Abstract
Purpose
Several studies have suggested that a combined approach of stress myocardial perfusion imaging (MPI) and coronary computed tomography angiography (CCTA) can provide diagnostic results with excellent accuracy. We aimed to explore whether the addition of CCTA to stress MPI provides incremental diagnostic value in intermediate-to-high cardiovascular risk patients.
Methods
A total of 106 consecutive patients (93 male, 65 ± 10.4 years) underwent coronary artery calcium scoring (CACS), CCTA and 201Thallium stress MPI before coronary angiography was reviewed. Thirty-seven patients (34.9%) had a history of proven coronary artery disease (CAD) or revascularization procedures, and four had documented non-significant CAD (3.8%). The remaining patients consisted of 17 (16.0%) classified as intermediate, and 48 (45.3%) as the high-risk groups.
Results
Obstructive CAD was diagnosed by invasive coronary angiography in 88 patients with 161 vessels. The sensitivity and specificity in a patient-based analysis for obstructive CAD were 99% and 17% for CCTA, 80% and 50% for MPI and 91% and 67% for the combined method, respectively. The per-vessel diagnostic sensitivity and specificity were 95% and 54% for CCTA, 59% and 75% for MPI and 84% and 76% for the combined method. There were significant differences (p < 0.05) when comparing the combined method with MPI or CCTA by areas under the curve in a patient- or vessel-based analysis. However, CACS of 400 or more could not further stratify the patients with obstructive CAD.
Conclusions
CCTA, not CACS, provided additional diagnostic values to stress MPI in patients with intermediate-to-high cardiovascular risk.
Keywords: Coronary artery disease (CAD), Coronary computed tomography angiography (CCTA), Myocardial perfusion imaging (MPI), Single-photon emission computed tomography (SPECT)
INTRODUCTION
Coronary artery disease (CAD) is one of the leading causes of morbidity and mortality worldwide, and accounts for a considerable number of deaths annually. More than 400,000 people in the United States and over 17,000 people in Taiwan die of CAD each year.1,2 This disease will continue to have a substantial impact on public health in aging populations as the prevalence of cardiovascular risk factors increases.
Noninvasive tests are crucial for accurately assessing disease status and tailoring further treatment strategies. Stress myocardial perfusion imaging (MPI) has become the most commonly utilized imaging modality in diagnosing the presence of CAD and evaluating its severity. It is a functional test and detects hemodynamically significant CAD by revealing inducible ischemia after physical or pharmacological stress. Numerous clinical guidelines have manifested significant evidence for the diagnostic accuracy, risk stratification, and prognostic value of MPI.3-5
In contrast, coronary computed tomography angiography (CCTA) is a recently developed imaging method. Unlike MPI, CCTA provides CAD anatomical information by directly visualizing vascular structures and luminal stenosis as well as characterizing plaque composition. The existing literature has demonstrated CCTA’s high diagnostic accuracy and negative predictive value in concordance with invasive coronary angiography (CAG) in patients with suspected or known CAD.6-8 The prognostic value and its appropriate use criteria have also been established.5,9
Several studies observed that, through complementary information, CCTA offers additional value compared to MPI alone for stratifying risks and guiding treatment strategy.10-14 However, additional radiation exposure and cost remain important considerations. In addition, there were limited data in terms of whether adding CCTA to MPI provides incremental information regarding CAD diagnosis in patients with intermediate-to-high CAD risk. Therefore, our study aimed to explore the diagnostic performance of MPI, CCTA and combined methods using CAG as the gold standard in this population.
MATERIALS AND METHODS
Study population
Our study examined 118 patients retrospectively who had both myocardial perfusion scintigraphy and CCTA within 6 months and elective invasive CAG. These individuals were chosen from 1747 patients who received CCTA for known or suspected CAD at the National Taiwan University Hospital between Jan. 2007 and March 2011. No patients underwent revasculari-zation between CCTA and MPI. Basic information was collected from the patients, including age, gender, body mass index, risk factors, Framingham risk scores and symptoms. There were 106 patients that were ultimately enrolled in this study, after exclusion criteria reduced the number of study participants by 12 including those who underwent MPI using treadmill stress mode and failed to reach 85% of age-predicted maximal heart rate (n = 7), and low CAD risk patients (n = 5) (Figure 1). In each patient, a diagnostic coronary angiogram was performed using the standard technique after pretreatment with intracoronary nitroglycerin to avoid vessel spasm; multiple coronary artery projections were recorded digitally. All angiograms were examined, and the degree of coronary stenoses was assessed using a computer-aided quantitative angiographic analysis system (DCI-S Automated Coronary Analysis, Philips Medical Systems, Eindhoven, Netherlands). An obstructive CAD was defined as ≥ 50% luminal stenosis in the left main coronary artery or in at least one of the primary coronary arteries and their major branches.15 During follow-up periods, there were surveys of both revascularization and major adverse cardiac events, defined as sudden cardiac death, congestive heart failure, fatal or non-fatal myocardial infarction. This study was approved by the institutional review board at National Taiwan University Hospital.
Figure 1.
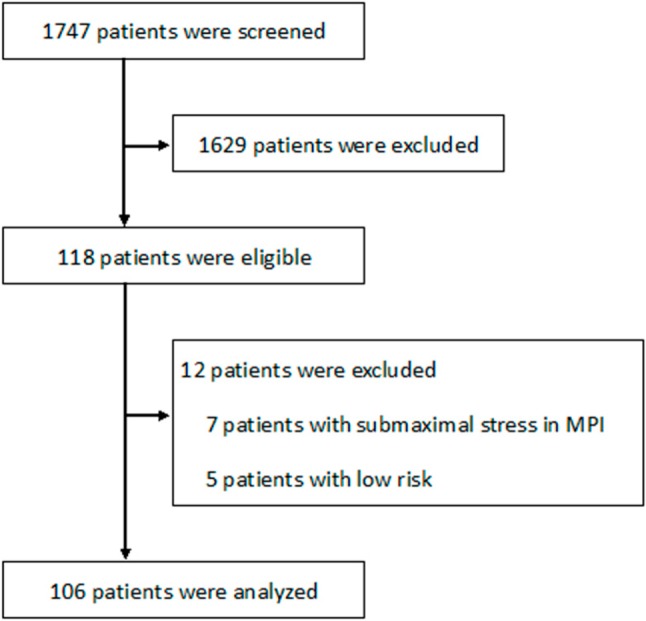
Flow diagram showing patient enrollment in the study.
