Abstract
Functional networks in resting-state fMRI are identified by characteristics of their intrinsic low-frequency oscillations, more specifically in terms of their synchronicity. With advanced aging and in clinical populations, this synchronicity among functionally linked regions is known to decrease and become disrupted, which may be associated with observed cognitive and behavioral changes. Previous work from our group has revealed that oscillations within the slow-5 frequency range (0.01–0.027 Hz) are particularly susceptible to disruptions in aging and following a stroke. In this study, we characterized longitudinally the changes in the slow-5 oscillations in stroke patients across two different time-points. We followed a group of ischemic stroke patients (n = 20) and another group of healthy older adults (n = 14) over two visits separated by a minimum of three months (average of 9 months). For the stroke patients, one visit occurred in their subacute window (10 days to 6 months after stroke onset), the other took place in their chronic window (> 6 months after stroke). Using a mid-order group ICA method on 10-minutes eyes-closed resting-state fMRI data, we assessed the frequency distributions of a component's representative time-courses for differences in regards to slow-5 spectral power. First, our stroke patients, in their subacute stage, exhibited lower amplitude slow-5 oscillations in comparison to their healthy counterparts. Second, over time in their chronic stage, those same patients showed a recovery of those oscillations, reaching near equivalence to the healthy older adult group. Our results indicate the possibility of an eventual recovery of those initially disrupted network oscillations to a near-normal level, providing potentially a biomarker for stroke recovery of the cortical system. This finding opens new avenues in infra-slow oscillation research and could serve as a useful biomarker in future treatments aimed at recovery.
Keywords: Resting-state fMRI, Low-frequency oscillations, fALFF, Slow-5 oscillations, Aging, Stroke
Highlights
-
•
Slow-5 oscillation amplitudes are reduced in stroke patients at the subacute stage.
-
•
Slow-5 oscillation amplitudes correlate with cognitive performance.
-
•
Slow-5 oscillations recover in the same patients at the chronic stage.
-
•
Findings support the high implication of slow-5 oscillations in network disruption.
-
•
Slow-5 oscillations may serve as a bio-marker of functional network health.
1. Introduction
Resting-state fMRI (rs-fMRI) is an imaging technique that is widely implemented in the study of clinical population. This technique allows for the investigation of cortical functional networks without the need of goal-directed behavior. This approach minimizes the influence of known confounding factors associated with goal-oriented task-fMRI design (e.g. attention, motivation, and ability to perform the task as instructed), which can be particularly challenging in clinical populations such as stroke, with patients exhibiting various deficits (e.g. language, attention, and motor). For the purpose of this study, we defined the “resting” condition as a state where neither task participation nor goal-directed behavior is required.
A current approach to rs-fMRI analysis is to study functional connectivity, a measure assessing the correlation, or synchronization, of oscillations among distal regions (Friston et al., 1993). Biswal and colleagues (Biswal et al., 1995) were the first to demonstrate that functionally similar regions presented synchronous fluctuations even in the absence of a task. This approach allows for functionally connected brain regions to be identified and investigated (Biswal et al., 2010, Zuo et al., 2010, Beckmann et al., 2005, Fox and Raichle, 2007, Patriat et al., 2013). In subjects experiencing functional and/or cognitive decline, such as in normal aging, or in clinical populations with neurological disorders, such as patients with Alzheimer's disease (Greicius et al., 2004, Sorg et al., 2007), autism spectrum disorders (Cherkassky et al., 2006, Kennedy and Courchesne, 2008), or schizophrenia (Bluhm et al., 2007, Zhou et al., 2007), synchrony in the intrinsic oscillations between regions have been shown to be disrupted. Detailed reviews on network dysfunction in mental disorder are available in the literature (Broyd et al., 2009, Greicius, 2008). One consistent finding of network impairments is the disruption of the “default-mode” network. Andrews-Hanna and colleagues (Andrews-Hanna et al., 2007) have shown that connectivity between the anterior and posterior nodes within the DMN system were most severely disrupted by age. Moreover, within-network co-activation (Sorg et al., 2007, Damoiseaux et al., 2008) and functional connectivity density (Tomasi & Volkow, 2012) were also found to be reduced. However, very few studies have investigated the resting-state intrinsic oscillations in stroke outside of the system directly impaired from the injury. Early evidence suggests the occurrence of a comparable reduction of functional connectivity within the DMN following a stroke when stroke lesion did not pertain to regions of the DMN (Tsai et al., 2013, Tuladhar et al., 2013, Park et al., 2014, Wang et al., 2014). The reduction of functional connectivity within the DMN is in addition to the disruption of networks directly affected by the stroke lesion itself (Ward et al., 2003, Saur et al., 2006, Grefkes et al., 2008, Wang et al., 2010, Park et al., 2011, Carter et al., 2012), part of a diaschisis effect (vonMonakow, 1914). However, because of the acute nature of the stroke injury, mechanisms under which the disruption of the DMN system happens may differ.
Synchronicity of the oscillations from distal regions (functional connectivity) is not the only extractable measure from rs-fMRI. Regional homogeneity (ReHo) (Zang et al., 2004) provides a measure of local synchronicity of neighboring voxel fluctuations representing a local coherence of neural firing. Alternatively, voxel-wise amplitude information of the intrinsic low-frequency oscillations (LFOs) can also be obtained by the sum of the amplitude spectra within a specific low-frequency band (i.e. amplitude of low-frequency fluctuation, ALFF) (Yang et al., 2007) and the proportion of low-frequency amplitude spectra in comparison to the spectra over the whole acquired frequency range (i.e. fractional ALFF or fALFF) (Zou et al., 2008). In an ischemic stroke population, Alzheimer's disease population and in subjects with mild cognitive impairment (MCI), these measures have similarly shown decreases within the regions of the DMN (Tsai et al., 2013, Zhang et al., 2012, Liu et al., 2014). Investigations of both regional homogeneity and ALFF address possible mechanisms of synchrony disruption. ReHo could be used to investigate whether the long distance dys-synchrony could be a consequence of localized dys-synchrony within a region, where fluctuations between neighboring voxels no longer coincide (Zang et al., 2004, He et al., 2007). In contrast, a reduction in the long distance correlation between regions of the same network (functional connectivity) due to a decrease in local intrinsic fluctuation amplitude which reduces the signaling of the communicated information in comparison to system noise could be addressed by measures of ALFF and fALFF (Di et al., 2013a). In a more global perspective, various studies have also demonstrated reduced cortical spectral power at the low frequencies in patients with schizophrenia and bipolar disorders within network components (Garrity et al., 2007, Calhoun et al., 2011), showing that amplitude hypothesis is plausible even at the level of the network. Previous work from our group (La et al., 2014, La et al., 2015a) presented comparable findings in an ischemic stroke population following the ischemic injury. The investigation of spectral power in an ischemic stroke population over time allows us to better characterize the longitudinal course. In contrast to schizophrenia or bipolar disorders, which are chronic and more persistent, patients surviving the acute stroke injury often show some level of spontaneous recovery that can be examined in a longitudinal study.
