Abstract
Voltage-gated sodium channels (VGSCs) are responsible for initiation and propagation of action potentials in excitable cells. VGSCs in mammalian brain are heterotrimeric complexes of α and β subunits. Originally called “auxiliary,” we now know that β subunit proteins are multifunctional signaling molecules that play roles in both excitable and non-excitable cell types, and with or without the pore-forming α subunit present. β subunits function in VGSC and potassium channel modulation, cell adhesion, and gene regulation, with particularly important roles in brain development. Mutations in the genes encoding β subunits are linked to a number of diseases, including epilepsy, sudden death syndromes like SUDEP and SIDS, and cardiac arrhythmia. While VGSC β subunit-specific drugs have not yet been developed, this protein family is an emerging therapeutic target.
Keywords: cell adhesion, development, epilepsy, arrhythmia, pain, neurodegenerative disease
I. Introduction
Voltage-gated sodium channels (VGSCs) are responsible for generation of the rising phase and propagation of the action potential (AP) in excitable cells, including neurons and cardiac myocytes (1). VGSCs have been purified as heterotrimeric complexes of α and β subunits from mammalian brain where a central α subunit forms the ion-conducting pore and is complexed with two different β subunits (2). This is postulated, but not proven, to be the case in other excitable tissues. Originally characterized as “auxiliary,” important only in modulation of VGSC function, the β subunit proteins are now known to be dynamic, multifunctional molecules that engage in diverse and essential roles throughout multiple tissues and systems, in both excitable and non-excitable cell types, and with or without VGSC α subunits (3). Their ability to participate in both conducting and non-conducting roles makes VGSC β subunits unique among voltage-gated ion channel subunits. During the more than two decades since the first VGSC β subunits were identified, a growing body of research has identified the importance of these proteins not only in normal physiology, but also in numerous pathophysiologies including channelopathies.
The breadth of β subunit function hinges on the key structural and functional motif common to all members of this family of proteins: an immunoglobulin (Ig) loop enabling them to function as cell adhesion molecules (CAMs) (3; 4). These adhesive functions are critical to brain development and thus to excitability, including the processes of neurite outgrowth, axon pathfinding, fasciculation, and cell migration, and are postulated to play similar roles in heart and peripheral nerve (3; 4). In their roles as ion channel modulators, not only of VGSCs but also voltage-gated K+ channels (VGKCs) (5–7), β subunits make important contributions to the regulation of neuronal firing. As substrates for sequential cleavage by β- (BACE) and γ-secretases, β subunits contribute to not only cell adhesion but also to the regulation of VGSC α subunit gene expression (8). Taking all of these roles into consideration, it is no wonder that mutations in the genes encoding VGSC β subunits are linked to pathophysiology. This review highlights the primary disease areas in which VGSC β subunit gene mutations are linked as well as some of the novel pathways and disorders with which β subunits are only now beginning to be associated, with the advent of powerful tools such as genome-wide association studies (GWAS) and whole exome sequencing.
II. Fundamentals: β Subunits 101
1. VGSC Genes and Structure
Nine VGSC α subunits have been identified, denoted Nav1.1-Nav1.9 and encoded by a gene family with nomenclature SCN(X)A (9). Sodium current (INa) flow down its concentration gradient occurs in response to changes in membrane potential, wherein VGSCs open, or activate, in response to a depolarizing stimulus, then inactivate via closure of an intracellular inactivation gate, becoming refractory to conduction until the channel reaches the closed state and is thus able to respond to another stimulus (1). VGSC α subunits are categorized into two groups based on their sensitivity to tetrodotoxin (TTX). TTX-sensitive (TTX-S) α subunits (Nav 1.1, Nav1.2, Nav1.3, Nav1.4, Nav1.6, and Nav1.7) are blocked by nanomolar concentrations of TTX, whereas TTX-resistant (TTX-R) α subunits are blocked by micromolar concentrations of TTX (9).
The five VGSC β subunit proteins are encoded by a family of four genes. β1 and its splice variant β1B are encoded by SCN1B (10–13), and β2, β3, and β4 are encoded by SCN2B (14), SCN3B (15), and SCN4A (16), respectively. All five VGSC β subunits contain an extracellular Ig domain homologous to V-type Ig loop motifs present in the Ig superfamily (IgSF) of CAMs (11; 15–18), an observation that provided key early evidence of the multifunctionality of these proteins. With the exception of β1B, β subunits have type 1 topology, consisting of a single polypeptide chain with an extracellular N-terminus, a single transmembrane-spanning segment, and an intracellular C-terminus. In the case of β1B (originally called β1A), normal splicing of the exon 3/intron 3 boundary does not occur, leading to in-frame retention of intron 3 and generation of an alternate C-terminal sequence that does not include a transmembrane domain (11). As a result, β1B is a developmentally regulated secreted protein (12).
