care Surg. 2014;76(3):601–608. doi: 10.1097/TA.0000000000000129. (discussion 8-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes J.M., Richardson V.R., Smith K.A. Complement C3 is a novel plasma clot component with anti-fibrinolytic properties. Diab. Vasc. Dis. Res. 2012;9(3):216–225. doi: 10.1177/1479164111432788. [DOI] [PubMed] [Google Scholar]
- Huber-Lang M., Sarma J.V., Zetoune F.S. Generation of C5a in the absence of C3: a new complement activation pathway. Nat. Med. 2006;12(6):682–687. doi: 10.1038/nm1419. [DOI] [PubMed] [Google Scholar]
- Kessel C., Nandakumar K.S., Peters F.B., Gauba V., Schultz P.G., Holmdahl R. A single functional group substitution in c5a breaks B cell and T cell tolerance and protects against experimental arthritis. Arthritis Rheum. 2014;66(3):610–621. doi: 10.1002/art.38237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M.A., Maasch C., Vater A. Targeting complement component 5a promotes vascular integrity and limits airway remodeling. Proc. Natl. Acad. Sci. U. S. A. 2013;110(15):6061–6066. doi: 10.1073/pnas.1217991110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisinger M.J., Goebeler V., Lu Z. Thrombin generates previously unidentified C5 products that support the terminal complement activation pathway. Blood. 2012;120(8):1717–1725. doi: 10.1182/blood-2012-02-412080. [DOI] [PubMed] [Google Scholar]
- Kyrle P.A., Mannhalter C., Beguin S. Clinical studies and thrombin generation in patients homozygous or heterozygous for the G20210A mutation in the prothrombin gene. Arterioscler. Thromb. Vasc. Biol. 1998;18(8):1287–1291. doi: 10.1161/01.atv.18.8.1287. [DOI] [PubMed] [Google Scholar]
- Langer F., Spath B., Fischer C. Rapid activation of monocyte tissue factor by antithymocyte globulin is dependent on complement and protein disulfide isomerase. Blood. 2013;121(12):2324–2335. doi: 10.1182/blood-2012-10-460493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machlus K.R., Cardenas J.C., Church F.C., Wolberg A.S. Causal relationship between hyperfibrinogenemia, thrombosis, and resistance to thrombolysis in mice. Blood. 2011;117(18):4953–4963. doi: 10.1182/blood-2010-11-316885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machlus K.R., Lin F.C., Wolberg A.S. Procoagulant activity induced by vascular injury determines contribution of elevated factor VIII to thrombosis and thrombus stability in mice. Blood. 2011;118(14):3960–3968. doi: 10.1182/blood-2011-06-362814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D., Benjamin E.J., Go A.S. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- Oikonomopoulou K., Ricklin D., Ward P.A., Lambris J.D. Interactions between coagulation and complement–their role in inflammation. Semin. Immunopathol. 2012;34(1):151–165. doi: 10.1007/s00281-011-0280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierangeli S.S., Girardi G., Vega-Ostertag M., Liu X., Espinola R.G., Salmon J. Requirement of activation of complement C3 and C5 for antiphospholipid antibody-mediated thrombophilia. Arthritis Rheum. 2005;52(7):2120–2124. doi: 10.1002/art.21157. [DOI] [PubMed] [Google Scholar]
- Raskob G.E., Angchaisuksiri P., Blanco A.N. Thrombosis: a major contributor to global disease burden. Arterioscler. Thromb. Vasc. Biol. 2014;34(11):2363–2371. doi: 10.1161/ATVBAHA.114.304488. [DOI] [PubMed] [Google Scholar]
- Rawal N., Pangburn M.K. C5 convertase of the alternative pathway of complement. Kinetic analysis of the free and surface-bound forms of the enzyme. J. Biol. Chem. 1998;273(27):16828–16835. doi: 10.1074/jbc.273.27.16828. [DOI] [PubMed] [Google Scholar]
- Rawal N., Pangburn M. Formation of high-affinity C5 convertases of the alternative pathway of complement. J. Immunol. 2001;166(4):2635–2642. doi: 10.4049/jimmunol.166.4.2635. [DOI] [PubMed] [Google Scholar]
- Ricklin D., Hajishengallis G., Yang K., Lambris J.D. Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 2010;11(9):785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon J.E., Girardi G., Holers V.M. Complement activation as a mediator of antiphospholipid antibody induced pregnancy loss and thrombosis. Ann. Rheum. Dis. 2002;61(Suppl. 2):ii46–ii50. doi: 10.1136/ard.61.suppl_2.ii46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims P.J., Faioni E.M., Wiedmer T., Shattil S.J. Complement proteins C5b-9 cause release of membrane vesicles from the platelet surface that are enriched in the membrane receptor for coagulation factor Va and express prothrombinase activity. J. Biol. Chem. 1988;263(34):18205–18212. [PubMed] [Google Scholar]
- van den Eijnden-Schrauwen Y., Kooistra T., de Vries R.E., Emeis J.J. Studies on the acute release of tissue-type plasminogen activator from human endothelial cells in vitro and in rats in vivo: evidence for a dynamic storage pool. Blood. 1995;85(12):3510–3517. [PubMed] [Google Scholar]
- von Bruhl M.L., Stark K., Steinhart A. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J. Exp. Med. 2012;209(4):819–835. doi: 10.1084/jem.20112322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada K., Takahashi H., Tatewaki W., Takizawa S., Shibata A. Plasmin-a2-plasmin inhibitor complex in plasma of patients with thromboembolic diseases. Thromb. Res. 1989;56(6):661–665. doi: 10.1016/0049-3848(89)90283-1. [DOI] [PubMed] [Google Scholar]
- Wakefield T.W., Myers D.D., Henke P.K. Mechanisms of venous thrombosis and resolution. Arterioscler. Thromb. Vasc. Biol. 2008;28(3):387–391. doi: 10.1161/ATVBAHA.108.162289. [DOI] [PubMed] [Google Scholar]
- Wat J., Foley J.H., Krisinger M.J. Polyphosphate suppresses complement via the terminal pathway. Blood. 2014;123(5):768–776. doi: 10.1182/blood-2013-07-515726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedmer T., Esmon C.T., Sims P.J. Complement proteins C5b-9 stimulate procoagulant activity through platelet prothrombinase. Blood. 1986;68(4):875–880. [PubMed] [Google Scholar]
- Wiggins R.C., Giclas P.C., Henson P.M. Chemotactic activity generated from the fifth component of complement by plasma kallikrein of the rabbit. J. Exp. Med. 1981;153(6):1391–1404. doi: 10.1084/jem.153.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolberg A.S., Aleman M.M., Leiderman K., Machlus K.R. Procoagulant activity in hemostasis and thrombosis: Virchow's triad revisited. Anesth. Analg. 2012;114(2):275–285. doi: 10.1213/ANE.0b013e31823a088c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong E.K., Kavanagh D. Anticomplement C5 therapy with eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome. Transl. Res. 2015;165(2):306–320. doi: 10.1016/j.trsl.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Yan C., Gao H. New insights for C5a and C5a receptors in sepsis. Front. Immunol. 2012;3:368. doi: 10.3389/fimmu.2012.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecher D., Cumpelik A., Schifferli J.A. Erythrocyte-derived microvesicles amplify systemic inflammation by thrombin-dependent activation of complement. Arterioscler. Thromb. Vasc. Biol. 2014;34(2):313–320. doi: 10.1161/ATVBAHA.113.302378. [DOI] [PubMed] [Google Scholar]