Abstract
Efficacy of lenalidomide was investigated in 103 patients with relapsed/refractory chronic lymphocytic leukemia (CLL) treated on the prospective, multicenter randomized phase-II CLL-009 trial. Interphase cytogenetic and mutational analyses identified TP53 mutations, unmutated IGHV, or del(17p) in 36/96 (37.5%), 68/88 (77.3%) or 22/92 (23.9%) patients. The overall response rate (ORR) was 40.4% (42/104). ORRs were similar irrespective of TP53 mutation (36.1% (13/36) vs 43.3% (26/60) for patients with vs without mutation) or IGHV mutation status (45.0% (9/20) vs 39.1% (27/68)); however, patients with del(17p) had lower ORRs than those without del(17p) (21.7% (5/22) vs 47.1% (33/70); P=0.049). No significant differences in progression-free survival and overall survival (OS) were observed when comparing subgroups defined by the presence or absence of high-risk genetic characteristics. In multivariate analyses, only multiple prior therapies (⩾3 lines) significantly impacted outcomes (median OS: 21.2 months vs not reached; P=0.019). This analysis indicates that lenalidomide is active in patients with relapsed/refractory CLL with unfavorable genetic profiles, including TP53 inactivation or unmutated IGHV. (ClinicalTrials.gov identifier: NCT00963105).
key-points
Overall response rate and survival outcomes are similar in relapsed/refractory chronic lymphocytic leukemia patients treated with lenalidomide irrespective of TP53 or IGHV mutations.
This suggests that lenalidomide activity may not be affected by loss of functional TP53 or IGHV status.
Introduction
Single-agent lenalidomide has clinical activity in chronic lymphocytic leukemia (CLL), both in treatment-naive patients,1, 2 and in those with relapsed and refractory disease3, 4, 5, 6 or unfavorable characteristics.2, 4, 5
Recent clinical trials in CLL patients demonstrated that unmutated IGHV is associated with unfavorable outcomes with conventional chemotherapy or chemoimmunotherapy regimens.7, 8, 9 Multivariate analysis established del(17p), TP53 mutation or unmutated IGHV were each important independent prognostic factors for survival.7, 9 TP53 mutation without del(17p) is also of prognostic importance, with both markers demonstrating independent prognostic significance in multivariate analyses.10
Patients with CLL having del(17p) had reduced overall response rate (ORR) and progression-free survival (PFS) in a study involving unselected CLL patients treated in routine clinical practice.11 Furthermore, the presence of del(17p) has been associated with significantly inferior outcome in the context of novel, non-cytotoxic treatments, such as ibrutinib.12
We investigated the efficacy of lenalidomide in subgroups of relapsed and refractory CLL patients with high-risk genetics and clinical characteristics at baseline.
Materials and methods
The study design and patient population are described elsewhere.13 In brief, patients were randomized 1:1:1 to receive a double-blinded starting dose of oral lenalidomide (5, 10 or 15 mg per day) on days 1–28 of each 28-day treatment cycle. Subject to tolerability, doses were escalated to a maximum of 25 mg per day, with dose modifications applied as required. All patients received appropriate prophylaxis for tumor lysis syndrome and thrombosis. Treatment was continued until disease progression or unacceptable toxicity. Institutional Investigational Review Board of each participating site approved this study, which was conducted according to good clinical practice and the ethical principles outlined in the Declaration of Helsinki. All patients provided written informed consent.
Several exploratory analyses were conducted as part of the trial. Clinical and demographic characteristics of interest were age, disease stage, number of prior treatments, presence of bulky disease or constitutional symptoms and purine analog response status.
