Abstract
Inland waters cover less than one percent of Earth’s surface, but harbor more than six percent of all insect species: nearly 100,000 species from 12 orders spend one or more life stages in freshwater. Little is known about how this remarkable diversity arose, although allopatric speciation and ecological adaptation are thought to be primary mechanisms. Freshwater habitats are exceptionally susceptible to environmental change, and exhibit marked ecological gradients. The amphibiotic lifestyles of aquatic insects result in complex contributions of extinction and allopatric and non-allopatric speciation in species diversification. In contrast to the lack of evolutionary studies, the ecology and habitat preferences of aquatic insects have been intensively studied, in part because of their widespread use as bio-indicators. The combination of phylogenetics with the extensive ecological data provides a promising avenue for future research, making aquatic insects highly suitable models for the study of ecological diversification.
Keywords: aquatic habitats, ecology, phylogenetics, adaptation, speciation
INTRODUCTION
Inland waters cover less than 1% of the Earth’s surface, yet harbor 10% of all known animal species. Of this diversity, over 60% is found in the aquatic insects, which today number close to 100,000 described species (11; Table 1). This is probably an underestimate, and with the taxonomic deficit being skewed toward the insects, we estimate that aquatic insects may well number > 200,000 and thereby make up 80% of aquatic animal diversity. Aquatic insects spend one or more stages of their life cycle in the water, with the majority moving to terrestrial habitats as adults. They play important ecological roles in both realms as primary consumers, detritivores, predators, and pollinators. The ecology of many groups is well studied, owing to their roles as bio-indicators or disease vectors, but freshwaters have been largely overlooked as a hotbed of diversification, despite their disproportionate contribution to global biodiversity. A recent review (74) explored the question of why there are so many insect species, but included very few aquatic examples. The investigation of aquatic insects is therefore timely, with freshwater habitats being widely recognized as the most threatened on Earth (131).
Table 1. The major aquatic insect lineages, their character, and their value for diversification research.
| radiation a | species b | tax c | lot d | diversity and ecology | diversification research potential | |
|---|---|---|---|---|---|---|
| Ephemeroptera (mayflies) | 3,046 | + | + | Rivals Plecoptera as 3rd largest
purely aquatic order (15). Differs notably from sister-group Odonata in that larvae feed mainly on algae and fine detritus and are morphologically diverse; adults do not feed, emerge synchronously, live for a short time, disperse poorly and are morphologically uniform. |
Comparatively well studied taxonomically; few
species-level phylogenies available. Palaeoptera provide interesting comparison of two ecologically very different radiations. Suitable for studying the role of life history in diversification, e.g. parthenogenesis and temporal isolation through reproductive synchronicity. |
|
| Odonata (dragon- & damselflies) | 5,952 | ++ | − | 2nd largest purely aquatic order;
4th largest aquatic insect radiation (61). All species are predators due
to excellent sight, adult flight and extrudable mouthparts of
larva. Broad range of dispersal capacities rivaled only by some Coleoptera. Most visible sexual behavior among aquatic insects. |
Best-researched group relative to species
numbers, with most work on sexual selection and conflict by
sperm-displacement (secondary genitalia), male visual courtship displays
(often with colored wings and body) and female color
forms. Taxonomy and distribution best known of any aquatic group. Only insects with global overview of species’ threat status (26). |
|
| Heteroptera | Nepomorpha (water bugs) | 2,404 | ++ | − | Two radiations, both largely
predatory, in the otherwise strictly terrestrial order Hemiptera: in
contrast to complex invasion history and extreme ecological diversity of
Diptera and Coleoptera, All life stages bound to water. Unlike most freshwater insects, ecology of larvae and adults notably similar and with a high frequency of flightless forms in adults (5). Gerromorpha were main animal group to invade freshwater surface. |
Moderately well studied, but the
potential to study sexual conflict in groups with sexual dimorphism
remains unexploited (6). Good potential for studying historical biogeography: despite many lentic species, allopatric diversification is thought to predominate due to hololimnic life cycle and low dispersal, which also offers unique potential for radiation in old lakes (100). |
| Gerromorpha (water striders) | 2,021 | + | − | |||
| Plecoptera (stoneflies) | 3,497 | − | ++ | 3rd largest purely aquatic order, most notable coldwater radiation with distinct northern and southern hemisphere radiations (42). | Very underworked. Ecologically sensitive and relatively uniform, with limited dispersal: mostly allopatric speciation, group suitable mainly for studies of historical biogeography, but also for temporal isolation. | |
| Diptera (flies) | Culicomorpha (mosquitoes, midges, black flies) | 19,618 | − | − | Only mainly terrestrial order with
large freshwater proportion, including 1st, 2nd
and 5th aquatic insect radiations (but see
Trichoptera). With >20 freshwater invasions up to 50% of aquatic insects may be Diptera and 30% of Diptera aquatic. Greatest ecological diversity and flexibility of any aquatic order. However, species’ ecologies often unknown (most notably in Tipulomorpha) and vast majority may live in moist substrates rather than in water (133). Specific pre-adaptation for multiple freshwater invasions and radiations unclear, but likely related to exceptionally diverse functional morphology, physiological adaptability (e.g. to extreme chemical and physical environments) and numerous feeding modes. |
A large body of work focuses on
disease vectors and medical applications, e.g. Simuliidae and Culicidae:
excellent process work on Anopheles gambiae (see
Sidebar), but notable lack of studies on patterns. Strong correlation of
aquatic larvae with blood-sucking adults, perhaps due to pre-adapted
mouthparts and host concentration near water. Research on most families hampered by lack of taxonomic and ecological knowledge. Only reasonably studied non-vectors are Chironomidae, which dominate aquatic communities in individual and species numbers, show extreme ecological diversity, e.g. survive from 5600 m height to 1000 m depth and −20°C in air to 40° in water; 7 day to 7 year life cycles; marine and Antarctic species (39). Often preserved as subfossils, making them an ideal group for geographic and diversification research (101). |
| Tipulomorpha (crane flies) | 15,770 | − − | ? | |||
| Tabanomorpha (horse flies etc.) | 5,373 | ? | ? | |||
| Psychodomorpha (moth flies etc.) | 3,412 | ? | ? | |||
| Ephydridae (shore flies) | 1,994 | ? | ? | |||
| Trichoptera (caddisflies) | 14,291 | − − | + | Largest purely aquatic order, currently
3rd most diverse taxonomically, but may be the largest
aquatic insect radiation with up to 50,000 species (33). Great diversity due to micro-habitat specialization, full array of feeding modes (probably second only to Diptera) facilitated by great diversity in silk-spinning strategies and case construction, and relatively low dispersal. |
Very underworked, especially relative to
species richness, as only 25% of species may be described. Few species
level phylogenies. Well-known sister-group Lepidoptera offers good aquatic/terrestrial comparison. Adaptive significance of case-building and feeding behavior only poorly studied at high taxonomic resolution, though both likely present key innovations. |
|
| Megaloptera (fish-, dobson- & alderflies) | 328 | + | + | Very small purely aquatic order. Relict distribution, mostly in Americas and Asia (28). Monophyly has been contested, but most recently supported (134). | Low species and ecological diversity, and limited distribution, suitable for historical biogeography | |
| Coleoptera (beetles) | Hydradephaga (diving b., whirligigs) | 5,126 | + | − | Largest group of animals on Earth
is 97% terrestrial, with >20 freshwater invasions, but diverse
life histories hard to define, aquatic and terrestrial behavior merge at
shoreline. Sealed ‘air tank’ under elytra was major pre-adaptation for frequent and flexible invasion of freshwater: individuals could ‘reinvade’ freshwater daily. Only 8% of aquatic species have a typically amphibiotic life cycle (submerged larva, shorter-lived terrestrial adult) as most groups aquatic in all life stages, some with terrestrial larva and aquatic adult (56). |
Prominent in the literature,
perhaps second only to Odonata, with most work on diversification of any
aquatic group. Much focus on habitat stability (105), providing good comparisons with strongly
lotic (Gyrinidae, Elmidae), lentic and dispersive (Dytiscoidea) or
specialized groups (Hydraenidae), as well as one of the best recent
fossil records (1, 40). Ecology often linked to notable key innovations like adult surface dwelling (Gyrinidae), swimming by simultaneous stroke of adult middle and hind legs (Dytiscidae), and antimicrobial exocrine secretion (Hydraenidae). |
| Hydrophyloidea (water scavenger b.) | 2,205 | + | − | |||
| Scirticidae (marsh b.) | 1,330 | − | − | |||
| Hydraenidae (minute moss b.) | 1,380 | − | + | |||
| Elmidae (riffle b.) | 900 | + | ++ | |||
Only groups of >300 species resulting from single freshwater invasion are shown; Culicomorpha and Psychodomorpha probably form one lineage of over 23,000 known species (137), as do Ephemeroptera and Odonata (Palaeoptera) of over 9,000 (126). Many smaller or partly aquatic groups are excluded, all in mostly terrestrial orders, thus only seventeen of over fifty aquatic invasions are considered. Groups tied closely to freshwater but lacking aquatic life stages are also excluded, e.g. Leptopodomorpha (shore bugs). The list of included Diptera is tentative, because the number of actually aquatic species (and thus separate invasions) is unclear. Other families with hundreds and possibly thousands of aquatic species are Dolichopodidae, Muscidae, Stratiomyidae and Syrphidae.
Numbers of described species taken from stated sources and updates (50, 92). Major (>300 species) constituents of Culicomorpha are Chironomidae (7,290), Ceratopogonidae (5,902), Culicidae (3,725) and Simuliidae (2,121); of Tipulomorpha are Limoniidae (10,777), Tipulidae (4,415) and Pediciidae (496); of Tabanomorpha are Tabanidae (4,434) and Rhagionidae (756); of Psychodomorpha are Psychodidae (3,026) and Blephariceridae (331); of Hydradephaga are Dytiscidae (3,908) and Gyrinidae (750); of Hydrophyloidea is Hydrophylidae (1,800).