In the investigations of functional intrinsic networks of the DMN and other resting-state networks defined by their synchronous oscillations, there have been few examinations making use of the LFO's amplitude information. Investigations of those amplitude information have been implemented by subdividing the spectra of these spontaneous oscillations into distinct infra-slow frequency ranges (i.e. slow-5: 0.01–0.027 Hz, slow-4: 0.027–0.073 Hz, slow-3: 0.073–0.198 Hz, slow-2: 0.198–0.25 Hz) (Zuo et al., 2010, Buzsáki and Draguhn, 2004, Penttonen and Buzsáki, 2003). Zuo et al (Zuo et al., 2010) found significant slow-4 and slow-5 oscillations to be primarily restricted to gray matter, while slow-2 and slow-3 oscillations were primarily restricted to white matter. Furthermore, they demonstrated that many areas exhibiting maximal low-frequency oscillation amplitudes were regions of the DMN with slow-5 more dominant than slow-4 in these areas in normal healthy young subjects. For this reason, our investigation focuses primarily on the oscillations within the slow-5 range. Furthermore, previous studies from our group demonstrated that a decrease in slow-5 amplitudes contributed to a reduction in slow-5 dominance and the interruption of the balance between slow-5 and slow-4 oscillation in the subacute phase of stroke (La et al., 2014, La et al., 2015a), consistent with the literature describing an extensive change in activity in the slow-5 band after stroke (Zhu et al., 2015). Though the DMN is a common candidate for exploring connectivity changes, the effect of a stroke may extend beyond the boundaries of the DMN. This reduction in slow-5 oscillation amplitude in the subacute stroke group was restricted to subnetworks of the DMN as was observed in the healthy older group, but this reduction also occurred in ‘task-positive’ networks (La et al., 2015b), suggesting a more generalized deficit of the cortical system following a stroke. With regards to resting-state brain oscillations, we suggest that slow-5 oscillations may play a pivotal role in general network health, but also in network disruption following an acute injury such as a stroke.
In this study, we provided a continuation of this assessment of the slow-5 spectral power, with an investigation of a possible recovery of those oscillations in a longitudinal observational study following a group of ischemic stroke patients from a subacute time-point to a chronic time-point.
2. Methods
2.1. Participants
Twenty stroke patients with mild deficits (mean NIH-Stroke Scale (NIH-SS) score of 1.7) with various stroke lesions were recruited and received MR scans at two different time-points. The lesions of the twenty stroke patients were mostly non-overlapping lesions, with no lesion pertaining to areas of the DMN. A lesion density map, derived from semi-automated segmentation from available T1 BRAVO, Cube T2 FLAIR and Diffusion Weighted Image (DWI) using Jim 7 (Xinapse, http://www.xinapse.com/), is provided in Fig. 1. Fourteen older healthy adults (OHAs) (ages between 50 and 75 years old) were also recruited as controls. Summary participant demographics and visits characteristics are provided in Table 1. More information regarding clinical, demographic, and session information for the 20 enrolled ischemic stroke patients can be found in Supplement material A (Suppl. mat. A). Participants recruited in the study were free of neurological or psychiatric disorders. Participants provided full written informed consent toward participation in compliance with the University of Wisconsin-Madison Health Sciences Institutional Review Board (IRB). Other exclusion criteria included contra-indications to MRI, claustrophobia or pregnancy, and intake of certain types of medications (e.g. antipsychotics, antidepressants, sedative hypnotics, etc.). Participants presented no sign of compromised capacity or ability to consent, as established by neurological examination.
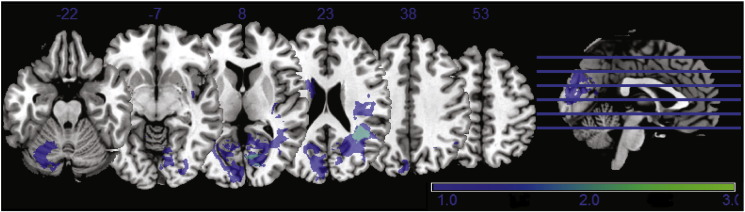
Fig. 1.
Lesion density map for the 20 ischemic stroke patients presenting minimal overlap. No lesion over regions of the DMN were observed.
Table 1.
Summary demographic data for healthy older adults and stroke patients. Subjects' age and time between visits displayed no difference. Gender difference between the groups were not significant as tested by two-tailed Fisher's exact test (p = 0.46).
| Sample size N (females) | Time (months) between visit (mean ± std. dev) | Age (mean ± std. dev.) in years | Gender (M:F) | Clinical NIH-SS (mean) | |
|---|---|---|---|---|---|
| Normal controls | 14 (6) | 9.7 ± 2.5 | 59.6 ± 4.9 | 8: 6 | n/a |
| Stroke patients | 20 (5) | 8.7 ± 6.3 | 59.2 ± 12.5 | 15: 5 | 1.79 |
2.2. MRI acquisition
Magnetic resonance images (MRI) were collected at two different time points. For the patients, the first visit (S1) occurred on an average of 3.2 months after stroke onset (between 10 days and 6 months, subacute phase of the stroke) and a second visit (S2) occurred on an average of 8.7 months after the initial visit (chronic phase of the stroke). For our control group of healthy old subjects, the two visits (N1 and N2) were separated on an average of 9.7 months [Table 1]. Neuroimaging data were collected at the University of Wisconsin-Madison, using a 3.0-Tesla GE Discovery MR750 scanner (GE Healthcare, Waukesha, WI) equipped with an 8-channel head coil. High resolution T1-weighted images were acquired using inversion-recovery prepared spoiled gradient recalled (SPGR) BRAVO sequence with 156 slices, isotropic 1 × 1 × 1 mm3, over a 256 × 256 matrix, TR = 8.132 ms, TE = 3.18 ms, TI = 450 ms, FOV = 256 mm, flip angle = 12°. The resting-state session consisted of a 10-minute eyes-closed, task-free scan where the participants were instructed to relax, but remain still and awake for the duration of the scan. The task-free, resting-state scan was acquired using a single-shot T2*-weighted gradient-echo echo planar imaging, with 40 sagittal slices, TR = 2.6 s, TE = 22 ms, FOV = 224 mm, flip angle = 60°, isotropic 3.5 × 3.5 × 3.5 mm3 voxel. Participants were provided earplugs to attenuate scanner noise and foam padding to reduce head motion. Participants were also reminded to keep their head still before each scan.
2.3. Data pre-processing
Pre-processing of the neuroimaging data was performed using SPM8 (Wellcome Trust Centre for Neuroimaging, University of College London, United Kingdom), in which the first 10 volumes were discarded to allow for magnetization to reach equilibrium. The images were then slice-time corrected and spatially re-aligned to correct for head motion. Data spikes were removed using AFNI's 3dDespike function (https://afni.nimh.nih.gov/pub/dist/doc/program_help/). The images were then normalized to MNI template place and smoothed with a 4-mm Gaussian kernel. The pre-processed images from the two visits were then introduced as paired-samples to a group ICA (GIFTv2.0, http://mialab.mrn.org/software/gift/), a blind source signal separation method. A mid-order 28 independent component model was implemented using Infomax algorithm (Bell & Sejnowski, 1995), standard PCA type, and back-reconstruction using GICA method. Reliability of the ICA algorithm was assessed using the ICASSO toolbox (http://www.cis.hut.fi/projects/ica/icasso/) (Himberg et al., 2004), with 20 iterations using RandInit and Bootstrap methods.
Head motion was assessed using a Euclidean norm (enorm—square root of the sum of squares) approach, computed with AFNI's 1d_tool.py (https://afni.nimh.nih.gov/pub/dist/doc/program_help/), to characterize the possible contribution of head motion to the observed effects. No difference was found between the populations in terms of head motion during the resting state scan (ANOVA: F-value = 1.316, p-value = 0.277) (Suppl. mat. B), providing assurance that any observed effect of slow-5 oscillation amplitude recovery would not have received significant contributions from head motion.