Blood samples for IGHV and TP53 mutation analysis, and fluorescence in situ hybridization studies for interphase cytogenetic assessment were collected pre-dose on day 1. All genetic analyses were performed centrally (Ulm University, Ulm, Germany, or University of California, San Diego, San Diego, USA), as described.9, 10, 14
Descriptive statistics were used to describe continuous demographic and baseline variables for each patient; categorical variables were summarized using frequency tabulations for treatment groups separately and combined. Efficacy analyses were performed on the intention-to-treat population and included all patients with genetic data available. For all efficacy end points, determination of responses (including progression of disease) was based on the investigator's assessment of CLL response data using International Workshop on CLL guidelines for diagnosis and treatment of CLL.15 Responses by presence or absence of pretreatment characteristics were compared using logistic regression stepwise selection. Differences were considered significant at the P<0.05 level. Logistic regression was done to assess the relationship of patient response (responder vs non-responder) using stepwise selection. The following baseline characteristics were included: relapsed vs refractory to last prior therapy; IGHV mutation status; bulky disease; del(17p) and del(11q) status; serum β2-microglobulin level; disease stage; and number of prior therapies (<3 vs ⩾3).
Role of the funding source
Celgene Corporation funded the study. All authors had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results and discussion
Of 104 patients enrolled, 103 received treatment; baseline characteristics are described elsewhere and the primary results demonstrated that lower starting doses of lenalidomide could facilitate dose escalation, with indication of improved efficacy in patients who escalated to higher doses.13
Based on the intent-to-treat safety population (n=103), data on TP53 mutations, IGHV mutational status or del(17p) were available for 96, 89 or 93 patients, respectively. TP53 mutations were identified in 36 (37.5%) patients, unmutated IGHV in 68 (77.5%) patients and del(17p) in 23 (24.7) patients.
Most patients with TP53 mutations also harbored unmutated IGHV (27/36; 75.0%), whereas around half had del(17p) (17/36; 47.2%). In the absence of TP53 mutation, del(17p) was found in 5/60 (8.3%) patients. A majority of patients with del(17p) also had TP53 mutations (17/22; 77.3%) or unmutated IGHV (16/22; 72.7% Supplementary Table 1). Patients with TP53 mutations, compared with those without, were more likely to be >65 years (55.6% vs 33.3%), have del(17p) (47.2% vs 8.3%), have Rai high-risk/Binet C disease (55.6% vs 39.3%) or to have a reduced (<1 50 000/mm3) platelet count (75.0% vs 45.0% Supplementary Table 1). Patients with unmutated IGHV were more likely than patients with mutated IGHV to have TP53 mutation (39.7% vs 20.0%) or bulky disease (45.6% vs 25.0%). Patients with del(17p), compared with those without, were more likely to have TP53 mutation (36.8% vs 15.0%), Rai high-risk/Binet C disease (45.6% vs 35.0%) or a reduced (<1 50 000/mm3) platelet count (77.3% vs 50.0% Supplementary Table 1).
Investigator-assessed ORR was 40.4% (42/104) for all patients (Supplementary Table 2). Median time to first response to lenalidomide for all patients was 3.3 months (range: 1.9–34.9). The median response duration was 22.8 months (range: 16.6–29.3). ORRs for patients with and without TP53 mutation were 36.1% (13/36) and 43.3% (26/60; P=0.526); for patients with and without mutated IGHV, ORRs were 45.0% (9/20) and 39.7% (27/68; P=0.796). ORR for patients with del(17p) was lower than for those without deletions with borderline significance, using Fisher's exact test (21.7% vs 47.1%, P=0.049; odds ratio: 0.31; 95% confidence interval: 0.10 and 0.93). No other significant differences were observed for any other characteristic assessed at baseline.
