Abstract
Bacteria play a vital role in various biogeochemical processes in lacustrine sediment ecosystems. This study is among the first to investigate the spatial distribution patterns of bacterial community composition in the sediments of Poyang Lake, the largest freshwater lake of China. Sediment samples were collected from the main basins and mouths of major rivers that discharge into the Poyang Lake in May 2011. Quantitative PCR assay and pyrosequencing analysis of 16S rRNA genes showed that the bacteria community abundance and compositions of Poyang Lake sediment varied largely among sampling sites. A total of 25 phyla and 68 bacterial orders were distinguished. Burkholderiales, Gallionellales (Beta-proteobacteria), Myxococcales, Desulfuromonadales (Delta-proteobacteria), Sphingobacteriales (Bacteroidetes), Nitrospirales (Nitrospirae), Xanthomonadales (Gamma-proteobacteria) were identified as the major taxa and collectively accounted for over half of annotated sequences. Moreover, correlation analyses suggested that higher loads of total phosphorus and heavy metals (copper, zinc and cadmium) could enhance bacterial abundance in the sediment.
Electronic supplementary material
The online version of this article (doi:10.1186/s40064-016-2026-7) contains supplementary material, which is available to authorized users.
Keywords: Bacterial community, Sediment, Poyang Lake, High-throughput sequencing
Background
Freshwater lakes are one of the most extensively altered ecosystems on earth due to changes of climate, hydrologic flow and human activities related processes, such as land-use and nutrient inputs (Carpenter et al. 2011). Lake sediments are important grounds for series of biogeochemical transformations of essential nutrients (carbon, nitrogen and phosphorus) and contaminants (Nealson 1997; Bouskill et al. 2010). Sediment microorganisms, especially bacteria, play a dominant role in these critical processes. Bacteria-mediated transformations in sediments lead to active exchange of energy and materials with the water column and intimately connect sedimentary processes with diverse aquatic ecosystem functions (Ranjard et al. 2000; Urakawa et al. 2000).
Bacterial community composition (BCC) in freshwater lakes has been extensively investigated, partly because its potentials in predicting major biogeochemical functions. Early studies have shown that lake sediment BCC may be shaped by physicochemical factors, such as temperature, stream flow (Bernhard et al. 2005), pH (Lindström et al. 2005) and nutrient concentrations (Bai et al. 2012; Zhang et al. 2015). In addition, BCC has been found to co-vary with metal concentrations in lake sediments (Cummings et al. 2003; Bouskill et al. 2010; Sauvain et al. 2014). However, the above studies and most available reports were obtained based on investigations of multiple isolated lakes. The relationship between BCC and environmental conditions within individual freshwater lakes, especially those with large volumes and surface areas has not been fully understood (Yannarell and Triplett 2004; Bouzat et al. 2013). Alternatively, whether environmental factors apply similar impacts on BCC in main lake area and estuarine zone remain unclear.
Poyang Lake (28°52′21″–29°06′46″N, 116°10′24″–116°23′50″E), located in northern Jiangxi Province, is the largest freshwater lake in China with a storage capacity of 2.95 billion m3 (Fig. 1). The lake covers an area of 4125 km2 and has an average depth of 5.1 m. Poyang Lake is a throughput type of lake, mainly collect freshwater from tributary rivers, including the Gan, Fu, Xiu, Xin and Rao Rivers, and discharging into the Yangtze River. In recent years, natural and anthropogenic inputs of nutrients and xenobiotics have consistently increased. As a result, a decreasing gradient of nutrients and heavy metals along transects from estuaries to main lake basins has established (Liu et al. 2012; Zhang et al. 2014; Wang and Liang 2015). This may result in spatial variations of sediment BCCs and their biogeochemical activities, which may in turn impact the lake ecosystem function. However, so far no study has been done on sediment BCC in Poyang Lake.
Fig. 1.
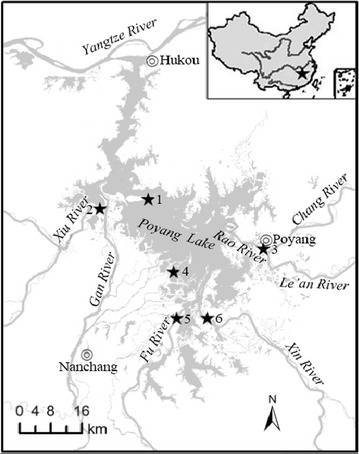
Sampling sites in Poyang Lake. 1 Songmenshan Region, 2 Xiu River Estuary, 3 Rao River Estuary, 4 Nanjishan Region, 5 Fu River Estuary, 6 Xin River Estuary
In this study, 16S rRNA gene-based quantitative PCR and pyrosequencing were used to examine sediment BCCs in Poyang Lake. Our specific goals were to (1) investigate horizontal dynamics of BCCs (i.e., relative abundance and diversity), (2) examine the potential correlations between BCCs and environmental factors.
Methods
Study sites and sample collection
Surface sediments (0–5 cm) were collected in May 2011 from six sites in Poyang Lake (Fig. 1). These sites were chosen to cover both of the main basins of the lake and the mouths of major rivers that discharge into Poyang Lake, including two sites from main basin of the lake, i.e., Site 1 in Songmenshan Region (29°12′26.9″N, 116°11′28.0″E), and Site 4 in Nanjishan Region (28°55′00.1″N, 116°16′44.4″E); four sites from the lake estuaries (mouths of influx rivers), i.e., Site 2 (29°11′27.4″N, 116°00′33.3″E), Site 3 (28°59′10.6″N, 116°40′10.6″E), Site 5 (28°39′54.4″N, 116°9′31.9″E) and Site 6 (28°43′42.2″N, 116°24′34.8″E) from Xiu, Rao, Fu and Xin River to Poyang Lake, respectively.