2.4. Analyses
We restricted our current investigation to five groupICA derived components: three components of the DMN (posterior DMN (pDMN), anterior DMN (aDMN), and ventral DMN (vDMN)), and two robust ‘task-positive’ network (TPN) components of the primary visual and sensorimotor networks. The five network components are identified by their high correlation to DMN, lower visual, and sensori-motor templates (Allen et al., 2011), and are illustrated in Fig. 2. Sub-networks of the DMN have been selected to be investigated primarily because of consistently reported DMN disruption found in clinical populations. Additionally, regions of the DMN are located deeper within the cortical structure with extensive vascularization, protecting those areas from direct stroke susceptibility. Despite lesions in the primary visual cortex in our stroke population, the primary visual and sensorimotor components were selected to represent the ‘task-positive’ networks because of their robust nature in the resting-state. Robustness of network during resting-state condition is assessed by the ratio (Power LF/Power HF).
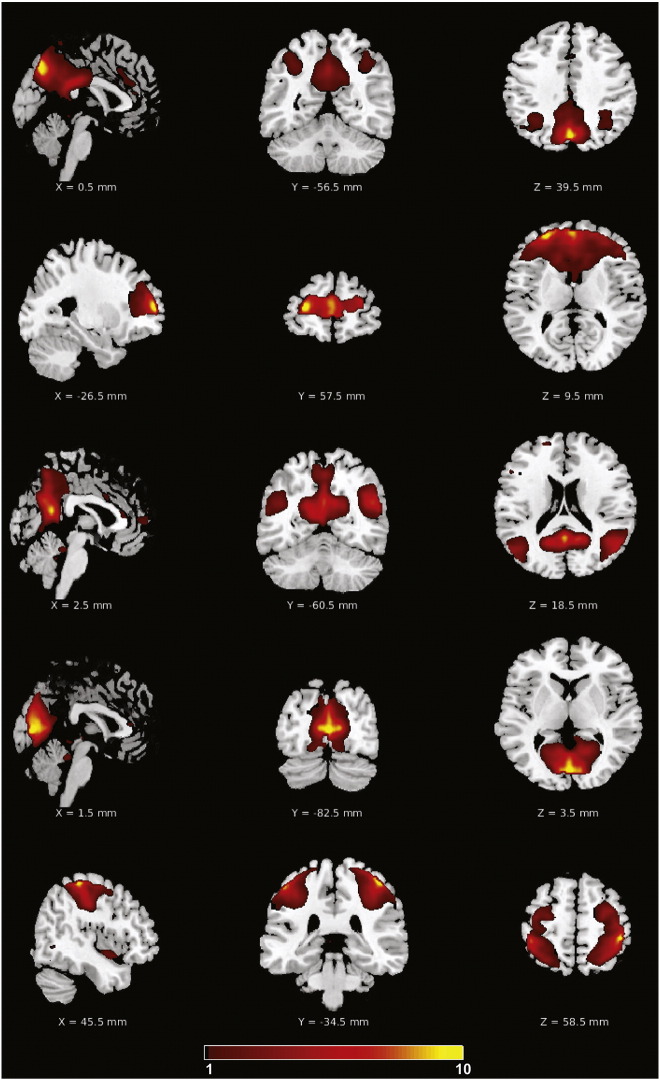
Fig. 2.
Consistent group ICA derived network components. From top to bottom: pDMN, aDMN, vDMN, primary visual and sensorimotor components.
For each network/subnetwork, corresponding independent component (IC) time-series was generated, followed by a Fast Fourier Transform to obtain its frequency distribution (or spectra) (Garrity et al., 2007, Allen et al., 2011, Gohel and Biswal, 2014, Calhoun et al., 2008). Component spectra were further smoothed with a Gaussian filter (σ = 2). Spectral characteristics of the low-frequency oscillations were assessed using two different approaches. We conducted an analysis of spectral power within the slow-5 (0.01–0.027 Hz) range of the group IC time-series for each of the five investigated components (pDMN, aDMN, vDMN, primary visual and sensorimotor) using a measure of fALFF applied to oscillations of an ICA-derived network component, similar to a method from La et al. (La et al., 2015a, La et al., 2015b). The current analysis was concentrated on the changes occurring in the slow-5 oscillation range. Linear mixed-effect analysis (with maximum likelihood method) was implemented on the measure of slow-5 component fALFF to account for the repeated time-points. Slow-5 component fALFF provides an estimate of the relative power of the oscillations within the slow-5 oscillation range in comparison to the oscillations over the whole assessed frequency range. We further reviewed the changes in frequency distribution by assessing the deviation of the spectra between the two scans within each of the participant by subtracting the spectra from the two different time-points, removing the effect of inter-subject differences. We proceeded with an analysis of the quantitative measure of the area under the curve (AUC) of those spectral differences within the slow-5 fluctuation range, which were tested for deviation from null hypothesis (mean = 0), with statistical significance assessed by one sample t-tests, equivalent to a paired two-sample t-test between the two visits.
3. Results
The 28 component mid-order group ICA generated 21 independent components, which shared spatial distribution resembling previously identified networks (DMN, motor, auditory, executive control, salience, etc.), and encompassed 86.9% of the total recorded variance. We focused on five of the components (3 components of the DMN, a primary visual component, and a sensorimotor component), and presented a longitudinal analysis of the frequency distribution of the intrinsic low-frequency oscillations in the two populations (OHAs and stroke patients) at two different time-points.
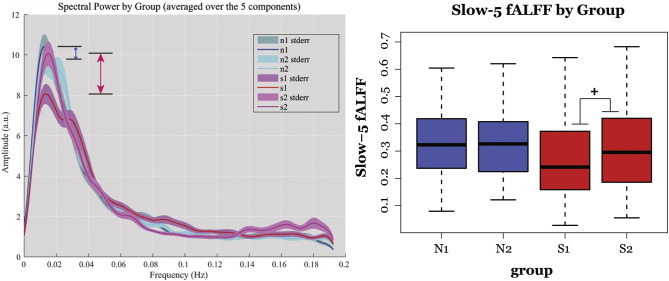
The measure of slow-5 fALFF of the IC provided a quantitative measure for the assessment of this reduction, describing the relative power of the oscillation within the slow-5 frequencies to that over the whole assessed frequency range. With the different network components combined [Fig. 3], similarly to Calhoun et al. (Calhoun et al., 2011), linear-mixed effects model by maximum likelihood on the composite component (combined DMN, visual, and sensorimotor) identified a difference between the two groups that is trending toward significance in a linear-mixed effect model (effect of group, LME: t32 = 1.934, p = 0.068; two-sample t-test S1-N1: t32 = 2.914, p = 0.004**). More interestingly, the linear mixed effect model revealed a significant trend in terms of Group × Time interaction in the stroke group in their progression toward their chronic stage (LME: t304 = 1.887, p = 0.060). While the mean slow-5 fractional spectral power values in the normal group remained stationary between the two sessions from 0.316 to 0.331 (a difference of 0.015, paired t-test: t12 = 0.092, p = 0.927), the stroke patient group transitioned from 0.270 in the subacute stage to 0.316 in their chronic stage (an increase of 0.047, paired t-test: t18 = 2.273, p = 0.004**), illustrated in the boxplots in Fig. 4 (right panel). After recovery, patients in their chronic phase reached a comparable level of slow-5 fALFF, similar to the normal healthy volunteers (two sample t-test: t32 = 0.667, p = 0.505).
Fig. 3.