VGSC α and β subunits interact via two distinct mechanisms. β1 and β3, which share 57% sequence homology (15), interact non-covalently with α subunits via their N- and C-termini (18–20). Although β1B is a secreted protein, it selectively associates with Nav1.5, but not with Nav1.1 or Nav1.3, in heterologous systems (12). β2 and β4 share 35% sequence homology (16) and engage in covalent interactions with α subunits via a single N-terminal cysteine in the extracellular Ig loop (2; 16; 21): Cys-26 in β2 (22) or Cys-58 in β4 (23).
Until recently, β subunits were modelled on the known structure of the extracellular domain of the IgSF CAM myelin P0, with which β subunits, especially β1 and β3, share a high degree of sequence homology (14; 15; 18). Structures for the β3 and β4 extracellular domains have now been published, confirming elements that had been deduced from the myelin P0 structure (23; 24) and providing useful and more directly comparable models for β1 and β2. Two cysteine residues in the extracellular domain of each β subunit are responsible for formation and maintenance of the Ig-fold and its multiple constituent β-sheets, and their positions are conserved across all five β subunits. For β3, a second intramolecular disulfide bond provides further stabilization. Interestingly, β3 associates with Nav1.5 in a trimeric complex that is capable of forming oligomers, rather than the canonical heterotrimeric complex of two non-identical β subunits with a single α subunit (24). The residues mediating this trimeric assembly are unique to β3 and not predicted to occur in β1 (24), supporting the distinct expression patterns and postulated functional roles of these proteins (reviewed in (4)). Thus, the canonical heterotrimeric VGSC structure, based on channel purification from brain, may not hold across tissues.
2. Expression and Localization
VGSC β subunits are expressed in a wide range of tissue and cell types, including excitable and non-excitable cells, and their expression patterns vary with development. VGSC α subunits are found not only in mammals but have orthologs in invertebrates, bacteria and electric eel, among others (4). β subunit proteins are expressed in a wide range of vertebrate species, but not invertebrates, suggesting that their evolution followed that of the pore-forming α subunit. In support of this, Drosophila VGSC α subunits associate with a non-pore forming subunit, tipE, that is not homologous to vertebrate β subunits but does modulate channel function, suggesting a separate evolutionary pathway (25). In mammalian brain, β1 and β2 predominate in postnatal development with peak levels in adult (10; 14), while expression of β1B and β3 are higher in embryonic development and early life (11; 12; 26). This developmental expression pattern is different in heart, where β1B and β3 expression persist into adulthood (11; 26). Details about the developmental profile of β4 expression are not yet known. In general, β subunit expression patterns are distinct and, in some locations, complementary, suggesting that each β subunit plays a unique non-compensatory role. Table I summarizes the current understanding of β subunit expression in selected cell types in vertebrate nervous system and cardiac tissue.
β subunits are localized to a number of important subcellular compartments in which they are components of macromolecular complexes, enabling them to engage in specialized functions. In brain and peripheral nerve, these include the axon initial segment (AIS) and nodes of Ranvier where VGSC α subunits are clustered at high density (21; 27–31) (Table 1). At both AIS and nodes, C-terminal association with the adaptor protein ankyrin-G is postulated to anchor β subunits to the cytoskeletal protein βIV spectrin (32). The specific components of VGSC macromolecular complexes differ based on their specific subcellular location. For example, at nodes of Ranvier, β subunits can associate with α subunits tightly clustered within the nodal gap and are thus positioned for channel modulation during rapid saltatory conduction (21; 27–29). In the paranodal subcompartment adjacent to the nodal gap, β1 is able to engage in adhesive functions that contribute to the close apposition of axo-glial membranes; here, β1 interacts with a trimeric complex of axo-glial paranodal proteins consisting of axonal contactin and Caspr and glial neurofascin-155 (Nf-155) (33; 34). β1 localization to this subcellular compartment is postulated to contribute to the establishment and maintenance of paranodes and formation of the nodal gap.
Table 1.
