B10 cell development and function
Keywords: antigen specificity, B lymphocyte, B10 cell, IL-10, regulatory B cell
Abstract
B cells are known to instigate and promulgate immune responses by producing antibodies and presenting antigens to T cells. However, a rare but potent B-cell subset in both humans and mice is capable of inhibiting immune responses through the production of the anti-inflammatory cytokine IL-10. Regulatory B cells do not express any unique combination of surface markers but instead represent a small population of B cells that have acquired the unique ability to produce IL-10. This numerically rare B-cell subset is therefore functionally referred to as ‘B10 cells’ to reflect both their molecular program and the fact that their anti-inflammatory effects in models of autoimmunity, infection and cancer are solely attributable to IL-10 production. As with most B cells, B10 cell development and function appear to be predominantly, if not exclusively, driven by antigen-receptor signals. Once generated, B10 cells respond to both innate and adaptive immune signals, with a requirement for antigen-specific local interactions with T cells to induce IL-10 production and to provide optimal immune suppression in mouse models of autoimmune disease. B10 cells therefore provide an antigen-specific mechanism for delivering IL-10 locally to sites of immune activation and inflammation. The ability of B10 cells to regulate innate and adaptive immune responses makes them an ideal therapeutic target for the treatment of many immune-related disorders.
Introduction
B cells are the origin of humoral immune responses and have thus historically been studied for their role in promoting inflammatory responses. However, more than a decade ago, ‘regulatory B cells’ that inhibit inflammatory immune responses were described in a mouse model of inflammatory bowel disease (IBD) (1). Since this initial observation, several groups have noted roles for regulatory B cells in a number of diverse autoimmune (2–10) and allergic conditions (11–13), as well as the existence of many distinct mechanisms through which different regulatory B cells can reduce inflammation. Although the characterization of regulatory B cells remains nascent, multiple regulatory B-cell subsets and their functional activities are currently being studied.
One of the most well-described mechanisms through which regulatory B cells suppress inflammation is through the secretion of the anti-inflammatory cytokine IL-10 (14–16). Given the overlapping phenotype of IL-10-producing B cells with multiple distinct B-cell subsets, and the probability that multiple regulatory B cell subsets would eventually be described, we labeled these numerically rare B cells as ‘B10 cells’ to reflect their unique functional program and the fact that their anti-inflammatory effects were solely attributable to IL-10 production (11). Although it is appreciated that other populations of regulatory B cells exist, this review is specifically focused on the development and significance of B10 cells and their potential for therapeutic intervention in cases of autoimmunity, infection and cancer.
B10 cell identification by function
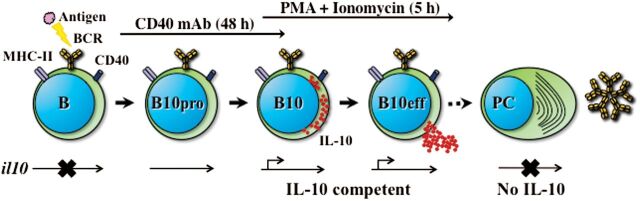
B10 cells were first characterized in mice using an induced model of contact hypersensitivity in which T cells and other immune cells instigate inflammation following sensitization and re-challenge with hapten immunogens (11). Relative to wild-type mice, CD19-deficient mice had heightened inflammatory responses, whereas mice over-expressing the B-cell-specific CD19 molecule, which results in up-regulated B-cell antigen-receptor (BCR) signaling, had reduced inflammation. The adoptive transfer of splenic B-cell subsets from wild-type mice into CD19-deficient mice inhibited T-cell-mediated inflammation following antigenic challenge, an effect that was found to be dependent on the presence of B10 cells. B cells that are actively producing IL-10, which are functionally defined as ‘B10 effector cells’ (B10eff, Fig. 1), are difficult to identify in vivo due to their very low numbers. However, B10 cells that have been functionally programmed to express IL-10 in vivo, and are thereby IL-10 competent, are identified by the production of IL-10 ex vivo following 5-h stimulation with phorbol 12-myristate 13-acetate (PMA) and ionomycin, which stimulate protein kinase C and calcium transport, respectively (Fig. 1).
Fig. 1.
B cell acquisition of IL-10 competence. Antigen–BCR interactions generating appropriate signals drive B-cell acquisition of the functional program that allows B cells to become IL-10-competent B10 cells. Select B cells that have received appropriate signals in vivo but that have not fully acquired IL-10 competence are called B10pro cells. Although the il10 locus is thought to be transcriptionally accessible in B10pro cells, they are not competent to express IL-10 after 5-h stimulation with PMA and ionomycin. However, B10pro cells can be induced to functionally mature into IL-10-competent B10 cells by agonistic CD40 mAb engagement for 48h. B10 cells are functionally defined by their ability to express IL-10 protein following brief (5h) ex vivo stimulation with PMA and ionomycin and therefore have a fully accessible and transcriptionally active il10 locus. B10pro plus B10 cells can be visualized after 48h of CD40 signaling plus 5h of PMA and ionomycin stimulation. B10eff cells derived from B10 cells actively secrete IL-10 for 24–48h in vivo. After terminating IL-10 transcription, a very small fraction of B10eff cells (<1.0%) differentiate into antibody-secreting plasma cells (PC) in vivo that predominantly secrete germline-encoded polyreactive, autoreactive or antigen-specific IgM antibodies. Plasma cells do not express measurable IL-10 transcripts and are therefore thought to have an inaccessible il10 locus.
