Abstract
Radionuclide myocardial perfusion imaging (MPI) with single photon emission computed tomography (SPECT) has been widely used clinically as one of the major functional imaging modalities for patients with coronary artery disease (CAD) for decades. Ample evidence has supported the use of MPI as a useful and important tool in the diagnosis, risk stratification and treatment planning for CAD. Although popular in the United States, MPI has become the most frequently used imaging modality among all nuclear medicine tests in Taiwan. However, it should be acknowledged that MPI SPECT does have its limitations. These include false-positive results due to certain artifacts, false-negative due to balanced ischemia, complexity and adverse reaction arising from current pharmacological stressors, time consuming nature of the imaging procedure, no blood flow quantitation and relatively high radiation exposure. The purpose of this article was to review the recent trends in nuclear cardiology, including the utilization of positron emission tomography (PET) for MPI, new stressor, new SPECT camera with higher resolution and higher sensitivity, dynamic SPECT protocol for blood flow quantitation, new software of phase analysis for evaluation of LV dyssynchrony, and measures utilized for reducing radiation exposure of MPI.
Keywords: Coronary artery disease, Myocardial flow reserve, Myocardial perfusion imaging, Phase analysis, PET, SPECT
INTRODUCTION
Myocardial perfusion imaging (MPI) has been widely used in the diagnosis, risk stratification, treatment planning and prognostic evaluation for patients with, or suspected of having, coronary artery disease (CAD) for over 30 years. It plays an important role in clinical decision-making as a gatekeeper for further intervention, and has been proven to be a cost-effective non-invasive imaging modality based on numerous randomized trials and clinical studies.
Myocardial perfusion imaging (MPI) with single photon emission tomography (SPECT) is an imaging procedure that utilizes an intravenously administered radiotracer to delineate the distribution of blood flow in myocardium during stress (either exercise or pharmacological stimulation) and at rest. Patients with hemodynamically significant coronary artery stenosis will have diminished radiotracer concentration in myocardial areas of diseased vessels, which constitute the basis of diagnosing CAD with MPI. If the perfusion abnormality shown on MPI is worse at stress than at rest, the so-called reversible defect, the myocardial segment is most likely due to ischemia. If the perfusion abnormality remains unchanged from stress to rest (fixed defect), the lesion usually indicates scarred myocardium.
In clinical practice, the major indications of MPI are obtained through the evaluation of patients with suspected or known CAD, including assessment of the presence, location, extent and severity of myocardial ischemia and scar, determination of the functional significance of anatomic stenosis detected by invasive or computed tomography (CT) coronary angiography, and assessment of myocardial viability in ischemic cardiomyopathy.1 MPI has been an integral part of clinical management for CAD patients.
Numerous published studies, most of which used MPI with SPECT (MPS), had previously confirmed the diagnostic accuracy of MPS in detecting obstructive CAD when using the diameter stenosis of diseased vessels demonstrated on coronary angiography.2 As a functional modality for assessing the hemodynamic significance of coronary lesion, MPS had also established its role in risk stratification and prognostic assessment in patients with CAD.3 The patients with normal or near-normal results on MPS would have an annually cardiac event rate of < 1% . In addition, those showing normal perfusion or only mild ischemia on MPS could be safely managed with conservative medical treatment rather than invasive revascularizations. For those with moderate to severe ischemia on MPS, however, revascularization significantly improves survival rates compared to patients treated with medication only.4 Therefore, MPS has been clinically considered as a gatekeeper of invasive procedure in the management of CAD.
However, there are still some limitations for current MPS. First, attenuation artifacts from the diaphragm or breast, especially in obese patients, might result in false-positive perfusion defect in the inferior and anterior territories.5 Second, patients with relatively balanced ischemia among three major vascular territories might result in a homogenous distribution of radiotracer in myocardium and thus underestimate the severity of ischemia or even show a falsely normal result.6 Third, pharmacological stress overall estimated about 50% of the MPS studies; but currently used stressors, including adenosine, dipyridamole or dobutamine, were procedurally time-consuming with a high incidence of adverse reactions.7 Finally, the radiation exposure was still relatively high, based on currently used hardware and software modalities.8
In this article, we aimed to update the recent trends and advances in nuclear cardiology that could further optimize the diagnostic accuracy of MPI and facilitate the management of CAD. Those topics reviewed included the utilization of positron emission tomography (PET) for MPI, a new pharmacological stressor, new SPECT cameras with higher resolution and higher sensitivity, dynamic SPECT protocol for blood for flow quantitation, new software of phase analysis for analyzing LV dyssynchrony, and the measures for reducing radiation exposure from MPI.
MYOCARDIAL PERFUSION IMAGING WITH PET
PET has been increasingly used for MPI over the past decade. In fact, MPI with PET has several advantages over MPS. First of all, PET images are derived from coincidence detection of two 511-keV photons of rapid annihilations of PET tracers in ring PET scanners, and thus have better spatial resolution and allow more accurate attenuation correction than SPECT techniques. In addition, PET has superior detection sensitivity and high temporal resolution, which allows for the acquisition of dynamic images for quantitation of myocardial blood flow (MBF) and myocardial flow reserve (MFR); it has been confirmed to be a strong and independent predictor of cardiac mortality in patients with known or suspected CAD,9 and to provide incremental value on risk stratification over established clinical variables and relative MPI.10
A recent meta-analysis including 19 studies using 15O-water, 13N-ammonia or 82Rb for PET MPI demonstrated weighted sensitivity, specificity, negative predictive value, and positive predictive value of 91%, 86%, 81%, and 93%, respectively, superior to those of 99mTc SPECT (sensitivity 90%, specificity 75%) and 201Tl SPECT (sensitivity 87%, specificity 78%).11
15O-water
As a freely diffusible tracer, 15O-water shows a perfectly linear relationship between myocardial blood flow and tracer uptake.12 However, the short half-life (2 minutes) limits its usage to institution with an on-site cyclotron. In addition, it is neither Food and Drug Administration (FDA)-approved nor is it clinically used in the United States. The detailed properties of PET perfusion tracers were summarized in Table 1.