At each sampling site, triplicate surface sediment samples were taken with a grab sampler to obtain a total of 18 samples. Samples were transferred into sterile polyethylene ziplock bags, put on ice and immediately transported to laboratory. Large organic debris was removed from the sediments with sterile forceps. Afterwards, samples were divided into two aliquots. One aliquot was processed immediately for measurements of sediment property variables; and the other aliquot was stored in sterile polypropylene tubes at −80 °C for molecular analysis.
Measurement of sediment properties
Sediment pH was measured on sediment slurry at a 1:2.5 (w/v) sediment: distilled water ratio using a FE20K pH meter (Mettler Toledo) (Rayment and Higginson 1992). For the measurement of organic matter, the dry matter content of sediment was determined after oven-dried at 105 °C for 24 h, then grinded using a mortar and pestle and sieved using a 0.25 mm mesh for the following measurements: sediment ash-free-dry-mass (AFDM) was obtained as the subsequent loss of weight after 4 h at 550 °C in a BF51800 muffle furnace (Thermal) (Hesse 1972). Total organic carbon (TOC), total nitrogen (TN) and total phosphorus (TP) contents were analyzed by the Walkley–Black wet oxidation procedure, the microkjeldahl method and the phosphomolybdic acid blue color method, respectively (Liu et al. 1996). The concentrations of heavy metals including copper (Cu), zinc (Zn), lead (Pb) and cadmium (Cd) were quantified using atomic absorption spectrophotometry after microwave digestion of samples. Briefly, 0.5 g of sieved and dried sediment was added into 9 ml concentrated nitric acid plus 3 ml concentrated hydrochloric acid at 175 °C for 10 min (US EPA 2007). After cooling down, the extracts were centrifuged at 3000 rpm for 5 min; supernatant was analyzed using an AA800 atomic absorption spectrophotometer (PerkinElmer).
DNA extraction and quantitative PCR
Microbial genomic DNA was extracted from 0.5 g sediment (wet-weight) using a Power Soil DNA extraction kit (MoBio) following the manufacturer’s instructions. The obtained DNA was used as templates for quantitative polymerase chain reaction (qPCR) to determine the copy numbers of bacterial 16S rRNA genes. qPCR was performed with the primer set Eub338F/Eub518R (Fierer et al. 2005). Standard curves ranging from 105 to 109 gene copies per μl were obtained by a tenfold serial dilution of linearized plasmids (Takara) containing cloned 16SrRNA genes that were amplified from Escherichia coli DNA. R2 value for the standard curves was 0.98, the slope was −3.15, which corresponded to an estimated amplification efficiency of 104 %. All DNA samples were processed along with negative controls and standards.
Pyrosequencing of bacterial 16S rRNA genes
The V4–V6 region of the 16S rRNA gene was PCR amplified from extracted DNA using universal primers 530F and 1100R (Turner et al. 1999). Both primers contained sequencing adaptor regions; the forward primers also contained sample barcodes (Lu et al. 2015). The PCR program included an initial denaturation at 95 °C for 3 min, followed by 30 cycles of denaturation at 95 °C for 30 s, annealing at 58 °C for 1 min and extension at 72 °C for 1 min and a final extension at 72 °C for 5 min. PCR amplicons were examined by gel electrophoresis (1 % agarose). Verified amplicons were excised from the gels and purified first with a QIAquick gel extraction kit (Qiagen) and then with an Agencourt AMPure XP system (Beckman Coulter). Purified PCR amplicons were quantified using a Quant-iT Picogreen dsDNA Assay kit (Life Technologies). Equimolar amounts of amplicons of different samples were pooled and pyrosequenced in one run using a GS 454 junior sequencing system with unidirectional Lib-L chemistry (Roche 454 Life Sciences) (Lu et al. 2015).
Obtained raw sequence reads were processed using the Pipeline Initial Process of the Ribosomal Database Project (RDP) to sort and rename sequences based on sample tags before trimming off the tags and primers from sequences (Cole et al. 2009). Trimmed sequences were processed using the Mothur software package for quality control and sequence annotations (Schloss et al. 2009). Briefly, sequences that were shorter than 100 bases or contained ambiguous base calls were excluded for further analysis. Subsequently, chimeric sequences were removed. After quality control steps, the remaining sequence reads were clustered into operational taxonomic units (OTUs) at 3 % divergence implemented in Mothur. The longest sequence within each OTU group was assigned as the representative sequence and blasted against the SILVA SSU database for taxonomic annotation (Pruesse et al. 2007). Since bacterial communities were being emphasized in this work, sequences annotated as chloroplasts and archaea were ignored in further analyses.
Pyrotag sequences were deposited in the NCBI Sequence Read Archive (SRA) under the project accession number SRP033375.
Statistical analysis
Physical and chemical variations among sediment samples were analyzed using principal components analysis (PCA) by Canoco 5.0 (Biometrics). Differences of sediment environmental variables and copy numbers of bacterial 16S rRNA genes among sampling sites were assessed using one-way ANOVA by SPSS 19.0 software package. The level of statistical significance was p < 0.05.
Based on taxonomic annotation, sequences were grouped at the order level to construct rarefaction curves and calculate diversity indices (Mou et al. 2013), including Chao1 and Shannon (H′) using the Mothur program (Heck et al. 1975). The ∫-Libshuff command in Mothur was used to compare BCCs between sequence libraries. To reveal the bacterial distribution patterns among sampling sites, a heatmap was generated at the order level by PC-ORD5 (MjM Software), based on the same matrix. To investigate relationships between sediment BCCs and environmental variables, distance based redundancy analysis (dbRDA) with Monte Carlo tests was carried out using the Canoco program for Windows 5.0. Furthermore, Pearson coefficient correlations between the major taxa including copy numbers of bacterial 16S rRNA genes, Shannon and Chao1 indices and sediment environmental variables were calculated using SPSS 19.0.