Stimulation with PMA and ionomycin to induce cytokine production in vitro is commonly used in T-cell studies to drive the transcription and translation of genes in an open configuration when the appropriate transcription factors are expressed. The addition of monensin, which is optimal for mice, or brefeldin-A, optimal for humans, to block protein secretion (together, PIM or PIB) allows for B10 cell cytoplasmic IL-10 visualization by flow cytometry. The addition of LPS modestly enhances IL-10 production versus stimulation with PIM alone (together, L+PIM) (17). Stimulating human B cells with PIB for 5h reveals average B10 cell frequencies of 0.8% among peripheral blood B cells (18). Most B cells are not induced to express IL-10 by even long-term PIM or PIB stimulation, indicating that the majority of B cells is not IL-10 competent. Thus, acute B-cell stimulation with PMA and ionomycin is a useful method for identifying IL-10-competent B10 cells.
In C57Bl/6 mice, B10 cells account for 1–3% of splenic B cells, though this number can increase significantly with inflammation and disease (7, 9–11, 19). A larger fraction of B cells can be induced to acquire IL-10 competence by prolonged stimulation through cell surface CD40 (19). These B cells have been labeled as B10 progenitor (B10pro) cells (Fig. 1). Although agonistic CD40 engagement for up to 48h does not induce IL-10 production by B10pro cells, subsequent 5-h L+PIM stimulation reveals B10pro cell acquisition of IL-10 competence. Together, B10+B10pro cells generally represent 3–8% of mouse splenic B cells. LPS stimulation similarly induces B10pro cell acquisition of IL-10 competence, although it also induces IL-10 production and secretion, thereby making B10+B10pro cell enumeration difficult. Importantly, the vast majority of B cells is not induced to express IL-10 following LPS stimulation. Human B10+B10pro cells are visualized similarly following 48-h CD40 stimulation and represent ~7% of blood B cells (18). Unlike B10 cells, mouse B10pro cell numbers remain relatively stable during inflammation and disease (7, 9–11, 19), whereas human B10+B10pro cell numbers can be elevated significantly in subjects with autoimmune disease (18).
That the majority of B cells do not acquire IL-10 competence in vitro, even with prolonged stimulation, argues that B10pro and B10 cells have both received appropriate programming signals in vivo to prime them for future IL-10 production (Fig. 1). Thus, the term ‘B10pro cell’ does not imply a developmental stage of B-cell maturation but instead reflects their relative stage of functional priming. At the molecular level, it is possible that the il10 gene in B10pro cells is open and has become accessible for transcription, but the appropriate factors required for productive gene transcription have yet to be induced (Fig. 1).
B10 cell phenotype and location
There is no specific transcription factor or cell surface protein phenotype unique to all B10 cells, although populations enriched for B10 cells have been identified. The phenotype of B10 cells can be readily observed ex vivo, as short-term stimulation with L+PIM does not dramatically alter the expression of most cell surface proteins (7, 9, 11, 19, 20). In general, spleen B10 cells are IgMhi IgDlo CD19hi MHC-IIhi CD21int/hi CD23lo CD24hi CD43+/− CD93− and therefore share overlapping phenotypic characteristics with multiple B-cell developmental subsets, including immature transitional and marginal zone B cells and peritoneal B1 cells. B10 cells are enriched within the splenic CD1dhi CD5+ subset, where B10 cells and B10+B10pro cells represent 15–20% and 50% of this phenotypic compartment, respectively; however, B10 and B10+B10pro cells can also be found in the CD1dlo and CD5− populations (11).
Spleen and peritoneal B10 cells have similar phenotypes, although peritoneal B cells do not express high levels of CD1d (19, 21). Spleen B10 cells are also enriched in the T-cell immunoglobulin domain and mucin domain protein 1 (TIM-1)+ B-cell population, in spite of the fact that B10 cells are additionally found in the TIM-1− population (22). Thus, there are no specific phenotypic markers for B10 cells other than their functional capacity for IL-10 production. Because B10 cells are only modestly increased within certain phenotypic subpopulations that predominantly contain non-B10 cells, experiments in which phenotypically defined B-cell subsets are adoptively transferred require the use of IL-10-deficient B cells as controls to understand the role of bona fide B10 cells in vivo. Moreover, in vitro and in vivo studies using mixed populations of B cells with potentially diverse functions and regulatory properties should be interpreted cautiously.