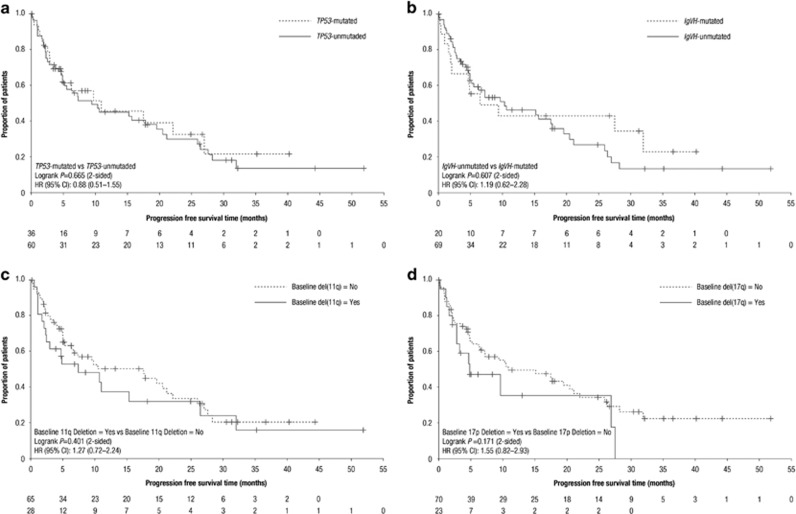
At a median follow-up time of 24 months, significant survival differences were found between responders and patients with stable disease (median PFS: 26.5 vs 7.2 months, P<0.001; median overall survival (OS): not estimable vs 19.8 months; P=0.011; Table 1). The median PFS and median OS were 9.7 and 33.0 months, respectively, in the overall population. Median PFS in patients with TP53 mutations, compared with those without, was short with 11.0 vs 9.5 months (P=0.665; Figure 1a); median OS was 19.4 vs 35.4 months (P=0.249; Table 1). For patients with mutated vs unmutated IGHV, median PFS was 6.5 vs 10.4 months (P=0.607; Figure 1b); median OS was 31.9 months vs not estimable (P=0.293). In patients with del(11q) vs those without, median PFS was 7.3 vs 17.6 months (P=0.401; Figure 1c); median OS was 21.3 vs 35.4 months (P=0.435). In patients with del(17p) vs those without, median PFS was 4.9 vs 11.0 months (P=0.171; Figure 1d); median OS was 18.9 vs 34.9 months (P=0.318; Table 1). Of note, although several of the observed differences between risk groups were sizeable, no significant differences were observed as the study was not powered to detect such differences between risk groups.
Table 1. PFS and OS according to pretreatment characteristicsa.
| Characteristic | N | Median PFS (months) | P-valueb | Median OS (months) | P-valueb |
|---|---|---|---|---|---|
| All patients | 104 | 9.7 | NE | 33.0 | NA |
| Responders | 42 | 26.5 | <0.001 | NE | 0.011 |
| Patients with SD | 31 | 7.2 | 19.8 | ||
| Binet stage | NA | ||||
| Binet stage A | 10 | 3.7 | 35.4 | ||
| Binet stage B | 28 | 15.3 | 37.7 | ||
| Binet stage C | 26 | 27.6 | 19.7 | ||
| RAI staging system score | NA | ||||
| Low-risk disease | 5 | 4.9 | 20.8 | ||
| Intermediate-risk disease | 14 | 19.6 | NE | ||
| High-risk disease | 21 | 8.0 | 28.5 | ||
| TP53 mutation | |||||
| Yes | 36 | 11.0 | 0.665 | 19.4 | 0.249 |
| No | 60 | 9.5 | 35.4 | ||
| del(17p) | |||||
| Yes | 23 | 4.9 | 0.171 | 18.9 | 0.318 |
| No | 70 | 11.0 | 34.9 | ||
| del(11q) | |||||
| Yes | 28 | 7.3 | 0.401 | 21.3 | 0.435 |
| No | 65 | 17.6 | 35.4 | ||
| IGHV mutation status | |||||
| Mutated | 20 | 6.5 | 0.607 | 31.9 | 0.293 |
| Unmutated | 69 | 10.4 | NE | ||
| Number of prior treatments | |||||
| <3 | 44 | 17.6 | 0.150 | NE | 0.019 |
| ⩾3 | 60 | 5.5 | 21.2 | ||
| Bulky disease | |||||
| Yes | 45 | 10.6 | 0.339 | 33.0 | 0.689 |
| No | 58 | 9.7 | 34.9 | ||
| Refractory to purine analogs | |||||
| Yes | 44 | 5.5 | 0.283 | 21.3 | 0.268 |
| No | 60 | 10.4 | 35.4 | ||
Abbreviations: NA, not applicable; NE, not estimable; OS, overall survival; PFS, progression-free survival; SD, stable disease.
Based on intent-to-treat population.