Table 1. Properties of PET perfusion tracers10,11 .
| 15O-water | 13N-ammonia | 82Rb | 18F-flurpiridaz | |
| Half-life | 2 min | 10 min | 76 s | 109 min |
| Cyclotron on-site needed | Yes | Yes | No | No |
| Average positron energy (MeV) | 0.74 | 0.49 | 1.48 | 0.25 |
| RMS positron range (mm) | 1.0 | 0.6 | 2.6 | 0.2 |
| Positron range | 4.1 | 2.5 | 8.6 | 1.0 |
| Myocardial extraction fraction | 100 | 80 | 65 | 94 |
| Image quality | Poor | Excellent | Good | Excellent |
| Image interpretation | No | Yes | Yes | Yes |
| Quantification of myocardial blood flow | Yes | Yes | Yes | Yes |
| Stress modality | Pharmacologic | Pharmacologic or exercise (feasible but not practical) | Pharmacologic | Pharmacologic or exercise |
PET, positron emission tomography; RMS, root-mean square.
13N-ammonia
Owing to the 10-minute half-life of 13N-ammonia, its production also requires an on-site cyclotron. Since its positron range is shorter than 15O-water and 82Rb, 13N-ammonia provides cardiac images with better resolution in comparison with 15O-water and 82Rb. The myocardial extraction fraction of 13N-ammonia is approximately 80%, which is less than 15O-water and 18F-flurpiridaz, but higher than those of 82Rb and 201Tl.13
82Rb
82Rb has an ultra-short half-life of 76 seconds. It is produced in a commercially available Strontium-82 (82Sr) generator equipped with a computer-controlled elution pump which is connected by intravenous tubing to the patient. Therefore, on-site cyclotron is not required. After administration, the generator gets 90% replenishment within 5 minutes and full replenishment in 10 minutes. Serial images can be performed every 5-6 minutes. Because of its ultra-short half-life, exercise stress is not feasible for 82Rb study because there remains substantial respiratory motion during the early post-stress period. With pharmacological stress, an entire rest/stress 82Rb study can be completed in 45 minutes. The other advantage of 82Rb PET is that it captures ventricular function close to peak stress, thus more sensitive for ischemia than SPECT in detecting post-ischemic stunning.13
18F-flurpiridaz
18F-labeled agents have two major advantages over other PET tracers. First, 18F has a half-life of 109 minutes, and may be supplied at regional cyclotrons in a similar way as 18F-FDG. The longer half-life of 18F also ensures that the radiotracer is present long enough to allow a patient injected at peak treadmill exercise then move to the camera with enough radioactivity for effectively imaging. Second, the root-mean-square (RMS) positron range of 18F (0.2 mm) is much shorter than that of 82Rb (2.6 mm), 15O-water (1.0 mm), and 13N-ammonia (0.6 mm), thus theoretically producing image resolution better than other PET tracers.
18F-flurpiridaz demonstrated a flow-independent extraction fraction of 94%, and a nearly linear relationship between uptake and stress MBF. The well linear relationship of myocardial extraction fraction of 18F-flurpiridaz with myocardial flow rates makes it an optimal candidate for absolute MBF quantitation. Recently, the first absolute quantitation of MBF with 18F-flurpiridaz in humans has been published.14 The results are in a similar range as those measured with other radiotracers such as 82Rb, 13N-ammonia, and 15O-water.
A2A AGONIST FOR PHARMACOLOGIC STRESS ON MPI
Adequate stress is essential for MPI. In addition to treadmill exercise, pharmaceutical stress is also used for patients unable to exercise. Dipyridamole and adenosine are the stressors currently used in clinical practices. These agents act as a coronary vasodilator, resulting in coronary flow heterogeneities between normal and diseased vessels. In addition to A2A adenosine receptor, both agents also act on A1, A2B, and A3 receptors which can cause adverse reactions such as shortness of breath, flushing, chest tightness, AV block, and bronchospasm. In 2008, the US Food and Drug Administration approved “regadenoson” for stress testing in conjunction with MPI for patients who could not tolerate exercise-stress. Regadenoson is the derivative of adenosine with a similar chemical structure (Figure 1), but is characterized by a 4-substituted pyrazole structure, which makes it more selective to A2A receptors than adenosine.15
Figure 1.
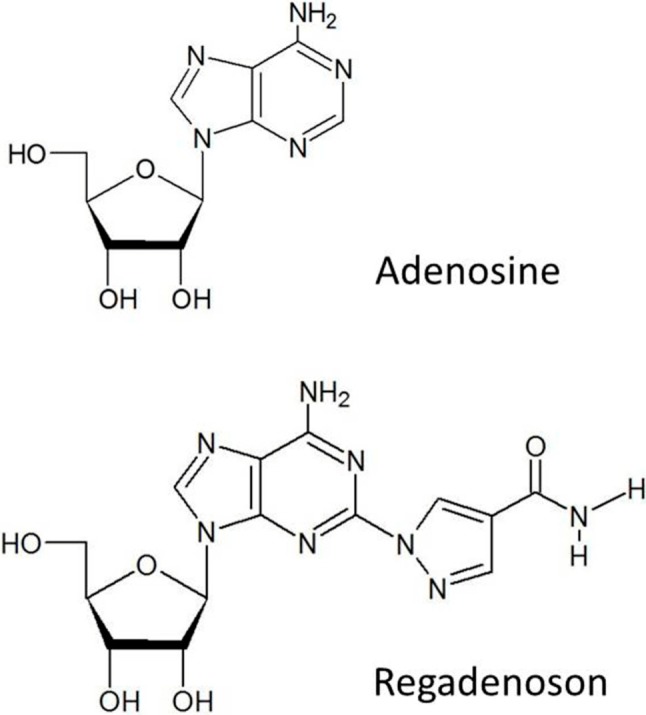
Chemical structures of adenosine and regadenoson.
Mechanism, pharmacodynamics and dosage
Regadenoson is highly selective but low affinity (Ki ≈ 1.3 μM) for an A2A adenosine receptor. It means that regadenoson is a potent and selective coronary vasodilator with rapid onset but short duration of action, which allow its administration with a fixed-dose bolus (not weight-based).16 Additionally, age, gender, and human race had minimal impacts on its pharmacokinetics. Even for those patients with hepatic and/or renal insufficiency, the observed blood concentration and distribution volume were not affected by administered dosage. Therefore, it was unnecessary to make dosage adjustments in these patients.17
Regadenoson are marketed and distributed in the form of a single-use vial and single-use pre-filled syringe with the same strength of 0.4 mg/5 mL (0.08 mg/mL). Using a No. 22 or larger gauge needle, regadenoson is administered by IV push in less than 10 seconds through peripheral vein, immediately followed by 5 mL of normal saline irrigation. Radiopharmaceutical should be administered 10 to 20 seconds thereafter.