Results
Sediment characteristics
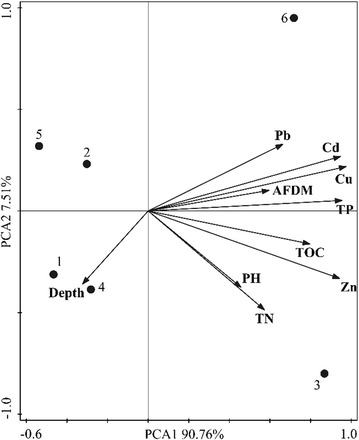
Among the ten tested parameters of Poyang lake sediments, six of which have no difference among samples, only AFDM % (ash free dried mass), TP (total phosphorus), the concentrations of Cu and Cd differentiated (ANOVA, p < 0.05) (Additional file 1: Table S1). Principal Components Analysis (PCA) of measured physical chemical variables grouped sites 1, 2, 4 and 5 away from sites 3 and 6. Generally, sites 3 and 6 had greater concentrations of AFDM, TP, Cu and Cd, but shallower water depths than sites 1, 2, 4 and 5 (Fig. 2; Additional file 1 : Table S1).
Fig. 2.
PCA ordination of sediment characteristics
16S rRNA gene abundance
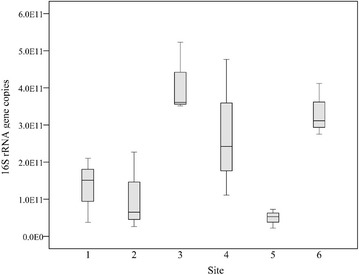
Quantitative PCR results showed that bacterial 16S rRNA gene abundance varied significantly among six sites (ANOVA, p = 0.008). The copy number varied between 4.96 × 1010 and 4.12 × 1011 copies per gram of dry sediment (Fig. 3), with the higher values found for sites 3 and 6, and the lower values for sites 1, 2, 4 and 5.
Fig. 3.
Histogram comparing the copy numbers of bacterial 16S rRNA gene in sediments of Poyang Lake
Pyrosequencing statistics and alpha-diversity
The average length of 16S rRNA gene pyrotag sequences (without the primers and adaptors) was 504 bp. A total of 19,892 bacterial 16S rRNA gene sequences were obtained and 18,242 remained after quality filtering and chimera removing. Sequences were grouped into 4394 OTUs (3 % divergence), with 276–1876 OTUs per sample (Table 1). Recovered OTU taxa were affiliated with 25 phyla, 68 orders and 196 species. Rarefaction analysis of bacterial communities was performed at the order level and all but the site 4 library were approaching plateau (Additional file 2: Fig. S1). Order-level Shannon index (H′) values were similar for all samples and ranged from 2.96 to 3.21 (Table 1, p > 0.05).
Table 1.
Library coverage estimations and sequence diversity of 16S rRNA genes pyrosequencing
| Sampling site | Reads | OTUs | Phylum | Order | Species | H′ | Chao1 |
|---|---|---|---|---|---|---|---|
| 1 | 3796 | 1680 | 22 | 54 | 105 | 3.21 | 57.00 |
| 2 | 5547 | 1326 | 22 | 54 | 81 | 3.02 | 55.67 |
| 3 | 6422 | 1876 | 24 | 57 | 115 | 3.18 | 59.63 |
| 4 | 427 | 276 | 16 | 40 | 40 | 3.02 | 45.00 |
| 5 | 1034 | 556 | 18 | 42 | 44 | 2.96 | 51.00 |
| 6 | 1016 | 572 | 20 | 44 | 41 | 3.07 | 59.60 |
| Total | 18,242 | 4394 | 25 | 68 | 196 |
H′, Shannon index
Bacterial community structure
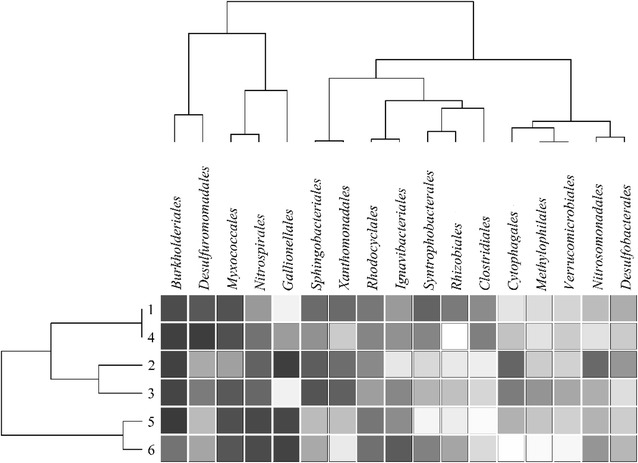
Over 83 % of the annotated sequences were affiliated with 17 bacterial orders of 6 phyla. Each of these 17 orders accounted for 2 % or more of the total sequences (Fig. 4) and was designated as major taxa. Except for Rhizobiales, all orders were found in each sequence library. Burkholderiales was the most abundant taxa and accounted for 12.61 % of sequences on average; it is followed by Myxococcales (7.81 %), Sphingobacteriales (7.18 %), Gallionellales (6.72 %), Nitrospirales (6.31 %), Xanthomonadales (5.60 %) and Desulfuromonadales (5.40 %). Burkholderiales represented the most abundant taxa in sediments of all sites except site 6, where Gallionellales was the most abundant. Desulfuromonadales in the main basin sites (sites 1 and 4; 15.79 and 9.23 %, respectively) were more abundant than those from estuaries (sites 2, 3, 5 and 6, ranged 2.80–4.89 %). Nitrospirales occurred mainly in the sites 5 (11.49 %) and 6 (11.09 %) (Fig. 4). Also, heatmap analysis based on relative abundance of major orders grouped samples into three clades: sites 1–4, sites 2–3, and sites 5–6 (Fig. 4). Furthermore, Libshuff analysis based on sequences revealed significant dissimilarities among sequence libraries of the six sites (p < 0.0085, with Bonferroni correction) (Additional file 3: Table S2).