Mean spectra and slow-5 fALFF for combined components (pDMN, aDMN, vDMN, visual and sensorimotor). Left: group mean spectra with arrows representing the difference between the two time-points within a group (Blue: healthy aged-matched controls, Red: stroke subjects between subacute to chronic stage). Right: boxplot of slow-5 fALFF per group with whiskers representing the upper and lower fence. The linear mixed-effect model revealed a trend toward significant difference between the groups (p < 0.08), and more importantly a trend toward significance in Group × Time interaction (+ p < 0.08).
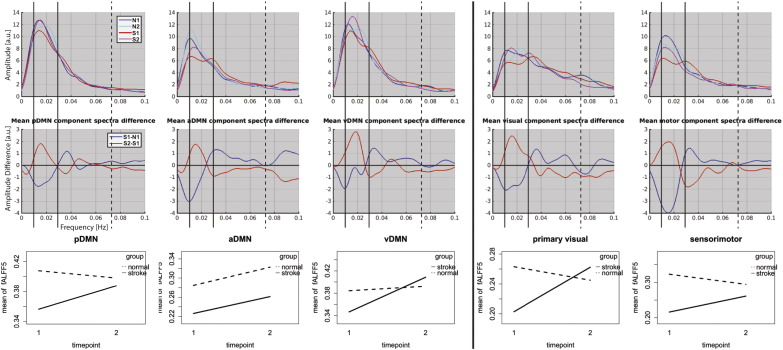
Fig. 4.
Change in frequency distribution between visit1 and visit2. [Top]: each mean spectra for normals at visit1 (blue—N1), visit2 (cyan—N2), stroke_visit1 (red—S1), and stroke_visit2 (magenta—S2). Solid vertical lines demark limits of the slow-5 oscillation range (0.01–0.027 Hz) and dotted vertical lines mark the upper limit of the slow-4 frequency range (0.027–0.073 Hz). [Middle]: Difference in the mean spectra with stroke_visit2 minus stroke_visit1 (red) and stroke_visit1 minus normal_visit1 (blue). Stroke patients in their subacute stage exhibited lower slow-5 oscillations amplitude compared to the age-matched controls. However, consistent increases in slow-5 oscillation relative power were revealed in each of the five networks in the later stage of the stroke (chronic). [Bottom]: Interaction plot of longitudinal recovery of slow-5 component fALFF between visit1 and visit2 in ischemic stroke patients (solid line) in comparison to normal controls (dotted line).
Differences were also seen within individual networks. Fig. 4 illustrates a deficit in the slow-5 oscillations in our stroke patients in their subacute stage compared to their age-matched normal counterparts indicated by a dip in the blue plot (S1-N1) [Fig. 4, middle row]. Reduced slow-5 oscillation amplitude consistently occurred in all network components in the subacute stroke patients. In the pDMN, the disparity amounted to 0.052 difference in the two groups (0.408 in normal old (N1), and 0.356 in subacute stroke patients (S1), versus 0.398 in normal old in their second time point (N2)) [Fig. 4, bottom row]. Other networks presented similar results of lower slow-5 fALFF in the subacute group: aDMN (0.285 in OHA vs. 0.226 in subacute stroke), vDMN (0.385 in OHA vs. 0.347 in subacute stroke), visual (0.263 in OHA vs. 0.202 in subacute stroke), and sensorimotor (0.323 in OHA vs. 0.216 in subacute stroke) [Fig. 4, bottom row], verifying the presence of a uniform reduction in slow-5 oscillation amplitude at the subacute time-point of the stroke across the different assessed networks. The review of the frequency distribution within each network component also revealed a consistent pattern of frequency distribution in the stroke patients during the recovery phase (between subacute to chronic time-points), primarily observed within the slow-5 fluctuations [Fig. 4, middle row, red]. The pDMN showed a change in slow-5 relative power (measured by component fALFF) from 0.356 to 0.388 between the two visits. Other components showed similar increases (aDMN: 0.226 to 0.261; vDMN: 0.347 to 0.409; visual: 0.203 to 0.262; motor: 0.216 to 0.261) [Fig. 4, bottom row]. In contrast, the aging control group displayed minimal change between the two time-points (pDMN: 0.408 to 0.398; aDMN: 0.285 to 0.323; vDMN: 0.384 to 0.393; visual: 0.263 to 0.245; motor: 0.323 to 0.294). These longitudinal changes for each of the components are illustrated as interaction plots (bottom panel of Fig. 3). After subtraction of the frequency distribution from time-point 2 to time-point 1, the area under the curve (AUC) of these subtractions were assessed against the null hypothesis of zero difference between the two time-points, equivalent to a paired-sample t-test. The resulting amplitude plot demonstrated a consistent positive deflection from the null hypothesis of zero near the 0.018 Hz frequency [Fig. 5]. A quantitative area under the curve measure was computed, and provided some evidence for the implication of the slow-5 oscillations during this stage, known for recovery. Components of the DMN exhibited trends toward significance (pDMN: t19 = 1.333, p = 0.099; vDMN: t19 = 1.611, p0.061, one tailed), while positive deflections in the primary visual (t19 = 2.494, p = 0.011, one tailed) and sensorimotor (t19 = 1.681, p = 0.055, one tailed) components reached stronger significance. No significant effect was found in the aDMN, possibly influenced by the earlier increase (i.e. lower frequency) of high slow-5 (0.024–0.027 Hz) in the second time-point, an increase normally observed in frequencies of the next higher fluctuation range (i.e. slow-4) [Fig. 5].
Fig. 5.
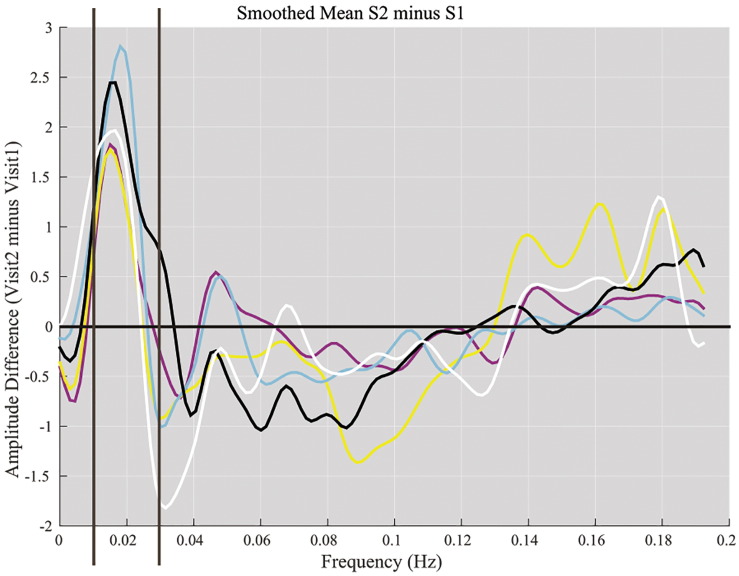
Average across subjects of the difference between time-point 1 (subacute) and time-point 2 (chronic) per component. [color coding: pDMN (magenta), aDMN (yellow), vDMN (cyan), visual (black), and motor (white)]. A consistent positive deflection was observed near 0.017–0.02 Hz range across all five network components.
The observed component fALFF in the slow-5 oscillation range showed associations with behavioral performance. When subjects were grouped into high versus low performers (using a median split) in a cognitive task of phonemic verbal fluency, differences were noted. Lower values of slow-5 oscillation fALFF were observed for stroke patients with lower verbal fluency scores in comparison with the group with higher scores (unpaired two-sample t-test: t98 = 2.763, p = 0.007**, Suppl. mat. C). However, regression analysis of the limited dataset of subjects with verbal fluency score on both visits (14 of the 20 stroke patients) did not find significant correlation between the level of behavior recovery and the recovery of slow-5 oscillations.