State of taxonomy is inferred from estimates of species described: under 30% (−−), and over 50% (−), 70% (+) or 80% (++), or unknown (?) and likely very low.
Proportion of lotic species is based on estimates from North America and Europe: almost all species strictly lotic (++), most lotic but good number lentic (+), >25% lentic (−), or knowledge deficient but many edge species and lotic/lentic distinction often unclear (?).
The fossil record suggests that all aquatic insect groups are the result of the invasion of freshwater by terrestrial groups (140). Although belonging to twelve orders, aquatic insects may represent more than fifty separate invasions (Table 1). Ephemeroptera, Odonata, Plecoptera, Trichoptera and Megaloptera are almost entirely aquatic and make up over 27,000 known species, over half of them Trichoptera. The remaining diversity includes over 10% of the hemipteran suborder Heteroptera, about 30% of Diptera, around 3% of Coleoptera, and very small proportions of Hymenoptera, Lepidoptera, Neuroptera and Orthoptera. Diptera are by far the largest group, containing nearly half of all aquatic insects. All major orders are cosmopolitan, with the notable exception of the Megaloptera, and have 50-75% of known species in the tropics, except Plecoptera with 65% Holarctic species (11). Freshwaters are highly diverse and include ponds, lakes, springs, streams, rivers, wetlands, reservoirs and ditches. The transition to freshwaters demanded adaptation in mechanisms of thermo- and osmo-regulation, respiration, feeding and locomotion. Among the most notable characteristics of freshwaters are their more stable daily and seasonal temperatures compared with air and soil. Freshwaters occupy a low position on the landscape where they accumulate nutrients and detritus. Aquatic autotrophs are smaller (often unicellular), grow faster, and have a higher nutritional quality than land plants. Aquatic habitats also exhibit marked spatio-temporal gradients of connectivity and permanence, ranging from stable to dynamic, and insular to connected: for example seasonal precipitation makes some habitats temporarily dry, turns small streams into large rivers, or reconnects previously separated wetlands. This heterogeneity is important to freshwater biodiversity because of the variety of life histories and ecological roles it enables. Because of their shape and size, freshwater systems have a large interface with adjacent terrestrial habitats. We estimate that over 70% of aquatic animal species including most insects have complex life cycles, providing great adaptability and the potential to disperse outside water. It is this potential to adjust and disperse, to access opportunity and evade extinction, that sets insects apart from less diverse life forms (74) and separates the freshwater majority from better-studied minorities like fish.
Here we review the important contributions to our understanding of aquatic insect diversification (see glossary). Our emphasis is on phylogenetic studies that infer processes that led to species diversification. Where these are lacking, we refer to studies of population divergence within species that suggest relevant mechanisms. Our arrangement of these mechanisms into discrete sections oversimplifies the diversification process, because mechanisms are not mutually exclusive and many studies address multiple factors. Our synthesis suggests that we have a good knowledge of general patterns of diversity, but that few studies explicitly investigate the processes responsible.
GEOGRAPHICAL DIVERSIFICATION
Habitat stability, dispersal and diversification
Freshwater habitats can be broadly grouped into running (lotic) and standing (lentic) water bodies and the majority of aquatic species is restricted to one or the other. The relatively short geological lifespan of standing waters make dispersal (see glossary) necessary for lentic species, while running water habitats are more geologically stable and therefore lotic species may be less dispersive (105). Indeed, lentic dragonflies and beetles have larger ranges than lotic species (2, 49) and dispersal ability rather than ecological tolerance accounts for this (10, 29). The contrasting persistence of habitats is likely to have implications for lineage diversification. Low dispersal may increase speciation through allopatry in stable habitats and indeed lotic insects demonstrate more genetic differentiation than lentic species (72, 91). However, smaller population sizes and immobility can also increase extinction (108). The net result is that greater species turnover is expected in lotic clades. The only explicit test of habitat stability on diversification showed no significant difference in beetle species turnover between lentic Ilybius and lotic Deronectes (106). This equivocal result may be because diversification operates at different spatial scales in the two groups with investigation of lentic taxa requiring more widespread sampling.
Homogenizing gene flow under widespread dispersal should suppress allopatric divergence, as supported by morphological stasis in the fossil record during periods of instability (117). Dispersal can also reduce extinction because of large population sizes and an ability to track environmental changes. Indeed, most African Odonata threatened with extinction are lotic (25). Dispersal also allows occupation of new habitat: lentic mayflies diverged after dispersal between Africa and Madagascar (83) and Megalagrion damselflies by colonizing new islands in Hawaii (59). Similar isolation by distance will occur in continental habitat refuges caused by changing climates and associated range shifts. Quaternary fossil records of 20% of 259 European water beetle species, especially lentic ones, fall outside their current range (1). The observations imply that freshwater species diverged and survived under very different impacts, timing and scales, and at very different rates of turnover, depending on their habitat and thus dispersal capacity (Figure 1). Nonetheless, net diversification may be similar: we estimate that about 40% of Holarctic species occur mainly in standing water, including over half the Odonata, Heteroptera and Coleoptera, as well as probably Diptera. The strictly lotic proportion is higher in the tropics, although 37% of African Odonata is not (25). Currently we lack quantitative data at appropriate scales to test these ideas. Also, habitat stability and dispersal ability are not truly dichotomous, but vary gradually. Aside from (but often as a result of) their stability, lotic and lentic systems differ in many other ways (structure, distribution, connectivity, chemistry, microclimate, seasonality, biotic interactions) with great potential for ecological diversification.
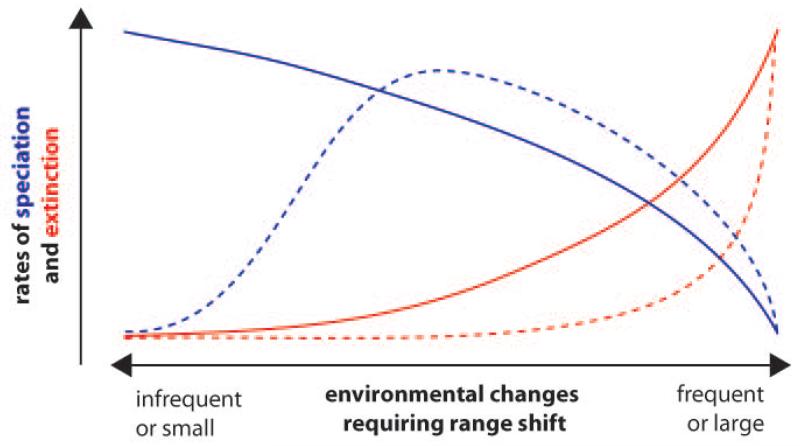
Figure 1. The habitat stability hypothesis of freshwater diversification.
The plot indicates speciation (blue) and extinction rates (red) under environmental change for immobile (solid) and highly mobile (dashed) groups. Note that because of the lower extinction rate, diversification is not necessarily highest when speciation is maximal in the mobile groups.
Environment and allopatry
The extensive population genetics literature on stream insects finds repeated evidence for intraspecific differentiation, demonstrating the potential for allopatric speciation. Differentiation is typically associated with restricted overland dispersal between mountain regions (51). Finer-scale differentiation between (sub-)catchments within mountain ranges is less prominent but also important (41). For example, surrounding forest probably impaired dispersal in the mayfly Ephemerella invaria (3), but facilitated it in the caddisfly Orthopsyche fimbriata (118). The mayfly Andesiops torrens and caddisfly Smicridea annulicornis differentiated within catchments as they are adapted to avoid drift in torrential streams (112), while the mayfly Baetis alpinus diverged across lakes in valleys that were ice-free since the Holocene (84).
Allopatric diversification under these conditions predicts parallel radiations of (largely) non-overlapping species that are ecologically similar. Examples are six simultaneous splits of New Zealand stoneflies by glaciations (75), the retreat into aquifers of Australian aquatic beetles with desertification (66) and intra- and interspecific diversification of European headwater caddisflies following Pleistocene range regression and expansion (95, 103). The Australian midge Echinocladius martini and European stonefly Arcynopteryx dichroa underwent strong allopatric processes in upland refuges, in response to drying and cooling climates respectively (62, 125). Other examples are parallel radiations of Hydropsyche caddisflies in upper, middle and lower stream reaches (87) and Hydraena beetle diversification following expansion and geographic fragmentation (107), both in the western Mediterranean. Ecological divergence in allopatry allows new species to remain segregated once barriers disappear: three lineages in the beetle Ochthebius glaber possess distinct environmental niches, suggesting this is in progress (114).
While geographic isolation under low dispersal is easily demonstrated, ongoing dispersal will obscure allopatric patterns in mobile groups. Dispersal to new habitat, divergence in isolation, and survival of sister species after re-expansion, predicts that recent sister taxa are allopatric and often ecologically similar. The radiation of Trithemis dragonflies into forty African species probably began in open temporary pools, with peak diversification occurring when forest expansion separated these populations (30). As open landscapes coalesced thereafter, species of those habitats expanded into huge largely overlapping ranges. These lineages thus barely radiated further, but three ecologically more constrained lineages (in cool, flowing and swampy habitats respectively) produced over half of the species, probably in allopatry. More evolutionary shifts occurred to forest and running waters than to open and standing ones, confirming Ribera’s (105) predictions that lentic species would be older and lotic species have a greater tendency to specialize and are less likely to revert to lentic habitat. In this scenario, overlapping sister species should show evidence of recent expansion and/or ecological segregation. Also, genetic differentiation is expected within species across current ecological barriers. Unfortunately, the few phylogeographic studies on lentic taxa at appropriate scales focus on migratory species (43). Despite strong differentiation of Anopheles scanloni mosquitoes in habitat islands, crossing experiments demonstrated that speciation had not yet occurred (89).