Mouse B10 cells predominantly localize in the spleen and peritoneal cavity but also exist in the peripheral and mesenteric lymph nodes and blood and at very low frequencies in gut-associated lymphoid tissues in wild-type mice (19, 21, 23, 24). B10 cells can also be found in the central nervous system and draining lymph nodes during experimental autoimmune encephalomyelitis (EAE) disease (10, 25) and in the draining lymph nodes of mice infected with Listeria monocytogenes (26). Within the peritoneal cavity, B10 cells are most abundant within the CD5+ CD11b+ B1a subset, representing close to 20% of this compartment. B10 cells can also be found at lower frequencies within the CD5− CD11b+ B1b and CD5− CD11b− B2 subsets, demonstrating that IL-10 competence is not restricted to a specific phenotypically defined B-cell subset. Similarly, B10pro cells comprise up to 40% of B1a cells and can also be found in the B1b and B2 compartments (21). Despite this high frequency of B10 cells within the B1a compartment, B10 cells are numerically higher in the spleen due to the low overall number of B cells in the peritoneal cavity. Likewise, B10+B10pro cells represent 1–4% of B cells in the mesenteric lymph nodes, lamina propia and Peyer’s patches and 3–8% of B cells in the peripheral blood and lymph nodes.
Blood B10 cells from adult humans express heightened levels of CD19, IgD, and the activation and memory markers CD27, CD48 and CD148 (18). Given their expression of memory markers, B10 cells are likely to have encountered their respective antigens in vivo. Relative to immature or transitional B cells, B10 cells express less IgM and do not express CD10. B10 and conventional B cells express similar levels of CD5, CD20, CD21, CD22, CD23, CD25, CD28 and CD40. Human B10 cells can represent close to 25% of total B cells within the CD24hi CD27+ blood B-cell subpopulation, although they are also found in the CD24lo and CD27− subpopulations. Some studies have reported that most B10 cells can be found in the naive CD27− subpopulation (27), whereas others have observed an enrichment of regulatory B cells within the CD24hi CD38hi subpopulation following 72-h culture (28).
Regardless, B10 cells are rare but present in numerous peripheral tissues. Human B10 cells have been identified in the spleen, tonsils and newborn cord blood, where they represent 0.3–0.8% of total B cells, and decrease in number with age (18). Thus, B10 cells can be in many phenotypically defined subpopulations, demonstrating that IL-10 competence remains the best marker for identifying this specific subset of regulatory B cells.
BCR signals drive B10 cell development and effector function
Early studies of B10 cell function in mouse models of inflammation indicated that B10 cells derived from antigen-experienced mice were more effective at suppressing disease upon adoptive transfer than were B10 cells derived from naive mice (3, 10, 11). Other evidence pointing to the relationship between BCR signaling and IL-10 production came from studies using human CD19 transgenic mice, which have increased BCR signaling and elevated B10 cell numbers. By contrast, CD19-deficient mice have impaired BCR signaling and few, if any, B10 cells (11). Spleen and peritoneal cavity B10 cells utilize a diverse repertoire of BCR genes that are predominantly germline and without somatic mutations (21, 29). This indicates that B10 cells are generated in response to an array of antigens without undergoing somatic hypermutation or BCR affinity maturation within germinal centers. In support of this, multiple strains of transgenic mice with a fixed BCR repertoire have limited B10 cell numbers, further suggesting a requirement for appropriate antigen-specific BCR signaling during B10 cell development (19).
BCR–antigen interactions and BCR-induced signals appear to be crucial for proper B10 cell function. In support of this, a small percentage of B10 effector cells differentiate into antibody-secreting cells after terminating IL-10 production in vivo and in vitro (29). The adoptive transfer of B10 effector cells into B-cell-deficient mice leads to their production of serum IgM and IgG antibodies. Further, B10 effector cells generate serum antibodies more quickly than do conventional B cells, indicating that B10 cells are already antigen-primed and activated in vivo prior to their adoptive transfer. Moreover, B10 effector cells produce self-reactive antibodies, whereas conventional B cells do not. Thus, the B10 cell BCR repertoire is enriched for reactivity with self-antigens, yet also expresses polyreactive natural antibodies and BCRs reactive with foreign antigen (21). Thus, B10 cells with varying antigen specificities rely on a diverse BCR repertoire for their development.
B10+B10pro cells develop normally in the absence of T cells. Furthermore, B-cell MHC class II, CD40, MyD88 and IL-10 receptor expressions are not required for B10 cell development, further highlighting the importance of BCR signaling in driving IL-10 competence (19). However, antigen-specific B10 cell function is demonstrated by the observation that B10 effector cell generation requires cognate MHC class II- and CD40-dependent interactions between B10 cells and CD4+ T cells for in vivo disease suppression (20). Antigen-specific B10 cells thus likely encounter antigens in vivo via germline BCRs and present antigen to the corresponding antigen-specific CD4+ T cells. Cognate interactions between T cells and B10pro cells likely drive B10 cell development and T-cell secretion of IL-21, which acts directly on B10 cells to induce IL-10 production within the local microenvironment. Given their immediate proximity to antigen-specific B10 effector cells, cognate CD4+ T-cell activation and effector cell functions are then down-regulated. Consequently, B10 cells derived from MHC class II-, CD40-, IL-21-R- or IL-10-deficient mice are unable to suppress autoimmunity or inflammation.