CCTA imaging
The patients received oral short-acting propranolol (10 mg, AstraZeneca, UK) or diltiazem (30 mg, Mitsubishi Tanabe Pharma, Japan) to reach a target heart rate of ≤ 65 bpm and 0.4 mg sublingual nitroglycerin (Pfizer, USA) before image acquisition if no contraindications were noted. Computed tomography (CT) examination was performed with a 64-slice multidetector row computed tomography (MDCT) scanner (LightSpeed VCT, GE Healthcare, Milwaukee, WI, USA) until May, 2010 and afterwards with a 256-slice MDCT scanner (Brilliance iCT, Philips Medical Systems, Eindhoven, Netherlands). Initial unenhanced axial cardiac CT imaging was obtained by use of a prospective electrocardiographic trigger for the purpose of coronary artery calcium scoring (CACS) (Agatston method). Contrast-enhanced CT imaging was performed with images obtained from the mid-ascending aorta to the diaphragm at 0.625 mm slice thickness, 120 kV tube voltage, 0.35 s tube rotation time in a 64-slice MDCT scanner, at 0.8 mm slice thickness, 120 kV tube voltage, and 0.27 s tube rotation time in 256-slice MDCT scanner. The mean tube current was 616 ± 140 mA, with 609 ± 126 mA (range 248-759 mA) for 64-slice MDCT scanner and 640 ± 185 mA (range 306-947 mA) for 256-slice MDCT scanner (p = 0.53). Nonionic contrast media (iopromide, Ultravist 370 mg I/ml, Bayer HealthCare, Tarrytown, NY, USA) was injected using a dual-barrel injector (Stellant D, Medrad, Warrendale, PA, USA).16 Image reconstruction was performed using retrospective cardiac gating. Image quality was assessed by both bolus timing and the demonstration of coronary arteries, and ranked as good (no artifact), satisfactory (moderate artifact, acceptable for clinical diagnosis) and suboptimal (heavy motion artifact impairing diagnostic accuracy) for CCTA.17 Coronary atherosclerosis with luminal stenosis ≥ 50% was considered positive for CAD. The definition of in-stent restenosis was the same. Image quality and interpretation was performed by an experienced radiologist without knowledge of clinical information. We also evaluated the added value of coronary artery CACS to MPI in diagnosing obstructive CAD with a cut-off point of 400.18
201Thallium (Tl) single-photon emission CT (SPECT)
During a one-day period, stress/rest protocols were performed using standardized Bruce treadmill or dipyridamole stress testing (0.14 mg/kg/min over 4 minutes) (Boehringer Ingelheim, San Cugat del Vallés, Spain). A dose of 111 megabecquerel (MBq) 201Tl was given at either the peak stress level of the treadmill test or 3 minutes after the completion of dipyridamole infusion.19 Those patients with severe hypoperfusion in post-stress images received an additional 37 MBq of 201Tl. Imaging acquisition was started at 5 minutes, and 4 hours later on one of three dual-head gamma cameras (Hawkeye, GE Healthcare, Milwaukee, WI, USA; SMV, GE Healthcare, Milwaukee, WI, USA; Symbia T2, Siemens Healthcare Systems, Erlangen, Germany). Low-energy, high resolution collimators were used, with energy windows setting at 72 keV (± 10%) and 167 keV (± 10%). After a noncircular 180° acquisition for 32 projections at 30 seconds/projection (at stress) and 40 seconds/projection (at redistribution), the image data were processed in the eNTEGRA Workstation (Version 2.5202) for Hawkeye and Vision Workstations (Version 6.0.0) for SMV, using OSEM (2 iterations, 8 subsets) with a Butterworth filter (order 4, cutoff 0.25; Hawkeye) and filter-back projection with a Butterworth filter (order 5, cutoff 0.24; SMV). As for Symbia T2, the imaging data were obtained with noncircular 180° acquisition for 64 projections at 22 seconds/projection (at stress) and 30 seconds/projection (at redistribution), and processed in Syngo Workstation (Version 7.0.7.14), using Flash 3D (8 iterations, 8 subsets) and a Gaussian filter. Images were next reconstructed into short-axis, vertical-long-axis and horizontal-long-axis views, with the slice thickness of 8.3 mm, 5.5 mm, 5.5 mm for Hawkeye, and 8.1 mm, 5.4 mm, 5.4 mm for SMV, and 8.1 mm, 5.3 mm, 5.3 mm for Symbia T2. There was no attenuation or scatter correction during imaging interpretation.
For assessing the location and severity of perfusion defects, 17 segmentation scores and a 5-point scale (0 = normal, 1 = mildly abnormal, 2 = moderately abnormal, 3 = severely abnormal, 4 = no uptake) were used.20 The summed stress score (SSS) was calculated by adding all scores in 17 segments on the stress image, and the summed rest score (SRS) was the sum of scores on the rest image. The summed difference score was the total difference of all the segments between SSS and SRS. A scan was considered abnormal if SSS ≥ 4.21 The perfusion defects were placed into several categories: the anterior and septal walls, the territory of left anterior descending artery (LAD); the lateral walls, the left circumflex artery (LCX); and the inferior wall of the right coronary artery (RCA).