ECOLOGICAL DIVERSIFICATION
Ecotones and habitat gradients
The linearity of stream systems provides a downstream succession of environmental conditions and communities, which could promote parapatric diversification (120). Illies (53) suggested that warm-adapted lineages of aquatic insects arose from cold-adapted ones, with evolution within river systems progressing downstream. This was contested by inferences that caddisflies arose in lentic or lotic-depositional habitats (135) and for mayflies, where some Malagasy species appear to have diversified from lowland ancestors to colder and faster-flowing upstream sections (132). This is similar to the upstream invasion and ecological diversification suggested for molluscs and fish (53). In net-spinning caddisflies, strong links exist between downstream changes in flow conditions, feeding behavior, and species distribution (4). Mey (79) described an endemic radiation of Hydropsyche on a mountain in Luzon, with ten related species in a succession from headwaters to lower reaches. Statzner & Dolédec (120) examined the distribution of ecological traits and phylogenetic relationships among Hydropsyche species in the Loire River in France. Their data indicate ecological specialization along the gradients (e.g. net-building behavior, net mesh size, respiration range) and provided some support for a headwater ancestor with primarily downstream evolution and progressive environmental adaptation, supporting the idea of environmentally driven parapatric speciation in streams. Habitat segregation between sister species of Epeorus mayflies, one occurring directly upstream from the other, was proposed to be the result of adaptation to colder water in the upstream species (88). In black flies, stream velocity and altitude differ among closely related species in Thailand (102) and river ancestors gave rise to distinct cascade populations and species on Pacific islands, with increased allopatric isolation in cascade habitats subsequently furthering diversification (60).
Thermal clines are an integral characteristic of freshwater habitats, where the mean and variance of temperatures change from source to mouth in streams and with depth in lakes. Extreme temperatures have invoked many adaptations (31, 104) and one of the most diverse groups of aquatic insects, the Chironomidae, exhibit some of the most extreme tolerances (39). Few studies have related thermal adaptation to diversification. Funk et al. (45) linked it to phenology shifts and ecological diversification of three closely related mayfly species. Other studies have linked thermal tolerance, rather inconclusively, to distribution. Water temperature was a poor predictor for species occurrence in Iberian Hydropsyche caddisflies, despite their strong longitudinal succession (87). While Calosi et al. (21) found that thermal tolerance of Deronectes beetles is a better predictor of range size than wing size, suggesting that ranges are determined by tolerance rather than dispersal, Arribas et al. (10) showed that thermal plasticity in Enochrus beetles is greater in lotic than in lentic species, and that wing size is the better predictor.
Shifts into distinct habitats may also invoke diversification. Hawaii's endemic Megalagrion damselflies radiated into all habitats available within islands, from ponds to streams, tree holes, seeps and even exhibit fully terrestrial development (59). The chironomid genus Sergentia comprises five species endemic to Lake Baikal that originated in the rivers feeding into the lake. More recent species inhabit increasingly deeper regions in the lake. The small size of this radiation is linked to the terrestrial adult that may limit the larvae from invading deeper habitats (93). Many water bugs have strictly aquatic life cycles and are thus not bound to shallow or shore habitats. For example, two poorly known endemic naucorid genera in Lake Tanganyika might represent lake radiations (100). Indeed examples of insect diversification in such habitats, so well-known from fish, are rare. Shifts into phytotelmata (small pockets of water held by plants) have received reasonable attention, for example in Aedes mosquitoes on Pacific islands (119) and diving beetles and giant damselflies in the Neotropics (12, 54), but apparently induced relatively minor diversification. The transition between water and land is mainly relevant in those freshwater groups with strongly terrestrial roots, like Diptera. While the initial aquatic invasions may have induced diversification, as in Coleoptera (52), reinvasion of land and proceeding secondary invasions of water appears to have resulted in relatively few new species in hydrophiloid beetles (40), Tetanocera flies (24) and Nothopsyche caddisflies (47). Truly amphibious (versus amphibiotic) larvae that can complete development both above and below water are only known from the Hawaiian Hyposmocoma moths. This habit evolved in parallel at least three times and led to speciation in one of these amphibious clades (110).
Chemical gradients
Water is an effective medium of dissolved chemicals and thus generates many different gradients in freshwater habitats, e.g. oxygen concentration from headwaters to river mouths (see above), or salinity degrees from freshwater to marine. Water striders, for example, invaded marine environments multiple times and diversified in these habitats through behavioral and physiological adaptations (7). Water chemistry is directly influenced by atmospheric conditions, bedrock geology, and biotic interactions, and has thus changed over evolutionary time, potentially affecting aquatic insect diversification. For example, Ivanov & Sukatsheva (55) hypothesized that an increase of foliage debris in freshwaters following the proliferation of angiosperms during the Cretaceous led to eutrophication and oxygen depletion, inducing extinction and large-scale range expansion in Trichoptera.
Two recent studies (20, 22) mapped tolerance to pollution on phylogenies in an attempt to identify the best taxonomic level for bio-indication. In most cases, cadmium uptake and elimination differed consistently among examined families but also among two congeneric mayfly species (20) and within families of Australian midges (22). Differences in pollution tolerance among closely related taxa may indicate ecological differentiation along chemical gradients. Unfortunately, the presence of locally resistant ecotypes may complicate patterns in nature, raising the question how conserved tolerance is over time (85).
A series of studies examined caddisfly diversification in relation to ultramafic geology on New Caledonia (37, 38). Ultramafic rocks lead to high pH values and mineral loads, including heavy metals. Several groups diversified upon adapting to these harsh conditions after arrival on the island. In all three examined groups diversification started on ultramafic rocks, associated with environmentally diverse and fragmented habitats. Subsequent diversification is associated with shifts to non-ultramafic rocks, as these geological layers were exposed. Such shifts are more frequent than the reverse and may reflect high fitness costs associated with persistence in inhospitable conditions. The studies show that diversification of New Caledonian caddisflies is associated with the underlying geology and that taxa retained their potential to persist in both chemical environments. Distinct lineages within the South African mayfly complex Baetis harrisoni probably originated in allopatry, but their continued separation is linked to catchment geology through different pH tolerance (97).
BEHAVIORAL FACTORS
Life History
Surprisingly few studies have related insect life history traits to their diversification (74). The concept of diversification by temporal isolation seems particularly suitable for scrutiny in freshwater insects with synchronized adult emergence, particularly Ephemeroptera and Plecoptera. Although considered important in some herbivorous insects (73), there is little evidence that it is a common mechanism for speciation. Schultheis et al. (115) tested the concept on the semivoltine stonefly Peltoperla tarteri, but found that gene flow occurred between cohorts, probably the result of some individuals increasing or decreasing their development rate so as to switch cohorts. In contrast, a small genetic difference was found between two populations of the damselfly Lestes virens that both emerge in spring but mate in summer and autumn (113). Three sympatric clades of the mayfly Baetis rhodani complex exhibit strong genetic divergence and striking differences in phenology, but temporal isolation probably only acts to restrict gene flow among previously differentiated lineages (68). Other observations suggesting the importance of temporal isolation include offspring of experimentally hybridized caddisflies that had different development rates and emergence periods (69), and two co-occurring Haliplus beetle species that exhibit growth and emergence expected under an avoidance strategy (23).
Some species of mayflies are occasionally parthenogenetic in some populations, while in other species only females are known. Funk et al. (44) studied two sister species where one exhibits both sexual and asexual populations and the other is purely asexual. The two are sympatric and morphologically indistinguishable, but genetic analysis and experimental hybridization shows they are clearly distinct. Speciation probably preceded development of obligate parthenogenesis, but the study shows that such shifts can reinforce isolation and thus promote diversification. The only known parthenogenetic populations of Odonata (Ischnura hastata on the Azores) arose from one recent long-distance dispersal event from North America (67). Although it is not clear where parthenogenesis evolved and whether it forced the extinction of sexual island populations, the parthenogenetic lineage has begun accumulating unique mutations.
Feeding ecology
Dietary specialization in herbivorous terrestrial insects is a strong correlate of diversification though other ecological and geographical factors may also play an important role (82). Co-evolution with angiosperms has not been scrutinized in aquatic insects, but probably plays a minor role in most groups because most larvae feed on algae or detritus and adults do not feed (Ephemeroptera, Trichoptera, Megaloptera) or both are largely predatory (Odonata, many Coleoptera). Carnivorous parasitism is also linked to terrestrial insect diversity, especially in Hymenoptera, but it is apparently a rare habit in freshwaters and its importance is contentious (32). Feeding ecology more generally, however, seems important although empirical evidence is rare. Both Trichoptera and Diptera, which include the largest radiations (Table 1), exhibit exceptional diversity of larval feeding types. In caddisflies this is linked to great diversity in silk-spinning and case-building behavior (70, 135). The latter has also been associated with respiration, prey avoidance, and desiccation protection (138, 141) and may generally have promoted ecological diversification (70). The diversification of a species-rich clade of the subfamily Drusinae of Limnephilidae was linked to the shift from shredding detritus to grazing phytobenthic algae (94), and that of diving beetles to the specialized mandibular sucking channels, which hinder dilution of feeding fluids in aqueous environments (13). Numerous dipteran invasions into freshwater are associated with hematophagy (137), but why blood-feeding adults seem correlated with aquatic larvae and whether it promoted diversification is unclear. Bataille et al. (16) show both habitat and host shifts associated with the Galapagos Islands colonization of Aedes taeniorhynchus, but population differentiation was detected among habitats and not hosts. Diversification in the African Simulium damnosum black fly complex did not reveal clear patterns relating feeding, vector prevalence or habitat preference (63).