This exquisite system for regulating T-cell-derived autoimmunity also extends to the control of innate immune responses, where CD4+ T-cell licensing of B10 cell function through cognate interactions is required for B10 cell inhibition of macrophage function (26). B10 cell development and effector function are therefore tightly regulated by antigen-specific receptors in vivo through molecularly distinct mechanisms in order to prevent generalized immunosuppression due to indiscriminant IL-10 secretion (20).
As with most B cells, B10 cells respond to TLR agonists such as LPS and CpG oligonucleotides. These TLR agonists do not induce IL-10 production by the majority of B cells, which have not been primed to express IL-10 but can induce IL-10-competent B10 cells to produce IL-10 in vitro (19, 30, 31). Consequently, polyclonal B10pro and B10 cell activation may lead to IL-10-induced suppression of immunity. Alternatively, the systemic administration of IL-10 has not been found to be therapeutic or immunosuppressive in clinical trials, suggesting that IL-10 produced within specific microenvironments or in the context of adaptive immune responses may be more physiologically relevant. Because B10 cells develop normally in MyD88-deficient and gnotobiotic mice, it is unlikely that these innate signals overtly regulate B10 cell development or IL-10 production during homeostasis or adaptive immune responses (19, 21).
In addition to IL-21, other cytokines such as IL-1β, IL-6, IL-33 and IL-35 are reported to play a role in expanding B10 cells in vivo and inducing their protective functions (24, 32–35). In the case of IL-35 and B cells, unresolved issues remain as to whether this cytokine is produced as a disulfide-linked heterodimer, whether it can be manufactured as a native protein ex vivo, the nature of receptor subunit composition on B cells, and whether IL-35 concentrations can be measured accurately, particularly given the absence of IL-35-specific mAb reagents (35–37). By contrast, TGF-β and IFN-γ inhibit B10pro cell maturation in vivo and may provide a means to negatively regulate B10 cell IL-10 production (18). Understanding the multiple pathways that regulate B10 cells and other regulatory B-cell subsets will be instrumental to developing novel therapeutics that exploit these unique B-cell subpopulations.
B10 cells as targets for autoimmune disease therapy
B10 cells have been most extensively studied in the context of autoimmune disease, where the potential for therapeutic expansion of this subset has great promise for clinical application. Specifically, the roles of B cells and B10 cells have been extensively studied in mouse models of contact hypersensitivity, IBD, lupus, EAE, collagen-induced arthritis (CIA) and graft-versus-host disease (GVHD). Although B-cell depletion during active disease can ameliorate symptomology (7, 9), B-cell deficiency prior to disease initiation due to either their congenital absence, as in µMT (3) and CD19-deficient mice (10, 12, 38, 39), or following CD20 mAb-induced B-cell depletion (7, 9) worsens disease severity.
Increased disease observed in the absence of B cells has been attributed to the loss of B10 cells, as the adoptive transfer of small numbers of B10 cells or B10 cell-enriched B-cell subpopulations can significantly attenuate disease onset and pathology, whereas the transfer of comparable IL-10-deficient B cells is without effect during contact hypersensitivity (11), IBD (15, 21, 23, 39), lupus (9, 12), EAE (3, 7, 10, 20), CIA (4, 6, 40) and GVHD (41). Thus, while most B cells can drive significant pathology during autoimmune disease (42, 43), altering the balance of regulatory and pathogenic B cells by the selective addition of syngeneic B10 cells can ameliorate early disease symptoms and progression in diverse mouse models.
In contrast to the above findings, lupus development in MRL.Faslpr mice was not worsened by a congenital B-cell-specific IL-10 deficiency, arguing against the importance of regulatory B cells (44). Although these genetic studies suggest that IL-10-dependent B-cell regulation does not restrain pathogenic B-cell-driven disease in this spontaneous lupus model, there are multiple potential explanations for these results. First, MRL.Faslpr mice represent an aggressive genetically driven autoimmune disease that is not amenable to most treatments. Second, B-cell-specific IL-10 deficiency was created by CD19 cre recombinase-driven excision of floxed il10 alleles (44); however, not all B cells express sufficient amounts of CD19-driven cre (45), and their congenital IL-10 defect likely results in compensation by other regulatory mechanisms.