The quality evaluation and imaging interpretation were performed by two experienced nuclear medicine physicians unaware of the clinical information. Image quality was ranked as excellent (perfectly suitable for diagnosis, 4 points), good (sufficiently suitable for diagnosis, 3 points), fair (difficult for evaluation, 2 points), and poor (impossible to evaluate due to severe noise or artifacts, 1 point) with consensus.22 Two readers (one radiologist, one nuclear medicine physician) performed further assessment of CAD with the information gained from both MPI and CCTA by careful correlation of perfusion abnormalities to the corresponding stenotic vessels by consensus.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation. Categorical variables were expressed as percentages. Differences between continuous variables were compared using Student t-test, and those between categorical variables were compared using Chi-square test or Fisher’s test, as appropriate. With findings on the CAG as the gold standard, the results of MPI, CCTA and combined methods were presented as sensitivity, specificity, positive predictive value, negative predictive value and accuracy data on both a per-patient basis and a per-vessel basis to assess the diagnostic efficacy of CAD. Next, receivers operating characteristic (ROC) curves were generated, and the areas under the curve (AUC) were calculated for identifying obstructive CAD using diagnostic criteria based on either or both imaging modalities and were compared by MedCalc Software (Version 12, Ostend, Belgium). The p values were 2-sided, and values ≤ 0.05 were considered statistically significant.
RESULTS
Patient characteristics and invasive coronary angiographic findings
The demographic characteristics of the 106 patients were listed in Table 1. There were 93 males (87.7%) and 13 females (12.3%), with a mean age of 65.0 ± 10.4 years (range: 42-87 years). Thirty-seven patients (34.9%) had a previous history of proven CAD and revascularization, either by catheterization (29 patients, 27.4%), bypass (2 patients, 1.8%), or both (6 patients, 5.7%). Twenty-four patients (22.6%) had a history of stent implantation. Four patients (3.8%) had documented non-significant CAD. The remaining 65 patients consisted of 17 (16.0%) individuals with intermediate risk and 48 (45.3%) with a high risk. The mean interval between MPI and CCTA was 2.0 ± 2.2 months (range: 0-6.0 months), and the mean interval between the first index study of MPI or CCTA and CAG was 3.7 ± 3.2 months (range 0.1-18.9, with the span > one year in 2 patients). Fifty-nine patients (55.7%) received MPI before CCTA.
Table 1. Patient characteristics .
| n | 106 |
| Age (years) | 65.0 ± 10.4 (42-87) |
| Males, n (%) | 93 (87.7%) |
| BMI | |
| Mean ± SD | 26.4 ± 4.4 |
| Range | 17.7-47.1 |
| BMI ≥ 30 | 17 |
| Risk factors | |
| Hypertension | 79 (74.5%) |
| Diabetes mellitus | 44 (41.5%) |
| Dyslipidemia | 59 (55.7%) |
| Current smoker | 25 (23.6%) |
| Clinical symptoms | |
| Chest pain | 76 (71.7%) |
| Dyspnea | 18 (17.0%) |
| No cardiac symptoms | 12 (11.3%) |
| Framingham risk score | |
| Intermediate | 17 (16.0%) |
| High | 48 (45.3%) |
| Known CAD | 37 (34.9%) |
| Documented nonsignificant CAD | 4 (3.8%) |
| Previous myocardial infarction | 5 (4.7%) |
| Invasive coronary angiographic results | |
| No significant CAD | 18 (17.0%) |
| 1-vessel-disease | 37 (34.9%) |
| 2-vessel-disease | 29 (27.4%) |
| 3-vessel-disease | 22 (20.7%) |
BMI, body mass index; CAD, coronary artery disease; SD, standard deviation.
Stress MPI was performed with standardized treadmill mode in 43 patients (40.6%) and dipyridamole in 63 patients (59.4%). The mean dosage of 201Tl administered was 118.4 ± 14.8 MBq, and the estimated effective absorbed radiation dose was 14.3 ± 1.8 millisieverts (mSv). Images were acquired using the Hawkeye gamma camera scanner for 42 patients, Symbia T2 gamma camera scanner for 5 patients and the SMV gamma camera scanner for the remaining 59 patients, with mean image quality scores as 3.3 ± 0.5, 3.6 ± 0.5, and 3.4 ± 0.6, respectively. There was no significant difference of image quality between different scanners.
The CCTA was performed with a 64-slice MDCT scanner (LightSpeed VCT, GE Healthcare, Milwaukee, WI, United States) for 85 patients and a 256-slice MDCT scanner (Brilliance iCT, Philips Medical Systems, Eindhoven, Netherlands) for the remaining 21 patients. The CCTA study quality was good or satisfactory in 94 patients (88.7%), including 75 patients with a 64-slice MDCT scanner and 19 patients with a 256-slice MDCT scanner. No significant difference in image quality was observed between the two scanners (p = 0.93).
The mean heart rate during scanning was 61.0 ± 9.8 bpm. The calcium scores were 860 ± 1075, which excluded the regions of stent implantation. The radiation burden was available in 88 patients, with a mean value of 17.8 ± 5.4 mSv, 17.7 ± 5.3 mSv for the 64-slice MDCT scanner (n = 67) and 18.0 ± 5.7 for the 256-slice scanner (n = 21), with no difference between the two scanners (p = 0.81). The radiation exposure from 256-slice scanner was reduced further lately due to the application of dose modulation technique. The left ventricular ejection fraction (LVEF) was normal (> 50%) in 100 patients (94%), mild to moderately impaired in 2 patients, and poor in 4 patients (LVEF ≤ 35%).
The indication for CAG in this study population included intermediate-risk stress MPI results (symptomatic, n = 14; asymptomatic, n = 1), high-risk stress MPI results with or without symptoms (n = 25), ≥ 50% luminal stenosis of coronary arteries in CCTA (symptomatic, n = 24; asymptomatic, n = 3), post-revascularization patients with intermediate- to high-risk noninvasive tests (symptomatic, n = 33; asymptomatic, n = 4), symptomatic LV systolic dysfunction (n = 1), discordant findings (low-risk imaging findings with ongoing symptoms, n = 1). Coronary angiography demonstrated obstructive CAD in 88 patients (83.0%), and in 161 vessels, including 67 LAD (63.2%), 44 LCX (41.5%) and 50 RCA lesions (47.2%). Percutaneous coronary intervention (PCI) was performed in 66 patients; two patients received coronary artery bypass surgery. In the remaining 20 individuals with obstructive CAD, PCI failed in 4 patients (one with proximal LAD total occlusion, one with 90% stenosis in the diagonal branch, one with total proximal RCA occlusion, and one with 2-vessel disease, specifically the total occlusion of mid-LAD and posterior descending arteries). Sixteen patients continued on medical therapy due to non-significant obstructive CAD (luminal stenosis ≥ 50% but < 70%) or a mild ischemic burden (n = 13), technique difficulties (n = 2), or patient preference (n = 1): one patient had 3-vessel disease, two patients had 2-vessel-disease, four patients had RCA lesion (three with total RCA occlusion and collateral formation), and 9 patients had LAD lesions (proximal LAD lesion in one patient). During a mean follow-up period of 36.0 ± 14.7 months (range: 6.0-64.7 months) after the first image study, only 3 patients experienced major adverse cardiac events; these included one patient who suffered repeat decompensated heart failure episodes followed by sudden cardiac death, and two patients who suffered non-fatal myocardial infarction.
