Abstract
Canine heartworm, Dirofilaria immitis, is a nematode parasite that infects dogs by way of mosquito bite. Rarely, humans play accidental hosts to this parasite and are not a suitable environment for the nematode to live. As the parasite dies in the pulmonary vessels it embolizes the vessels causing infarction and eventual nodule formation in the lungs. In the right clinical context, a nodule can be considered malignant prompting invasive tissue sampling. We describe a case of a 48-year-old man who was found to have multiple asymptomatic scattered pulmonary nodules during imaging workup for an insulinoma. Fine needle biopsy of the largest nodule revealed a necrotic granuloma, lab testing and culture ruled out fungal and bacterial causes. Clinically, this picture was consistent with D. immitis infection.
Keywords: Zoonosis, Parasite, Dirofilaria immitis, Canine host
Introduction
Heartworm (Dirofilaria immitis) is a roundworm parasite that is spread to dogs by mosquito bites, causing heart failure in the definitive host canine [1]. Other hosts may include cats, wolves, foxes and rarely humans [1]. The nematode is found throughout the world and in the United States is endemic to the east, southeastern seaboard, and the southern coast [2]. In the event that a human is bitten by an infected mosquito, the nematode travels from the subcutaneous tissue into the vessels, and eventually enters the right ventricle [2].
The majority of patients are asymptomatic, however non-specific signs and symptoms that have been reported include cough, hemoptysis, chest pain and wheezing [3]. Although Dirofilaria is a parasite, peripheral eosinophilia is only found in about 6.5–15% of the cases [2], [4]. In the right clinical setting, these lesions are presumed to be neoplastic and require the appropriate workup [5].
Case report
We describe a 48-year-old gentleman with a 30 pack-year smoking history who was admitted for refractory hypoglycemia secondary to a suspected insulinoma. Biochemical work up proved positive warranting imaging studies with computed tomography (CT) chest/abdomen/pelvis to look for an underlying insulin-secreting tumor. His complete blood count showed a white blood cell count of 9.9 × 103/μL, hemoglobin of 13.8 g/dL, platelet count of 442 K/μL with an absolute eosinophil count of 0.1 K/μL. Complete metabolic panel revealed a mildly low sodium level and a glucose level of 34 mg/dL but was otherwise unremarkable. His liver functional tests were normal. His insulin level was elevated at 133 mIU/mL, as well as pro-insulin of 46.5 pmol/L, random cortisol of 129.1 mcg/dL, and c-peptide of 9.3 ng/mL. His vitals signs were normal and he was afebrile.
Imaging studies revealed multiple pulmonary nodules, the largest measuring 1.4 cm × 1.2 cm in left lung (Fig. 1). Further serologic work up was negative for fungal and mycobacterial causes of pulmonary nodules as antibodies for histoplasma, aspergillus, coccidioides, and blastomyces were negative by immuno-diffusion and complement fixation. Interferon-gamma release assay for tuberculosis was negative.
Fig. 1.
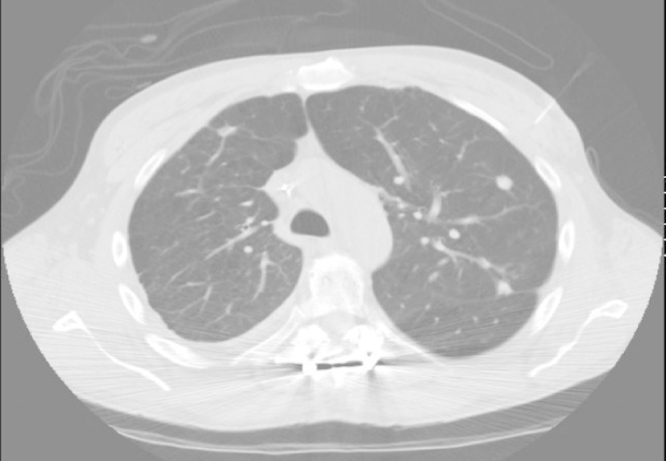
CT scan of the thorax showing multiple pulmonary nodules.