Compensatory mechanisms are clearly demonstrated in the EAE model of autoimmunity, where total B-cell depletion prior to disease initiation markedly exacerbates acute disease because of the removal of B10 cells (7). By contrast, B-cell depletion after the development of disease symptoms ameliorates chronic disease. This dichotomy results from the fact that regulatory T (Treg) cell expansion within the central nervous system occurs after disease manifestations are apparent; in this context, B10 cell depletion has no apparent pathogenic effect, but the removal of antigen-presenting B cells ameliorates disease. However, the adoptive transfer of B10 cells into EAE mice during disease progression or established disease is therapeutic (20). Disease manifestations are therefore reciprocally balanced by both B10 cells and Treg cells (10, 46).
B10 cell numbers expand in multiple inflammatory disease models, including delayed-type contact hypersensitivity, non-obese diabetic and lupus-prone NZB/W and MRL.lprfas mouse strains relative to wild-type C57Bl/6 mice (7, 9–11, 19). Similarly, humans with autoimmune disease, including those with multiple sclerosis, systemic lupus, rheumatoid arthritis, Sjögren’s syndrome and autoimmune vesiculobullous skin disease, have significantly expanded B10+B10pro cell numbers (18). Presumably, this represents clonal B10pro and B10 cell expansion because of increased autoantigen exposure as a result of tissue pathology, but this expansion is obviously insufficient to prevent disease progression. Similarly, the adoptive transfer of B10 cell-enriched B-cell subpopulations during active EAE does not ablate disease progression due to the relatively low numbers of appropriately antigen-specific B10 cells (10). However, B10 cells derived from antigen-experienced mice suppress disease to a greater extent than do B10 cells from naive mice (10), indicating that B10 cells expand and likely function in an antigen-specific manner. Thus, small increases in the total B10pro and/or B10 cell population may be inadequate, unless there is a sufficient increase in the number of antigen-specific B10 cells that can directly suppress the corresponding antigen-specific T cells that are driving disease pathology.
Given that antigen-specific B cells are relatively rare within the B-cell compartment, it is likely that antigen-specific B10 cells are also extremely rare. Although it would be fundamentally impossible to isolate sufficient numbers of B10 cells for autologous therapy given their rarity in blood (18), these rare cells may be able to expand sufficiently using therapeutic intervention to specifically suppress disease driven by diverse autoantigens (29). In support of this, the adoptive transfer of a few hundred-thousand B10 cells from mice with active disease can dramatically inhibit EAE initiation when the B10 cells are also activated with agonistic CD40 mAb prior to adoptive transfer (10). Additionally, the selective targeting of B cells with agonistic anti-CD40 antibody in MRL.Faslpr mice is reported to generate induced regulatory T2-like B cells that can suppress lupus (8).
The discovery that CD40 and IL-21 signals drive B10 cell development and expansion in vivo (20, 47) facilitated the development of an ex vivo culture system that expands B10 effector cell numbers by several million-fold (20). Remarkably, small numbers of these ex vivo expanded B10 effector cells are able to prevent disease initiation and progression and even reverse established disease in mice with EAE (20). Consequently, sufficient numbers of B10 cells can now be generated from one mouse to be able to effectively treat 21000 mice with EAE. These observations demonstrate that B10 cells can not only regulate disease initiation but also are able to effectively treat established disease when given at sufficient numbers. Thereby, the expansion and reintroduction of autologous B10 cell populations could be an exciting therapeutic option for patients with autoimmune and inflammatory disease.
B10 cell depletion for therapy of infection and cancer
Whereas B10 cell-mediated immune suppression is generally advantageous during autoimmune disease, therapeutic inhibition of B10 cell function can have beneficial effects, particularly for pathogen clearance and anticancer immune responses (48, 49). As one example, select anti-mouse B-cell mAbs can preferentially deplete B10 cells while leaving 95% of tissue B cells intact, significantly enhancing humoral immune responses to model antigens even in the absence of adjuvants (47). Furthermore, B10 cell depletion during mouse infection with Listeria monocytogenes dramatically accelerates macrophage activation, which leads to immediate bacteria clearance (26). In fact, Listeria are cleared so rapidly in mice without B10 cells that adaptive T-cell responses and memory do not develop in the absence of B10 cells. Thus, the normal course of adaptive and innate responses to bacterial infection is dictated in part by the regulatory activities of B10 cells.
B10 cell suppression of monocyte activation and bacterial clearance remains dependent on cognate B10 cell interactions with T cells, as B10 cells deficient in MHC-II, IL-10 or IL-21R expression do not regulate monocyte activation and Listeria clearance (26). Additionally, B-cell MyD88-dependent IL-10 production suppresses neutrophil recruitment and activation and natural killer cell and T-cell cytokine production in response to infection with Salmonella typhimurium, resulting in impaired bacterial clearance and reduced host survival (50). B10 cells can therefore suppress inflammatory responses against infectious agents, whereas therapeutic B10 cell depletion can accelerate immune responses and expedite the clearance of infectious organisms.
