Abstract
Few studies have evaluated the prevalence of replicating hepatitis C virus (HCV) infection in sub-Saharan Africa. Among 1812 individuals infected with human immunodeficiency virus, no patient in rural Mozambique and 4 patients in urban Zambia were positive for anti-HCV antibodies. Of these, none had confirmed HCV replication.
Keywords: hepatitis C infection, HIV infection, sub-Saharan Africa, screening
Hepatitis C virus (HCV) infection is a major cause of liver-related complications and mortality in human immunodeficiency virus (HIV)-infected populations in high-income settings [1]. However, its importance seems to vary widely across the African continent. Although the prevalence of anti-HCV antibody-positivity lies between 4% and 7% in most of sub-Saharan Africa (SSA) [2], there are large differences in the reported prevalence of replicating (ribonucleic acid [RNA]-positive) HCV infections, which ranges from <1% in southern Africa to >10% in Central Africa [3–5]. Only a few studies have confirmed their HCV screening findings with RNA testing.
Based on anti-HCV antibody prevalence estimates, the World Health Organization (WHO) recommends HCV screening of all risk groups, including HIV-infected populations [6]. Due to the high rates of false-positive HCV serological tests reported from SSA, this costly strategy may not prove universally efficient. In order to inform the implementation of HCV-testing guidelines, more data on the prevalence of replicating HCV infections in SSA are needed. We screened antiretroviral therapy (ART)-naive HIV-infected individuals in large outpatient clinics in Zambia and Mozambique for the presence of anti-HCV antibodies, confirmed results with HCV viral load testing, and examined potential risk factors for blood-borne infections.
METHODS
Consecutive HIV-infected adults initiating ART in 2 urban clinics in Lusaka, Zambia, and 4 rural clinics in Ancuabe, Mozambique, were enrolled between May 2013 and November 2014. Alongside HCV-related testing, patients received a clinical examination and laboratory screening including a full blood count, transaminases, serum creatinine, and a CD4 cell count. In addition, a detailed questionnaire on risk factors for liver disease was administered in face-to-face interviews with all patients in Zambia and a subsample of the cohort in Mozambique. This survey included questions on alcohol and drug consumption, risk factors for blood-borne infections, and past history of sexually transmitted infections. All data were entered into an electronic database for clinical care, monitoring, evaluation, and reporting purposes. All patients provided written informed consent to participate in a prospective substudy within the framework of the International epidemiological Databases to Evaluate AIDS in Southern Africa (IeDEA-SA) [7]. The Biomedical Research Ethics Committee of University of Zambia School of Medicine, the Institutional Review Board of University of North Carolina at Chapel Hill, and the Comité Nacional de Bioética para a Saúde, República de Moçambique, approved the study.
All patients were screened for anti-HCV antibodies using the oral Oraquick point-of-care test (OraSure Technologies Inc., Bethlehem, PA), which is known to have good diagnostic accuracy in individuals infected with HIV [8]. In a random selection of 118 patients in Lusaka, we performed a second screening test on serum samples using an anti-HCV enzyme-linked immunosorbent assay (ELISA) assay (Access 2 Analyzer, Beckman Coulter). Blood was collected in serum separator tubes, centrifuged within 6 hours, and serum was aliquoted into 2 mL microtainer tubes for storage at −80°C until the date of testing. All patients with a positive antibody test were retested with Oraquick in whole blood via finger-prick sampling. A confirmation quantitative HCV-RNA test (Roche COBAS AmpliPrep/COBAS TaqMan HCV Test) was performed in all patients with any positive antibody screening test result. All tests were performed according to the manufacturer's instructions.
Hepatitis C virus prevalence was expressed as a percentage with 95% confidence intervals (CIs). Baseline characteristics were presented as median values and interquartile range (IQR) for continuous variables and absolute numbers and percentages for categorical ones. All statistical analyses were performed using Stata 13.1 (StataCorp, College Station, TX).