Adverse reactions and contraindications
The most frequent reported adverse reactions of regadenoson were dyspnea, flushing, and headache. Other reactions included chest pain, angina, abdominal pain, palpitation, dizziness, nauseas, and ventricular conduction abnormalities. However, most patients demonstrated good tolerance and most of the adverse reactions disappeared within 15 minutes. Regadenoson should not be used in patients with severe hypotension (systolic blood pressure less than 80 mmHg), symptomatic and persistent second or third degree AV block, active wheezing, and severe chest pain (ST depression ≥ 2 mm), or impaired cardiac perfusion (pale, cyanosis, and cold skin). Regadenoson is not absolutely contraindicated for patients with asthma and chronic obstructive pulmonary disease, but must be carefully monitored.
Whether regadenoson can be safely used during pregnancy had not yet been firmly established, and the use of regadenoson in pregnant patients should be subject to a risk/benefit analysis. It is also unknown whether or not regadenoson will be secreted into breast milk. Because it takes approximately 10 hours to completely eliminate from the body, breast feeding should be avoided at least 10 hours after regadenoson administration. Besides, the incidence of hypotension is higher in the elderly, and blood pressure should be carefully monitored when used in elderly patients.18
Current clinical application
Regadenoson was first marketed in the United States in 2008, and thereafter approved in Europe in 2011. Most clinical experiences with regadenoson, however, were from the United States, and few were from England. In a recent report on regadenoson involving more than 1200 patients, only a few patients developed severe adverse reactions, including 2 transient heart blocks; no patient had asthma or severe hypotension.19 Besides, it was also demonstrated that the diagnostic performance of MPS in detecting CAD by regadenoson was similar with adenosine and the image qualities were of comparable utility.17
NEW GENERATION SPECT CAMERA FOR MPI
Three cardiac SPECT imaging systems are commercially available and have been widely utilized in recent years (Figure 2 and Table 2). All of them use a re-designed cardiac-centric collimating system, and two utilize solid-state semiconductor cadmium-zinc-telluride (CZT) technology. These systems optimize geometric efficiency, enabling ultra-fast scan or reduction of radiation dose to patients.20
Figure 2.
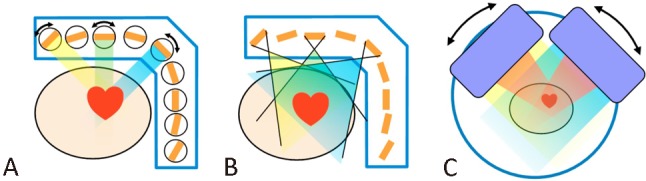
Illustration of three cardiac SPECT systems: the D-SPECT system (A), the DNM system (B), and the IQ-SPECT system (C). DNM, Discovery NM530c; SPECT, single photon emission computed tomography
Table 2. Comparison of recent cardiac SPECT imaging systems20,21 .
| Parameters | D-SPECT | DNM | IQ-SPECT | Conventional |
| Collimation | Wide-angle parallel hole | Multiple pinhole | Multifocal (astigmatic) | Parallel hole |
| Number of detectors | 9 | 19 | 2 | 1 or 2 |
| Type of detector | CZT | CZT | NaI | NaI |
| Acquisition | Rotation (individual) | Stationary | Rotation (cardiac-centric) | Rotation (circular, ellipsoid, etc) |
| Attenuation correction | Not available | Optional CTAC1 | CTAC | Optional CTAC or transmission scan |
| Other position | Upright | Prone | Prone | Prone |
| Perform general SPECT | No | No | Yes | Yes |
1 Available with Discovery NM570c; CTAC, CT attenuation correction; CZT, cadmium-zinc-telluride; SPECT, single photon emission computed tomography.
D-SPECT
The D-SPECT gamma cameras (Spectrum Dynamics, Caesarea, Israel) use nine CZT detectors with square parallel-hole tungsten collimators.21 These mobile detector columns are mounted on a L-shaped gantry, rotating in synchrony, focusing on the heart during image acquisition (Figure 2A). This system enables high-speed MPI scanning with up to 8 times increased sensitivity and higher resolution than conventional SPECT camera. With 4-minute stress and 2-minute rest acquisitions, this high-speed SPECT system provides high-quality images, perfusion abnormality highly correlated to conventional SPECT, and an equivalent level of diagnostic confidence. In a multicenter study, this high-speed SPECT system appears to have superior diagnostic accuracy compared to conventional SPECT cameras.21
This high-sensitivity system also leads to significantly reduced radiation dose. In a recent multicenter study, high-quality ultra-low-dose MPI can be performed in non-obese individuals with an effective dose of 1 mSv for a single injection.22 Although this system is not capable of attenuation correction, it allows sequential supine and upright acquisitions to deal with the problem of attenuation artifacts. In obese patients, D-SPECT has a high image quality and high diagnostic accuracy for detection of CAD by both quantitative and visual analysis.23
The improved energy resolution of the CZT detectors allows discrimination of 159 keV photo-peak of 123I from 140 keV photo-peak of 99 mTc. Simultaneous assessment of myocardial perfusion and fatty acid metabolism by 99mTc-MIBI and 123I-BMIPP is then feasible. Taking the advantage of high count sensitivity and linearity, the D-SPECT system is inherently suitable for dynamic SPECT studies. Dynamic tomographic imaging and quantification in humans are found to be feasible and reproducible using this novel SPECT camera.24
Discovery NM530c
The Discovery NM530c (DNM) gamma camera (GE Medical System Israel, Tirat Hacarmel, Israel) also utilizes CZT detectors. The miniature detector and multi-pinhole collimator design enables simultaneous acquisition of angular data in 19 projections without any moving parts during acquisition (Figure 2B). With pinhole collimation, septal penetration can be avoided, resulting in better image resolution as compared with the D-SPECT camera.20 CT-based attenuation correction (CTAC) is optionally available on this system.