B10 cells also suppress antitumor immune responses, and so their therapeutic depletion can be advantageous during anticancer therapy. Endogenous B10 cells profoundly impair lymphoma depletion by CD20 mAb in mice, leading to increased tumor volumes and decreased survival following tumor development (51). Furthermore, the adoptive transfer of CD20-deficient B10 cells inhibits CD20 mAb-dependent lymphoma depletion, whereas IL-10-deficient B10 cells do not impair tumor clearance by CD20 mAb. B10 cell regulation of lymphoma clearance by CD20 mAb is due at least in part to B10 cell inhibition of macrophage TNF-α and nitrous oxide production. B10 cell numbers also increase in mice with lymphoma (51), which may occur with other tumors as well.
In humans, malignant cells from 90% of patients with chronic lymphocytic leukemia (CLL) retain the capacity to produce IL-10 (52). In patients with IL-10-competent CLL cells, there were 18–30 times more IL-10-competent CLL cells than there are B10 cells present in the blood of healthy donors. Similarly, patients with CLL cells that functioned like B10pro cells had 100-fold more IL-10-competent CLL cells than there are B10pro cells present in the blood of healthy donors.
These IL-10-competent CLL cells also express cell surface phenotypes similar to nonmalignant B10 cells, further suggesting a functional relationship between CLL cells and B10 cells. Indeed, in the T-cell leukemia protein 1 (TCL1)-transgenic mouse model of CLL, where mice have an age-associated B10 cell expansion that precedes the development of overt malignant CLL (53), IL-10-competent CLL cells and nonmalignant B10 cells share similar phenotypes. Importantly, systemic inflammation induced by LPS injection results in IL-10 production by CLL cells in vivo, suggesting that these cells may be immunosuppressive and contribute to heightened serum IL-10 levels in TCL1-transgenic mice. Thus, B10 cells can impair anticancer immune responses and therapies and might induce general immune suppression in patients with CLL. B10 cells are therefore promising potential targets for therapeutic depletion in patients with such malignancies.
Conclusions
Both the pro-inflammatory and regulatory functions of B cells are key components of normal adaptive and innate immune responses. It is now widely appreciated that B cells can also negatively regulate immune responses, in part through the secretion of anti-inflammatory IL-10. Regulatory B10 cells are identified by their functional capacity to express IL-10. Although numerically rare, B10 cells are easily identified following short-term ex vivo stimulation but are not specific to any B-cell developmental stage or tissue. Endogenous B10 cells potently suppress inflammation at the early stages of disease and can now be expanded ex vivo to numbers sufficient to alter the balance between regulatory and pathogenic B cells and halt aggressive disease. The ex vivo expansion and adoptive transfer of autologous B10 cells to treat autoimmune disease offer one potential pathway to the clinic, but their preferential depletion in vivo also offers the possibility of invigorating ongoing immune responses.
Determining precisely how B10 cell il10 transcription and immune suppression are regulated at the molecular level may also make pharmacologic B10 cell manipulation feasible. Although B10 cell function requires cognate interactions with CD4+ T cells, whether B10 cell immune suppression is generalized to any inflammatory insult or is tailored to select antigen-specific responses remains to be conclusively demonstrated. Similarly, the durability and localization of endogenous and adoptively transferred B10 cells will need to be better understood before this unique population can be utilized optimally for therapeutic benefit. Determining whether and how B10 cells and other regulatory B-cell subsets intersect and/or cooperate to regulate immune responses will undoubtedly reveal considerable functional complexities within the B-cell lineage.
Conflict of interest statement: The authors declared no conflict of interests.