Fig. 4.
Heatmap analysis of bacterial community composition at order level
Species level annotation was obtained for 3940 sequences (23.5 % of total sequences). Out of the 196 recovered species, Sideroxydans lithotrophicus (10.36 %), Albidiferax ferrireducens (8.86 %), Gallionella capsiferriformans (7.28 %), Methylobacillus flagellatus (5.13 %) and Nitrosospira multiformis (3.58 %) of Beta-proteobacteria, and Geobacter bemidjiensis (7.89 %), Anaeromyxobacter dehalogenans (7.44 %) and Geobacter lovleyi (3.88 %) of Delta-proteobacteria were generally found in all sites (Table 2).
Table 2.
Main bacterial community compositions in the sediments of Poyang Lake at species level
| Sample site | 1 (%) | 2 (%) | 3 (%) | 4 (%) | 5 (%) | 6 (%) |
|---|---|---|---|---|---|---|
| Sideroxydans lithotrophicus | 3.04 | 14.68 | 2.97 | 8.21 | 26.67 | 35.67 |
| Albidiferax ferrireducens | 1.45 | 16.12 | 4.77 | 5.97 | 15.19 | 2.34 |
| Geobacter bemidjiensis | 11.88 | 5.87 | 9.07 | 13.43 | 3.70 | 1.75 |
| Anaeromyxobacter dehalogenans | 11.74 | 1.00 | 10.16 | 14.18 | 8.89 | 14.62 |
| Gallionella capsiferriformans | 0.00 | 19.77 | 0.23 | 0 | 1.85 | 1.75 |
| Methylotenera mobilis | 1.01 | 4.23 | 10.09 | 1.49 | 1.85 | 0 |
| Geobacter lovleyi | 8.70 | 1.07 | 4.22 | 7.46 | 2.22 | 4.68 |
| Nitrosospira multiformis | 0.00 | 9.24 | 0.94 | 0 | 0.00 | 0 |
| Aquabacterium | 4.49 | 1.79 | 4.61 | 2.24 | 4.07 | 0 |
| Thiobacillus denitrificans | 0.72 | 0.21 | 8.60 | 0.75 | 0.37 | 4.09 |
| Arenimonas oryziterrae | 0.72 | 4.58 | 3.83 | 0.75 | 0 | 0 |
| Geothrix fermentans | 2.32 | 1.43 | 1.80 | 0 | 4.81 | 0.58 |
| Methylobacillus flagellatus | 4.20 | 0.72 | 1.33 | 2.24 | 4.07 | 1.17 |
| Desulfuromonas acetoxidans | 5.94 | 0.29 | 0.70 | 8.96 | 0.74 | 0.58 |
| Sulfuritalea hydrogenivorans | 2.75 | 0.93 | 2.35 | 1.49 | 0 | 0.58 |
| Opitutus terrae | 1.45 | 2.08 | 0.86 | 1.49 | 2.22 | 0 |
| Acidovorax avenae | 0.29 | 2.01 | 0.39 | 0.75 | 6.67 | 0 |
Influential factors on bacterial communities
Pearson correlation analysis revealed that bacterial 16S rRNA gene abundance was significantly correlated with several sediment property variables, including pH (r = 0.54, p = 0.02), TP (r = 0.70, p = 0.001), Cu (r = 0.69, p = 0.002), Zn (r = 0.50, p = 0.035) and Cd (r = 0.65, p = 0.004) (Table 3). Bacterial diversity (Shannon index) or richness (Chao1) was not significantly correlated with any of measured sediment variables (Table 3).
Table 3.
Correlations between major taxa and environmental variables based on Pearson’s product momentum correlation coefficient
| Variables | PH | SM | AFDM | TOC | TN | TP | Depth | Cu | Zn | Pb | Cd |
|---|---|---|---|---|---|---|---|---|---|---|---|
| qPCR | 0.537 | 0.702 | 0.615 | 0.761 | 0.639 | 0.698 | – | 0.685 | 0.500 | 0.285 | 0.649 |
| H′ | 0.179 | 0.089 | −0.306 | 0.466 | 0.603 | 0.560 | 0.435 | 0.284 | 0.517 | −0.339 | 0.303 |
| Chao1 | −0.039 | 0.500 | −0.209 | 0.269 | 0.249 | 0.766 | 0.195 | 0.655 | 0.536 | 0.329 | 0.658 |
Values in italics are different from 0 with a significance level alpha = 0.05
H′, Shannon index
To determine the effect of sediment properties on BCC, the property variables were analyzed using dbRDA (Fig. 5), where TN, Pb, Cu and Cd were the most contribution factors as environmental input. Main basin sites (1 and 4) and tributary sites (2, 3, 5 and 6) were mainly separated along the first dbRDA axis, which explained 38.79 % of fitted variation and correlated with TN and Pb contents of the sediment. In addition, the second axis of dbRDA explained 27.20 % of total variation. However, no environmental factors passed the Monte Carlo significance test.
Fig. 5.