RESULTS
In total, 1812 individuals infected with HIV were included in the study (Mozambique, n = 1057; Zambia, n = 755). Median age was 32 years (IQR, 26–39) and 65.5% were female. Median pre-ART CD4 count was 255 cells/µL (IQR, 133–370), and 37.8% of patients had WHO stage 3 or 4 HIV diseases. Table 1 describes the main demographic characteristics of the study populations and potential risk factors for blood-borne infections, by country. In both countries, traditional risk factors for blood-borne infections were very common: over 50% of the study population had a history of body piercing, whereas tattoos or traditional scarification were observed in 85% of Zambians and 93% of Mozambicans. However, the proportion of participants who had a history of blood transfusions was low in both countries. No history of past or present injection drug use was reported in any of the clinics.
Table 1.
Baseline Characteristics of HIV-Infected Patients Screened for Hepatitis C Virus Coinfection, by Country
| Characteristics | Zambia (n = 755) | Mozambique (n = 1057) |
|---|---|---|
| Median age in years (IQR) | 34 (29–40) | 30 (24–38) |
| Female sex (%) | 410 (54.3) | 777 (73.5) |
| Median CD4 count in cells/µL (IQR) | 228 (118–336) | 288 (156–437) |
| Advanced HIV disease (%) | 334 (44.8) | 345 (32.8) |
| History of risk factors for blood-borne infections | (n = 755) | (n = 104) |
| Incarceration (%) | 122 (16.2) | 3 (2.9) |
| Piercing (%) | 418 (55.4) | 59 (56.7) |
| Tattoo or scarification (%) | 644 (85.3) | 97 (93.3) |
| Tooth extraction/surgery (%) | 202 (26.8) | 58 (55.8) |
| Blood transfusion (%) | 52 (6.9) | 11 (10.6) |
Abbreviations: HIV, human immunodeficiency virus; IQR, interquartile range.
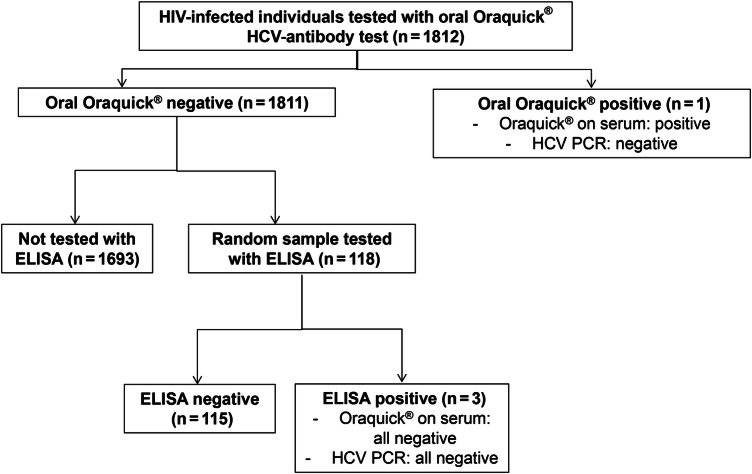
In Mozambique, zero of 1057 patients tested had a positive anti-HCV Oraquick test. Among 755 HIV-infected Zambians screened, 1 was identified as positive using the Oraquick test, resulting in a seroprevalence of 0.13% (95% CI, .02–.94). The only patient with a positive test had a negative HCV-RNA confirmation test (Figure 1). Among the 118 patients in Zambia who underwent a second screening test based on the ELISA assay, 3 were positive for anti-HCV antibodies. Two of them had a negative HCV viral load, and 1 had a first HCV viral load of 50 IU/mL but a negative result in a second sample. Thus, in the sample of patients tested with both screening assays, anti-HCV antibody seroprevalence was 2.5%. Oraquick was repeated in the serum of the 4 patients with any positive antibody test and confirmed the result from the oral test in all of them.
Figure 1.