References
- 1. Mizoguchi E.Mizoguchi A.Preffer F. I. and Bhan A. K. 2000. Regulatory role of mature B cells in a murine model of inflammatory bowel disease. Int. Immunol. 12:597. [DOI] [PubMed] [Google Scholar]
- 2. Tian J.Zekzer D.Hanssen L.Lu Y.Olcott A. and Kaufman D. L. 2001. Lipopolysaccharide-activated B cells down-regulate Th1 immunity and prevent autoimmune diabetes in nonobese diabetic mice. J. Immunol. 167:1081. [DOI] [PubMed] [Google Scholar]
- 3. Fillatreau S.Sweenie C. H.McGeachy M. J.Gray D. and Anderton S. M. 2002. B cells regulate autoimmunity by provision of IL-10. Nat. Immunol. 3:944. [DOI] [PubMed] [Google Scholar]
- 4. Mauri C.Gray D.Mushtaq N. and Londei M. 2003. Prevention of arthritis by interleukin 10-producing B cells. J. Exp. Med. 197:489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gray M.Miles K.Salter D.Gray D. and Savill J. 2007. Apoptotic cells protect mice from autoimmune inflammation by the induction of regulatory B cells. Proc. Natl Acad. Sci. USA 104:14080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Evans J. G., Chavez-Rueda K. A., Eddaoudi A., et al. 2007. Novel suppressive function of transitional 2 B cells in experimental arthritis. J. Immunol. 178:7868. [DOI] [PubMed] [Google Scholar]
- 7. Matsushita T.Yanaba K.Bouaziz J. D.Fujimoto M. and Tedder T. F. 2008. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J. Clin. Invest. 118:3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blair P. A., Chavez-Rueda K. A., Evans J. G., et al. 2009. Selective targeting of B cells with agonistic anti-CD40 is an efficacious strategy for the generation of induced regulatory T2-like B cells and for the suppression of lupus in MRL/lpr mice. J. Immunol. 182:3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haas K. M., Watanabe R., Matsushita T., et al. 2010. Protective and pathogenic roles for B cells during systemic autoimmunity in NZB/W F1 mice. J. Immunol. 184:4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matsushita T.Horikawa M.Iwata Y. and Tedder T. F. 2010. Regulatory B cells (B10 cells) and regulatory T cells have independent roles in controlling EAE initiation and late-phase immunopathogenesis. J. Immunol. 185:2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yanaba K.Bouaziz J. D.Haas K. M.Poe J. C.Fujimoto M. and Tedder T. F. 2008. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity 28:639. [DOI] [PubMed] [Google Scholar]
- 12. Watanabe R., Ishiura N., Nakashima H., et al. 2010. Regulatory B cells (B10 cells) have a suppressive role in murine lupus: CD19 and B10 cell deficiency exacerbates systemic autoimmunity. J. Immunol. 184:4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Amu S.Saunders S. P.Kronenberg M.Mangan N. E.Atzberger A. and Fallon P. G. 2010. Regulatory B cells prevent and reverse allergic airway inflammation via FoxP3-positive T regulatory cells in a murine model. J. Allergy Clin. Immunol. 125:1114. [DOI] [PubMed] [Google Scholar]
- 14. O’Garra A.Chang R.Go N.Hastings R.Haughton G. and Howard M. 1992. Ly-1 B (B-1) cells are the main source of B cell-derived interleukin 10. Eur. J. Immunol. 22:711. [DOI] [PubMed] [Google Scholar]
- 15. Mizoguchi A.Mizoguchi E.Smith R. N.Preffer F. I. and Bhan A. K. 1997. Suppressive role of B cells in chronic colitis of T cell receptor α mutant mice. J. Exp. Med. 186:1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mizoguchi A. and Bhan A. K. 2006. A case for regulatory B cells. J. Immunol. 176:705. [DOI] [PubMed] [Google Scholar]
- 17. Matsushita T. and Tedder T. F. 2011. Identifying regulatory B cells (B10 cells) that produce IL-10. Methods Mol. Biol. 677:99. [DOI] [PubMed] [Google Scholar]
- 18. Iwata Y., Matsushita T., Horikawa M., et al. 2011. Characterization of a rare IL-10-competent B cell subset in humans that parallels mouse regulatory B10 cells. Blood 117:530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yanaba K.Bouaziz J. D.Matsushita T.Tsubata T. and Tedder T. F. 2009. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J. Immunol. 182:7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yoshizaki A., Miyagaki T., DiLillo D. J., et al. 2012. Regulatory B cells control T cell autoimmunity through IL-21-dependent cognate interactions. Nature 491:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maseda D., Candando K. M., Smith S. H., et al. 2013. Peritoneal cavity regulatory B cells (B10 cells) modulate IFN-ϒ + CD4+ T cell numbers during colitis development in mice. J. Immunol. 191:2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ding Q., Yeung M., Camirand G., et al. 2011. Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J. Clin. Invest. 121:3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mizoguchi A.Mizoguchi E.Takedatsu H.Blumberg R. S. and Bhan A. K. 2002. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity 16:219. [DOI] [PubMed] [Google Scholar]
- 24. Rosser E. C., Oleinika K., Tonon S., et al. 2014. Regulatory B cells are induced by gut microbiota-driven interleukin-1beta and interleukin-6 production. Nat. Med. 20:1334. [DOI] [PubMed] [Google Scholar]
- 25. Matsumoto M., Baba A., Yokota T., et al. 2014. Interleukin-10-producing plasmablasts exert regulatory function in autoimmune inflammation. Immunity 41:1040. [DOI] [PubMed] [Google Scholar]