The DNM system requires significantly shorter scan time, providing better image quality and good correlation with conventional Anger cameras, coronary angiography, and fractional flow reserve.25 The effective dose can also be reduced to as low as 1.4 mSv in a stress-only protocol and 5.8 mSv in a one-day stress-rest protocol.26 Combined supine/prone position imaging and real-time breath-hold triggering have been used as an alternative to CTAC. This camera is also capable of dynamic SPECT acquisition for absolute flow quantification. The measurement is validated against microsphere method in porcine model.27 Taking advantage of the high sensitivity, respiration motion can be tracked and compensated without an external gating device.28 In comparison with the D-SPECT system, D-SPECT has a markedly higher count sensitivity than DNM system due to the different collimator design, whereas the DNM system has superior spatial resolution and contrast-to-noise ratio.
IQ-SPECT
The IQ-SPECT system (Figure 2C) developed by Siemens consists of three main components: 1) a multifocal collimator (SmartzoomTM) which is focusing at its center, magnifying the heart, but morphs to near-parallel collimation at its edges, avoiding truncation; 2) a cardio-centric orbit, keeping the heart in the region of four-fold magnification; and 3) an advanced reconstruction engine with precise modeling of the system.29 This design provides cardiac-centric magnification and four-fold increased myocardial count rate while avoiding truncation artifacts. There is relatively minor modification of the imaging system. General SPECT can be performed with a collimator change, and CT-based attenuation correction is also available for this system.
This IQ-SPECT system allows quarter-time acquisition with minimal disagreement in comparison to full time acquisition on conventional imaging system.30 Regarding dose reduction, the only evidence from a phantom study is available to date.1 Image quality and quantitative contrast were preserved with as low as 18% of conventionally administered dose. Although the IQ-SPECT system provides enhanced sensitivity while it has similar spatial resolution and contrast-to-noise ratio as compared to conventional Anger camera.
MBF QUANTITATION WITH CONVENTIONAL SPECT
As previously mentioned, the perfusion abnormalities on MPI may be unremarkable or even be false normal due to relatively balanced hypoperfusion between the stenosed vessels in multi-vessel CAD. A previous study found that the performance of MPI in detecting flow-limited CAD was very limited in patients with stenoses in multiple coronary vessels; thereby the diagnostic sensitivity was as low as only 48%.32 Therefore, absolute quantitation of MBF with PET has been considered as the solution to overcome this drawback.33 However, the main challenge comes from the fact that the production of PET tracers demands either high start-up costs to install an on-site cyclotron within the hospital (for 15O-water and 13N-Ammonia), or relatively high ongoing costs to purchase commercial generators (for 82Rb) in monthly basis.
Dynamic SPECT protocol for flow quantitation
Recently, MBF and MFR quantitation with SPECT have been determined to be feasible.34 The investigators used conventional dual-head SPECT/CT scanner, which was capable of performing fast dynamic SPECT (DySPECT) acquisitions with a total of 12 minutes of multiple back-and-forth gantry rotations during injections of 99mTc-sestamibi at rest or dipyridamole-stress. Using commercially available software for PET flow quantitation (FlowQuant, University of Ottawa Hospital, Ottawa, Canada),35 this DySPECT method were found to have very good inter-operator and intra-operator reproducibility for measuring MBF and MFR.36 Using invasive coronary angiography as the reference standard, stress MBF and MFR by DySPECT were found to have significantly higher diagnostic accuracies than traditional perfusion parameters, such as sum stress score (SSS) and sum difference score (SDS).36 An example image was shown on Figure 3.
Figure 3.
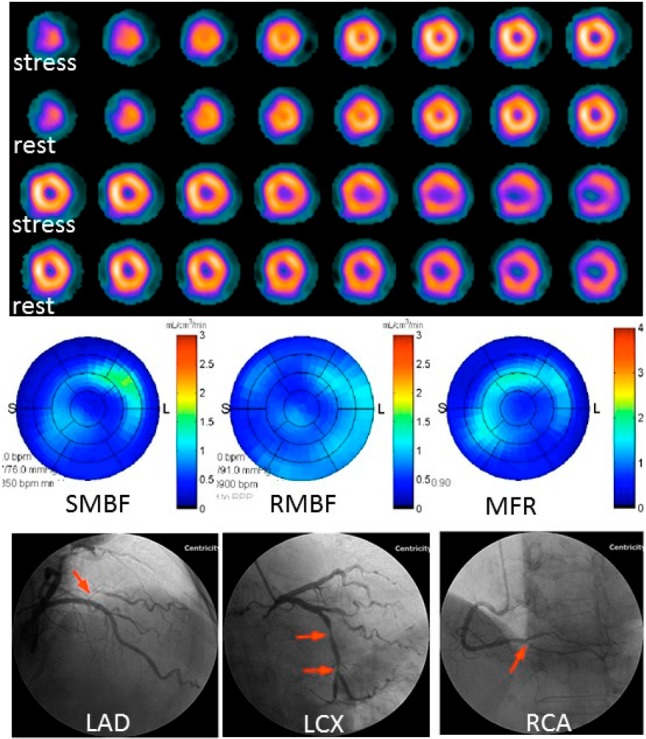
Dynamic SPECT (DysSPECT) imaging for a 71-year-old male patient with hyperlipidemia and no history of coronary artery disease (CAD). He complained of chest pain unrelated to physical exertion. Conventional perfusion images revealed no definite perfusion abnormality on the stress/rest images, and no transient ischemia dilatation was found (top). The quantitative flow values from DySPECT were stress myocardial blood flow (SMBF) = 0.54 ml/min/g, rest myocardial blood flow (RMBF) = 0.53 ml/min/g and myocardial flow reserve (MFR) = SMBF/RMBF = 1.0, which were extensively lower than the CAD thresholds (SMBF = 1.96 and MFR = 2.0) (middle). Coronary angiography performed 31 days after the SPECT imaging showed severe stenosis in all three coronary arteries (bottom).
PHASE ANALYSIS FOR LV DYSSYNCHRONY
Phase analysis for gated MPS was first developed by Chen et al. in 2005 for measuring left-ventricular (LV) dyssynchrony.37 Previous studies have shown that quantitative indices given by phase analysis, such as phase standard deviation (PSD) and phase histogram bandwidth (PHB), were well-correlated with LV dyssynchrony measured by tissue Doppler imaging and speckle tracking echocardiography.38 In addition, PSD and PHB were also found to be predictive of response to cardiac resynchronization therapy (CRT) in heart failure patients.