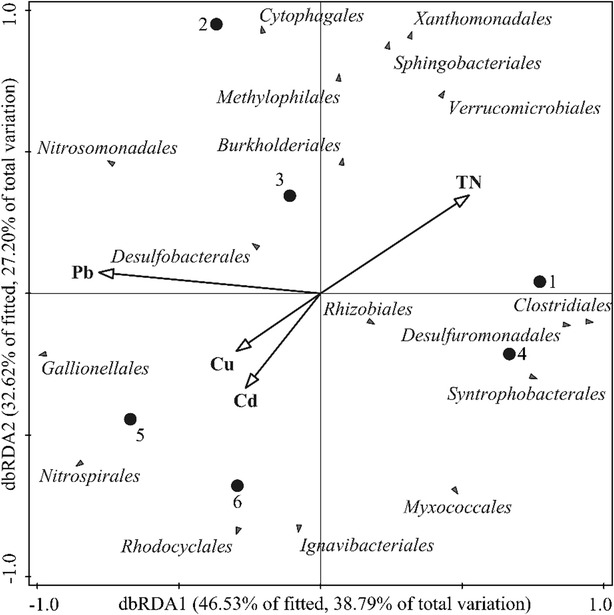
Distance based redundancy analysis (dbRDA) biplots of the sediment bacterial communities associated with environmental variables. Black and solid circles indicate different samples; grey triangles indicate different taxa
Discussion
As one of the largest freshwater lakes in China, Poyang Lake provides a number of important ecological services, such as flood storage, regulation of the local climate and habitats for migratory birds. Moreover, it is extremely rich in biodiversity (Wu et al. 2011). However, in recent years, the Poyang Lake has been experiencing the problems of water quality deteriorating and water-level lowering, largely due to the excessive external nutrient loading caused by rapid economic development and agricultural intensification (Wang et al. 2013). The lake ecosystem is facing the degradation trend.
Our study revealed spatial heterogeneity of environmental variables in the sediment of Poyang Lake. For example, contents of TP, Cu and Cd from sites 3 and 6 (Rao River and Xin River estuaries) were significantly higher than other sites (sites 1 and 4 from main basins of lake, sites 2 and 5 from Xiu River and Fu River estuaries) (Fig. 2). This finding is similar to a previous report, in which the concentrations of TP in sediments collected from Rao and Xin River estuaries were higher than those in the center of the Poyang Lake (Wang and Liang 2015). The observed high concentrations of TP, Cu and Cd from Rao and Xin River estuaries are consistent with their locations, which serve as bases for multiple industrial plants such as copper and phosphate mines (Zhang et al. 2014). Our results indicated that Rao River and Xin River were the main input sources of nutrients and metal pollutants of Poyang Lake among the 6 sampling sites and the together appearance of nutrients and metal pollutants were simultaneous.
In accordance with variations in sediment conditions, bacterial abundance and community structure also showed spatial heterogeneity. The 16S rRNA gene copy numbers was 2.31 × 1011 copies per gram of dry sediment on average in this study (Fig. 3), similar as those of Taihu Lake, another large and eutrophic lake in China (Ye et al. 2009). Samples of sites 3 and 6 (Rao River and Xin River estuaries) had higher values of 16S rRNA gene copy numbers than the other sites; and this pattern was likely shaped by sediment chemical properties, especially contents of TP, Cu and Cd (Table 3). These findings indicated that high loads of organic compounds and heavy metals may relate to the increases in bacterial abundance. This is in agreement with the results reported in previous studies, which showed positive correlations between sedimentary bacterial abundance and levels of organic matter and nutrients (Steger et al. 2011; Zhang et al. 2015). In addition, bacterial abundance, also recorded using quantitative PCR, found the highest bacterial population within the most heavy metal polluted sediments (Bouskill et al. 2010).
For the spatial distribution of BCCs, heatmap analysis revealed that BCCs of two sites 1 and 4 (Songmenshan and Nanjishan regions) were similar to each other, suggesting similar sediment conditions in these two sites (Fig. 4). This result may be explained by their close positions (both belong to main basin of Poyang Lake) and the sufficient mixing of water current throughout the main basin. However, the BCCs from sites 2 and 3 (Xiu River and Rao River estuaries) were clustered together in Fig. 4, even though their sediment characteristics were significantly different, which were likely affected by riparian inputs or other unknown factors. This result is consistent with the idea that local adaptation maybe is favoring particular lineages in specific regions (Bouzat et al. 2013). Furthermore, our data supported that spatial distribution difference of bacterial communities within a lake is due to shifts in the relative abundance of OTUs rather than variation in presence/absence of some vital species (Staley et al. 2015).
Previous studies reported that nitrogen concentration may have a direct impact on the bacterial composition in both lake water column and sediment samples (Haukka et al. 2006; Zhao et al. 2012). Low levels of Pb contamination in anoxic freshwater sediment (Rush Lake in USA) may impact the community structure of the culturable fraction of the indigenous microbes (Grandlic et al. 2006). In addition, BCCs in Lake Geneva were significantly different between contaminated (High contents of Pb, Cu and Cd) and uncontaminated stations, where sulphate-reducing bacteria and Fe(III)-reducing bacteria (Geobacter sp.) were more abundant in the contaminated sediments (Haller et al. 2011). In this work, among pH value, certain compounds (AFDM, TOC, TN, TP, Cu, Zn, Pb and Cr) and water depth, TN and Pb were found to be the most important factors that affected variability of bacterial communities (Fig. 5). However, no significant correlation was observed between sediment physicochemical variables and BCCs. This suggests that BCCs in the sediment of Poyang Lake may be synergistically regulated by multiple factors.