Diagnostic performance of MPI, CCTA, or combined methods
Table 2 showed the diagnostic performance of MPI, CCTA and combined methods on a patient- and vessel-based analysis in reference to CAG results. No significant difference existed between the AUC of ROC curves generated by MPI and CCTA results on a per-patient basis. On a vessel-based analysis, similar results were found in LAD territory, whereas in RCA territory, CCTA showed borderline superior performance, and in LCX territory, CCTA significantly outperformed MPI study (p = 0.008), and Figure 2 is an example. Combination of two imaging modalities had better diagnostic performance than standalone MPI, both on patient-based and vessel-based analyses. As compared to CCTA, combined method also yielded significant improvement in diagnosing CAD on per-patient and per-vessel bases, except for evaluating LCX lesions. A large proportion of study subjects had positive CCTA (n = 102, Figure 3), reflecting its limited specificity in this population. In these patients, 15 patients were false positive (Table 2), and could be reduced to 6 patients in combined method. Among 27 patients with negative MPI findings, 18 patients (66.7%) had obstructive CAD. This consisted of 6 patients with multivessel disease and 12 with single vessel disease (proximal LAD lesion in one patient). Coronary CT angiography was positive in 26 patients, except for one with suboptimal imaging quality due to respiratory artifact and with 70% stenosis in diagonal branch shown in CAG.
Table 2. Diagnostic performance of CCTA and MPI using obstructive CAD detected in CAG as gold standard .
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | FP/FN/TN/TP | Accuracy (%) | AUC | p value* | |
| Patient based analysis | ||||||||
| MPI | 80 | 50 | 89 | 33 | 9/18/9/70 | 75 | 0.648 | |
| CCTA | 99 | 17 | 85 | 75 | 15/1/3/87 | 85 | 0.578 | 0.40 |
| Combination | 91 | 67 | 93 | 60 | 6/8/12/80 | 87 | 0.788 | 0.03# |
| Vessel-based analysis | ||||||||
| Overall MPI | 59 | 75 | 70 | 64 | 39/66/118/95 | 67 | 0.668 | |
| CCTA | 95 | 54 | 68 | 91 | 72/8/85/153 | 75 | 0.746 | 0.02 |
| Combined | 84 | 76 | 78 | 83 | 38/25/119/136 | 80 | 0.801 | < 0.001# |
| LAD MPI | 70 | 54 | 72 | 51 | 18/20/21/47 | 64 | 0.620 | |
| CCTA | 97 | 31 | 71 | 86 | 27/2/12/65 | 74 | 0.639 | 0.77 |
| Combined | 90 | 62 | 80 | 77 | 15/7/24/60 | 79 | 0.755 | 0.007# |
| LCX MPI | 32 | 94 | 78 | 66 | 4/30/58/14 | 68 | 0.627 | |
| CCTA | 93 | 60 | 62 | 93 | 25/3/37/41 | 74 | 0.764 | 0.008 |
| Combined | 75 | 74 | 67 | 81 | 16/11/46/33 | 75 | 0.746 | 0.02 |
| RCA MPI | 68 | 68 | 65 | 70 | 18/16/38/34 | 68 | 0.679 | |
| CCTA | 94 | 64 | 70 | 92 | 20/3/36/47 | 78 | 0.791 | 0.051 |
| Combined | 86 | 88 | 86 | 88 | 7/7/49/43 | 87 | 0.867 | < 0.001# |
* Compared to MPI by AUC. # Significant difference when compared to CCTA by AUC.
AUC, area under curve; CAD, coronary artery disease; CAG, coronary artery angiography; CCTA, coronary computed tomographic angiography; FP, false positive; FN, false negative; LAD, left anterior descending artery; LCX, left circumflex artery; MPI, myocardial perfusion imaging; NPV, negative predictive value; PPV, positive predictive value; RCA, right coronary artery; TN, true negative; TP, true positive.
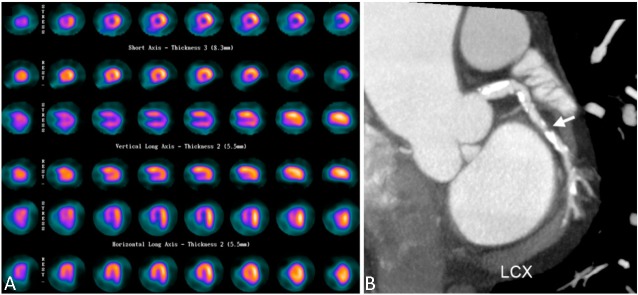
Figure 2.
Representative images of LCX lesion not recognized in myocardial perfusion image (MPI). A 71-year-old man suffered from shortness of breath for six months. (A) Myocardial perfusion imaging demonstrated inducible ischemia at the apex, septal, anterior, and apical inferior walls, indicating LAD and RCA territories. (B) Coronary CT angiography performed 20 days later showed heavy calcification (calcium score 2087) and significant coronary stenosis over three major coronary arteries, including the partially calcified plague at LCX (arrow). This finding was confirmed by invasive coronary angiography performed 48 days after MPI. CT, computed tomography; LAD, left anterior descending artery; LCX, left circumflex artery; RCA, right coronary artery.