4. Discussion
Neuronal communication is mechanistically sub-served by the coherence of neuronal signal (Fries, 2005). Activated neuronal groups oscillate and undergo rhythmic excitability fluctuations that produce viable temporal windows for communication (Canolty and Knight, 2010, Siegel et al., 2012). Functional networks in resting-state fMRI are defined by such characteristic of their intrinsic fluctuations. In the event of disruption of those oscillations, synchronization between regions would likely be impaired, ultimately having detrimental effects on the functionality of the network. This was previously demonstrated in various clinical populations, where functional connectivity (or long-distance synchronization) and network co-activation (within network synchronization) decreases were observed in many of the cortical networks, including the DMN (Broyd et al., 2009, Greicius, 2008).
Based on the initial identification by Pentonnen and Buzsaki (Penttonen & Buzsáki, 2003), various groups have investigated the resting-state oscillations with the distinction of slow-5 (0.01–0.027 Hz) and slow-4 (0.027–0.073 Hz). Beyond demonstrating that those slow-5 and slow-4 oscillations were primarily restricted to gray matter, Zuo and colleagues (Zuo et al., 2010) established that slow-5 oscillations were the major contributors of resting state fluctuations in healthy normal volunteers, consistent with other works as well (Garrity et al., 2007, Calhoun et al., 2011, Calhoun et al., 2008). Previous work from our group showed that this dominance of the slow-5 oscillations, however, may not persist in clinical populations such as ischemic stroke patients (La et al., 2014, La et al., 2015a). In a cross-sectional study with four groups (43 healthy young, 42 healthy old, 14 acute stroke, and 18 subacute stroke), we have presented results showing alteration of the resting-state fluctuation in the subacute stage of stroke, with such an alteration potentially arising specifically from a decrease in slow-5 oscillations, in agreement with other studies reporting similar decrease in spectral power in schizophrenia and bipolar disorders (Garrity et al., 2007, Calhoun et al., 2011, Calhoun et al., 2008).
In this present study, we performed a longitudinal analysis in a stroke population to assess the viability of a return to normal of the intrinsic slow-5 oscillation power in the later chronic phase of stroke, 6 months or greater after initial onset. Additionally, a longitudinal investigation of stroke patients also allows this investigation to control for between-subject cerebro-vascular reactivity (CVR) differences. It has previously been demonstrated that CVR variability in the later stage, after the initial disruption from the acute stroke injury, is rather limited, resulting in a stable CVR in the subacute to the later stage of the stroke (chronic) within a subject (Geranmayeh et al., 2015). Our results have presented a characterization of disrupted network oscillations in the subacute stroke population and demonstrated that recovery of slow-5 oscillation power and return to normal spectral power level are possible during the chronic stage of stroke.
It has been demonstrated in an earlier study from our group (La et al., 2015b) that impairment from ischemic stroke onset may impact/disrupt various cortical networks other than the network directly disrupted by the stroke-induced lesion, possibly due to a diaschisis effect resulting in a globalized deficit. This statement is supported by Honey & Sporns (Honey & Sporns, 2008), where they hypothesized that lesion effects extend beyond the immediate neighbors of the lesioned site with lesions of hub regions with long-range connections like parietal and frontal areas leading to wider and greater widespread disturbance. Seitz et al. (Seitz et al., 1999) also demonstrated that recovery of stroke-induced impairment is sub-served by brain structures in locations remote from the stroke lesion. Electroencephalography coherence studies have similarly shown that recovery of function was associated with an increase in cortico-cortical coherence from remote regions (Strens et al., 2004). Additionally, Nair et al. (Nair et al., 2015) reported impaired resting-state functional connectivity in the language network in stroke patients in the early stage of stroke that returned to near normalcy in latter stages of stroke. These studies independently suggest an impairment from stroke remotely that extends beyond directly overlapping regions involving the stroke lesion. The observed similarity in the pattern of alteration (disruption in the subacute stage and recovery in the chronic stage) in individual networks remote from the stroke lesion justifies the combining (averaging) of the network component spectra.
Analogous to the method implemented by Calhoun and colleagues (Calhoun et al., 2011), we combined component spectra from various components to form a composite component of the five assessed systems before assessing for statistical significance. The combination of spectra (Fig. 4) illustrated a consistency of the spectral deficit in the slow-5 band, in agreement with our previous findings. This lower level of slow-5 oscillation amplitude was also in correlation to lower cognitive scores as recorded as recorded in a verbal fluency task. We extended our analysis of the power spectra from the prior study with the addition of a longitudinal component investigating the observed difference between two time-points separated, on average, by a period of 9 months. Through a linear mixed-effects model, we observed a trend toward significance of the Group × Time interactions on amplitude of the slow-5 fALFF in the stroke population. Though it is difficult to determine whether recovery of slow-5 oscillations is part of the functional recovery due in part to the lack of substantial behavioral measures, the observed slow-5 recovery in our ischemic stroke population occurred in a time window where recovery is known to occur (Cramer, 2004). This finding is also in concordance with previous work by Tuladhar et al. (Tuladhar et al., 2013), where connectivity within the DMN was near complete restoration at 6 months post injury.
The recovery of fluctuation amplitudes occurred prominently at oscillations of approximately 0.017–0.021 Hz. Subtraction of the amplitude of oscillations in the chronic stage from the subacute stage showed that deflections in stroke subjects were the most prominent in that range, with ‘task-positive’ networks showing the highest significance [Fig. 5]. Posterior and ventral sub-components of the DMN drew trends toward significance, with significance of the observed effect potentially limited by our restricted sample size. The disruption in our stroke population may be more readily influencing sub-networks of DMN, but recovery of those oscillations within the DMN sub-components may be less consistent. Our current results are also consistent with findings of a previous study (Zhu et al., 2015), which showed activity changes were more extensive in the slow-5 band within a stroke population. The significance of those oscillations is, however, currently unknown. Our results suggest that these fluctuations, which exhibited maximal amplitude in healthy individuals, can be disrupted in the subacute phase and may recover in the later chronic phase of stroke, serving as a marker of the integrity of the functional network. Deflections from the null hypothesis of zero difference between the two time-points were present at other frequencies (i.e. higher frequencies) as well, but demonstrated little consistency among the networks.
Comparatively, an earlier EEG study demonstrated an alpha (7–13 Hz) power peak frequency decrease in stroke patients, with this decrease in alpha band coherence between a given node and the rest of the brain being highly predictive of deficits, independent of anatomical lesions in the area (Dubovik et al., 2012). Though alpha rhythms and the infra-slow oscillations in slow-5 are on very different scales in terms of oscillations, different rhythms are associated with different spatiotemporal scales, with low-frequency oscillations presenting long time windows useful for synchronizing distant network areas with large conduction delays, and high-frequency oscillations presenting short time windows for the synchronization of small, nearby, groups of neurons (Buzsáki and Draguhn, 2004, Canolty and Knight, 2010, Siegel et al., 2012). Phase synchronization between alpha oscillations in different brain areas may allow for effective network communication in active task-relevant neuronal processing (Von Stein et al., 2000, Palva and Palva, 2011), synchronization that is potentially disrupted in the event of a stroke.