Expression profiles of VGSC β subunits in brain, peripheral nerve, and heart. Adapted from (4).
| β1 | β1B | β2 | β3 | β4 | |
|---|---|---|---|---|---|
| Nervous system | |||||
| Hippocampal neurons | (28) | (16; 27) | (15; 26) | (16; 21) | |
| Cortical neurons | (5) | (11; 13) | (16; 27) | (15; 26; 152) | (16; 21) |
| Purkinje neurons | (30) | (11; 13) | (16; 27) | (26) | (16; 21) |
| Cerebellar granule neurons | (30; 39) | ||||
| Astrocytes | (87) (reactive) (153; 154)(cultured) | (153; 154)(cultured) | (153)(cultured) | ||
| Bergmann glia | (39) | (39) | |||
| Nodes of Ranvier | (28; 43) | (27) | (21) | ||
| Dorsal root ganglia | (117–119) | (11; 13) | (16; 117; 118; 122) | (15; 26; 118; 152) | (16; 21; 118) |
| Heart | |||||
| Ventricular myocytes | (100) | (100) | (100) | (100) | |
| Atrial myocytes | (97; 98) | (98) | (98) | (98) | |
| Sino-atrial node | (155) | (155) | (155) | (155) |
3. Post-Translational Modification
VGSC β subunit function, localization, and expression are regulated by multiple post-translational modifications, including phosphorylation, glycosylation, and proteolytic cleavage (reviewed in (4)). The β1 intracellular domain (ICD) contains a tyrosine residue at position 181 that is a substrate for phosphorylation (32; 35), likely through the src family of kinases, particularly fyn kinase (36; 37). In vitro, phosphorylation of β1-Y181 is essential for β1-mediated recruitment of ankyrin to points of cell-cell contact; mutation of this site abolishes ankyrin recruitment without abolishing channel modulatory function (32). Phosphorylation also plays a role in β subunit trafficking to the correct subcellular compartments, as evidenced by differential localization of phosphorylated and non-phosphorylated β1 in heart (35). β subunit proteins are highly glycosylated, containing 3–4 N-linked glycosylation sites (10; 18; 38), which accounts for approximately 1/3 of the molecular weight of the mature proteins. These modifications contribute to both surface expression and channel modulation (38). β subunits are also substrates for proteolytic cleavage by BACE1 and γ-secretase, sequentially shedding first the extracellular domain, resulting in generation of a soluble Ig loop-containing protein similar to β1B (12) or an engineered soluble Fc fusion protein (39; 40), and then releasing the shorter ICD (8). These cleavage events may have important functional roles: γ-secretase cleavage is required for β1-mediated neurite outgrowth in vitro (3), and γ-secretase cleavage of β2 in vitro leads to translocation of the ICD to the nucleus where it increases SCN1A expression (41).
4. β Subunits in Channel Modulation
Canonically, VGSC β subunits function in concert with α subunits to promote channel trafficking to the plasma membrane and to modulate VGSC biophysical properties. Co-expression of β subunits in vitro and in vivo leads to increased α subunit expression at the plasma membrane. During VGSC biosynthesis, the final step before plasma membrane insertion is covalent association of α with β2 (42), such that hippocampal neurons from Scn2b null mice show ~50% reductions in INa density and in the number of α subunits at the plasma membrane (27). Deletion of β subunits can also lead to alterations in the complement of α subunits expressed in specific brain regions; in Scn1b null hippocampus, CA3 neurons display decreased levels of Nav1.1 and increased levels of Nav1.3 (28). Additionally, these interactions display functional reciprocity: in Scn1b null cerebellar granule neurons (CGNs), AIS expression of Nav1.6 is reduced in favor of increased expression of Nav1.1 and Nav1.2, and conversely, expression of Nav1.6 is required for β1-mediated neurite outgrowth (30).
β subunits also modulate α subunit function, with effects including alterations in peak INa density, voltage-dependence of activation and inactivation, rate of inactivation, and persistent and resurgent INa in cell- and tissue-specific patterns (reviewed in (4)). Early observations indicated that co-expression of β1 with Nav1.2 led to increases in peak INa, increased rate of inactivation, and hyperpolarizing shifts in activation and inactivation (10). Since that time, extensive study of β subunit function in heterologous systems and mouse models has shown that β subunit effects vary based on the α subunit being modulated as well as the specific cell type (reviewed in (4)). As the biophysical properties of each α subunit differ, at least in vitro, this offers the potential to finely tailor excitability to specific organ, cellular, or subcellular requirements in a dynamic fashion via specific combinations of α and β subunits. Importantly, β subunits also interact with and functionally modulate some VGKCs, particularly the Kv4 channels and Kv1.1 (5–7). The association of β subunits, especially β1, with VGSCs and VGKCs provides a means for channel cross-talk during the AP. Taking this multifunctionality into account, it is no surprise that mutations in β subunit genes result in varied and severe channelopathies.