Species interactions
Predation and parasitism can influence diversification by divergent selection, especially in lentic habitats as important predators like fish cope poorly with seasonal instability (129). For example, parasitic mite loads and thus potential fitness in the North American damselfly Ischnura verticalis differed by habitat (57). Selection by predation under different visibility (plant densities, transparency) is influenced by water beetles’ size and color (139). Two forms considered incipient species within the mosquito Anopheles gambiae (see Sidebar) out-compete one another in their preferred habitat without predation, but in the presence of a predator the permanent water form has an advantage over that of temporary water in both habitats (46). In a contrasting case, three congeneric dragonfly pairs in Namibia, each with one species in temporary water and one in perennial water, had growth rates correlated with habitat but conserved anti-predator behavior, i.e. in accordance with ancestral habitat (122). Stoks & McPeek (121) described two North American damselfly diversifications, both shaped by changes in anti-predator behavior and growth rates, but filling ecological space by habitat shifts from opposite ends of the pond permanence gradient: Lestes began in temporary ponds with only dragonfly predators, while Enallagma started in lakes with fish. The phylogeny of Chaoborus midges showed multiple shifts between habitats with and without fish, with evasive behavior adjusting each time (17). Plasticity in defensive strategies possibly enabled the Holarctic dragonfly genus Leucorrhinia to diversify in habitats with different types of predators (99). Variable habitat stability and dispersal will affect the intensity of interspecific competition locally too. For example, two genetically close and ecologically identical Enallagma damselfly species can coexist in the mosaic distribution of lentic habitats, thus helping to maintain diversity (19), whereas competitive exclusion among congeners may be structuring populations of montane caddisflies (95).
Sexual selection and conflict
Sexual selection probably had a major impact on insect diversification (74). Among aquatic insects this has been studied best in the Odonata, particularly Calopteryx damselflies, which use their colorful wings in territorial displays. Strong genetic divergence between Swedish C. splendens populations was linked to selection for male wing markings (124). Moreover, aggression of sympatric C. virgo males, which have darker wings, resulted in selection for smaller wing markings in C. splendens (128). Similarly, C. aequabilis has smaller spots in sympatry with the dark-winged C. maculata and while this was considered an example of speciation by reinforcement there was no support for this (86). Despite the general focus on selection for male characters, Wellenreuther et al. (136) showed that gene flow between ecologically dissimilar populations of C. splendens is restricted by males’ preference for immigrant females from populations with similar predation and competition pressures as their own. Also, mating success in C. splendens was lower for immigrant than local males (123), while it is linked to male behavior (perching vs. hovering) in different habitats (shaded vs. sunny) in the neotropical damselfly Protoneura amatoria (64). Furthermore, local variation in genital morphology within Calopteryx species suggest that post-mating sexual selection and sperm competition can reinforce speciation in allopatry (27). Strong phylogenetic variation of complex sperm traits in diving water beetles may have a similar impact (48). Genetic divergence across an altitude gradient in European Agabus beetles was partly attributed to sexual selection on elytra reticulation (36) and a similar potential case of reinforcement was suggested for two parapatric Euphaea damselfly species (65).
Sexual conflict may also induce diversification in insects (8), as can co-evolution of male and female traits (18). Miller (80) reported a single origin of male suckers in diving beetles and five subsequent appearances of anti-sucker sculpturing in females. Similar sexual arms races occur in water striders, e.g. in species of the genera Aquarius (29) and Rheumatobates (109) and even within populations of Gerris incognitus (98). Arnqvist et al. (9) suggested that two female forms of Phoreticovelia bugs may lead to evolutionary divergence: wingless females carry and nourish diminutive males on their backs, while winged forms do not. McPeek et al. (77, 78) inferred that male claspers and corresponding female structures evolved synchronously in Enallagma damselflies and are important for species recognition, but not under sexual selection. However, McPeek & Gavrilets (76) proposed that speciation is promoted by females’ mating preferences and their risk to mate unsuccessfully with closely related species, which applies especially in radiations like Enallagma where many recently derived species coexist (127).
These examples highlight the potential importance of sexual selection and conflict, and also show their complex interaction with external factors, but whether they really increase diversification has not been tested sufficiently. Misof (81) found some support that two possible proxies of sexual selection (sexual dimorphism and large body size) induced higher speciation rates in the odonate suborder Anisoptera. Serrano-Meneses et al. (116) confirmed that proportionately larger males in larger species (Rensch's rule) are linked to territoriality, at least in damselflies. Wing shape is also related to mate-guarding and dispersal behavior in Anisoptera (58) and the shape of Calopteryx hindwings, which have a greater role in displays, evolved faster than forewing shape (90). Thus morphology can be applied as a proxy of sexual behavior, but also dispersal, in diversification studies. Similarly, the evolution of female color forms in damselflies, linked to sexual conflict, requires rigorous phylogenetic testing (130).
SYNTHESIS
With their gradients and contrasts, freshwaters are an extraordinary environment for biodiversity evolution. By evaporating and precipitating, buffering and absorbing, and eroding and depositing, water has created perhaps the most chemically, physically, climatologically and geologically variable of biomes. Inland waters are simultaneously stable and dynamic, and isolated and connected. They form ubiquitous veins, archipelagoes and pulses of life with an endless interface with land, sea and air that insects perpetually straddle. While water is known as the source of life, it is also life’s most endangered home. This diversity and dynamic make freshwaters and their insects excellent models to understand why life is rich: how stability and mobility, gradients and barriers, and adaptations and interactions govern evolution and shape biodiversity. There is tremendous potential for reconstructing species-level phylogenies, integrating ecological knowledge, and studying diversification rates and changing adaptations (Table 1).
Firstly, we need a better understanding of the impact of habitat stability and dispersal ability on diversification (Figure 1). During periods of environmental (e.g. climatic) stasis, speciation across physical barriers will be maximal in immobile groups and negligible in mobile ones. Speciation in mobile groups will increase when environmental change induces range reconfigurations and isolation, but crashes when changes become too great or rapid. Extinction will increase more rapidly with environmental change in less mobile groups. Consequently, allopatric diversification must occur at very different scales and periods for the two ecological extremes: lotic diversification peaks under greatest environmental stasis, while lentic diversification requires substantial change. The model requires further refinement, incorporating the ecological differences correlated with stability and the impact of dispersal on competition. Ribera (105) noted that while species from unstable habitats must be good dispersers, those from stable habitats can be sedentary, but do not have to be. Thus widespread species may arise, seeding diversification pulses when its offspring reverts to specialization in stable habitats: a Eurasian ‘supertramp’ diving beetle that arose in the New Guinean highlands may represent the onset of such an event (14).
Secondly, we must quantify the relative importance of the identified mechanisms and key innovations, as each group diversified very differently (Table 1). The habitat stability model predicts higher species turnover in predominantly lotic groups (e.g. Ephemeroptera, Plecoptera, Trichoptera, Hydraenidae) than in groups with more good dispersers (Odonata, Hydradephaga, Hydrophiloidea). The occupation of diverse microhabitats accommodates more parapatric lineages, increasing diversification in groups that easily invade small and peripheral environments, notably Diptera. By contrast, ecological constraints may have limited diversification in groups largely confined to the water surface (Gerromorpha), cooler habitats (Plecoptera) or a relict range (Megaloptera). Feeding niche diversity allows for more sympatric lineages: while inhibiting the richness of mostly generalist predatory or scavenging groups (Odonata, Gerromorpha, many Coleoptera) this may explain why Diptera and Trichoptera are so rich. Reinforcing factors such as sexual selection, important in Odonata, are probably also relevant to other groups, but remain poorly studied.
SUMMARY POINTS
1. Freshwaters cover < 1% of the Earth’s surface, but > 6% of all insect species spend one or more life stages in aquatic habitats. This diversity occurs across 12 orders and has arisen following > 50 terrestrial invasions of freshwaters and subsequent diversification. The myriad adaptations to life in freshwater comprise a remarkable example of convergence.
2. Diversification is poorly understood, largely because we lack species-level phylogenies suitable for hypothesis testing in most groups. Nonetheless, evidence for allopatry, sexual selection, and ecological diversification into microhabitats and feeding modes suggest these have been critical processes.
3. Aquatic insects provide excellent models for research on diversification. Their habitats exhibit marked spatial and temporal gradients in stability and their amphibiotic lifestyles link strong habitat dependence with response to change via dispersal. This has likely led to varying contributions of extinction as well as allopatric and ecological speciation.
4. The habitat stability hypothesis was developed for macro-ecology and has been successfully applied to predict ecological and population-genetic patterns. How it applies exactly to diversification is not clear, although it should lead to higher speciation and extinction rates (i.e. greater turnover) in stable habitats because the species there are less dispersive. The single test of this hypothesis to date found equivocal evidence.
5. Highly dispersive (mostly lentic) species probably respond better to environmental changes than lotic species. Subsequently lower extinction rates and more frequent and extensive geographic range reconfigurations are probably the main drivers of their diversification. However, genetic isolation may occur more erratically, over larger spatial scales, and be obscured by recolonization patterns, making comparative work difficult.
FUTURE ISSUES
1. The foremost research priorities are to obtain a better understanding of the impact of habitat stability and dispersal ability on freshwater diversification (Figure 1) and to quantify the relative importance of the diversification mechanisms and key innovations in the insect groups (Table 1).
2. The large number of studies of the aquatic insects, stimulated by basic research on lake and stream ecosystems as well as by their use as bio-indicators, has produced an enormous amount of ecological trait data. These are appropriate for studying the evolution of ecological characters in combination with species-level phylogenies.
3. Population-genetic studies should be used to integrate intraspecific and species-level examination of diversity patterns and to infer diversification mechanisms from both historic (phylogeny, trait evolution) and contemporary (dispersal limitation, genetic diversity patterns) perspectives.
4. The ecology and evolutionary biology of aquatic insects are rarely studied in both the aquatic and terrestrial stages. Research should focus on whether their amphibiotic life cycles place different evolutionary constraints and selective pressures on aquatic and terrestrial life history stages, i.e. whether ecological segregation in one or both stages enhances diversification.
SIDEBAR.