These same slow alpha fluctuations have also been demonstrated to contribute to resting BOLD connectivity through mode of cross-frequency coupling (Wang et al., 2012), with BOLD signals providing amplitude envelopes of concurrent EEG range oscillations (Leopold et al., 2003, Goldman et al., 2002, Mantini et al., 2007). These distinct amplitude envelopes provide a modulation of alpha frequency signaling. As the dominant oscillations of the infra-slow rs-fMRI resting-state fluctuations, slow-5 oscillations may provide such distinct amplitude envelopes underlying the opening and closing of temporal windows at the global, cortical scale, for coherently oscillating neuronal groups. Furthermore, Schölvinck and colleagues (Schölvinck et al., 2010) demonstrated a correlation between the intrinsic fluctuations of the resting fMRI signal and local field potentials (LFPs) at the lower frequencies (2–15 Hz) with no lag, and LFPs at the upper gamma-range frequencies (40–80 Hz) with a lag of 6–8 s (greater functional distance), demonstrating a tight coupling of resting state oscillations with the underlying neural activity.
In stroke, the number of investigations of the post-stroke effects using electro-encephalography has been very limited, but quantification of abnormal EEG in the affected and unaffected hemisphere has been demonstrated by Giaquinto et al. (Giaquinto et al., 1994). In addition, they showed significant improvement of the power spectrum in the first 3 months, showing that quantified EEG undergoes early and subtle changes in the follow-up of stroke. However, no correlation between their measure of quantified EEG and clinical measures was found. Another study (Juhasz et al., 1997) has similarly demonstrated improvement of alpha activity over the affected hemisphere, with no recovery of alpha peak frequency in patients with poor indication of recovery in their neurologic status. With the proposed association between EEG frequencies of alpha and the infra-slow oscillations recorded by fMRI, it possible that our observations of reduced slow-5 oscillations and recovery of those in the later stage, are in support of findings from those quantified EEG studies, but in the infra-slow oscillations detectable by fMRI.
In regard to the influence of intrinsic slow-5 oscillations on network functional connectivity, we argue that these oscillations may have a role in the modulation of functional connectivity, a hypothesis introduced by Di et al. (Di et al., 2013a). However, while they have suggested that ALFF may be related to functional connectivity as an increase in the physiological noise, since physiological noise is known to influence both measures (functional connectivity (Birn et al., 2006, Birn et al., 2008); and ALFF (Biswal et al., 2007, Di et al., 2013b)), the present results suggest that the correlation of ALFF to functional connectivity may be due to the amplitude of the slow-5 oscillations, dominant in the resting state underlying neural synchronization, and these are the oscillations that are disrupted following the event of an ischemic stroke. The influence of physiological noise on functional connectivity and power spectra should not be understated. The current study lacked physiological signal recording of cardiac and respiratory cycles. However, such contributions have been suggested to influence fluctuations outside of the slow-5 oscillation range (~ 0.03 Hz for respiratory variations (Birn et al., 2006) and ~ 0.04 Hz for hypercapnic conditions (Biswal et al., 2007)) and would have influenced the resting-state oscillations equally among the populations, hence not contributing to group differences in the oscillation amplitudes within the slow-5 range but within the slow-4 band. Furthermore, ICA has been found to be relatively robust to respiration-related fluctuations, separating respiration-volume variations into separate components (Birn et al., 2008). Thorough exploration of the slow-4 oscillations would have benefitted the study, despite the non-dominance of those oscillations in the healthy adult resting-state. But, because of this lack of physiological recording, we were not able to evaluate changes observed within that frequency range.
Another limitation is the absence of a full-length neuro-psychological dataset, limiting the interpretation of our results. Clinical assessment with NIH-SS (National Institutes of Health Stroke Scale) has been collected for each participant. Most of our stroke patients had mild strokes as characterized by their NIH-SS (scale from 0 to 42, with 0 denoting no stroke symptoms and 42 a severe stroke, our stroke population mean NIH-SS score = 1.79), therefore the values of these scores were limited as small variability was seen between our subjects. Cognitive scores were collected for only some of the participants (14 of the 20 stroke patients) as subjects opted out of the behavioral assessment or the assessment were not completed for both sessions for the recording of longitudinal cognitive changes. The cognitive evaluations were also limited in the number of assessments. Therefore in this study, we were limited in the ability to characterize brain-behavior correlations and limit our discussion to a recovery of the slow-5 oscillation over the time window known to present spontaneous recovery.
The non-uniformity of the stroke-induced lesions (demonstrated in lesion density map [Fig. 1]) also limits the consistency of the functional and structural deficits across the stroke populations in the study. Despite this limitation, we were able to investigate selected networks and subnetworks to assess remote effects of stroke irrelevant to lesion location (see method section). Sub-networks of the DMN with regions residing deeper cortically and being highly vascularized, presented lesser vulnerability to common strokes than other systems, and were therefore good candidates for the investigation of a stroke diaschisis effect. Additionally, the DMN has repeatedly been shown to be implicated in cortical disruption in various neurological disorders. We also investigated the effect of stroke on two task-positive networks, early visual network and a sensori-motor network, two robust networks during resting-state, which allowed for the investigation of the effect of stroke on networks other than the DMN. It is important to note that 5 lesions belonged to regions of the visual cortex or part of the visual system, and 3 lesions belonged to the regions involved in motor and somatosensory association cortex, likely having a contribution on oscillation distribution, potentially influencing the results and limiting our interpretation. Yet, the effect of recovery of slow-5 oscillation amplitude was observed in all of networks rather than only lesioned networks, suggesting the effect to be much more global and not restricted to a specific network.
The large variability in the time of first assessment and time-delay between the subacute (S1) and chronic (S2) could have influenced the level of recovery observed. However, as shown in Fig. 4, oscillation amplitudes in the slow-5 band were able to recover to a near-normal level at the second time-point, suggesting that variation between time-points may not have played an influential role in the recovery. Instead, other factors, such as stroke severity or baseline component fALFF at time of injury may play a larger role in determining the level of power spectrum recovery. The present population in the study, however, did not permit this distinction as most of our stroke patients were very mildly impaired.
This current investigation also ran into the issue of power, with only 20 ischemic stroke subjects to be assessed longitudinally. However, a longitudinal study does allow us to somewhat address the issue of the effects of CVR, as although there are between-subject variability in CVR, within-subject CVR over time has been demonstrated to remain stable after initial injury (Geranmayeh et al., 2015). Also, combining the different network components and creating the composite component allowed for an improvement in the power of the analysis, minimizing contributions from CVR and other physiological responses. Yet, further studies with larger cohort are needed to validate our current findings.
Despite those limitations, findings from this study support and reinforce the novel hypothesis of an implication of the slow-5 oscillations in network integrity, disrupted in clinical population (Liu et al., 2014, La et al., 2015a, Yu et al., 2014), but also suggest that those specific oscillations in the slow-5 frequency range may have a role in the recovery of those functional networks after injury. Though the significance of these oscillations is not currently clearly defined, those oscillations may play a distinct role in the synchronization between distant network nodes, and offer an amplitude envelop for the coordination of local neuronal processing. For clinical population, this opens up new avenues of therapy-oriented research, with impaired oscillation potentially being targeted to enhance recovery of cortical systems or networks, and limit cortical disruption following injury.