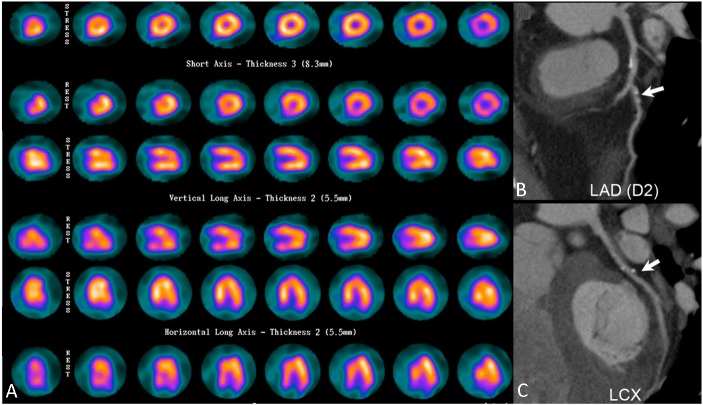
Figure 3.
Representative images of overestimation of disease severity in coronary CT angiography (CCTA). A 51-year-old man with a previous history of percutaneous occlusive balloon angioplasty to the left anterior descending artery (LAD) and left circumflex artery (LCX) recently presented with intermittent chest tightness. (A) Myocardial perfusion imaging (MPI) yielded a negative result. (B) and (C), Nonetheless, CCTA subsequently showed a calcium score of 168, luminal stenosis > 50% at the diagonal branch (arrow), and about 50% stenosis at proximal LCX (arrow), mid LAD and first obtuse marginal branch (not shown). A coronary angiography later showed no definite evidence of obstructive coronary artery disease.
The incremental value of CACS to MPI
Figure 4 demonstrated the prevalence of obstructive CAD further stratified by results of MPI and CACS. In patients with negative MPI (n = 27), subgroup with CACS < 400 (n = 17, average 163 ± 120, range 2-369) had similar prevalence of obstructive CAD as compared to subgroup with CACS ≥ 400 (n = 10, average 1120 ± 492, range 458-1810) (65% versus 70%, respectively, p = 1.0). Obstructive CAD was still proven in 11 patients with negative MPI and CACS < 400. In subgroup of CACS < 400 and negative MPI, no significant difference was observed between patients with and without obstructive CAD, regarding age, gender, body mass index, Framingham score, hypertension, diabetes, hyperlipidemia, current cigarette usage, or symptoms. In patients with positive MPI (n = 79), the prevalence of obstructive CAD was similar between two subgroups stratified by CACS of 400 (CACS < 400, n = 41, average 152 ± 121, range 0-386; CACS ≥ 400, n = 38, average 1694 ± 1125, range 586-4649) (85% versus 92%, respectively, p = 0.48).
Figure 4.
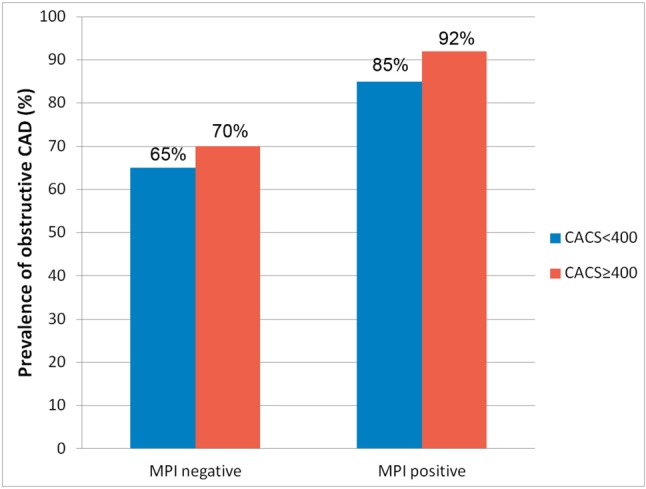
Prevalence of obstructive coronary artery disease evaluated by myocardial perfusion imaging (MPI) and coronary artery calcium score (CACS) categories.
DISCUSSION
There are several findings in this study. First of all, MPI and CCTA have similar diagnostic power in detecting obstructive CAD on a per-patient basis. However, on a per-vessel basis, MPI has an inferior ability to demonstrate LCX lesions as compared to CCTA. Second, false negative MPI results are not uncommon in this population. We confirmed that CCTA aided in diagnostic accuracy for obstructive CAD to standalone MPI in intermediate-to-high risk patients. Third, CACS of 400 did not show significant incremental value in stratification in this small population. Additional CCTA, not CACS, should be considered first when clinical suspicion of CAD is high, even MPI is negative.