One-stop shop evaluation for CRT implantation
A comprehensive model for CRT implantation has recently been established as follows: CRT should be considered for patients with baseline LV mechanical dyssynchrony and performed with the LV lead placed at the site of latest activation with viable myocardium. Since phase analysis with MPI can assess LV mechanical dyssynchrony, latest activation site and myocardial scar in the same imaging procedure, this imaging modality thus can provide a one-stop shop evaluation of the parameters for optimizing the implantation of CRT.39 An example image was shown on Figure 4.
Figure 4.
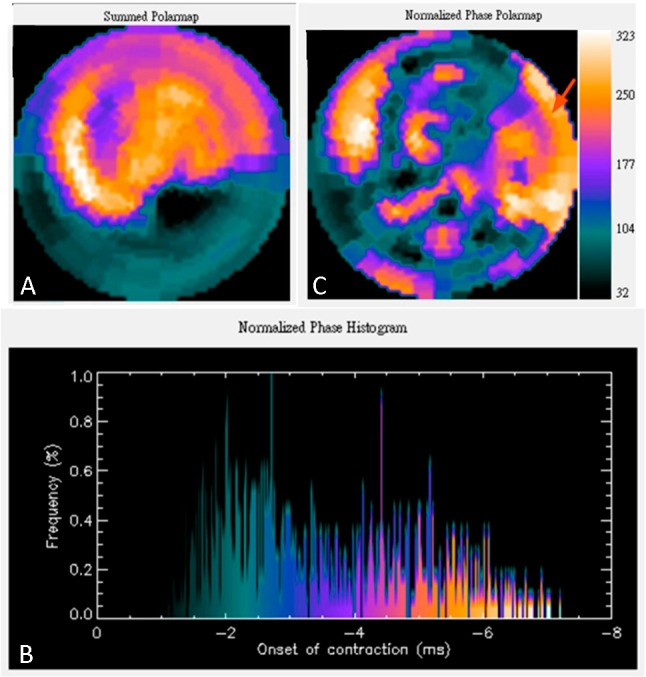
A 44-year-old male patient with ischemic cardiomyopathy was referred for cardiac resynchronization therapy (CRT) due to continuously deteriorated LV dysfunction even treated with revascularization. The pre-CRT assessments revealed NYHA class III, LVEF = 30% and QRS = 150 ms, which were fulfilled with the indication for CRT. For further assessment for guiding the LV lead implantation of CRT, he was also referred for ECG-gated SPECT MPI. (A) The perfusion images revealed an extensive scar in the inferior, inferoseptal and inferolateral walls. (B) Phase analysis showed greatly widening histogram bandwidth, compatible with significant LV mechanical dyssynchrony. (C) Phase polar map showed the latest mechanical activation site mainly in anterolateral and inferolateral walls. Since inferolateral wall was considered as scarred myocardium, the optimal site for LV lead placement should be located at the anterolateral wall (arrow).
Ischemia-induced LV dyssynchrony
Since the development of phase analysis of gated SPECT MPI, all of the above studies were done using Tc-99m labeled radiotracers (sestamibi or tetrofosmin). Chen et al. first validated the performance of Tl-201 MPI for phase analysis and found a good correlation with Tc-99m sestamibi.40 As we known, gated MPI with Tl-201 acquires data immediately post injection of radiotracer but as long as one hour or more for Tc-99m tracers. Therefore, Tl-201 MPI theoretically had a better chance of detecting stress-induced functional change than Tc-99m tracers. The same group further investigated whether stress-induced myocardial ischemia is associated with LV mechanical dyssynchrony.41 This study demonstrated that stress-induced myocardial ischemia caused dyssynchronous contraction in the ischemic region, deteriorating LV synchrony. Normal myocardium had more synchronous contraction at stress, but scarred myocardium had no change.
Huang et al. recently studied the relation of early post-stress LV dyssynchrony and the extent of angiographic CAD on Tl-201 MPI.42 They found that the patients with multi-vessel CAD (≥ 70% stenosis on invasive coronary angiography) had significantly more global dyssynchrony than the patients without CAD. The patients with multi-vessel CAD showed significantly more global and territorial dyssynchrony on stress images than on rest. More patients with 3-vessel CAD were correctly classified as multi-vessel disease, when combining both visual interpretation and dyssynchrony assessment. An example image was shown on Figure 5.
Figure 5.
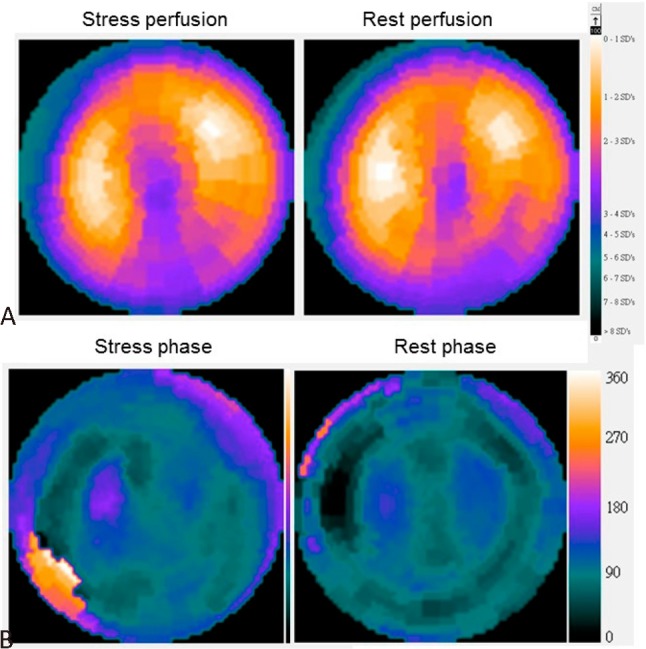
Phase analysis for a patient with balanced 3-vessel disease (LAD stenosis 70%, LCX stenosis 80%, RCA stenosis 70%, respectively). (A) The perfusion polar map revealed only mild grade of perfusion abnormality with partial reversibility in apex and inferior wall. (B) Phase polar map revealed remarkable deterioration in global synchrony from rest to stress, with phase SD and bandwidth of 18° and 65° at rest, and 31° and 85° at stress, respectively. LAD, left anterior descending artery; LCX, left circumflex artery; RCA, right coronary artery.