Diversification in action – Anopheles gambiae
Research on incipient speciation in the malaria mosquito Anopheles gambiae exemplifies the use of an integrative approach in the study of diversification and “provides us with an exceptional opportunity to observe evolution in action” (34). Studies have revealed two molecular forms, M and S, with strong but incomplete barriers to gene flow between them (35). The forms are maintained by asymmetric introgression where hybrids exhibit low fitness outside zones of intensive hybridization, leading to geographic mosaics of reproductive isolation. Prezygotic isolation between forms has been linked to mate recognition and choice of wing-beat frequency and flight-tone (96), as well as timing of swarming behavior (111). The diversification likely began in western Africa in response to human land use over the last few thousand years (35). M is associated with permanent and anthropogenic conditions, particularly irrigation, and is better adapted to predation (46). While S developed insecticide resistance first, introgression presumably transferred this to M (35). Another form, Bamako, is also differentiating in laterite rock pools in Mali (71).
ACKNOWLEDGEMENTS
This review benefited from conversations with Michael Balke, Wolfram Graf, Herman de Jong, Vincent Kalkman, Mike May and Nico Nieser. MTM’s research is partially supported by the European Union FP7 Project BioFresh (freshwaterbiodiversity.eu). SUP’s research is funded by the “LOEWE - Landes-Offensive zur Entwicklung wissenschaftlich-ökonomischer Exzellenz” program of Hesse’s Ministry of Higher Education, Research, and the Arts, DFG grant PA1617/2-1, and FWF grant P 23687-B17.
GLOSSARY
- Amphibiotic
having aquatic larvae and terrestrial adults
- Dispersal
the ability of a species to establish a new population in a non-contiguous habitat patch (105)
- Diversification
the net result of speciation and extinction; the formation of species or the increase in taxonomic diversity
LITERATURE CITED
- 1.Abellán P, Benetti CJ, Angus RB, Ribera I. A review of Quaternary range shifts in European aquatic Coleoptera. Global Ecol. Biogeogr. 2011;20:87–100. [Google Scholar]
- 2.Abellán P, Ribera I. Geographic location and phylogeny are the main determinants of the size of the geographical range in aquatic beetles. BMC Evol. Biol. 2011;11:344. doi: 10.1186/1471-2148-11-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander LC, Hawthorne DJ, Palmer MA, Lamp WO. Loss of genetic diversity in the North American mayfly Ephemerella invaria associated with deforestation of headwater streams. Freshwat. Biol. 2011;56:1456–67. [Google Scholar]
- 4.Alstad DN. Current speed and filtration rate link caddisfly phylogeny and distributional patterns on a stream gradient. Science. 1982;216:533–34. doi: 10.1126/science.216.4545.533. [DOI] [PubMed] [Google Scholar]
- 5.Andersen NM. The evolution of wing polymorphism in water striders (Gerridae): a phylogenetic approach. Oikos. 1993;67:433–43. [Google Scholar]
- 6.Andersen NM. A phylogenetic analysis of the evolution of sexual dimorphism and mating systems in water striders (Hemiptera: Gerridae) Biol. J. Linn. Soc. 1997;61:345–68. [Google Scholar]
- 7.Andersen NM. The evolution of marine insects: phylogenetic, ecological and geographical aspects of species diversity in marine water striders. Ecography. 1999;22:98–111. [Google Scholar]
- 8.Arnqvist G, Edvardsson M, Friberg U, Nilsson T. Sexual conflict promotes speciation in insects. Proc. Natl. Acad. Sci. U. S. A. 2000;97:10460–64. doi: 10.1073/pnas.97.19.10460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnqvist G, Jones TM, Elgar MA. The extraordinary mating system of Zeus bugs (Heteroptera: Veliidae: Phoreticovelia sp.) Aust. J. Zool. 2007;55:131. [Google Scholar]
- 10.Arribas P, Velasco J, Abellán P, Sánchez-Fernández D, Andújar C, et al. Dispersal ability rather than ecological tolerance drives differences in range size between lentic and lotic water beetles (Coleoptera: Hydrophilidae) J. Biogeogr. 2012;39:984–94. [Google Scholar]
- 11.Balian EV, Lévêque C, Segers H, Martens K, editors. Freshwater Animal Diversity Assessment. Developments in Hydrobiology 198. Hydrobiologia. 2008;595:1–637. [Balian et al 2008: Essential overview of freshwater biodiversity with detailed chapters on each major insect group.] [Google Scholar]
- 12.Balke M, Gómez-Zurita J, Ribera I, Viloria A, Zillikens A, et al. Ancient associations of aquatic beetles and tank bromeliads in the Neotropical forest canopy. Proc. Nat. Acad. Sci. U. S. A. 2008;105:6356–61. doi: 10.1073/pnas.0710368105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balke M, Ribera I, Beutel RG. The systematic position of Aspidytidae, the diversification of Dytiscoidea (Coleoptera, Adephaga) and the phylogenetic signal of third codon positions. J. Zool. Syst. Evol. Res. 2005;43:223–42. [Google Scholar]
- 14.Balke M, Ribera I, Hendrich L, Miller MA, Sagata K, et al. New Guinea highland origin of a widespread arthropod supertramp. Proc. Roy. Soc. B. 2009;276:2359–67. doi: 10.1098/rspb.2009.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barber-James HM, Gattolliat J-L, Sartori M, Hubbard MD. Global diversity of mayflies (Ephemeroptera, Insecta) in freshwater. Hydrobiologia. 2008;595:339–50. [Google Scholar]
- 16.Bataille A, Cunningham AA, Cedeno V, Patino L, Constantinou A, et al. Natural colonization and adaptation of a mosquito species in Galapagos and its implications for disease threats to endemic wildlife. Proc. Nat. Acad. Sci. U. S. A. 2009;106:10230–35. doi: 10.1073/pnas.0901308106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berendonk TU, Barraclough TG, Barraclough JC. Phylogenetics of pond and lake lifestyles in Chaoborus midge larvae. Evolution. 2003;57:2173–78. doi: 10.1111/j.0014-3820.2003.tb00395.x. [DOI] [PubMed] [Google Scholar]
- 18.Bergsten J, Miller KB. Phylogeny of diving beetles reveals a coevolutionary arms race between the sexes. PLoS One. 2007;2:e522. doi: 10.1371/journal.pone.0000522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bourret A, McPeek MA, Turgeon J. Regional divergence and mosaic spatial distribution of two closely related damselfly species (Enallagma hageni and Enallagma ebrium) J. Evol. Biol. 2012;25:196–209. doi: 10.1111/j.1420-9101.2011.02418.x. [DOI] [PubMed] [Google Scholar]
- 20.Buchwalter DB, Cain DJ, Martin Ca, Xie L, Luoma SN, Garland T. Aquatic insect ecophysiological traits reveal phylogenetically based differences in dissolved cadmium susceptibility. Proc. Nat. Acad. Sci. USA. 2008;105:8321–6. doi: 10.1073/pnas.0801686105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calosi P, Bilton DT, Spicer JI, Votier SC, Atfield A. What determines a species' geographical range? Thermal biology and latitudinal range size relationships in European diving beetles (Coleoptera: Dytiscidae) J. Anim. Ecol. 2010;79:194–204. doi: 10.1111/j.1365-2656.2009.01611.x. [DOI] [PubMed] [Google Scholar]
- 22.Carew ME, Miller AD, Hoffmann AA. Phylogenetic signals and ecotoxicological responses: potential implications for aquatic biomonitoring. Ecotoxicology. 2011;20:595–606. doi: 10.1007/s10646-011-0615-3. [DOI] [PubMed] [Google Scholar]
- 23.Cayrou J, Céréghino R. Life-cycle phenology of some aquatic insects: implications for pond conservation. Aquat. Conserv.: Mar. Freshwat. Ecosyst. 2005;15:559–71. [Google Scholar]
- 24.Chapman EG, Foote BA, Malukiewicz J, Hoeh WR. Parallel evolution of larval morphology and habitat in the snail-killing fly genus Tetanocera. J. Evol. Biol. 2006;19:1459–74. doi: 10.1111/j.1420-9101.2006.01132.x. [DOI] [PubMed] [Google Scholar]
- 25.Clausnitzer V, Dijkstra K-DB, Koch R, Boudot J-P, Darwall WR, et al. Focus on African freshwaters: hotspots of dragonfly diversity and conservation concern. Front. Ecol. Environ. 2012;10:129–34. [Google Scholar]
- 26.Clausnitzer V, Kalkman VJ, Ram M, et al. Odonata enter the biodiversity crisis debate: the first global assessment of an insect group. Biol. Conserv. 2009;142:1864–69. [Google Scholar]
- 27.Cordero Rivera A, Andrés JA, Córdoba-Aguilar A, Utzeri C. Postmating sexual selection: allopatric evolution of sperm competition mechanisms and genital morphology in calopterygid damselflies (Insecta: Odonata) Evolution. 2004;58:349–59. doi: 10.1111/j.0014-3820.2004.tb01650.x. [DOI] [PubMed] [Google Scholar]
- 28.Cover MR, Resh VH. Global diversity of dobsonflies, fishflies, and alderflies (Megaloptera; Insecta) and spongillaflies, nevrorthids, and osmylids (Neuroptera; Insecta) in freshwater. Hydrobiologia. 2008;595:409–17. [Google Scholar]
- 29.Damgaard J, Andersen NM, Sperling FAH. Phylogeny of the water strider genus Aquarius Schellenberg (Heteroptera: Gerridae) based on nuclear and mitochondrial DNA sequences and morphology. Insect Syst. Evol. 2000;31:71–90. [Google Scholar]