Despite variation among sampling locations, in general, Burkholderiales (Beta-proteobacteria) was the most abundant taxa in the surface sediments of Poyang Lake. This finding is consistent with our previous report in which Burkholderiales was found to be dominant in the water column of Poyang Lake (Wu et al. 2012). This taxon is common in freshwater environments (Newton et al. 2011; Staley et al. 2015) and has been found to be able to degrade several aromatic compounds (Pérez-Pantoja et al. 2012). In addition, the observation of abundant Myxococcales, Desulfuromonadales (Delta-proteobacteria), Sphingobacteriales (Bacteroidetes), Gallionellales (Beta-proteobacteria) and Nitrospirales (Nitrospirae) in the sediment of Poyang Lake may reflect the metabolic versatility of these groups. Gallionellales was proved to participate in the iron cycle of various waterbody environments (Emerson et al. 2010; Krepski et al. 2012). Therefore, the existence of many iron-oxidizing bacteria affiliated to Gallionellales (Sideroxydans lithotrophicus and Gallionella capsiferriformans, Table 2) demonstrated that the active geological cycle of iron may occur in sediment of Poyang Lake (Blothe and Roden 2009). Nevertheless, the iron content remains to be determined before we are able to draw this conclusion. The prevalence of Nitrosospira multiformis in this study revealed active ammonia-oxidizing process, while the presence of Nitrospirales members have been known as nitrite-oxidizing bacteria, therefore, nitrification is intensively involved in the nitrogen cycle of lake sediment (Feng et al. 2013; Shen et al. 2013). Methylobacillus flagellates occupied almost all samples in this study, and suggested the methyl utilization and carbon cycling in the sediments (Chistoserdova et al. 2007). The predominant Geobacter bemidjiensis and Geobacter lovleyi represented acetate or tetrachloroethene (PCE) utilization, as well as the reduction of heavy metals (Wagner et al. 2012; Merkley et al. 2015).
Conclusion
In this study, bacterial abundance and community composition displayed large spatial variations in the sediment of Poyang Lake. Bacterial community abundance was mainly affected by estuaries inputs of both nutrients and pollutants, while bacterial community composition might be affected by the process of biogeochemical transformation. The gradient of nutrients and pollutants, geographically distributed from estuaries inlet to the main basin of Poyang Lake, was formed by estuaries inputs, and afterwards the microbial transformation process followed. Sediment bacterial communities in Poyang Lake were mainly composed of taxa that are typical to freshwater sediment, including Burkholderiales, Myxococcales, Sphingobacteriales, Gallionellales, Nitrospirales, Xanthomonadales and Desulfuromonadales.
Authors’ contributions
WBK analyzed sequencing data, performed the statistical analysis and drafted the manuscript. JZ collected the samples and carried out the experiments. XXL performed the 454 sequencing. YTM drafted the manuscript. XZM participated in the design of experiment and helped to draft of manuscript. LW designed the experiment and drafted manuscript. All authors read and approved the final manuscript.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 31060082, 31260110), Jiangxi Natural Science Foundation of China (2007GZN1927) and the Kent State University Research Council (to X. Mou). The authors would like to thank Dr. Z. Y. Kong and other members who provided their valuable and constructive suggestions in our lab.
Competing interests
The authors declare that they have no competing interests.
Additional files
10.1186/s40064-016-2026-7 Physicochemical variables of sediments in Poyang Lake.
10.1186/s40064-016-2026-7 Rarefaction curves of bacterial pyrosequencing.
10.1186/s40064-016-2026-7 Libshuff comparison between the sequences libraries of samples from six sites.
Contributor Information
Wenbo Kou, Email: 393055359@qq.com.
Jie Zhang, Email: zhangjie880124@foxmail.com.
Xinxin Lu, Email: xlv@kent.edu.
Yantian Ma, Email: mayt05@163.com.
Xiaozhen Mou, Email: xmou@kent.edu.
Lan Wu, Email: wl690902@hotmail.com.
References
- Bai Y, Shi Q, Wen D, Li Z, Jefferson WA, Feng C, Tang X. Bacterial communities in the sediments of Dianchi Lake, a partitioned eutrophic waterbody in China. PLoS One. 2012;7:e37796. doi: 10.1371/journal.pone.0037796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhard AE, Colbert D, McManus J, Field KG. Microbial community dynamics based on 16S rRNA gene profiles in a Pacific Northwest estuary and its tributaries. FEMS Microbiol Ecol. 2005;52:115–128. doi: 10.1016/j.femsec.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Blothe M, Roden EE. Composition and activity of an autotrophic Fe(II)-oxidizing, nitrate-reducing enrichment culture. Appl Environ Microbiol. 2009;75(21):6937–6940. doi: 10.1128/AEM.01742-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouskill NJ, Barker-Finkel J, Galloway TS, Handy RD, Ford TE. Temporal bacterial diversity associated with metal-contaminated river sediments. Ecotoxicology. 2010;1:317–328. doi: 10.1007/s10646-009-0414-2. [DOI] [PubMed] [Google Scholar]
- Bouzat JL, Hoostal MJ, Looft T. Spatial patterns of bacterial community composition within Lake Erie sediments. J Great Lakes Res. 2013;39:344–351. doi: 10.1016/j.jglr.2013.03.003. [DOI] [Google Scholar]