RADIATION EXPOSURE FROM MPI
The ranges of the radiation doses for agents used in the SPECT system are around 10 to 20 mSv, depending on the radiopharmaceutical used and its relevant imaging protocol.43-45 Table 3 lists estimated effective doses for adults with various radiopharmaceuticals. The use of positron-emitting radiopharmaceuticals, such as 82Rb and 13N-ammonia in this table, can greatly reduce the radiation burden for patients. However, the availability of PET or PET/CT scanner and logistics of PET radiopharmaceuticals are still less common and convenient than those with SPECT or SPECT/CT system, preventing the popularity of PET-based MPI in everyday practices.
Table 3. Effective doses for adults from myocardial perfusion imaging with various radiopharmaceuticals .
| Radiopharmaceutical | Protocol | Effective dose (mSv) |
| 99mTc-sestamibi | One-day rest (10 mCi)/stress (30 mCi) | 12.10* |
| Two-day stress (30 mCi)/rest (30 mCi) | 18.76* | |
| 99mTc-tetrofosmin | One-day rest (10 mCi)/stress (30 mCi) | 10.62#† |
| Two-day stress (30 mCi)/rest (30 mCi) | 16.54#† | |
| 201Tl | Stress (2.5 mCi)/redistribution | 12.95† |
| 82Rb | Rest (30 mCi)/stress (30 mCi) | 7.55* |
| 13N-ammonia | Rest (20 mCi)/stress (20 mCi) | 2.96* |
* Derived from ICRP publication 80. # Derived from 4th addendum to ICRP publication 53. † Derived from ICRP publication 106.
The historical observation of atomic bomb survivors in Hiroshima and Nagasaki found an increased incidence of cancer in survivors receiving whole-body doses of 100 mSv or more. However, such trend could not be identified in survivors exposed to less than 100 mSv compared with the normal incidence of cancer.46 Thus the risk of developing malignancy related to low level radiation exposure, such as used in most medical diagnostic procedures, is still debated. The American Association of Physicists in Medicine made their position statement on radiation risks from medical imaging procedures in 2011, mentioning that the risks of medical imaging at doses below 50 mSv for a single procedure or 100 mSv for multiple procedures over short time periods were too low to be detectable and might be nonexistent. They also considered that predictions of hypothetical cancer incidence and deaths in patient populations exposed to such low doses were highly speculative and should be discouraged.47
Despite the existing controversies about the radiation effect, several methods have been advocated to further reduce the radiation dose with MPI. For instance, a stress-only protocol can be used if the stress images appear to be normal. The rest of the images in this setting usually do not provide additional information and thus can be omitted, sparing additional radiation exposure.48 The new advent of semiconductor cardiac SPECT system, as mentioned earlier in this article, provides another option for lowering the radiation dose.
CONCLUSIONS
In recent years, the continuing development of hardware, software, stressor and protocol have remarkably optimized the utilizations of MPI for evaluating patients with CAD. MPI with PET or PET/CT has shown a superior sensitivity and specificity than SPECT in the detection of flow-limiting CAD and also can routinely provide quantitative information, such as MBF and MFR. It currently has been widely utilized in western countries as a clinical routine. 13N-Ammonia is the tracer of choice for institutes with on-site cyclotron. For institutes without on-site cyclotron, 82Rb is currently a practical choice if the patient volumes are big enough to maintain the cost of 82Sr generator. Although 18F-flurpiridaz is still undergoing phase III clinical trial, it seems a promising PET tracer for MPI for the units without cyclotron.
The new vasodilator stressor – regadenoson – is easy to use and well tolerable for patients, even with hepatic and/or renal insufficiency. It has rapidly replaced conventional vasodilator agents, such as adenosine or dipyridamole, in western countries. However, the major barrier for introducing regadenoson into other Asian countries or Taiwan is the high price.
New CZT SPECT or conventional SPECT have also preliminarily shown the feasibility for quantitation of MBF and MFR, although further clinical validation is still needed. In addition, phase analysis provides one-stop-shop evaluation for heart failure patients potentially indicated for CRT and also opens a new window for nuclear cardiology research on CAD.
Finally, it is always important for nuclear medicine professionals to reduce the radiation exposure of MPI to patients as to make it as low as reasonable. If MPI with PET is not available, we may use stress-only SPECT protocol, especially for patients with relatively lower pre-test likelihood. For institutes with large volume of patients, rapid CZT camera cannot only provide high throughput but also equally good quality of images using a lower administered dose.