Based on unstratified log-rank test.
Figure 1.
Progression-free survival curves. Patients with and without (a) TP53 mutations; (b) IGHV mutations; (c) del(11q); and (d) del(17p).
Multivariable analyses were performed for PFS and OS including baseline del(11q), del(17p), TP53 mutation, unmutated IGHV, disease stage, relapse/refractory to prior purine analog therapy, baseline β2 microglobulin, bulky disease and number of prior CLL treatments as potential variables. Backward deletion was performed at a significance level of 0.05 and the main effects with P-values of ⩽0.05 were retained in the final model and were identified as independent prognostic factors. Regarding PFS, none of the factors were selected into the final model. Regarding OS, only extensive pretreatment (⩾3 lines) significantly impacted outcomes (median OS: 21.2 months vs not reached; hazard ratio: 0.51; 95% confidence interval: 0.28–0.90; P=0.019).
Our data reveal that ORR and survival outcomes are similar and relatively poor in relapsed and refractory patients with CLL following lenalidomide treatment irrespective of the presence of TP53 or IGHV mutations, suggesting that lenalidomide activity may not be affected by loss of functional TP53 or unmutated IGHV. Purine analog refractory status and disease stage, both the clinical features associated with high-risk disease, did not appear to impact ORR or survival outcomes following lenalidomide treatment (Supplementary Table 2; Table 1).
In conclusion, our data indicate that a relatively modest lenalidomide activity is seen in relapsed and refractory CLL patients with unfavorable cytogenetic profiles, with ORRs of 36.1% and 39.1% observed in patients with TP53 mutations and unmutated IGHV, respectively. In patients with del(17p), ORR was lower (21.7%) yet still apparent. However, in some patients, these responses were durable as highlighted in the PFS and OS curves (Figure 1). PFS and OS outcomes were similar irrespective of high-risk genetic characteristics. The trial was not powered to detect subtle differences between small subgroups, for example, with del(17p) vs TP53 mutation. The pleiotropic effects of lenalidomide observed on the tumor microenvironment16 or leukemia cell proliferation17 and new insights into the various mechanisms of action of lenalidomide are of increasing interest. These insights may provide a rationale for specific combination regimens, including lenalidomide plus ibrutinib, or other agents with distinct mechanisms of action.
Acknowledgments
We received editorial support from Excerpta Medica (Ronald van Olffen, PhD, CMPP) in the preparation of this manuscript, funded by Celgene Corporation. We are fully responsible for all content and editorial decisions for this manuscript. The work was partly funded by the DFG (SFB1074, project B2). This study was funded by Celgene Corporation.
Author contributions
All authors participated in the clinical trial reported in this paper, or in the analysis of data from this study. All authors directed development, review and approval of this manuscript, and are fully responsible for all content and editorial decisions.
Footnotes
Supplementary Information accompanies this paper on Blood Cancer Journal website (http://www.nature.com/bcj)
C-MW receives research funding, consultancy and honoraria by Celgene; MH receives research support from Celgene; TJK has served as an advisor to Celgene and received research funding from Celgene; GAMF has received honoraria from Celgene; PH receives honoraria from Celgene; JD has received a research grant and honoraria from Celgene; JGG receives honoraria from Celgene, Roche, Pharmacyclics, Mundipharma and Abbvie, and has received research support grant funding from Celgene; BP is an employee of Celgene and has equity; JZ is an employee of Celgene and has equity; SDB is an employee of Celgene and has equity; JM is an employee of Celgene and has equity; SS has received a research grant and honoraria from Celgene; the remaining authors declare no conflict of interest.