A CT guided core needle biopsy of the largest lung mass was done to rule out neoplasm and revealed a necrotic granuloma on microscopy (Fig. 2). This is not pathognomonic for parasitic disease in this case so other infectious etiologies were considered. The specimen was sent for fungal and mycobacterium culture which were negative at 4 and 6 weeks respectively. No sputum cultures or smears were performed on this patient in the acute setting, this is one of the limitations of our workup.
Fig. 2.
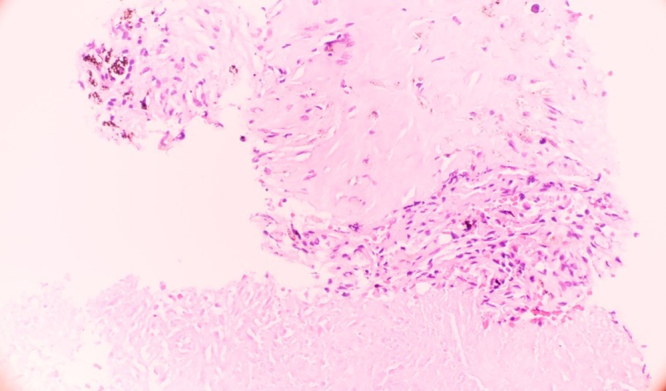
Parasitic nodule demonstrating necrotizing granuloma with histiocytes and fibrosis at the edge.
Further inquiry of patient's social history revealed that the patient has three dogs, one of which was ill with fatigue, weight loss, and hair loss. He could not recall whether he had his dogs were treated for heartworm. The patient denied any travel history to suggest tropical disease and granulomatous diseases such as granulomatosis with polyangiitis and sarcoidosis were ruled out based on the appearance of the biopsy and the absence of any systemic signs or symptoms consistent with inflammatory disease. There were no fevers throughout his hospitalization and lab testing for autoimmune disease was not performed during the workup. While there was not a confirmatory test or obvious parasite on biopsy, clinically the patient was diagnosed with an indolent infection secondary to D. immitis.
The patient was still experiencing repetitive, symptomatic episodes of hypoglycemia while on maximum medical therapy and was transferred to a quaternary care center for management of his biochemical insulinoma. The patient was discharged from the other facility without evidence of insulinoma.
Discussion
In the United States, heartworm infection in dogs and humans are endemic in the east and southeast regions. It is believed that the organism is inoculated into humans via a mosquito bite. From there the microfilaria migrates into subcutaneous tissues, where they mature for 80–120 days. They then migrate into the heart via the capillary system in order to mature for another 6 months. The human body is an unsuitable environment for heartworm and as the nematode dies it embolizes the pulmonary vessels causing infarction and eventual nodule formation that can present as a solitary or multiple pulmonary nodules [1]. The first case of human Dirofilariasis was documented in a Brazilian boy in 1887 by De Magelhaes when he discovered a filarial worm in the left ventricle of the patient [6].
The majority of patients infected with D. immitis are asymptomatic and most commonly present with a solitary pulmonary nodule incidentally found on chest radiography [7]. Occasionally there can be multiple pulmonary nodules mimicking metastatic disease or fungal or mycobacterial infection [7]. Regardless of the number of nodules, these asymptomatic nodules should be evaluated for possible underlying malignancy. In our patient, given his significant smoking history, invasive testing was necessary in order to prove his lesions were not neoplastic.
Diagnosis of Dirofilariasis can be made by a Dirofilaria-specific antibody, through enzyme-linked immunosorbent assay (ELISA). However, the assay is not commonly available [8]. In addition, the ELISA assays are not known to be particularly accurate with problems of cross-reactivity between D. immitis and other filariases [8]. The majority of cases are diagnosed by microscopy which shows a central core of necrosis surrounded by a granulomatous zone of tissues [9].
One feature we would typically expect to see in patients with invasive parasites is peripheral eosinophilia. However, as in our case, many other cases have not shown any eosinophilia. It is believed that the incidence of eosinophilia in infected patients is between 6.5% and 15% [2], [4]. Few of the case reports also document eosinophils on microscopy as part of the inflammatory process [7]. It is not known if there is a trend in the eosinophil level throughout the infectious process or if it trends either way.
