The telomerase inhibitor imetelstat (Janssen Biotech Inc., Raritan, NJ, USA) is a 13-mer lipid-conjugated oligonucleotide that targets the RNA template of human telomerase reverse transcriptase. In a pilot study,1 imetelstat produced complete clinical and molecular remissions in myelofibrosis patients with SF3B1 or U2AF1 mutations; this provided the rationale for the current study (Clinicaltrials.gov NCT01731951), as such spliceosome mutations are closely associated with refractory anemia with ring sideroblasts with (RARS-T) or without (RARS) thrombocytosis.2
The current signal-seeking study included nine patients with RARS-T (n=5), RARS (n=3) or myelodysplastic/myeloproliferative neoplasm with spliceosome mutation (n=1). Diagnoses were according to the World Health Organization criteria.3 Because of concern regarding myelosuppression,1 the 2-h intravenous infusion of imetelstat was administered at a reduced dose level (7.5 mg/kg every 4 weeks instead of 9.4 mg/kg every 3 weeks). Drug activity was monitored by both formal response criteria4 and effect on spleen size, thrombocytosis and leukocytosis. Adverse events were monitored by Common Terminology Criteria for Adverse Events (Version 4.03; US National Cancer Institute, Bethesda, MD, USA). Laboratory correlative studies included analysis of mutations.
As of 10 May 2015, accrual to the study was complete (total nine patients) and median time from study registration was 17.1 months (range, 16.1–20.1). Median age of the study population was 70 years (78% males); eight patients were transfusion-dependent and one patient with RARS-T had marked splenomegaly that was palpable at 16 cm below the left costal margin. Three patients each had leukocytosis and thrombocytosis. Seven patients had received prior treatments, including erythropoiesis-stimulating agents in six patients. Karyotype was abnormal in one patient, with loss of Y chromosome.
Four (44%) patients remain on active treatment, and the five treatment discontinuations were due to death during active therapy (unrelated to imetelstat), discovery of second malignancy, progression into acute leukemia and insufficient response (n=2). Median treatment duration was 13.7 months (range, 6.6–17.9). Treatment-emergent grade 4 neutropenia and thrombocytopenia were seen in 2 (22%) and 1 (11%) patients, respectively; grade 3 anemia, neutropenia and thrombocytopenia were seen in 6 (67%), 4 (44%) and 2 (22%) patients, respectively. None of the grade ⩾3 hematologic toxicities lasted ⩾4 weeks, and all demonstrated reversibility. The grade 3 non-hematologic events of aspiration, fatigue and lipase increase occurred in one patient, and a patient with preexisting cardiovascular disease experienced a grade 5 heart failure. Treatment-emergent liver function test abnormalities affected the following: alanine aminotransferase (grade 1, 56%/grade 2, 11%); aspartate transaminase (grade 1, 56%/grade 2, 11%); alkaline phosphatase (grade 1, 33%); and total bilirubin (grade 2, 11%). For patients who discontinued treatment with imetelstat, these events were mostly reversible.
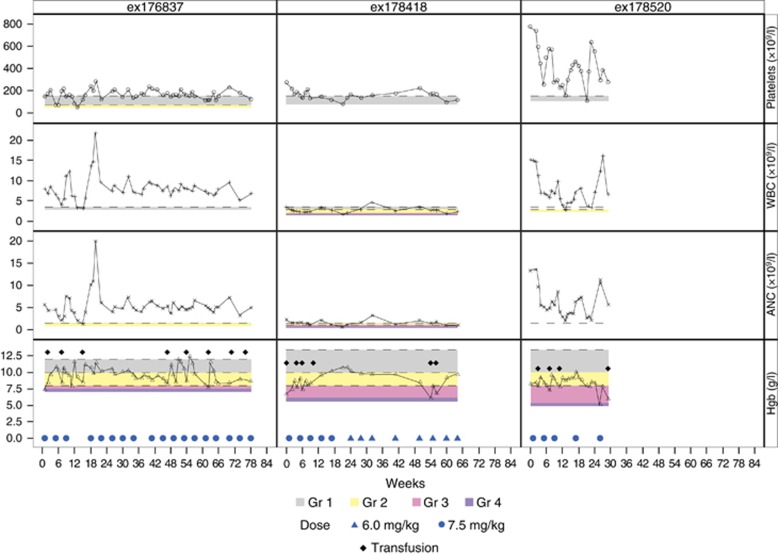
Three (38%) of eight transfusion-dependent patients became transfusion-independent at a median time of 11 weeks (range, 9–14) and their response lasted for a median of 28 weeks (range, 9–37; Figure 1); one of the three patients also had resolution of leukocytosis and thrombocytosis. A fourth patient experienced >50% decrease in palpable spleen size (16 cm at baseline) and a decrease in transfusion need. Two additional patients with thrombocytosis and leukocytosis experienced normalization of counts. Patients were screened before and after imetelstat therapy for mutations involving JAK2, SF3B1, U2AF1 and SRSF2. Three patients were mutated for JAK2, seven for SF3B1 (4 K700E, 2 H662Q and 1 K666N) and one for U2AF1 (Q157P). All three anemia responders (H662Q, K700E and K666N) and the fourth patient with spleen response (K700E) were SF3B1 mutated. Post-treatment analysis showed no effect on mutations.
Figure 1.
Effect of imetelstat on hemoglobin (Hgb), platelet and leukocyte counts in three patients with refractory anemia with ring sideroblasts with (ex178520) or without (ex176837 and ex178418) thrombocytosis. ex176837: T1 duration=28 weeks and treatment ongoing; ex178418: T1 duration=37 weeks and treatment ongoing; ex178520: T1 duration=9 weeks and off-study July 2014. ANC, absolute neutrophil; WBC, white blood cell.
The current study suggests drug activity in imetelstat-treated patients with RARS or RARS-T. The meager depth of response in the current study, compared with the aforementioned report in myelofibrosis,1 might reflect either different disease biology or suboptimal drug dose. Further investigation on the therapeutic benefit of imetelstat in RARS/RARS-T is warranted.
Acknowledgments
This research was supported by Janssen Research & Development, LLC, Raritan, NJ, USA, which also supported editorial assistance provided by PAREXEL, Hackensack, NJ, USA.
Footnotes
AR and YW are employees of and hold stock in Janssen. The remaining authors declare no conflict of interest.
References
- Tefferi A, Lasho TL, Begna KH, Patnaik MM, Zblewski DL, Finke CM et al. A pilot study of the telomerase inhibitor imetelstat for myelofibrosis. N Engl J Med 2015; 373: 908–919. [DOI] [PubMed] [Google Scholar]
- Broseus J, Alpermann T, Wulfert M, Florensa Brichs L, Jeromin S, Lippert E et al. Age, JAK2(V617F) and SF3B1 mutations are the main predicting factors for survival in refractory anaemia with ring sideroblasts and marked thrombocytosis. Leukemia 2013; 27: 1826–1831. [DOI] [PubMed] [Google Scholar]
- Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 2009; 114: 937–951. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Bennett JM, Kantarjian H, Pinto A, Schiffer CA, Nimer SD et al. Report of an international working group to standardize response criteria for myelodysplastic syndromes. Blood 2000; 96: 3671–3674. [PubMed] [Google Scholar]