- Carpenter SR, Stanley EH, Vander Zanden MJ. State of the world’s freshwater ecosystems: physical, chemical, and biological changes. Annu Rev Environ Resour. 2011;36:75–99. doi: 10.1146/annurev-environ-021810-094524. [DOI] [Google Scholar]
- Chistoserdova L, Lapidus A, Han C, Goodwin L, Saunders L, Brettin T, Tapia R, Gilna P, Lucas S, Richardson PM, Lidstrom ME. Genome of Methylobacillus flagellatus, molecular basis for obligate methylotrophy, and polyphyletic origin of methylotrophy. J Bacteriol. 2007;189(11):4020–4027. doi: 10.1128/JB.00045-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JR, Wang Q, Cardenas E, Fish J, Chai B, Farris RJ, et al. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009;37:D141–D145. doi: 10.1093/nar/gkn879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings DE, Snoeyenbos-West OL, Newby DT, Niggemyer AM, Lovley DR, Achenbach LA, Rosenzweig RF. Diversity of Geobacteraceae species inhabiting metal-polluted freshwater lake sediments ascertained by 16S rDNA analyses. Microb Ecol. 2003;46:257–269. doi: 10.1007/s00248-005-8002-3. [DOI] [PubMed] [Google Scholar]
- Emerson D, Fleming EJ, McBeth JM. Iron-oxidizing bacteria: an environmental and genomic perspective. Annu Rev Microbiol. 2010;64:561–583. doi: 10.1146/annurev.micro.112408.134208. [DOI] [PubMed] [Google Scholar]
- Feng S, Chen C, Wang Q, Zhang X, Yang Z, Xie S. Characterization of microbial communities in a granular activated carbon–sand dual media filter for drinking water treatment. Int J Environ Sci Technol. 2013;10:917–922. doi: 10.1007/s13762-013-0188-1. [DOI] [Google Scholar]
- Fierer N, Jackson JA, Vilgalys R, Jackson RB. Assessment of soil microbial community structure by use of taxon-specific quantitative PCR assays. Appl Environ Microbiol. 2005;71:4117–4120. doi: 10.1128/AEM.71.7.4117-4120.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandlic CJ, Geib I, Pilon R, Sandrin TR. Lead pollution in a large, prairie-pothole lake (Rush Lake, WI, USA): effects on abundance and community structure of indigenous sediment bacteria. Environ Pollut. 2006;144:119–126. doi: 10.1016/j.envpol.2005.12.029. [DOI] [PubMed] [Google Scholar]
- Haller L, Tonolla M, Zopfi J, Peduzzi R, Wildi W, Poté J. Composition of bacterial and archaeal communities in freshwater sediments with different contamination levels (Lake Geneva, Switzerland) Water Res. 2011;45:1213–1228. doi: 10.1016/j.watres.2010.11.018. [DOI] [PubMed] [Google Scholar]
- Haukka K, Kolmonen E, Hyder R, Hietala J, Vakkilainen K, Kairesalo T, Haario H, Sivonen K. Effect of nutrient loading on bacterioplankton community composition in lake mesocosms. Microb Ecol. 2006;51:137–146. doi: 10.1007/s00248-005-0049-7. [DOI] [PubMed] [Google Scholar]
- Heck KL, Van Belle G, Simberloff D. Explicit calculation of the rarefaction diversity measurement and the determination of sufficient sample size. Ecology. 1975;56:1459–1461. doi: 10.2307/1934716. [DOI] [Google Scholar]
- Hesse PR. A textbook of soil analysis. New York, NY: Chemical Publishing Company; 1972. [Google Scholar]
- Krepski ST, Hanson TE, Chan CS. Isolation and characterization of a novel biomineral stalk-forming iron-oxidizing bacterium from a circumneutral groundwater seep. Environ Microbiol. 2012;14:1671–1680. doi: 10.1111/j.1462-2920.2011.02652.x. [DOI] [PubMed] [Google Scholar]
- Lindström ES, Kamst-Van Agterveld MP, Zwart G. Distribution of typical freshwater bacterial groups is associated with pH, temperature, and lake water retention time. Appl Environ Microbiol. 2005;71:8201–8206. doi: 10.1128/AEM.71.12.8201-8206.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Jiang N, Zhang L. Soil physical, chemical analysis and description of soil profiles. Bejjing: Standards Press of China; 1996. pp. 33–37. [Google Scholar]
- Liu Q, Hu W, Ge G, Xiong Y, Lai J, Wu L. Contents of nutrients and heavy metals in the Poyang Lake during dry season. Resour Environ Yangtze Basin. 2012;21:1230–1235. [Google Scholar]
- Lu X, Sun S, Zhang Y, Hollibaugh JT, Mou X. Temporal and vertical distributions of bacterioplankton at the Gray’s Reef national marine sanctuary. Appl Environ Microbiol. 2015;81:910–917. doi: 10.1128/AEM.02802-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkley ED, Wrighton KC, Castelle CJ, Anderson BJ, Wilkins MJ, Shah V, Arbour T, Brown JN, Singer SW, Smith RD, Lipton MS. Changes in protein expression across laboratory and field experiments in Geobacter bemidjiensis. J Proteome Res. 2015;14(3):1361–1375. doi: 10.1021/pr500983v. [DOI] [PubMed] [Google Scholar]
- Mou XZ, Jacob J, Lu XX, Robbins S, Sun S, Ortiz JD. Diversity and distribution of free-living and particle-associated bacterioplankton in Sandusky Bay and adjacent waters of Lake Erie Western Basin. J Great Lakes Res. 2013;39:352–357. doi: 10.1016/j.jglr.2013.03.014. [DOI] [Google Scholar]
- Nealson KH. Sediment bacteria: who’s there, what are they doing, and what’s new? Annu Rev Earth Planet Sci. 1997;25:403–434. doi: 10.1146/annurev.earth.25.1.403. [DOI] [PubMed] [Google Scholar]
- Newton RJ, Jones SE, Eiler A, McMahon KD, Bertilsson S. A guide to the natural history of freshwater lake bacteria. Microbiol Mol Biol Rev. 2011;75:14–49. doi: 10.1128/MMBR.00028-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Pantoja D, Donoso R, Agulló L, Córdova M, Seeger M, Pieper DH, González B. Genomic analysis of the potential for aromatic compounds biodegradation in Burkholderiales. Environ Microbiol. 2012;14:1091–1117. doi: 10.1111/j.1462-2920.2011.02613.x. [DOI] [PubMed] [Google Scholar]