It is believed that dog ownership itself is not a risk factor [1]. Based on previous case series, other risk factors include the size of the dog population in the area, the prevalence of D. immitis infection in those dogs, the density of the mosquito population and the degree of human exposure to bites by said mosquitoes [1]. It is possible, though, that the patient's dog was sick due to D. immitis infection without treatment. We hypothesize, therefore, that the patient was exposed to a mosquito that also bit this dog.
One of the limitations of our study was the lack of ELISA antibody testing to help establish a diagnosis. In addition, the fine needle aspiration (FNA) that was performed showed only necrotizing granuloma consistent with a parasitic infection but did not show the actual parasite. However, we are confident in our diagnosis in that we have ruled out other granulomatous infections and conditions.
In conclusion, human pulmonary Dirofilariasis should be considered as a differential diagnosis in patients presenting with asymptomatic solitary or multiple pulmonary nodules in the appropriate epidemiologic and clinical setting. We determine the right clinical setting to be one that has a high rate of D. immitis infected dogs (both stray and domesticated) in addition to having a high mosquito population in order to be able to transfer the parasite from canines to humans.
Conflict of interest
This paper received no funding or grants from commercial or non-commercial sources. There is no conflict of interest in this report.
References
- 1.Milanez de Campos J.R., Barbas C.S., Filomeno L.T., Fernandez A., Minamoto H., Filho J.V. Human pulmonary dirofilariasis: analysis of 24 cases from Sao Paulo, Brazil. Chest. 1997;112:729–733. doi: 10.1378/chest.112.3.729. [DOI] [PubMed] [Google Scholar]
- 2.Asimacopoulos P.J., Katras A., Christie B. Pulmonary dirofilariasis. The largest single-hospital experience. Chest. 1992;102:851–855. doi: 10.1378/chest.102.3.851. [DOI] [PubMed] [Google Scholar]
- 3.Flieder D.B., Moran C.A. Pulmonary dirofilariasis: a clinicopathologic study of 41 lesions in 39 patients. Hum Pathol. 1999;30:251–256. doi: 10.1016/s0046-8177(99)90001-1. [DOI] [PubMed] [Google Scholar]
- 4.Sato M., Koyama A., Iwai K., Kawabata Y., Kojima S. Human pulmonary dirofilariasis with special reference to the ELISA for the diagnosis and follow-up study. Z Parasitenkd. 1985;71:561–563. doi: 10.1007/BF00928360. [DOI] [PubMed] [Google Scholar]
- 5.Pampiglione S., Rivasi F., Paolino S. Human pulmonary dirofilariasis. Histopathology. 1996;29:69–72. doi: 10.1046/j.1365-2559.1996.d01-487.x. [DOI] [PubMed] [Google Scholar]
- 6.Sakurai A., Gohara H., Tajiri N., Ando Y., Maruyama S., Yokoyama S. Human pulmonary dirofilariasis: a case report and a review of 117 cases in Japan (in Japanese with English abstract) Jpn J Clin Radiol. 2006;51:169–173. [Google Scholar]
- 7.Rena O., Leutner M., Casadio C. Human pulmonary dirofilariasis: uncommon cause of pulmonary coin-lesion. Eur J Cardiothorac Surg. 2002;22:157–159. doi: 10.1016/s1010-7940(02)00221-x. [DOI] [PubMed] [Google Scholar]
- 8.So T., Mitsueda R., Miyata T., Sekimura T., Yoshimatsu T., Nose N. Pulmonary dirofilariasis in a 59-year-old man. J Surg Case Rep. 2014 doi: 10.1093/jscr/rju082. pii:rju082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang H.J., Park Y.S., Lee C.H., Yim S.M., Yoo C.G., Kim Y.W. A case of human pulmonary dirofilariasis in a 48-year-old Korean man. Korean J Parasitol. 2013;51:569–572. doi: 10.3347/kjp.2013.51.5.569. [DOI] [PMC free article] [PubMed] [Google Scholar]