- 26. Horikawa M., Weimer E. T., DiLillo D. J., et al. 2013. Regulatory B cell (B10 cell) expansion during Listeria infection governs innate and cellular immune responses in mice. J. Immunol. 190:1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Duddy M., Niino M., Adatia F., et al. 2007. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J. Immunol. 178:6092. [DOI] [PubMed] [Google Scholar]
- 28. Blair P. A., Norena L. Y., Flores-Borja F., et al. 2010. CD19+CD24hiCD38hi B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity 32:129. [DOI] [PubMed] [Google Scholar]
- 29. Maseda D., Smith S. H., DiLillo D. J., et al. 2012. Regulatory B10 cells differentiate into antibody-secreting cells after transient IL-10 production in vivo . J. Immunol. 188:1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Saraiva M. and O’Garra A. 2010. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 10:170. [DOI] [PubMed] [Google Scholar]
- 31. Miles K., Heaney J., Sibinska Z., et al. 2012. A tolerogenic role for Toll-like receptor 9 is revealed by B-cell interaction with DNA complexes expressed on apoptotic cells. Proc. Natl Acad. Sci. USA 109:887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shen P., Roch T., Lampropoulou V., et al. 2014. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature 507:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sattler S., Ling G. S., Xu D., et al. 2014. IL-10-producing regulatory B cells induced by IL-33 (BregIL-33) effectively attenuate mucosal inflammatory responses in the gut. J. Autoimmun. 50:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang R.-X., Yu C.-R., Dambuza I. M., et al. 2014. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat. Med. 20:633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tedder T. F. and Leonard W. J. 2014. Autoimmunity: regulatory B cells–IL-35 and IL-21 regulate the regulators. Nat. Rev. Rheumatol. 10:452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jones L. L. and Vignali D. A. 2011. Molecular interactions within the IL-6/IL-12 cytokine/receptor superfamily. Immunol. Res. 51:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Aparicio-Siegmund S., Moll J. M., Lokau J., et al. 2014. Recombinant p35 from bacteria can form Interleukin (IL-)12, but Not IL-35. PLoS One 9:e107990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Matsushita T., Fujimoto M., Hasegawa M., et al. 2006. Inhibitory role of CD19 in the progression of experimental autoimmune encephalomyelitis by regulating cytokine response. Am. J. Pathol. 168:812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yanaba K.Yoshizaki A.Asano Y.Kadono T.Tedder T. F. and Sato S. 2011. IL-10-producing regulatory B10 cells inhibit intestinal injury in a mouse model. Am. J. Pathol. 178:735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yang M., Deng J., Liu Y., et al. 2012. IL-10-producing regulatory B10 cells ameliorate collagen-induced arthritis via suppressing Th17 cell generation. Am. J. Pathol. 180:2375. [DOI] [PubMed] [Google Scholar]
- 41. Le Huu D., Matsushita T., Jin G., et al. 2013. Donor-derived regulatory B cells are important for suppression of murine sclerodermatous chronic graft-versus-host disease. Blood 121:3274. [DOI] [PubMed] [Google Scholar]
- 42. Tedder T. F. 2009. CD19: a promising B cell target for rheumatoid arthritis. Nat. Rev. Rheumatol. 5:572. [DOI] [PubMed] [Google Scholar]
- 43. DiLillo D. J.Horikawa M. and Tedder T. F. 2011. B-lymphocyte effector functions in health and disease. Immunol. Res. 49:281. [DOI] [PubMed] [Google Scholar]
- 44. Teichmann L. L.Kashgarian M.Weaver C. T.Roers A.Müller W. and Shlomchik M. J. 2012. B cell-derived IL-10 does not regulate spontaneous systemic autoimmunity in MRL.Fas(lpr) mice. J. Immunol. 188:678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hobeika E., Thiemann S., Storch B., et al. 2006. Testing gene function early in the B cell lineage in mb1-cre mice. Proc. Natl Acad. Sci. USA 103:13789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Matsushita T. and Tedder T. F. 2009. B-lymphocyte depletion for the treatment of multiple sclerosis: now things really get interesting. Expert Rev. Neurother. 9:309. [DOI] [PubMed] [Google Scholar]
- 47. Poe J. C., Smith S.-H., Haas K. M., et al. 2011. Amplified B lymphocyte CD40 signaling drives regulatory B10 cell expansion in mice. PLoS One 6:e22464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Candando K. M.Lykken J. M. and Tedder T. F. 2014. B10 cell regulation of health and disease. Immunol. Rev. 259:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tedder T. F. 2015. B10 cells: a functionally defined regulatory B cell subset. J. Immunol. 194:1395. [DOI] [PubMed] [Google Scholar]
- 50. Neves P., Lampropoulou V., Calderon-Gomez E., et al. 2010. Signaling via the MyD88 adaptor protein in B cells suppresses protective immunity during Salmonella typhimurium infection. Immunity 33:777. [DOI] [PubMed] [Google Scholar]
- 51. Horikawa M.Minard-Colin V.Matsushita T. and Tedder T. F. 2011. Regulatory B cell production of IL-10 inhibits lymphoma depletion during CD20 immunotherapy in mice. J. Clin. Invest. 121:4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. DiLillo D. J., Weinberg J. B., Yoshizaki A., et al. 2012. Chronic lymphocytic leukemia and regulatory B cells share IL-10 competence and immunosuppressive function. Leukemia 27:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bichi R., Shinton S. A., Martin E. S., et al. 2002. Human chronic lymphocytic leukemia modeled in mouse by targeted TCL1 expression. Proc. Natl Acad. Sci. USA 99:6955. [DOI] [PMC free article] [PubMed] [Google Scholar]