Flow chart of patients according to hepatitis C virus (HCV) tests performed. Abbreviations: ELISA, enzyme-linked immunosorbent assay; HIV, human immunodeficiency virus; PCR, polymerase chain reaction.
DISCUSSION
Among individuals infected with HIV in 2 southern African cohorts, HCV seroprevalence ranged from 0.1% to 2.5% depending on the antibody test used. However, no active HCV infections were observed despite the presence of traditional risk factors for percutaneous transmission of blood-borne infections in a majority of patients. These data underscore the importance of confirming active HCV infection and challenge the recommendation to scale up HCV screening for all individuals infected with HIV in the region.
Although numerous studies have shown a relatively high prevalence of positive anti-HCV serology results in HIV-infected populations, several recent reports underlined the very low prevalence of confirmed, chronic HCV infection in Southern and East Africa. Similar to our study, the prevalence of replicating HCV infection was below 1% in a large clinical trial in Malawi and in an observational cohort in Uganda [3, 4]. A previous study among hospitalized individuals infected with HIV in Zambia also showed similar results [9]. Several hypotheses have been proposed to explain the gap between the prevalence of anti-HCV antibody and RNA-positivity, including cross-reactions of HCV serological assays to auto-antibodies or antibodies produced in the context of the polyclonal B-cell activation triggered by other infections [10]. For instance, previous reports have shown a link between the presence of antibodies to schistosomal infections and false-positive anti-HCV serologies [4, 11]. Of note, 1 patient in our study population had a negative Oraquick, a positive ELISA, and a first viral load of 50 IU/mL. However, HCV viral load was not detectable in a second measurement, which argues for a false-positive ELISA and a contamination during the first HCV amplification procedure.
Although HCV transmission patterns have been widely described in high-income countries, few data are available from the African continent. Because injection drug use is uncommon in many African countries, and no report has yet shown HCV epidemics in men who have sex with men in SSA, most HCV transmissions are thought to occur through the vertical or percutaneous routes [12, 13]. In Cameroon, where the prevalence of HCV infection reaches 12% in patients infected with HIV [5], molecular clock analyses showed that medical interventions before 1960, and to a lesser extent traditional practices, were linked to the transmission of HCV [14]. Such associations have not been described in southern Africa to date. In our study, HCV coinfection was very rare even though most patients had 1 or more potential risk factors such as tattoos, scarifications, and piercings. These results contrast with those of a recent report from Ghana, where Layden et al [15] showed that confirmed HCV infection was present in at least 74% of those with a positive antibody test and that the most important risk factors included traditional scarring and circumcision. Thus, despite the widespread presence of traditional risk factors for blood-borne infections across the continent, the prevalence of HCV infection in the general population seems to differ widely across regions, being generally higher in parts of Central and West Africa compared with East and Southern Africa. The main reasons for these epidemiological trends, including the near absence of HCV infection in the 2 countries included in our study, remain unclear.
Our study is one of few large and detailed assessments of active HCV infection among HIV-infected populations in SSA. Unfortunately, we were unable to perform both screening tests in all patients. Furthermore, the presence of injection drug use might have been underreported because patients might have felt uncomfortable disclosing this information. Sampling bias, a potential explanation for the low prevalence of HCV infection in our study setting, seemed unlikely because we included consecutive ART-eligible patients at primary care outpatient clinics. Finally, due to the low number of confirmed HCV infections, we were unable to identify related risk factors in our cohort.
CONCLUSIONS
Due to the limitations of anti-HCV assays, illustrated in our study by the 2.5% prevalence of anti-HCV antibodies with the ELISA test, and until Oraquick and other similar tests have been validated in the setting of HIV-HCV coinfection in SSA, epidemiological estimates of HCV prevalence should be confirmed by the detection of HCV RNA. Resources to screen for and treat HCV are limited in most SSA settings; therefore, national governments should assess local epidemiological data before implementing HCV testing in all individuals infected with HIV.