5. β Subunits are CAMs
Channel modulation represents only one facet of β subunit function. The presence of an Ig loop in the structure of all five β subunit proteins predicted a role in cell adhesion and placed β subunits within the IgSF of CAMs, one of the four primary superfamilies of adhesion molecules along with integrins, cadherins, and selectins (17). As CAMs, β subunits engage in both trans-homophilic and heterophilic adhesion. Importantly, β subunit-mediated cell adhesion can occur with or without expression of a VGSC α subunit in vitro, although adhesive interactions are critical to the promotion of α subunit cell surface expression (33; 34). β1 engages in extracellular homophilic cell adhesion as well as heterophilic adhesive interactions with VGSC β2, contactin-1, Nf-186, Nf-155, NrCAM, N-cadherin, and the extracellular matrix protein tenascin-R in vitro (33; 35; 43–45). It is predicted that β1B, which shares the N-terminal domain including the Ig loop with β1, engage in similar adhesive interactions as β1. Because β1B is a secreted molecule that can participate in long distance adhesive interactions, it may play roles in novel signaling functions in development, including acting as a soluble antagonist for cell adhesive interactions between cells. The cell adhesive functions of the other β subunits are less extensively characterized. β2 can interact with β1 and tenascin-C, but not contactin, in vitro (34; 45; 46). The ability of β3 to engage in adhesive events is controversial, with studies demonstrating that β3 does (47) and does not (48) homophilically induce cellular aggregation in vitro. Thus, the adhesive capacity of β3 may depend on the expression system and the presence or absence of other CAMs. The role of β4 in cell adhesion remains unclear, although β4 was not observed to mediate neurite outgrowth compared to β1 in vitro (39).
Side Bar.
VGSC β subunits were the first non-pore forming ion channel subunits discovered to be CAMs that are able to function both in the presence and absence of the ion-conducting pore. Could this be a trend among other ion channel subunits? A promising candidate for future investigation is calcium channel α2δ. This non-pore-forming VGCC subunit is a transmembrane protein with an extensive extracellular domain. The presence of a Von Willebrand factor-A domain in α2δ, similar to some integrins, suggests that it may participate in adhesive events (156). Another potential candidate is the Kv4 accessory subunit, DPP6. Similar to VGSC β1, this transmembrane protein plays roles in cell adhesion and motility, in addition to ion channel modulation, with subsequent effects on hippocampal synaptic development and function (157).
β subunit-mediated adhesion plays a role in multiple physiological and developmental processes. Adhesion is critical in axonal fasciculation and axon pathfinding during development. Scn1b null mice display defective fasciculation in the corticospinal tract and cerebellar parallel fibers, disorganization of axons in the cerebellar molecular layer, reduced neuron density in the dentate gyrus granule cell layer, increased proliferation of granule cell precursors in the hilus, and defective axonal extension and misorientation of somata and processes of inhibitory neurons in the dentate gyrus and CA1 (37; 49). The mechanism of trans β1-β1-mediated neurite outgrowth requires fyn kinase activity and TTX-S INa can be mimicked by the binding of secreted β1B to β1 on the neuronal cell surface (12; 30; 37; 39).
III. Channelopathies and Pathophysiology of VGSC β Subunits
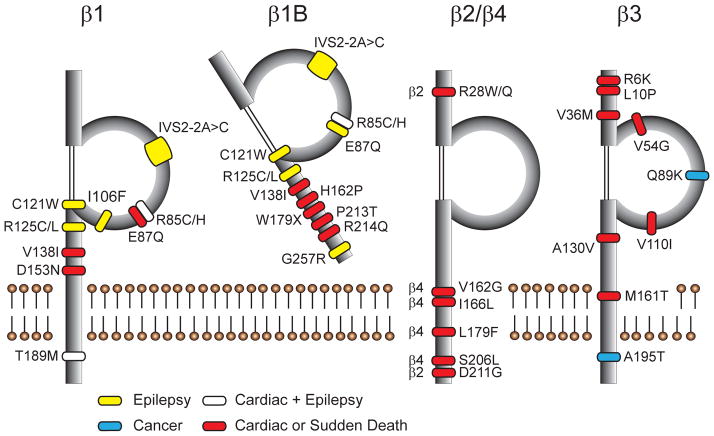
To summarize to this point, VGSC β subunits are multifunctional, with the capacity to signal through multiple pathways in multiple tissues on multiple timescales. Thus, it is not surprising that a growing number of β subunit gene mutations have been linked to various neuronal and cardiac diseases (Fig. 1).