5. Conclusions
Injury to the cortical system often elicits a global reorganization of the brain, from changes in patterns of activation to cortical network synchronization. Previously, disruption of the system has been associated with a reduction of spectral power in the frequency distribution of the low intrinsic oscillations in the resting state. More precisely, oscillations in the range of 0.01–0.027 Hz have been cited as having a role in the reduction of network co-activation. Our study not only provides evidence that such oscillations are disrupted in the event of a stroke, but that such oscillations have a potential for recovery after the initial injury as demonstrated in our ischemic stroke population in their chronic phase. Across the different networks assessed, all networks exhibited a rebound in those fluctuations in the later stage post-stroke, confirming our hypothesis of an implication of the slow-5 oscillations in normal functioning networks. Ultimately, our results also suggest slow-5 oscillations could serve as a potential bio-marker of functional network health, oscillations that could potentially be targeted for an enhancement of network recovery in the event of disruption.
The following are the supplementary data related to this article.
Clinical, Demographic, and Session Information for the 20 Ischemic Stroke patients enrolled. Note: Temp-Occ, Temporal-Occipital; Temp-Par, Temporal-Parietal.
Estimation of Head Motion per Population groups. Using the Euclidean norm (enorm) approach, no difference were found between the population in terms of head motion during the resting state scan (ANOVA: F-value = 1.316, p-value = 0.277).
Verbal Fluency Performance correspondence to slow-5 fALFF. With discriminations of high versus low performer (using a median split) in a cognitive task of phonemic verbal fluency, differences were noted. Lower values of slow-5 oscillation fALFF were observed for stroke patients with lower verbal fluency scores (unpaired two-sample t-test: t98 = 2.763, p = 0.007**).
Disclosures
The authors of this manuscript declare no existence of conflicting financial interest or any other possibly conflicting interest.
Acknowledgement
This study was supported by grants RC1MH090912, K23NS086852, and AHA Innovation Grant and Midwest Affiliate Grant-in-Aid funding 2015 (VP). CL was supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS) grant UL1TR000427, the NTP training grant T32-GM007507, and the CNTP training grant T32EB011434. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- Allen E.A. A baseline for the multivariate comparison of resting-state networks. Front. Syst. Neurosci. 2011;5 doi: 10.3389/fnsys.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J.R. Disruption of large-scale brain systems in advanced aging. Neuron. 2007;56(5):924–935. doi: 10.1016/j.neuron.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann C.F. Investigations into resting-state connectivity using independent component analysis. Philos. Trans. R. Soc., B. 2005;360(1457):1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell A.J., Sejnowski T.J. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995;7(6):1129–1159. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- Birn R.M. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI. NeuroImage. 2006;31(4):1536–1548. doi: 10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- Birn R.M., Murphy K., Bandettini P.A. The effect of respiration variations on independent component analysis results of resting state functional connectivity. Hum. Brain Mapp. 2008;29(7):740–750. doi: 10.1002/hbm.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Biswal B.B., Kannurpatti S.S., Rypma B. Hemodynamic scaling of fMRI-BOLD signal: validation of low-frequency spectral amplitude as a scalability factor. Magn. Reson. Imaging. 2007;25(10):1358–1369. doi: 10.1016/j.mri.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B.B. Toward discovery science of human brain function. Proc. Natl. Acad. Sci. 2010;107(10):4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluhm R.L. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr. Bull. 2007;33(4):1004–1012. doi: 10.1093/schbul/sbm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyd S.J. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci. Biobehav. Rev. 2009;33(3):279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Buzsáki G., Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304(5679):1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Calhoun V.D., Kiehl K.A., Pearlson G.D. Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Hum. Brain Mapp. 2008;29(7):828–838. doi: 10.1002/hbm.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun V.D. Exploring the psychosis functional connectome: aberrant intrinsic networks in schizophrenia and bipolar disorder. Frontiers in Psychiatry. 2011;2 doi: 10.3389/fpsyt.2011.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canolty R.T., Knight R.T. The functional role of cross-frequency coupling. Trends Cogn. Sci. 2010;14(11):506–515. doi: 10.1016/j.tics.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter A.R., Shulman G.L., Corbetta M. Why use a connectivity-based approach to study stroke and recovery of function? NeuroImage. 2012;62(4):2271–2280. doi: 10.1016/j.neuroimage.2012.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkassky V.L. Functional connectivity in a baseline resting-state network in autism. Neuroreport. 2006;17(16):1687–1690. doi: 10.1097/01.wnr.0000239956.45448.4c. [DOI] [PubMed] [Google Scholar]
- Cramer S.C. Functional imaging in stroke recovery. Stroke. 2004;35(11 Suppl. 1):2695–2698. doi: 10.1161/01.STR.0000143326.36847.b0. [DOI] [PubMed] [Google Scholar]
- Damoiseaux J. Reduced resting-state brain activity in the “default network” in normal aging. Cereb. Cortex. 2008;18(8):1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- Di X. The influence of the amplitude of low-frequency fluctuations on resting-state functional connectivity. Front. Hum. Neurosci. 2013;7 doi: 10.3389/fnhum.2013.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di X. Calibrating BOLD fMRI activations with neurovascular and anatomical constraints. Cereb. Cortex. 2013;23(2):255–263. doi: 10.1093/cercor/bhs001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovik S. The behavioral significance of coherent resting-state oscillations after stroke. NeuroImage. 2012;61(1):249–257. doi: 10.1016/j.neuroimage.2012.03.024. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Raichle M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn. Sci. 2005;9(10):474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Friston K. Functional connectivity: the principal-component analysis of large (PET) data sets. J. Cereb. Blood Flow Metab. 1993;13:5. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- Garrity A.G. 2007. Aberrant “Default Mode” Functional Connectivity in Schizophrenia. [DOI] [PubMed] [Google Scholar]
- Geranmayeh F. Measuring vascular reactivity with breath-holds after stroke: a method to aid interpretation of group-level BOLD signal changes in longitudinal fMRI studies. Hum. Brain Mapp. 2015;36(5):1755–1771. doi: 10.1002/hbm.22735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinto S. EEG recordings in the course of recovery from stroke. Stroke. 1994;25(11):2204–2209. doi: 10.1161/01.str.25.11.2204. [DOI] [PubMed] [Google Scholar]
- Gohel S.R., Biswal B.B. 2014. Functional Integration Between Brain Regions at Rest Occurs in Multiple-Frequency Bands. Brain connectivity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R.I. Simultaneous EEG and fMRI of the alpha rhythm. Neuroreport. 2002;13(18):2487. doi: 10.1097/01.wnr.0000047685.08940.d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefkes C. Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Ann. Neurol. 2008;63(2):236–246. doi: 10.1002/ana.21228. [DOI] [PubMed] [Google Scholar]
- Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr. Opin. Neurol. 2008;21(4):424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- Greicius M.D. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc. Natl. Acad. Sci. U. S. A. 2004;101(13):4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y. Regional coherence changes in the early stages of Alzheimer's disease: a combined structural and resting-state functional MRI study. NeuroImage. 2007;35(2):488–500. doi: 10.1016/j.neuroimage.2006.11.042. [DOI] [PubMed] [Google Scholar]