- 30.Damm S, Dijkstra K-DB, Hadrys H. Red drifters and dark residents: the phylogeny and ecology of a Plio-Pleistocene dragonfly radiation reflects Africa's changing environment (Odonata, Libellulidae, Trithemis) Mol. Phylogen. Evol. 2010;54:870–82. doi: 10.1016/j.ympev.2009.12.006. [Damm et al 2010: Study of a full lotic-lentic freshwater insect radiation in a tropical continent.] [DOI] [PubMed] [Google Scholar]
- 31.Danks HV. How aquatic insects live in cold climates. Can. Entomol. 2007;139:443–71. [Google Scholar]
- 32.Davis RB, Baldauf SL, Mayhew PJ. The origins of species richness in the Hymenoptera: insights from a family-level supertree. BMC Evol. Biol. 2010;10:109. doi: 10.1186/1471-2148-10-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Moor FC, Ivanov VD. Global diversity of caddisflies (Trichoptera: Insecta) in freshwater. Hydrobiologia. 2008;595:393–407. [Google Scholar]
- 34.della Torre A, Costantini C, Besansky NJ, Caccone A, Petrarca V, et al. Speciation within Anopheles gambiae - the glass is half full. Science. 2002;298:115–7. doi: 10.1126/science.1078170. [DOI] [PubMed] [Google Scholar]
- 35.della Torre A, Tu Z, Petrarca V. On the distribution and genetic differentiation of Anopheles gambiae s.s. molecular forms. Insect Biochem. Mol. Biol. 2005;35:755–69. doi: 10.1016/j.ibmb.2005.02.006. [della Torre et al 2005: Comprehensive review of distribution of molecular forms of A. gambiae with a summary of the evidence for ecological differentiation among the forms.] [DOI] [PubMed] [Google Scholar]
- 36.Drotz MK, Brodin T, Saura A, Giles BE. Ecotype differentiation in the face of gene flow within the diving beetle Agabus bipustulatus (Linnaeus, 1767) in northern Scandinavia. PloS One. 2012;7:e31381. doi: 10.1371/journal.pone.0031381. [Drotz et al 2012: Genetic differentiation across ecological gradient, possibly reinforced by sexual selection.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Espeland M, Johanson KA, Hovmöller R. Early Xanthochorema (Trichoptera, Insecta) radiations in New Caledonia originated on ultrabasic rocks. Mol. Phylogen. Evol. 2008;48:904–17. doi: 10.1016/j.ympev.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Espeland M, Johanson KA. The diversity and radiation of the largest monophyletic animal group on New Caledonia (Trichoptera: Ecnomidae: Agmina) J. Evol. Biol. 2010;23:2112–22. doi: 10.1111/j.1420-9101.2010.02072.x. [DOI] [PubMed] [Google Scholar]
- 39.Ferrington LC. Global diversity of non-biting midges (Chironomidae; Insecta-Diptera) in freshwater. Hydrobiologia. 2008;595:447–55. [Google Scholar]
- 40.Fikáček M, Prokin A, Angus RB, Ponomarenko A, Yue Y, et al. Phylogeny and the fossil record of the Helophoridae reveal Jurassic origin of extant hydrophiloid lineages (Coleoptera: Polyphaga) Syst. Entomol. 2012;37:420–47. [Google Scholar]
- 41.Finn DS, Bonada N, Múrria C, Hughes JM. Small but mighty: headwaters are vital to stream network biodiversity at two levels of organization. J. N. Am. Benthol. Soc. 2011;30:963–80. [Google Scholar]
- 42.Fochetti R, Tierno de Figueroa JM. Global diversity of stoneflies (Plecoptera; Insecta) in freshwater. Hydrobiologia. 2008;595:365–77. [Google Scholar]
- 43.Freeland JR, May M, Lodge R, Conrad KF. Genetic diversity and widespread haplotypes in a migratory dragonfly, the common green darner Anax junius. Ecol. Entomol. 2003;28:413–21. [Google Scholar]
- 44.Funk DH, Jackson JK, Sweeney BW. Taxonomy and genetics of the parthenogenetic mayfly Centroptilum triangulifer and its sexual sister Centroptilum alamance (Ephemeroptera: Baetidae) J. N. Am. Benthol. Soc. 2006;25:417–29. [Google Scholar]
- 45.Funk DH, Sweeney BW, Jackson JK. A taxonomic reassessment of the Drunella lata (Morgan) species complex (Ephemeroptera: Ephemerellidae) in northeastern North America. J. N. Am. Benthol. Soc. 2008;27:647–63. [Google Scholar]
- 46.Gimonneau G, Bouyer J, Morand S, Besansky NJ, Diabate A, Simard F. A behavioral mechanism underlying ecological divergence in the malaria mosquito Anopheles gambiae. Behav. Ecol. 2010;21:1087–92. doi: 10.1093/beheco/arq114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayashi F, Kamimura Y, Nozaki T. Origin of the transition from aquatic to terrestrial habits in Nothopsyche caddisflies (Trichoptera: Limnephilidae) based on molecular phylogeny. Zool. Sci. 2008;25:255–60. doi: 10.2108/zsj.25.255. [DOI] [PubMed] [Google Scholar]
- 48.Higginson DM, Miller KB, Segraves KA, Pitnick S. Convergence, recurrence and diversification of complex sperm traits in diving beetles (Dytiscidae) Evolution. 2012;66:1650–61. doi: 10.1111/j.1558-5646.2011.01532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hof C, Brandle M, Brandl R. Lentic odonates have larger and more northern ranges than lotic species. J. Biogeogr. 2006;33:63–70. [Google Scholar]
- 50.Holzenthal RW, Morse JC, Kjer KM. Order Trichoptera Kirby, 1813. In: Zhang Z-Q, editor. Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness. Zootaxa; 2011. pp. 209–11. [DOI] [PubMed] [Google Scholar]
- 51.Hughes JM, Schmidt DJ, Finn DS. Genes in streams: Using DNA to understand the movement of freshwater fauna and their riverine habitat. BioScience. 2009;59:573–83. [Google Scholar]
- 52.Hunt T, Bergsten J, Levkanicova Z, Papadopoulou A, John OS, et al. A comprehensive phylogeny of beetles reveals the evolutionary origins of a superradiation. Science. 2007;318:1913–16. doi: 10.1126/science.1146954. [DOI] [PubMed] [Google Scholar]
- 53.Illies J. Versuch einer allgemeinen biozönotischen Gliederung der Fließgewässer. Internationale Revue der gesamten Hydrobiologie und Hydrographie. 1961;46:205–13. [Google Scholar]
- 54.Ingley SJ, Bybee SM, Tennessen KJ, Whiting MF, Branham MA. Life on the fly: phylogenetics and evolution of the helicopter damselflies (Odonata, Pseudostigmatidae) Zool. Scr. 2012;41:637–50. [Google Scholar]
- 55.Ivanov VD, Sukatcheva ID. Trichoptera (Phryganeida) In: Rasnitsyn AP, Quicke LJ, editors. History of Insects. Kluwer Academic Publishers; Dordrecht, Boston, London: 2002. pp. 199–220. [Google Scholar]
- 56.Jäch M, Balke M. Global diversity of water beetles (Coleoptera) in freshwater. Hydrobiologia. 2008;595:419–42. [Google Scholar]
- 57.James J, Bert DG, Forbes MR. Wetland type differentially affects ectoparasitic mites and their damselfly hosts. Ecography. 2009;32:800–06. [Google Scholar]
- 58.Johansson F, Söderquist M, Bokma F. Insect wing shape evolution: independent effects of migratory and mate guarding flight on dragonfly wings. Biol. J. Linn. Soc. 2009;97:362–72. [Google Scholar]
- 59.Jordan S, Simon C, Polhemus D. Molecular systematics and adaptive radiation of Hawaii's endemic damselfly genus Megalagrion (Odonata: Coenagrionidae) Syst. Biol. 2003;52:89–109. doi: 10.1080/10635150390132803. [Jordan et al 2003: Ecological diversification into all available freshwater habitats on a volcanic archipelago.] [DOI] [PubMed] [Google Scholar]
- 60.Joy DA, Craig DA, Conn JE. Genetic variation tracks ecological segregation in Pacific island black flies. Heredity. 2007;99:452–59. doi: 10.1038/sj.hdy.6801023. [DOI] [PubMed] [Google Scholar]
- 61.Kalkman VJ, Clausnitzer V, Dijkstra K-DB, Orr AG, Paulson DR, van Tol J. Balian E, Lévêque C, Segers H, Martens K, editors. Global diversity of dragonflies (Odonata; Insecta) in freshwater. Hydrobiologia. 2008;595:351–63. Freshwater animal diversity assessment. [Google Scholar]
- 62.Krosch MN. Phylogeography of Echinocladius martini Cranston (Diptera: Chironomidae) in closed forest streams of eastern Australia. Aust. J. Entomol. 2011;50:258–68. [Google Scholar]
- 63.Krueger A, Hennings IC. Molecular phylogenetics of blackflies of the Simulium damnosum complex and cytophylogenetic implications. Mol. Phylogen. Evol. 2006;39:83–90. doi: 10.1016/j.ympev.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 64.Larison B. Impacts of environmental heterogeneity on alternative mating tactics in the threadtail damselfly. Behav. Ecol. Sociobiol. 2008;63:531–36. [Google Scholar]
- 65.Lee Y-H, Lin C-P. Pleistocene speciation with and without gene flow in Euphaea damselflies of subtropical and tropical East Asian islands. Mol. Ecol. 2012;21:3739–56. doi: 10.1111/j.1365-294X.2012.05654.x. [DOI] [PubMed] [Google Scholar]
- 66.Leys R, Watts CHS, Cooper SJB, Humphreys WF. Evolution of subterranean diving beetles (Coleoptera: Dytiscidae: Hydroporini, Bidessini) in the arid zone of Australia. Evolution. 2003;57:2819–34. doi: 10.1111/j.0014-3820.2003.tb01523.x. [DOI] [PubMed] [Google Scholar]