Stress MPI is a widely adopted imaging method for CAD assessment. It is recommended in stable symptomatic patients with intermediate-to-high CAD risk and selected patients after revascularization.5 The diagnostic performance indicated in meta-analysis showed a pooled sensitivity of 88% and specificity of 61% on a per-patient basis; it showed a pooled sensitivity of 69% and specificity of 79% on a coronary territory basis. However, the values can be influenced by many factors, such as different stressors, tracers, attenuation correction applications, CAD cutoff point on CAG, and prevalence of CAD and 3-vessel disease.3 Our study showed similar specificity on a patient basis as those in the higher CAD prevalence (≥ 72%) and 3-vessel disease (≥ 18%) (49% and 47%, respectively), and lower value as compared to those using ≥ 50% stenosis as CAD cutoff points on angiography (69%). Nonetheless, the sensitivity on a patient basis, vessel-based overall sensitivity and vessel-based overall specificity showed slightly lower results than those reported (80% versus 89-93%, 59% versus 69%, 75% versus 79%, respectively), which could be attributed to aforementioned factors and lack of nonperfusion abnormality information in the current study.23 We also noticed an unequal MPI detection rate of individual coronary artery stenosis. A relatively lower sensitivity was noted in LCX lesions, consistent with previous medical literature.24 One possible explanation may be that perfusion defects located in the least-attenuated lateral wall were not as apparent as those in the rest of the myocardium.24
CCTA offers morphological data of CAD, and the reported pooled sensitivity, specificity, median positive predictive value, negative predictive value in patient-based detection were 99%, 89%, 93% (range 64-100%) and 100% (range 86%-100%), respectively, in a meta-analysis involving 28 studies using 64-slice CT to evaluate suspected or known CAD. In segment-based detection, the values were 90%, 97%, 76% (range 44-93%) and 99% (range 95-100%), respectively.6 The excellent negative predictive value indicated its reliability to exclude obstructive CAD. Nonetheless, the wide range of PPV was suggestive of the possibility to overestimate disease severity. Especially in populations with a higher cardiovascular risk, it may be more likely associated with imaging modality challenges when interpreting CCTA, such as heavy calcification, motion artifacts caused by rapid, irregular heartbeats or respiration, and the presence of metallic stent material; all of these lead to limitations in performance of this modality.17,25 For example, the specificity can drop from approximate 90% to 50.6% as the calcium scores elevated from 0-400 to 401-1000.26 Therefore, in symptomatic patients without previous intervention, the appropriate use of CCTA is reserved for those with intermediate pre-test probability, along with uninterpretable ECG or inability to exercise. In patients after revascularization, it is considered “maybe appropriate” in evaluation of ischemic equivalent and those with prior left main coronary stent; other conditions are rarely appropriate and none of them is considered appropriate.5 In our study, the CCTA sensitivity exceeded 90% in both patient- and vessel-based analysis, and the PPV ranged from 62-85%, in line with many other studies, but the specificity was rather unsatisfactory.6-8 This is because more subjects with higher CAD risk were included (patients with intermediate risk constituted only 16%). Therefore, 11.3% of CCTA scans in this study were classified as unsatisfactory imaging quality, where the average calcium scores were as high as 1680 ± 1394; 24 patients (22.6%) had a history of stent implantation. This study did not exclude these patients in order to reflect the real-world clinical scenarios. As a result, nearly all of the subjects (102 patients, 96.2%) had positive scans, while only 88 patients (83.0%) were found to have obstructive CAD.
The MPI and CCTA findings, representing anatomical and functional aspects of CAD, may only share a modest relationship.27,28 That is, abnormal CCTA is a poor predictor of ischemia, and a normal MPI study can also correspond to various types and severities of coronary atherosclerosis. However, by providing complementary information and compensating the limitations of two methods, emerging evidence has demonstrated the usefulness of combined modalities in CAD diagnosis, treatment planning and risk stratification. Patel et al. studied 241 low-to-intermediate risk participants with equivocal MPI results and discovered that CCTA can effectively exclude significant obstructive CAD in 175 patients, thus avoiding unnecessary CAG by almost 75%.10 Gaemperli et al. reported that the combined non-invasive approach of CCTA and MPI in 78 patients with known or suspected CAD has excellent accuracy (100%) in the detection of flow-limiting coronary stenosis, defined as revascularization, using quantitative coronary angiography and MPI as standard of reference; therefore it can be a promising gatekeeper before CAG.11 Several studies with hybrid SPECT/CCTA have also shown incremental value. Schaap et al. demonstrated the reliability of treatment decisions made from hybrid CCTA/SPECT results in 107 patients with stable angina and intermediate-to-high pretest likelihood for CAD, using conventional reference information obtained from SPECT and CAG.12 In patients with either known or suspected CAD, Pazhenkottil et al. found significantly different revascularization rates among normal, unmatched, and abnormally matched results; the latter was also a strong predictor for MACE.13,14 Regarding diagnostic accuracy, Schaap et al. showed improved overall performance of the hybrid method compared to standalone SPECT and CCTA in 98 patients, with a reference standard of fractional flow reserve.29 In this study, although CCTA seemed to have higher sensitivity and lower specificity for CAD diagnosis when compared to MPI, overall diagnostic performance did not reveal significant differences between these two methods in both per-patient and per-vessel analyses, except for evaluation of LCX lesions, known to be the weak point of MPI. Despite all the challenging characters in the study population when reading CCTA, additional anatomical information showed benefit, as indicated by previous literature, and the combined approach significantly improved diagnostic accuracy of standalone MPI on both a patient and vessel bases. In patients with high clinical suspicion of CAD and negative MPI, CCTA could provide valuable noninvasive structural assessment of coronary arteries. On the other hand, CCTA scan has relatively high false positive results, and additional MPI can help reduce 60% of them in this study (from 15 patients to 6 patients).
Previous evidence also showed that knowledge of CACS can aid in diagnostic certainty when interpreting MPI, and help identify patients with significant CAD and negative MPI.30,31 Additionally, combination of CACS and MPI allows risk stratification before noncardiac surgery.32 In our study, CACS < 400 cannot further stratify patients in both positive and negative MPI groups. This could be attributed to the small number of each subgroup, and the relatively high prevalence of CAD in the study population; therefore, evaluation of more participants will be needed.
Radiation exposure from medical tests is an important issue. There is a trend towards the increasing utility of diagnostic procedures, along with increased exposure to radiation. Among various imaging modalities, CT and scintigraphy scanners contribute to 50.8% and 13.6% of the radiation dosage in Taiwan, respectively; similar results were also observed in the United States.33,34 Myocardial perfusion imaging is the fourth-leading cause of radiation exposure among all medical procedures, and the leading cause of radiation among all nuclear medicine procedures, with estimated effective doses of 14.3 ± 1.8 mSv on average in this study, consistent with those in the medical literature.33,34 The effective CCTA scan dose is in the same magnitude in this study (17.8 ± 5.9 mSv), similar to another multicenter research study (12 mSv, interquartile range 8-18 mSv).35 In response to initial concerns about radiation dose, many technical advances have permitted a dramatic reduction in patient radiation exposure with preserved image quality, such as cadmium-zinc-telluride (CZT) scanner for MPI or dose-saving CCTA algorithms.35,36 In addition, CCTA is not covered by the National Health Insurance program in Taiwan. The potential benefit of cardiac imaging has to be justified in the context of radiation exposure and cost before proceeding to another examination.