REFERENCES
- 1.Strauss HW, Miller DD, Wittry MD, et al. Procedure guideline for myocardial perfusion imaging. J Nucl Med. 1998;39:918–923. [PubMed] [Google Scholar]
- 2.Underwood SR, Anagnostopoulos C, Cerqueira M, et al. Myocardial perfusion scintigraphy: the evidence. Eur J Nucl Med Mol Imaging. 2004;31:261–291. doi: 10.1007/s00259-003-1344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaw LJ, Iskandrian AE. Prognostic value of gated myocardial perfusion SPECT. J Nucl Cardiol. 2004;11:171–185. doi: 10.1016/j.nuclcard.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Hachamovitch R, Hayes SW, Friedman JD, et al. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation. 2003; 107:2900–2907. doi: 10.1161/01.CIR.0000072790.23090.41. [DOI] [PubMed] [Google Scholar]
- 5.Baron JM, Chouraqui P. Myocardial single-photon emission computed tomographic quality assurance. J Nucl Cardiol. 1996;3:157–166. doi: 10.1016/s1071-3581(96)90008-2. [DOI] [PubMed] [Google Scholar]
- 6.Berman DS, Kang X, Slomka PJ, et al. Underestimation of extent of ischemia by gated SPECT myocardial perfusion imaging in patients with left main coronary artery disease. J Nucl Cardiol. 2007;14:521–528. doi: 10.1016/j.nuclcard.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Thomas GS, Prill NV, Majmundar H, et al. Treadmill exercise during adenosine infusion is safe, results in fewer adverse reactions, and improves myocardial perfusion image quality. J Nucl Cardiol. 2000;7:439–446. doi: 10.1067/mnc.2000.108030. [DOI] [PubMed] [Google Scholar]
- 8.Einstein AJ, Moser KW, Thompson RC, et al. Radiation dose to patients from cardiac diagnostic imaging. Circulation. 2007;116:1290–1305. doi: 10.1161/CIRCULATIONAHA.107.688101. [DOI] [PubMed] [Google Scholar]
- 9.Gould KL, Johnson NP, Bateman TM, et al. Anatomic versus physiologic assessment of coronary artery disease. Role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision-making. J Am Coll Cardiol. 2013;62:1639–1653. doi: 10.1016/j.jacc.2013.07.076. [DOI] [PubMed] [Google Scholar]
- 10.Murthy VL, Naya M, Foster CR, et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation. 2011;124:2215–2224. doi: 10.1161/CIRCULATIONAHA.111.050427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parker MW, Iskandar A, Limone B, et al. Diagnostic accuracy of cardiac positron emission tomography versus single photon emission computed tomography for coronary artery disease:a bivariate meta-analysis. Circ Cardiovasc Imaging. 2012;5:700–707. doi: 10.1161/CIRCIMAGING.112.978270. [DOI] [PubMed] [Google Scholar]
- 12.Lalonde L, Ziadi MC, Beanlands R. Cardiac positron emission tomography: current clinical practice. Cardiol Clin. 2009;27:237–255. doi: 10.1016/j.ccl.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Yoshinaga K, Klein R, Tamaki N. Generator-produced rubidium-82 positron emission tomography myocardial perfusion imaging-from basic aspects to clinical applications. J Cardiol. 2010;55:163–173. doi: 10.1016/j.jjcc.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Packard RR, Huang SC, Dahlbom M, et al. Absolute quantitation of myocardial blood flow in human subjects with or without myocardial ischemia using dynamic flurpiridaz F 18 PET. J Nucl Med. 2014;55:1438–1444. doi: 10.2967/jnumed.114.141093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao JG, Chuang TL, Wang YF. Regadenoson: a novel pharmacological stress agent for myocardial perfusion imaging. Ann Nucl Med Sci. 2010;23:123–220. [Google Scholar]
- 16.Trochu JN, Zhao G, Post H, et al. Selective A2A adenosine receptor agonist as a coronary vasodilator in conscious dogs: potential for use in myocardial perfusion imaging. J Cardiovasc Pharmacol. 2003;41:132–139. doi: 10.1097/00005344-200301000-00017. [DOI] [PubMed] [Google Scholar]
- 17.Cerqueira MD, Nguyen P, Staehr P, et al. Effects of age, gender, obesity, and diabetes on the efficacy and safety of the selective A2a agonist regadenoson versus adenosine in myocardial perfusion imaging: integrated ADVANCEMPI trial results. JACC Cardiovasc Imaging. 2008;1:307–316. doi: 10.1016/j.jcmg.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Hendel RC, Bateman TM, Cerqueira MD, et al. Initial clinical experience with regadenoson, a novel selective A2A agonist for pharmacologic stress single-photon emission computed tomography myocardial perfusion imaging. J Am Coll Cardiol. 2005;46:2069–2075. doi: 10.1016/j.jacc.2005.05.097. [DOI] [PubMed] [Google Scholar]
- 19.Gemignani AS, Abbott BG. The emerging role of the selective A2A agonist in pharmacologic stress testing. J Nucl Cardiol. 2010;17:494–497. doi: 10.1007/s12350-010-9211-9. [DOI] [PubMed] [Google Scholar]
- 20.DePuey EG. Advances in SPECT camera software and hardware: currently available and new on the horizon. J Nucl Cardiol. 2012;19:551–581; quiz 585. doi: 10.1007/s12350-012-9544-7. [DOI] [PubMed] [Google Scholar]
- 21.Neill J, Prvulovich EM, Fish MB, et al. Initial multicentre experience of high-speed myocardial perfusion imaging: comparison between high-speed and conventional single-photon emission computed tomography with angiographic validation. Eur J Nucl Med Mol Imaging. 2013;40:1084–1094. doi: 10.1007/s00259-013-2399-6. [DOI] [PubMed] [Google Scholar]
- 22.Einstein AJ, Blankstein R, Andrews H, et al. Comparison of image quality, myocardial perfusion, and left ventricular function between standard imaging and single-injection ultra-low-dose imaging using a high-efficiency SPECT camera:the MILLISIEVERT study. J Nucl Med. 2014;55:1430–1437. doi: 10.2967/jnumed.114.138222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakazato R, Slomka PJ, Fish M, et al. Quantitative high-efficiency cadmium-zinc-telluride SPECT with dedicated parallel-hole collimation system in obese patients:results of a multi-center study. J Nucl Cardiol. 2014;22:266–275. doi: 10.1007/s12350-014-9984-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ben-Haim S, Murthy VL, Breault C, et al. Quantification of myocardial perfusion reserve using dynamic SPECT imaging in humans: a feasibility study. J Nucl Med. 2013;54:873–879. doi: 10.2967/jnumed.112.109652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mouden M, Ottervanger JP, Knollema S, et al. Myocardial perfusion imaging with a cadmium zinc telluride-based gamma camera versus invasive fractional flow reserve. Eur J Nucl Med Mol Imaging. 2014;41:956–962. doi: 10.1007/s00259-013-2630-5. [DOI] [PubMed] [Google Scholar]