- 67.Lorenzo-Carballa MO, Hadrys H, Cordero-Rivera A, Andrés JA. Population genetic structure of sexual and parthenogenetic damselflies inferred from mitochondrial and nuclear markers. Heredity. 2012;108:386–95. doi: 10.1038/hdy.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lucentini L, Rebora M, Puletti ME, Gigliarelli L, Fontaneto D, et al. Geographical and seasonal evidence of cryptic diversity in the Baetis rhodani complex (Ephemeroptera, Baetidae) revealed by means of DNA taxonomy. Hydrobiologia. 2011;673:215–28. [Google Scholar]
- 69.Malicky H, Pauls SU. Cross-breeding of Chaetopteryx morettii and related species, with molecular and eidonomical results (Trichoptera, Limnephilidae) Ann Limnol-Int J Lim. 2012;48:13–19. [Google Scholar]
- 70.Malm T, Johanson KA, Wahlberg N. The evolutionary history of Trichoptera (Insecta): A case of successful adaptation to life in freshwater. Syst. Ent. 2013 in press. [Malm et al 2013: Phylogeny of Trichoptera explicitly linking the role of case-building for respiration, the evolution of case-building, and invasion of various freshwater habitats.] [Google Scholar]
- 71.Manoukis NC, Powell JR, Touré MB, Sacko A, Edillo FE, et al. A test of the chromosomal theory of ecotypic speciation in Anopheles gambiae. Proc. Nat. Acad. Sci. U. S. A. 2008;105:2940–5. doi: 10.1073/pnas.0709806105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marten A, Brändle M, Brandl R. Habitat type predicts genetic population differentiation in freshwater invertebrates. Mol. Ecol. 2006;15:2643–51. doi: 10.1111/j.1365-294X.2006.02940.x. [DOI] [PubMed] [Google Scholar]
- 73.Matsubayashi KW, Ohshima I, Nosil P. Ecological speciation in phytophagous insects. Entomol. Exp. Appl. 2010;134:1–27. [Google Scholar]
- 74.Mayhew PJ. Why are there so many insect species? Perspectives from fossils and phylogenies. Biol. Rev. 2007;82:425–54. doi: 10.1111/j.1469-185X.2007.00018.x. [Mayhew 2007: Essential review of insect diversification hypotheses; reveals the deficiency of knowledge on aquatic groups.] [DOI] [PubMed] [Google Scholar]
- 75.McCulloch GA, Wallis GP, Waters JM. Onset of glaciation drove simultaneous vicariant isolation of Alpine insects in New Zealand. Evolution. 2010;64:2033–43. doi: 10.1111/j.1558-5646.2010.00980.x. [DOI] [PubMed] [Google Scholar]
- 76.McPeek MA, Gavrilets S. The evolution of female mating preferences: differentiation from species with promiscuous males can promote speciation. Evolution. 2006;60:1967–80. [PubMed] [Google Scholar]
- 77.McPeek MA, Shen L, Torrey JZ, Farid H. The tempo and mode of three-dimensional morphological evolution in male reproductive structures. Am. Nat. 2008;171:E158–78. doi: 10.1086/587076. [DOI] [PubMed] [Google Scholar]
- 78.McPeek MA, Symes LB, Zong DM, McPeek CL. Species recognition and patterns of population variation in the reproductive structures of a damselfly genus. Evolution. 2011;65:419–28. doi: 10.1111/j.1558-5646.2010.01138.x. [DOI] [PubMed] [Google Scholar]
- 79.Mey W. Insular radiation of the genus Hydropsyche (Insecta, Trichoptera: Hydropsychidae) Pictet, 1834 in the Philippines and its implications for the biogeography of Southeast Asia. J. Biogeogr. 2003;30:227–36. [Google Scholar]
- 80.Miller KB. The phylogeny of diving beetles (Coleoptera: Dytiscidae) and the evolution of sexual conflict. Biol. J. Linn. Soc. 2003;79:359–88. [Google Scholar]
- 81.Misof B. Diversity of Anisoptera (Odonata): inferring speciation processes from patterns of morphological diversity. Zoology. 2002;105:355–65. doi: 10.1078/0944-2006-00076. [DOI] [PubMed] [Google Scholar]
- 82.Mitter C, Farrell BD, Wiegmann BM. The phylogenetic study of adaptive zones: has phytophagy promoted insect diversification? Am. Nat. 1988;132:107–28. [Google Scholar]
- 83.Monaghan MT, Gattolliat J-L, Sartori M, Elouard J-M, James H, et al. Transoceanic and endemic origins of the small minnow mayflies (Ephemeroptera, Baetidae) of Madagascar. Proc. R. Soc. Lond. B. 2005;272:1829–36. doi: 10.1098/rspb.2005.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Monaghan MT, Spaak P, Robinson CT, Ward JV. Genetic differentiation of Baetis alpinus Pictet (Ephemeroptera: Baetidae) in fragmented alpine streams. Heredity. 2001;86:395–403. doi: 10.1046/j.1365-2540.2001.00843.x. [DOI] [PubMed] [Google Scholar]
- 85.Morgan AJ, Kille P, Sturzenbaum SR. Microevolution and ecotoxicology of metals in invertebrates. Environ. Sci. Technol. 2007;41:1085–96. doi: 10.1021/es061992x. [DOI] [PubMed] [Google Scholar]
- 86.Mullen SP, Andrés JA. Rapid evolution of sexual signals in sympatric Calopteryx damselflies: reinforcement or 'noisy-neighbour' ecological character displacement? J. Evol. Biol. 2007;20:1637–48. doi: 10.1111/j.1420-9101.2007.01297.x. [DOI] [PubMed] [Google Scholar]
- 87.Múrria C, Bonada N, Arnedo MA, Zamora-Muñoz C, Prat N, Vogler AP. Phylogenetic and ecological structure of Mediterranean caddisfly communities at various spatio-temporal scales. J. Biogeogr. 2012;39:1621–32. [Google Scholar]
- 88.Ogitani M, Sekiné K, Tojo K. Habitat segregation and genetic relationship of two heptageniid mayflies, Epeorus latifolium and Epeorus l-nigrus, in the Shinano-gawa River basin. Limnology. 2010;12:117–25. [Google Scholar]
- 89.O'Loughlin SM, Somboon P, Walton C. High levels of population structure caused by habitat islands in the malarial vector Anopheles scanloni. Heredity. 2007;99:31–40. doi: 10.1038/sj.hdy.6800959. [DOI] [PubMed] [Google Scholar]
- 90.Outomuro D, Bokma F, Johansson F. Hind wing shape evolves faster than front wing shape in Calopteryx damselflies. Evol. Biol. 2011;39:116–25. [Google Scholar]
- 91.Papadopoulou A, Bergsten J, Fujisawa T, Monaghan MT, Barraclough TG, Vogler AP. Speciation and DNA barcodes: testing the effects of dispersal on the formation of discrete sequence clusters. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008;363:2987–96. doi: 10.1098/rstb.2008.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pape T, Blagoderov V, Mostovski MB. Order Diptera Linnaeus, 1758. In: Zhang Z-Q, editor. Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness. Zootaxa; 2011. pp. 222–29. [DOI] [PubMed] [Google Scholar]
- 93.Papoucheva E, Proviz V, Lambkin C, Goddeeris B, Blinov A. Phylogeny of the endemic Baikalian Sergentia (Chironomidae, Diptera) Mol. Phylogen. Evol. 2003;29:120–25. doi: 10.1016/s1055-7903(03)00075-7. [DOI] [PubMed] [Google Scholar]
- 94.Pauls SU, Graf W, Haase P, Lumbsch HT, Waringer J. Grazers, shredders and filtering carnivores - the evolution of feeding ecology in Drusinae (Trichoptera: Limnephilidae): insights from a molecular phylogeny. Mol. Phylogen. Evol. 2008;46:776–91. doi: 10.1016/j.ympev.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pauls SU, Theissinger K, Ujvárosi L, Bálint M, Haase P. Patterns of population structure in two closely related, partially sympatric caddisflies in Eastern Europe: historic introgression, limited dispersal, and cryptic diversity. J. N. Am. Benthol. Soc. 2009;28:517–36. [Google Scholar]
- 96.Pennetier C, Warren B, Dabiré KR, Russell IJ, Gibson G. “Singing on the wing” as a mechanism for species recognition in the malarial mosquito Anopheles gambiae. Curr. Biol. 2010;20:131–6. doi: 10.1016/j.cub.2009.11.040. [DOI] [PubMed] [Google Scholar]
- 97.Pereira-da-Conceicoa LL, Price BW, Barber-James HM, Barker NP, de Moor FC, Villet MH. Cryptic variation in an ecological indicator organism: mitochondrial and nuclear DNA sequence data confirm distinct lineages of Baetis harrisoni Barnard (Ephemeroptera: Baetidae) in southern Africa. BMC Evol. Biol. 2012;12:26. doi: 10.1186/1471-2148-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Perry JC, Rowe L. Sexual conflict and antagonistic coevolution across water strider populations. Evolution. 2011;66:544–57. doi: 10.1111/j.1558-5646.2011.01464.x. [DOI] [PubMed] [Google Scholar]
- 99.Petrin Z, Schilling EG, Loftin CS, Johansson F. Predators shape distribution and promote diversification of morphological defenses in Leucorrhinia, Odonata. Evol. Ecol. 2010;24:1003–16. [Google Scholar]
- 100.Polhemus JT, Polhemus DA. Global diversity of true bugs (Heteroptera; Insecta) in freshwater. Hydrobiologia. 2008;595:379–91. [Google Scholar]
- 101.Porinchu DF, MacDonald GM. The use and application of freshwater midges (Chironomidae: Insecta: Diptera) in geographical research. Prog. Phys. Geogr. 2003;27:378–422. [Google Scholar]
- 102.Pramual P, Kuvangkadilok C, Jitklang S, Tangkawanit U, Adler PH. Geographical versus ecological isolation of closely related black flies (Diptera: Simuliidae) inferred from phylogeny, geography, and ecology. Org. Divers. Evol. 2012;12:183–95. [Google Scholar]
- 103.Previsić A, Walton C, Kucinić M, Mitrikeski PT, Kerovec M. Pleistocene divergence of Dinaric Drusus endemics (Trichoptera, Limnephilidae) in multiple microrefugia within the Balkan Peninsula. Mol. Ecol. 2009;18:634–47. doi: 10.1111/j.1365-294X.2008.04046.x. [DOI] [PubMed] [Google Scholar]