This study had some limitations. First, the study population was small and heterogeneous. Second, only patients who underwent catheterization were included for analysis, resulting in a high CAD prevalence rate and variable specificity of imaging studies. In addition, ejection fraction and wall motion obtained from gated SPECT were not routinely performed, which inevitably affect diagnostic performance of MPI.3,23 Therefore, a large-scale prospective study is warranted to further elucidate our findings.
CONCLUSIONS
In conclusion, our study showed that CCTA and MPI had similar diagnostic performance in patients with intermediate-to-high risk CAD on a patient-based analysis. Standalone CCTA outperformed MPI in evaluation of LCX lesion, whereas the combined method showed significantly better diagnostic performance in both patient- and vessel-based analyses. CACS of 400 did not show additional value in stratification of obstructive CAD in such small patient group. CCTA can be considered first in selective clinical settings after MPI in this population. As for CCTA, additional MPI can also reduce a considerable amount of false positive results.
Acknowledgments
This study was supported in part both by grants NSC 100-2314-B-002-158 and NSC 101-2314-B-002-151-MY3 from the National Science Council of Taiwan.
DISCLOSURE STATEMENT
The publication costs associated with this article were defrayed in part by page charge payments. Therefore, and solely to indicate this fact, this article is hereby marked an “advertisement” in accordance with 18 USC section 1734. No other potential conflicts of interest were reported relevant to this article.
CONFLICT OF INTEREST
The authors state that they have no conflicts of interest.
REFERENCES
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Executive summary: heart disease and stroke statistics—2012 update. A report from the American Heart Association. Circulation. 2012;125:188–197. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 2.Statistics on causes of death. ROC: Department of Health, Executive Yuan; 2013. [Google Scholar]
- 3.Jaarsma C, Leiner T, Bekkers SC, et al. Diagnostic performance of noninvasive myocardial perfusion imaging using single-photon emission computed tomography, cardiac magnetic resonance,and positron-emission tomography imaging for the detection of obstructive coronary artery disease. A meta-analysis. J Am Coll Cardiol. 2012;59:1719–1728. doi: 10.1016/j.jacc.2011.12.040. [DOI] [PubMed] [Google Scholar]
- 4.Hachamovitch R, Berman DS, Shaw LJ, et al. Incremental prognostic value of myocardial perfusion single photon emission computed tomography for the prediction of cardiac death—differential stratification for risk of cardiac death and myocardial infarction. Circulation. 1998;97:535–543. doi: 10.1161/01.cir.97.6.535. [DOI] [PubMed] [Google Scholar]
- 5.Wolk MJ, Bailey SR, Doherty JU, et al. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;63:380–406. doi: 10.1016/j.jacc.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Beck T, Bergstahler C, Reimann A. Technology insight:possible applications of multislice computed tomography on clinical cardiology. Nat Clin Pract Cardiovasc Med. 2005;7:361–368. doi: 10.1038/ncpcardio0240. [DOI] [PubMed] [Google Scholar]
- 7.Mowatt G, Cook JA, Hillis GS, et al. 64-slice computed tomography angiography in the diagnosis and assessment of coronary artery disease:systemic review and meta-analysis. Heart. 2008; 94:1386–1393. doi: 10.1136/hrt.2008.145292. [DOI] [PubMed] [Google Scholar]
- 8.Meijboom WB, Meijs MFL, Schuijf JD, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol. 2008; 52:2135–2144. doi: 10.1016/j.jacc.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 9.Hulten EA, Carbonaro S, Petrillo SP, et al. Prognostic value of cardiac computed tomography angiography: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;57:1237–1247. doi: 10.1016/j.jacc.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Patel N, Pal RS, Flores F, Budoff M. Utility of cardiac computed tomography angiography to exclude clinically significant obstructive coronary artery disease in patients after myocardial perfusion. Am J Cardiol. 2012;109:165–168. doi: 10.1016/j.amjcard.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 11.Gaemperli O, Husmann L, Schepis T, et al. Coronary CT angiography and myocardial perfusion imaging to detect flow-limiting stenosis: a potential gatekeeper for coronary revascularization? Eur Heart J. 2009;30:2921–2929. doi: 10.1093/eurheartj/ehp304. [DOI] [PubMed] [Google Scholar]
- 12.Schaap J, Groot JAH, Nieman K, et al. Hybrid myocardial SPECT/CT coronary angiography and invasive coronary angiography in patients with stable angina pectoris lead to similar treatment decisions. Heart. 2013;99:188–194. doi: 10.1136/heartjnl-2012-302761. [DOI] [PubMed] [Google Scholar]
- 13.Pazhenkottil AP, Nkoulou RN, Ghadri JR, et al. Impact of cardiac hybrid single-photon emission computed tomography/computed tomography imaging on choice of treatment strategy in coronary artery disease. Eur Heart J. 2011;32:2824–2829. doi: 10.1093/eurheartj/ehr232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pazhenkottil AP, Nkoulou RN, Ghadri JR, et al. Prognostic value of cardiac hybrid imaging integrating single-photon emission computed tomography with coronary computed tomography angiography. Eur Heart J. 2011;32:1465–1471. doi: 10.1093/eurheartj/ehr047. [DOI] [PubMed] [Google Scholar]
- 15.Winther S, Svensson M, Jørgensen HS, et al. Diagnostic performance of coronary CT angiography and myocardial perfusion imaging in kidney transplantation candidates. JACC Cardiovasc Imaging. 2015;8:553–562. doi: 10.1016/j.jcmg.2014.12.028. [DOI] [PubMed] [Google Scholar]