Supplementary Material
References
- Chen CI, Bergsagel PL, Paul H, Xu W, Lau A, Dave N et al. Single-agent lenalidomide in the treatment of previously untreated chronic lymphocytic leukemia. J Clin Oncol 2011; 29: 1175–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badoux XC, Keating MJ, Wen S, Lee BN, Sivina M, Reuben J et al. Lenalidomide as initial therapy of elderly patients with chronic lymphocytic leukemia. Blood 2011; 118: 3489–3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanan-Khan A, Miller KC, Musial L, Lawrence D, Padmanabhan S, Takeshita K et al. Clinical efficacy of lenalidomide in patients with relapsed or refractory chronic lymphocytic leukemia: results of a phase II study. J Clin Oncol 2006; 24: 5343–5349. [DOI] [PubMed] [Google Scholar]
- Ferrajoli A, Lee BN, Schlette EJ, O'Brien SM, Gao H, Wen S et al. Lenalidomide induces complete and partial remissions in patients with relapsed and refractory chronic lymphocytic leukemia. Blood 2008; 111: 5291–5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher T, Miller KC, Lawrence D, Whitworth A, Hernandez-Ilizaliturri F, Czuczman MS et al. Efficacy of lenalidomide in patients with chronic lymphocytic leukemia with high-risk cytogenetics. Leuk Lymphoma 2010; 51: 85–88. [DOI] [PubMed] [Google Scholar]
- Wendtner CM, Hillmen P, Mahadevan D, Bühler A, Uharek L, Coutré S et al. Final results of a multicenter phase 1 study of lenalidomide in patients with relapsed or refractory chronic lymphocytic leukemia. Leuk Lymphoma 2012; 53: 417–423. [DOI] [PubMed] [Google Scholar]
- Oscier D, Wade R, Davis Z, Morilla A, Best G, Richards S et al. Prognostic factors identified three risk groups in the LRF CLL4 trial, independent of treatment allocation. Haematologica 2010; 95: 1705–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulian P, Rossi D, Forconi F, Del Poeta G, Bertoni F, Zucca E et al. IGHV gene mutational status and 17p deletion are independent molecular predictors in a comprehensive clinical-biological prognostic model for overall survival prediction in chronic lymphocytic leukemia. J Transl Med 2012; 10: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stilgenbauer S, Schnaiter A, Paschka P, Zenz T, Rossi M, Döhner K et al. Gene mutations and treatment outcome in chronic lymphocytic leukemia: results from the CLL8 trial. Blood 2014; 123: 3247–3254. [DOI] [PubMed] [Google Scholar]
- Zenz T, Kröber A, Scherer K, Häbe S, Bühler A, Benner A et al. Monoallelic TP53 inactivation is associated with poor prognosis in chronic lymphocytic leukemia: results from a detailed genetic characterization with long-term follow-up. Blood 2008; 112: 3322–3329. [DOI] [PubMed] [Google Scholar]
- Zaja F, Mian M, Volpetti S, Visco C, Sissa C, Nichele I et al. Bendamustine in chronic lymphocytic leukemia: outcome according to different clinical and biological prognostic factors in the everyday clinical practice. Am J Hematol 2013; 88: 955–960. [DOI] [PubMed] [Google Scholar]
- Byrd JC, Furman RR, Coutre SE, Burger JA, Blum KA, Coleman M et al. Three-year follow-up of treatment-naïve and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood 2015; 125: 2497–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendtner CM, Hallek M, Fraser GAM, Michallet A-S. Safety and efficacy of different lenalidomide starting doses in patients with relapsed or refractory chronic lymphocytic leukemia: results of an international multicenter double-blinded randomized phase II trial. Leuk Lymphoma 2016; 14: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröber A, Seiler T, Benner A, Bullinger L, Brückle E, Lichter P et al. V(H) mutation status, CD38 expression level, genomic aberrations, and survival in chronic lymphocytic leukemia. Blood 2002; 100: 1410–1416. [PubMed] [Google Scholar]
- Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H et al. International Workshop on Chronic Lymphocytic Leukemia. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 2008; 111: 5446–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kater AP, Tonino SH, Egle A, Ramsay AG. How does lenalidomide target the chronic lymphocytic leukemia microenvironment? Blood 2014; 124: 2184–2189. [DOI] [PubMed] [Google Scholar]
- Fecteau JF, Corral LG, Ghia EM. Lenalidomide inhibits the proliferation of CLL cells via a cereblon/p21WAF1/Cip1-dependent mechanism independent of functional p53. Blood 2014; 124: 1637–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.