- 104.Pritchard G, Harder LD, Mutch RA. Development of aquatic insect eggs in relation to temperature and strategies for dealing with different thermal environments. Biol. J. Linn. Soc. 1996;58:221–44. [Google Scholar]
- 105.Ribera I. Habitat constraints and the generation of diversity in freshwater macroinvertebrates. In: Lancaster J, Briers RA, editors. Aquatic Insects: Challenges to Populations. CAB International; Wallingford, UK: 2008. pp. 289–311. [Ribera 2008: Review of research carried out on the habitat stability hypothesis.] [Google Scholar]
- 106.Ribera I, Barraclough TG, Vogler AP. The effect of habitat type on speciation rates and range movements in aquatic beetles: inferences from species-level phylogenies. Mol. Ecol. 2001;10:721–35. doi: 10.1046/j.1365-294x.2001.01218.x. [DOI] [PubMed] [Google Scholar]
- 107.Ribera I, Castro A, Díaz JA, Garrido J, Izquierdo A, et al. The geography of speciation in narrow-range endemics of the ‘Haenydra’ lineage (Coleoptera, Hydraenidae, Hydraena) J. Biogeogr. 2011;38:502–16. [Google Scholar]
- 108.Ribera I, Vogler AP. Habitat type as a determinant of species range sizes: the example of lotic-lentic differences in aquatic Coleoptera. Biol. J. Linn. Soc. 2000;71:33–52. [Google Scholar]
- 109.Rowe L, Westlake KP, Currie DC. Functional significance of elaborate secondary sexual traits and their evolution in the water strider genus Rheumatobates. Can. Entomol. 2006;138:568–77. [Google Scholar]
- 110.Rubinoff D, Schmitz P. Multiple aquatic invasions by an endemic, terrestrial Hawaiian moth radiation. Proc. Nat. Acad. Sci. U.S.A. 2010;107:5903–06. doi: 10.1073/pnas.0912501107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rund SSC, Lee SJ, Bush RD, Duffield GE. Strain- and sex-specific differences in daily flight activity and the circadian clock of Anopheles gambiae mosquitoes. J. Insect Physiol. 2012;58:1609–19. doi: 10.1016/j.jinsphys.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 112.Sabando MC, Vila I, Peñaloza R, Véliz D. Contrasting population genetic structure of two widespread aquatic insects in the Chilean high-slope rivers. Mar. Freshwat. Res. 2011;62:1–10. [Google Scholar]
- 113.Samraoui B, Weekers PHH, Dumont HJ. Two taxa within the North African Lestes virens complex (Zygoptera: Lestidae) Odonatologica. 2003;32:131–142. [Google Scholar]
- 114.Sánchez-Fernández D, Lobo JM, Abellán P, Millán A. Environmental niche divergence between genetically distant lineages of an endangered water beetle. Biol. J. Linn. Soc. 2011;103:891–903. [Google Scholar]
- 115.Schultheis AS, Hendricks AC, Weigt LA. Genetic evidence for 'leaky' cohorts in the semivoltine stonefly Peltoperla tarteri (Plecoptera: Peltoperlidae) Freshwat. Biol. 2002;47:367–76. [Google Scholar]
- 116.Serrano-Meneses MA, Córdoba-Aguilar A, Azpilicueta-Amorín M, González-Soriano E, Székely T. Sexual selection, sexual size dimorphism and Rensch's rule in Odonata. J. Evol. Biol. 2008;21:1259–73. doi: 10.1111/j.1420-9101.2008.01567.x. [DOI] [PubMed] [Google Scholar]
- 117.Sheldon P. Plus ça change - a model for stasis and evolution in different environments. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1996;127:209–27. [Google Scholar]
- 118.Smith PJ, Smith BJ. Small-scale population-genetic differentiation in the New Zealand caddisfly Orthopsyche fimbriata and the crayfish Paranephrops planifrons. N. Z. J. Mar. Freshwat. Res. 2009;43:723–34. [Google Scholar]
- 119.Sota T, Mogi M. Origin of pitcher plant mosquitoes in Aedes (Stegomyia): A molecular phylogenetic analysis using mitochondrial and nuclear gene sequences. J. Med. Entomol. 2006;43:795–800. doi: 10.1603/0022-2585(2006)43[795:ooppmi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 120.Statzner B, Dolédec S. Phylogenetic, spatial, and species-trait patterns across environmental gradients: the case of Hydropsyche (Trichoptera) along the Loire River. Int. Rev. Hydrobiol. 2011;96:121–40. [Google Scholar]
- 121.Stoks R, McPeek MA. A tale of two diversifications: reciprocal habitat shifts to fill ecological space along the pond permanence gradient. Am. Nat. 2006;168(Suppl):S50–72. doi: 10.1086/509045. [Stoks & McPeek 2006: Review of ecological and genetic work on two lentic damselfly radiations under variable predation pressures in North American.] [DOI] [PubMed] [Google Scholar]
- 122.Suhling F, Sahlén G, Kasperski J, Gaedecke D. Behavioural and life history traits in temporary and perennial waters: comparisons among three pairs of sibling dragonfly species. Oikos. 2005;108:609–17. [Google Scholar]
- 123.Svensson EI, Eroukhmanoff F, Friberg M. Effects of natural and sexual selection on adaptive population divergence and premating isolation in a damselfly. Evolution. 2006;60:1242–53. [PubMed] [Google Scholar]
- 124.Svensson EI, Kristoffersen L, Oskarsson K, Bensch S. Molecular population divergence and sexual selection on morphology in the banded demoiselle (Calopteryx splendens) Heredity. 2004;93:423–33. doi: 10.1038/sj.hdy.6800519. [DOI] [PubMed] [Google Scholar]
- 125.Theissinger K, Bálint M, Feldheim KA, Haase P, Johannesen J, et al. Glacial survival and post-glacial recolonization of an arctic-alpine freshwater insect (Arcynopteryx dichroa, Plecoptera, Perlodidae) in Europe. J. Biogeo. 2013;40:236–48. [Google Scholar]
- 126.Thomas Ja, Trueman JWH, Rambaut A, Welch JJ. Relaxed phylogenetics and the Palaeoptera problem: resolving deep ancestral splits in the insect phylogeny. Syst. Biol. 2013;62:285–97. doi: 10.1093/sysbio/sys093. [DOI] [PubMed] [Google Scholar]
- 127.Turgeon J, Stoks R, Thum RA, Brown JM, McPeek MA. Simultaneous Quaternary radiations of three damselfly clades across the Holarctic. Am. Nat. 2005;165:E78–107. doi: 10.1086/428682. [DOI] [PubMed] [Google Scholar]
- 128.Tynkkynen K, Kotiaho JS, Luojumaki M, Suhonen J. Interspecific aggression causes negative selection on sexual characters. Evolution. 2005;59:1838–43. [PubMed] [Google Scholar]
- 129.Vamosi SM. On the role of enemies in divergence and diversification of prey: a review and synthesis. Can. J. Zool. 2005;83:894–910. [Google Scholar]
- 130.Van Gossum H, Mattern M. A phylogenetic perspective on absence and presence of a sex-limited polymorphism. Animal Biology. 2008;58:257–73. [Google Scholar]
- 131.Vörösmarty CJ, McIntyre PB, Gessner MO, Dudgeon D, Prusevich A, et al. Global threats to human water security and river biodiversity. Nature. 2010;467:555–61. doi: 10.1038/nature09440. [DOI] [PubMed] [Google Scholar]
- 132.Vuataz L, Sartori M, Gattolliat J-L, Monaghan MT. Endemism and diversification in freshwater insects of Madagascar revealed by coalescent and phylogenetic analysis of museum and field collections. Mol. Phylogen. Evol. 2013;66:979–91. doi: 10.1016/j.ympev.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 133.Wagner R, Barták M, Borkent A, Courtney G, Goddeeris B, et al. Global diversity of dipteran families (Insecta Diptera) in freshwater (excluding Simulidae, Culicidae, Chironomidae, Tipulidae and Tabanidae) Hydrobiologia. 2008;595:489–519. [Google Scholar]
- 134.Wang Y, Liu X, Winterton SL, Yang D. The first mitochondrial genome for the fishfly subfamily Chauliodinae and implications for the higher phylogeny of Megaloptera. PloS One. 2012;7:e47302. doi: 10.1371/journal.pone.0047302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Weaver JS, III, Morse JC. Evolution of feeding and case-making behavior in Trichoptera. J. N. Am. Benthol. Soc. 1986;5:150–58. [Google Scholar]
- 136.Wellenreuther M, Vercken E, Svensson EI. A role for ecology in male mate discrimination of immigrant females in Calopteryx damselflies? Biol. J. Linn. Soc. 2010;100:506–18. [Google Scholar]
- 137.Wiegmann BM, Trautwein MD, Winkler IS, Barr NB, Kim J-W, et al. Episodic radiations in the fly tree of life. Proc. Nat. Acad. Sci. U. S. A. 2011;108:5690–95. doi: 10.1073/pnas.1012675108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Williams DD, Tavares AF, Bryant E. Respiratory device or camouflage?: A case for the caddisfly. Oikos. 1987;50:42–52. [Google Scholar]
- 139.Wohlfahrt B, Vamosi SM. Antagonistic selection or trait compensation? Diverse patterns of predation-induced prey mortality due to the interacting effects of prey phenotype and the environment. Evol. Biol. 2009;36:386–96. [Google Scholar]
- 140.Wootton RJ. The historical ecology of aquatic insects: An overview. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1988;62:477–92. [Google Scholar]
- 141.Zamora-Munoz C, Svensson BW. Survival of caddis larvae in relation to their case material in a group of temporary and permanent pools. Freshwat. Biol. 1996;36:23–31. [Google Scholar]