- Pruesse E, Quast C, Knittel K, Fuchs BM, Ludwig W, Peplies J, Glöckner FO. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007;35:7188–7196. doi: 10.1093/nar/gkm864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjard L, Poly F, Nazaret S. Monitoring complex bacterial communities using culture-independent molecular techniques: application to soil environment. Res Microbiol. 2000;151:1–11. doi: 10.1016/S0923-2508(00)00136-4. [DOI] [PubMed] [Google Scholar]
- Rayment GE, Higginson FR (1992) Australian laboratory handbook of soil and water chemical methods. Inkata Press Pty Ltd, Melbourne, Australia
- Sauvain L, Bueche M, Junier T, Masson M, Wunderlin T, Kohler-Milleret R, Diez EG, Loizeau JL, Tercier-Waeber ML, Junier P. Bacterial communities in trace metal contaminated lake sediments are dominated by endospore-forming bacteria. Aquat Sci. 2014;76:S33–S46. doi: 10.1007/s00027-013-0313-8. [DOI] [Google Scholar]
- Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, et al. Introducing mothur: open source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen T, Stieglmeier M, Dai J, Urich T, Schleper C. Responses of the terrestrial ammonia-oxidizing archaeon Ca. Nitrososphaera viennensis and the ammonia-oxidizing bacterium Nitrosospira multiformis to nitrification inhibitors. FEMS Microbiol Lett. 2013;344(2):121–129. doi: 10.1111/1574-6968.12164. [DOI] [PubMed] [Google Scholar]
- Staley C, Gould TJ, Wang P, Phillips J, Cotner JB, Sadowsky MJ. Species sorting and seasonal dynamics primarily shape bacterial communities in the Upper Mississippi River. Sci Total Environ. 2015;505:435–445. doi: 10.1016/j.scitotenv.2014.10.012. [DOI] [PubMed] [Google Scholar]
- Steger K, Premke K, Gudasz C, Sundh I, Tranvik LJ. Microbial biomass and community composition in boreal lake sediments. Limnol Oceanogr. 2011;56:725–733. doi: 10.4319/lo.2011.56.2.0725. [DOI] [Google Scholar]
- Turner S, Pryer KM, Miao VP, Palmer JD. Investigating deep phylogenetic relationships among cyanobacteria and plastids by small subunit rRNA sequence analysis. J Eukaryot Microbiol. 1999;46:327–338. doi: 10.1111/j.1550-7408.1999.tb04612.x. [DOI] [PubMed] [Google Scholar]
- Urakawa H, Yoshida T, Nishimura M, Ohwada K. Characterization of depth-related population variation in microbial communities of a coastal marine sediment using 16S rDNA-based approaches and quinone profiling. Environ Microbiol. 2000;2:542–554. doi: 10.1046/j.1462-2920.2000.00137.x. [DOI] [PubMed] [Google Scholar]
- US EPA (2007) Microwave assisted acid digestion of sediments, sludges, soils, and oil. http://www.epa.gov/epawaste/hazard/testmethods/sw846/pdfs/3051a.pdf
- Wagner DD, Hug LA, Hatt JK, Spitzmiller MR, Padilla-Crespo E, Ritalahti KM, Edwards EA, Konstantinidis KT, Loffler FE. Genomic determinants of organohalide-respiration in Geobacter lovleyi, an unusual member of the Geobacteraceae. BMC Genom. 2012;13:200. doi: 10.1186/1471-2164-13-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Liang T. Distribution characteristics of phosphorus in the sediments and overlying water of Poyang Lake. PLoS One. 2015;10:e0125859. doi: 10.1371/journal.pone.0125859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Shu J, Ni Z. Investigation on pollution situation and countermeasures in Poyang Lake. J Environ Eng Technol. 2013;3:333–349. [Google Scholar]
- Wu L, Li M, Guo Y, Yang X. Influence of three gorges project on water quality of Poyang Lake. Proc Environ Sci. 2011;10:1496–1501. doi: 10.1016/j.proenv.2011.09.238. [DOI] [Google Scholar]
- Wu L, Ge G, Zhu G, Gong S, Li S, Wan J. Diversity and composition of the bacterial community of Poyang Lake (China) as determined by 16S rRNA gene sequence analysis. World J Microb Biotechnol. 2012;28:233–244. doi: 10.1007/s11274-011-0812-5. [DOI] [PubMed] [Google Scholar]
- Yannarell AC, Triplett EW. Within- and between-lake variability in the composition of bacterioplankton communities: investigations using multiple spatial scales. Appl Environ Microbiol. 2004;70:214–223. doi: 10.1128/AEM.70.1.214-223.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye W, Liu X, Lin S, Tan J, Pan J, Li D, Yang H. The vertical distribution of bacterial and archaeal communities in the water and sediment of Lake Taihu. FEMS Microbiol Ecol. 2009;70:263–276. doi: 10.1111/j.1574-6941.2009.00761.x. [DOI] [PubMed] [Google Scholar]
- Zhang J, Chen X, Liu Q, Wu L. Distribution and potential risk assessment of heavy metals in main estuaries of Lake Poyang’s tributaries. Resour Environ Yangtze Basin. 2014;23:95–100. [Google Scholar]
- Zhang J, Yang Y, Zhao L, Xie S, Liu Y. Distribution of sediment bacterial and archaeal communities in plateau freshwater lakes. Appl Microbiol Biotechnol. 2015;99:3291–3302. doi: 10.1007/s00253-014-6262-x. [DOI] [PubMed] [Google Scholar]
- Zhao D, Huang R, Zeng J, Yan W, Wang J, Ma T, Wang M, Wu Q. Diversity analysis of bacterial community compositions in sediments of urban lakes by terminal restriction fragment length polymorphism (T-RFLP) World J Microbiol Biotechnol. 2012;28:3159–3170. doi: 10.1007/s11274-012-1126-y. [DOI] [PubMed] [Google Scholar]