- Himberg J., Hyvärinen A., Esposito F. Validating the independent components of neuroimaging time series via clustering and visualization. NeuroImage. 2004;22(3):1214–1222. doi: 10.1016/j.neuroimage.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Honey C.J., Sporns O. Dynamical consequences of lesions in cortical networks. Hum. Brain Mapp. 2008;29(7):802–809. doi: 10.1002/hbm.20579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhasz C., Kamondi A., Szirmai I. Spectral EEG analysis following hemispheric stroke. Acta Neurol. Scand. 1997;96(6):397–400. doi: 10.1111/j.1600-0404.1997.tb00305.x. [DOI] [PubMed] [Google Scholar]
- Kennedy D.P., Courchesne E. The intrinsic functional organization of the brain is altered in autism. NeuroImage. 2008;39(4):1877–1885. doi: 10.1016/j.neuroimage.2007.10.052. [DOI] [PubMed] [Google Scholar]
- La C. Resting-State Brain Connectivity Conference. 2014. Characteristics of low-frequency oscillations in the impairment of the DMN in the elderly and ischemic stroke patients. Boston, MA. [Google Scholar]
- La C. 2015. Implication of the Slow-5 Oscillations in the Disruption of the Default-Mode Network in Healthy Aging and Stroke. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La C. 2015. Differing Patterns of Altered Slow-5 Oscillations in Healthy Aging and Ischemic Stroke. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold D.A., Murayama Y., Logothetis N.K. Very slow activity fluctuations in monkey visual cortex: implications for functional brain imaging. Cereb. Cortex. 2003;13(4):422–433. doi: 10.1093/cercor/13.4.422. [DOI] [PubMed] [Google Scholar]
- Liu X. Abnormal amplitude of low-frequency fluctuations of intrinsic brain activity in Alzheimer's disease. J. Alzheimer's Dis. 2014;40(2):387–397. doi: 10.3233/JAD-131322. [DOI] [PubMed] [Google Scholar]
- Mantini D. Electrophysiological signatures of resting state networks in the human brain. Proc. Natl. Acad. Sci. 2007;104(32):13170–13175. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair V.A. Functional connectivity changes in the language network during stroke recovery. Ann.Clin. and Transl.Neurol. 2015;2(2):185–195. doi: 10.1002/acn3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva S., Palva J.M. Functional roles of alpha-band phase synchronization in local and large-scale cortical networks. Front. Psychol. 2011;2 doi: 10.3389/fpsyg.2011.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C.H. Longitudinal changes of resting-state functional connectivity during motor recovery after stroke. Stroke. 2011;42(5):1357–1362. doi: 10.1161/STROKEAHA.110.596155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.Y. Significance of longitudinal changes in the default-mode network for cognitive recovery after stroke. Eur. J. Neurosci. 2014;40(4):2715–2722. doi: 10.1111/ejn.12640. [DOI] [PubMed] [Google Scholar]
- Patriat R. The effect of resting condition on resting-state fMRI reliability and consistency: a comparison between resting with eyes open, closed, and fixated. NeuroImage. 2013;78:463–473. doi: 10.1016/j.neuroimage.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penttonen M., Buzsáki G. Natural logarithmic relationship between brain oscillators. Thalamus Relat. Syst. 2003;2(02):145–152. [Google Scholar]
- Saur D. Dynamics of language reorganization after stroke. Brain. 2006;129(6):1371–1384. doi: 10.1093/brain/awl090. [DOI] [PubMed] [Google Scholar]
- Schölvinck M.L. Neural basis of global resting-state fMRI activity. Proc. Natl. Acad. Sci. 2010;107(22):10238–10243. doi: 10.1073/pnas.0913110107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz R.J. The role of diaschisis in stroke recovery. Stroke. 1999;30(9):1844–1850. doi: 10.1161/01.str.30.9.1844. [DOI] [PubMed] [Google Scholar]
- Siegel M., Donner T.H., Engel A.K. Spectral fingerprints of large-scale neuronal interactions. Nat. Rev. Neurosci. 2012;13(2):121–134. doi: 10.1038/nrn3137. [DOI] [PubMed] [Google Scholar]
- Sorg C. Selective changes of resting-state networks in individuals at risk for Alzheimer's disease. Proc. Natl. Acad. Sci. 2007;104(47):18760–18765. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strens L. Corticocortical coupling in chronic stroke: its relevance to recovery. Neurology. 2004;63(3):475–484. doi: 10.1212/01.wnl.0000133010.69694.f8. [DOI] [PubMed] [Google Scholar]
- Tomasi D., Volkow N.D. Aging and functional brain networks. Mol. Psychiatry. 2012;17(5):471. doi: 10.1038/mp.2011.81. 549-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai Y.-H. Disruption of brain connectivity in acute stroke patients with early impairment in consciousness. Front. Psychol. 2013;4 doi: 10.3389/fpsyg.2013.00956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuladhar A.M. Default mode network connectivity in stroke patients. PLoS One. 2013;8(6) doi: 10.1371/journal.pone.0066556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Stein A., Chiang C., König P. Top-down processing mediated by interareal synchronization. Proc. Natl. Acad. Sci. 2000;97(26):14748–14753. doi: 10.1073/pnas.97.26.14748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vonMonakow, Die Lokalisation im Grosshirn und de Abbau der Funktionen durch corticale Herde. Springer, 1914.
- Wang L. Dynamic functional reorganization of the motor execution network after stroke. Brain. 2010;133(Pt 4):1224–1238. doi: 10.1093/brain/awq043. [DOI] [PubMed] [Google Scholar]
- Wang L. Electrophysiological low-frequency coherence and cross-frequency coupling contribute to BOLD connectivity. Neuron. 2012;76(5):1010–1020. doi: 10.1016/j.neuron.2012.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. Altered functional organization within and between resting-state networks in chronic subcortical infarction. J.Cereb. Blood Flow Metab. 2014;34(4):597–605. doi: 10.1038/jcbfm.2013.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward N.S. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain. 2003;126(11):2476–2496. doi: 10.1093/brain/awg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. Amplitude of low frequency fluctuation within visual areas revealed by resting-state functional MRI. NeuroImage. 2007;36(1):144–152. doi: 10.1016/j.neuroimage.2007.01.054. [DOI] [PubMed] [Google Scholar]
- Yu R. Frequency-specific alternations in the amplitude of low-frequency fluctuations in schizophrenia. Hum. Brain Mapp. 2014;35(2):627–637. doi: 10.1002/hbm.22203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang Y. Regional homogeneity approach to fMRI data analysis. NeuroImage. 2004;22(1):394–400. doi: 10.1016/j.neuroimage.2003.12.030. [DOI] [PubMed] [Google Scholar]
- Zhang Z. Altered spontaneous activity in Alzheimer's disease and mild cognitive impairment revealed by Regional Homogeneity. NeuroImage. 2012;59(2):1429–1440. doi: 10.1016/j.neuroimage.2011.08.049. [DOI] [PubMed] [Google Scholar]
- Zhou Y. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophr. Res. 2007;97(1):194–205. doi: 10.1016/j.schres.2007.05.029. [DOI] [PubMed] [Google Scholar]
- Zhu J. Frequency-dependent changes in the regional amplitude and synchronization of resting-state functional MRI in stroke. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0123850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Q.-H. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J. Neurosci. Methods. 2008;172(1):137–141. doi: 10.1016/j.jneumeth.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X.-N. The oscillating brain: complex and reliable. NeuroImage. 2010;49(2):1432–1445. doi: 10.1016/j.neuroimage.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical, Demographic, and Session Information for the 20 Ischemic Stroke patients enrolled. Note: Temp-Occ, Temporal-Occipital; Temp-Par, Temporal-Parietal.
Estimation of Head Motion per Population groups. Using the Euclidean norm (enorm) approach, no difference were found between the population in terms of head motion during the resting state scan (ANOVA: F-value = 1.316, p-value = 0.277).
Verbal Fluency Performance correspondence to slow-5 fALFF. With discriminations of high versus low performer (using a median split) in a cognitive task of phonemic verbal fluency, differences were noted. Lower values of slow-5 oscillation fALFF were observed for stroke patients with lower verbal fluency scores (unpaired two-sample t-test: t98 = 2.763, p = 0.007**).