- 16.Lee BC, Lee WJ, Hsu HC, et al. Using clinical cardiovascular risk scores to predict coronary artery plaque severity and stenosis detected by CT coronary angiography in asymptomatic Chinese subjects. Int J Cardiovasc Imaging. 2011;27:669–678. doi: 10.1007/s10554-011-9874-6. [DOI] [PubMed] [Google Scholar]
- 17.Mir-akbari H, Ripsweden J, Jensen J, et al. Limitations of 64-detector-row computed tomography coronary angiography: calcium and motion but not short experience. Acta Radiol. 2009; 50:174–180. doi: 10.1080/02841850802647013. [DOI] [PubMed] [Google Scholar]
- 18.Manudu HM, Paul TK, Veeranki SP, Budoff M. The effects of coronary artery calcium screening on behavioral modification, risk perception, and medication adherence among asymptomatic adults:a systemic review. Atherosclerosis. 2014;234:338–350. doi: 10.1016/j.atherosclerosis.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 19.Wu YW, Yen RF, Chieng PU, Huang PJ. Tl-201 myocardial SPECT in differentiation of ischemic from nonischemic dilated cardiomyopathy in patients with left ventricular dysfunction. J Nucl Cardiol. 2003;10:369–374. doi: 10.1016/s1071-3581(03)00456-2. [DOI] [PubMed] [Google Scholar]
- 20.Holly TA, Abbott BG, Al-Mallah M, et al. Single photon-emission computed tomography. J Nucl Cardiol. 2010;17:941–973. doi: 10.1007/s12350-010-9246-y. [DOI] [PubMed] [Google Scholar]
- 21.Zellweger MJ, Hachamovitach R, Kang X, et al. The threshold, incidence, and predictors of prognostically high-risk silent ischemia in asymptomatic patients without prior diagnosis of coronary artery disease. J Nucl Cardiol. 2009;16:193–200. doi: 10.1007/s12350-008-9016-2. [DOI] [PubMed] [Google Scholar]
- 22.Matsuo S, Nakajima K, Onoguchi M, et al. Nuclear myocardial perfusion imaging using thallium-201 with a novel multifocal collimato SPECT/CT: IQ-SPECT versus conventional protocols in normal subjects. Ann Nucl Med. 2015;29:452–459. doi: 10.1007/s12149-015-0965-7. [DOI] [PubMed] [Google Scholar]
- 23.Berman DS, Kang X, Slomka PJ, et al. Underestimation of extent of ischemia by gated SPECT myocardial perfusion imaging in patients with left main coronary artery disease. J Nucl Cardiol. 2007;14:521–528. doi: 10.1016/j.nuclcard.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 24.Huang PJ, Ho YL, Wu CC, et al. Simultaneous dobutamine stress echocardiography and thallium-201 perfusion imaging for the detection of coronary artery disease. Cardiology. 1997;88:556–562. doi: 10.1159/000177419. [DOI] [PubMed] [Google Scholar]
- 25.Schroeder S, Achenbach S, Bengel F, et al. Cardiac computed tomography: indications, application, limitations, and training requirements. Eur Heart J. 2008;29:531–556. doi: 10.1093/eurheartj/ehm544. [DOI] [PubMed] [Google Scholar]
- 26.den Dekker MA, de Smet K, de Bock GH, et al. Diagnostic performance of coronary CT angiography for stenosis detection according to calcium score: systematic review and meta-analysis. Eur Radiol. 2012;22:2688–2698. doi: 10.1007/s00330-012-2551-x. [DOI] [PubMed] [Google Scholar]
- 27.Sato A, Hiroe M, Tamura M, et al. Quantitative measures of coronary stenosis severity by 64-slice CT angiography and relationship to physiologic significance perfusion in nonobese patients:comparison with stress myocardial perfusion imaging. J Nucl Med. 2008;49:564–572. doi: 10.2967/jnumed.107.042481. [DOI] [PubMed] [Google Scholar]
- 28.Gaemperli O, Schepis T, Valenta I, et al. Functionally relevant coronary artery disease: comparison of 64-slice CT angiography with myocardial perfusion SPECT. Radiology. 2008;248:414–423. doi: 10.1148/radiol.2482071307. [DOI] [PubMed] [Google Scholar]
- 29.Schaap J, Kauling RM, Boekholdt SM, et al. Incremental diagnostic accuracy of hybrid SPECT/CT coronary angiography in a population with an intermediate to high pre-test likelihood of coronary artery disease. Eur Heart J Cardiovasc Imaging. 2013;14:642–649. doi: 10.1093/ehjci/jes303. [DOI] [PubMed] [Google Scholar]
- 30.Mouden M, Ottervanger JP, Timmer JR, et al. The influence of coronary calcium score on the interpretation of myocardial perfusion imaging. J Nucl Cardiol. 2014;21:368–374. doi: 10.1007/s12350-013-9825-9. [DOI] [PubMed] [Google Scholar]
- 31.Schepis T, Gaemperli O, Koepfli P, et al. Added value of coronary artery calcium score as an adjunct to gated SPECT for the evaluation of coronary artery disease in an intermediate-risk population. J Nucl Med. 2007;48:1424–1430. doi: 10.2967/jnumed.107.040758. [DOI] [PubMed] [Google Scholar]
- 32.Ghadri JR, Fiechter M, Veraguth K, et al. Coronary calcium score as an adjunct to nuclear myocardial perfusion imaging for risk stratification before noncardiac surgery. J Nucl Med. 2012;53:1081–1086. doi: 10.2967/jnumed.111.100206. [DOI] [PubMed] [Google Scholar]
- 33.Chen TR, Tyan YS, Teng PS, et al. Population dose from medical exposure in Taiwan for 2008. Med Phys. 2011;38:3139–3148. doi: 10.1118/1.3592936. [DOI] [PubMed] [Google Scholar]
- 34.Scott-Moncrieff A, Yang J, Levine D, et al. Real-world estimated effective radiation doses from commonly used cardiac testing and procedure modalities. Can J Cardiol. 2011;27:613–618. doi: 10.1016/j.cjca.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 35.Hausleiter J, Meyer T, Hermann F, et al. Estimated radiation dose associated with cardiac CT angiography. JAMA. 2009;301:500–507. doi: 10.1001/jama.2009.54. [DOI] [PubMed] [Google Scholar]
- 36.Mouden M, Timmer JR, Ottervanger JP, et al. Impact of a new ultrafast CZT camera for myocardial perfusion imaging: fewer equivocal results and lower radiation dose. Eur J Nucl Med Mol Imaging. 2012;39:1048–1055. doi: 10.1007/s00259-012-2086-z. [DOI] [PubMed] [Google Scholar]