Acknowledgments
We thank all patients, doctors, and nurses who supported the implementation of the study at the Centre for Infectious Disease Research in Zambia (Lusaka, Zambia) and SolidarMed in Mozambique.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This study was funded by the National Institutes of Health (IeDEA-Southern Africa, grant U01AI069924). G. W. was supported by an Ambizione-PROSPER fellowship from the Swiss National Science Foundation (PZ00P3_154730). M. J. V. was supported by the Fogarty International Center of the National Institutes of Health (K01TW009998).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Ly KN, Xing J, Klevens RM et al. . The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med 2012; 156:271–8. [DOI] [PubMed] [Google Scholar]
- 2.Rao VB, Johari N, du Cros P et al. . Hepatitis C seroprevalence and HIV co-infection in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Infect Dis 2015; 15:819–24. [DOI] [PubMed] [Google Scholar]
- 3.Chasela CS, Wall P, Drobeniuc J et al. . Prevalence of hepatitis C virus infection among human immunodeficiency virus-1-infected pregnant women in Malawi: the BAN study. J Clin Virol 2012; 54:318–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mullis CE, Laeyendecker O, Reynolds SJ et al. . High frequency of false-positive hepatitis C virus enzyme-linked immunosorbent assay in Rakai, Uganda. Clin Infect Dis 2013; 57:1747–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laurent C, Bourgeois A, Mpoudi-Ngole E et al. . High rates of active hepatitis B and C co-infections in HIV-1 infected Cameroonian adults initiating antiretroviral therapy. HIV Med 2010; 11:85–9. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Guidelines for the screening, care and treatment of persons with hepatitis C infection, WHO, Geneva, April 2014. http://www.who.int/hiv/pub/hepatitis/hepatitis-c-guidelines/en/. [PubMed]
- 7.Egger M, Ekouevi DK, Williams C et al. . Cohort profile: the international epidemiological databases to evaluate AIDS (IeDEA) in sub-Saharan Africa. Int J Epidemiol 2012; 41:1256–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith BD, Teshale E, Jewett A et al. . Performance of premarket rapid hepatitis C virus antibody assays in 4 national human immunodeficiency virus behavioral surveillance system sites. Clin Infect Dis 2011; 53:780–6. [DOI] [PubMed] [Google Scholar]
- 9.Oshitani H, Kasolo F, Luo NP et al. . Low prevalence of hepatitis C virus infection in Lusaka, Zambia. Trans R Soc Trop Med Hyg 1995; 89:380. [DOI] [PubMed] [Google Scholar]
- 10.Bogdanos DP, Mieli-Vergani G, Vergani D. Virus, liver and autoimmunity. Dig Liver Dis 2000; 32:440–6. [DOI] [PubMed] [Google Scholar]
- 11.Agha S, El-Mashad N, El-Malky M et al. . Prevalence of low positive anti-HCV antibodies in blood donors: Schistosoma mansoni co-infection and possible role of autoantibodies. Microbiol Immunol 2006; 50:447–52. [DOI] [PubMed] [Google Scholar]
- 12.Ocama P, Seremba E. Management of HIV and hepatitis C virus infections in resource-limited settings. Curr Opin HIV AIDS 2011; 6:539–45. [DOI] [PubMed] [Google Scholar]
- 13.Layden JE, Phillips R, Opare-Sem O et al. . Hepatitis C in sub-Saharan Africa: urgent need for attention. Open Forum Infect Dis 2014; 1:ofu065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pepin J, Lavoie M, Pybus OG et al. . Risk factors for hepatitis C virus transmission in colonial Cameroon. Clin Infect Dis 2010; 51:768–76. [DOI] [PubMed] [Google Scholar]
- 15.Layden JE, Phillips RO, Owusu-Ofori S et al. . High frequency of active HCV infection among seropositive cases in west Africa and evidence for multiple transmission pathways. Clin Infect Dis 2015; 60:1033–41. [DOI] [PubMed] [Google Scholar]