- 26.Oddstig J, Hedeer F, Jögi J, et al. Reduced administered activity, reduced acquisition time, and preserved image quality for the new CZT camera. J Nucl Cardiol. 2013;20:38–44. doi: 10.1007/s12350-012-9634-6. [DOI] [PubMed] [Google Scholar]
- 27.Wells RG, Timmins R, Klein R, et al. Dynamic SPECT measurement of absolute myocardial blood flow in a porcine model. J Nucl Med. 2014;55:1685–1691. doi: 10.2967/jnumed.114.139782. [DOI] [PubMed] [Google Scholar]
- 28.Ko CL, Wu YW, Cheng MF, et al. Data-driven respiratory motion tracking and compensation in CZT cameras: a comprehensive analysis of phantom and human images. J Nucl Cardiol. 2014; 22:308–318. doi: 10.1007/s12350-014-9963-8. [DOI] [PubMed] [Google Scholar]
- 29.Rajaram R, Bhattacharya M, Xinhong D, et al. Tomographic performance characteristics of the IQ-SPECT system. Paper presented at: Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC), 2011 IEEE; 23-29 Oct. 2011 [Google Scholar]
- 30.Caobelli F, Pizzocaro C, Paghera B, Guerra UP. Evaluation of patients with coronary artery disease. IQ-SPECT protocol in myocardial perfusion imaging: preliminary results. Nuklearmedizin. 2013;52:178–185. doi: 10.3413/Nukmed-0570-13-03. [DOI] [PubMed] [Google Scholar]
- 31.Caobelli F, Kaiser SR, Thackeray JT, et al. IQ SPECT allows a significant reduction in administered dose and acquisition time for myocardial perfusion imaging: evidence from a phantom study. J Nucl Med. 2014;55:2064–2070. doi: 10.2967/jnumed.114.143560. [DOI] [PubMed] [Google Scholar]
- 32.Bateman TM, Heller GV, McGhie AI, et al. Diagnostic accuracy of rest/stress ECG-gated Rb-82 myocardial perfusion PET: comparison with ECG-gated Tc-99m sestamibi SPECT. J Nucl Cardiol. 2006;13:24–33. doi: 10.1016/j.nuclcard.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 33.Parkash R, deKemp RA, Ruddy TD, et al. Potential utility of rubidium 82 PET quantification in patients with 3-vessel coronary artery disease. J Nucl Cardiol. 2004;11:440–449. doi: 10.1016/j.nuclcard.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Hsu B, Chen WJ, Chen FC, et al. Quantitation of myocardial blood flow with quantitative dynamic SPECT/CT. Ann Nucl Med Mol Img. 2012;25:190–200. [Google Scholar]
- 35.Klein R, Renaud JM, Ziadi MC, et al. Intra- and inter-operator repeatability of myocardial blood flow and myocardial flow reserve measurements using rubidium-82 PET and highly automated analysis program. J Nucl Cardiol. 2010;17:600–616. doi: 10.1007/s12350-010-9225-3. [DOI] [PubMed] [Google Scholar]
- 36.Hsu B, Chen FC, Wu TC, et al. Quantitation of myocardial blood flow and myocardial flow reserve with 99mTcsestamibi dynamic SPECT/CT to enhance detection of coronary artery disease. Eur J Nucl Med Mol Imaging. 2014;41:2294–2306. doi: 10.1007/s00259-014-2881-9. [DOI] [PubMed] [Google Scholar]
- 37.Chen J, Garcia EV, Folks RD, et al. Onset of left ventricular mechanical contraction as determined by Phase analysis of ECG-gated myocardial perfusion SPECT imaging: development of a diagnostic tool for assessment of cardiac mechanical dyssynchrony. J Nucl Cardiol. 2005;12:687–695. doi: 10.1016/j.nuclcard.2005.06.088. [DOI] [PubMed] [Google Scholar]
- 38.Hsu TH, Huang WS, Chen CC, et al. Left ventricular systolic and diastolic dyssynchrony assessed by phase analysis of gated SPECT myocardial perfusion imaging: a comparison with speckle tracking echocardiography. Ann Nucl Med. 2013;27:764–771. doi: 10.1007/s12149-013-0744-2. [DOI] [PubMed] [Google Scholar]
- 39.Chen J, Garcia EV, Bax JJ, et al. SPECT myocardial perfusion imaging for the assessment of left ventricular mechanical dyssynchrony. J Nucl Cardiol. 2011;18:685–694. doi: 10.1007/s12350-011-9392-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen CC, Huang WS, Hung GU, et al. Left ventricular dyssynchrony evaluated by Tl-201 gated SPECT myocardial perfusion imaging: a comparison with Tc-99m sestamibi. Nucl Med Commun. 2013;34:229–232. doi: 10.1097/MNM.0b013e32835c91b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen CC, Shen TY, Chang MC, et al. Stress-induced myocardial ischemia is associated with early post-stress left ventricular mechanical dyssynchrony as assessed by phase analysis of Tl-201 gated SPECT myocardial Perfusion Imaging. Eur J Nucl Med Mol Imaging. 2012;39:1904–1909. doi: 10.1007/s00259-012-2208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang WS, Huang CH, Lee CL, et al. Relation of early post-stress left ventricular dyssynchrony and the extent of engiographic coronary artery Disease. J Nucl Cardiol. 2014;21:1048–1056. doi: 10.1007/s12350-014-9980-7. [DOI] [PubMed] [Google Scholar]
- 43.ICRP. Radiation dose to patients from radiopharmaceuticals. Addendum 3 to ICRP Publication 53. ICRP Publication 106. Approved by the Commission in October 2007. 2008;38:1–197. doi: 10.1016/j.icrp.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 44.Radiation dose to patients from radiopharmaceuticals (addendum 2 to ICRP publication 53) Ann ICRP. 1998;28:1–126. doi: 10.1016/s0146-6453(99)00006-8. [DOI] [PubMed] [Google Scholar]
- 45.Depuey EG, Mahmarian JJ, Miller TD, et al. Patient-centered imaging. J Nucl Cardiol. 2012;19:185–215. doi: 10.1007/s12350-012-9523-z. [DOI] [PubMed] [Google Scholar]
- 46.Stabin MG. Radiopharmaceuticals for nuclear cardiology: radiation dosimetry, uncertainties, and risk. J Nucl Med. 2008;49:1555–1563. doi: 10.2967/jnumed.108.052241. [DOI] [PubMed] [Google Scholar]
- 47.American Association of Physicists in Medicine. AAPM Position Statement on Radiation Risks from Medical Imaging Procedures. https://www.aapm.org/policies/. Accessed December 5, 2014. 2011. [Google Scholar]
- 48.Chang SM, Nabi F, Xu J, et al. Normal stress-only versus standard stress/rest myocardial perfusion imaging: similar patient mortality with reduced radiation exposure. J Am Coll Cardiol. 2010;55:221–230. doi: 10.1016/j.jacc.2009.09.022. [DOI] [PubMed] [Google Scholar]