Figure 1.
Disease-linked mutations in VGSC β subunits. Sites of mutated amino acids for each of the five β subunit protein products are denoted, including epilepsy (yellow), cardiac disease and non-SUDEP sudden death (red), cancer (blue), and mutations which have been identified in patients with multiple diagnoses (white). In β1/β1B, epilepsy-linked mutations cluster around the Ig loop, while no mutations have so far been identified in the Ig loop of β2 or β4, but instead occur within the transmembrane domain and C-terminus. Mutations in β3 occur throughout the protein.
1. Epilepsy
Epilepsy is characterized by aberrant electrical activity in brain leading to seizures that are recurrent and unprovoked (50). It is estimated that 1% of the U.S. population is afflicted with some form of epilepsy, which can be a consequence of genetic mutation or brain injury (50). Genes encoding VGSCs, including the β subunits, have been implicated in multiple forms of epileptic encephalopathies. The first SCN1B mutation identified in epilepsy was p.C121W, which disrupts a key disulfide bond involved in maintaining the Ig fold (51). p.C121W is causative in Generalized Epilepsy with Febrile Seizures plus (GEFS+), in which patients first experience febrile seizures which then progress to persistent afebrile seizures. GEFS+ is one of multiple epilepsies on the pediatric genetic epilepsy spectrum, ranging from mild idiopathic epilepsies to severe forms such as Dravet Syndrome (52).
Dravet Syndrome (DS), formerly known as Severe Myoclonic Epilepsy of Infancy, is a devastating pediatric epileptic encephalopathy (53). Similar to GEFS+, patients typically present with febrile seizures beginning under one year of age that do not resolve, eventually converting to recurrent afebrile seizures of multiple etiologies that are frequently pharmacoresistant. Major comorbidities of DS include early developmental delay and regression, cognitive impairment, high risk of sudden unexpected death in epilepsy (SUDEP), sleep impairment, and ataxia (54). Although the incidence of severe seizures decreases over time, DS leads to a persistent and debilitating phenotype that extends throughout the life of the patient. Many patients continue to experience generalized tonic-clonic seizures and ataxia. Cognitive impairment does not resolve with age, and there may be a correlation between the severity of cognitive impairment and the severity and frequency of persistent seizures. Many patients are unable to live independently and must rely on support from caregivers or institutionalization (55).
Classically, DS is considered to be a SCN1A-linked disease, with over 80% of DS patients carrying heterozygous de novo mutations in SCN1A that lead to Nav1.1 haploinsufficiency (52). However, a small but growing number of DS patients have mutations in SCN1B. The first homozygous SCN1B mutation identified in DS was p.R125C, which prevents normal trafficking of β1 to the cell surface, resulting in a functional null phenotype. Interestingly, normal surface localization can be rescued at lowered temperatures in vitro, suggesting possible therapeutic interventions (56). A second homozygous SCN1B DS mutation, p.I106F, was subsequently described (57). Heterozygous mutations (p.R85C, p.R85H, p.R125L), as well as a five amino acid deletion (IVS2-2A>C), in SCN1B are associated with other epilepsies, including GEFS+ and other febrile seizure disorders (51; 58–60). A mutation specific to β1B, p.G257R, linked to idiopathic epilepsy, was also found to be trafficking-deficient in vitro (12). The majority of SCN1B epilepsy mutations identified to date, with the exception of p.G257R, cluster around and within the Ig loop, suggesting that the cell adhesive functions of β1/β1B are clinically relevant (Fig. 1).
Mouse models and in vitro studies have contributed to a clearer understanding of the molecular mechanisms underlying SCN1B-linked epilepsy. Scn1b null mice have frequent spontaneous generalized seizures, display aberrant neuronal excitability, and have defects in neuronal development (28; 37; 49). Importantly, although seizure onset in these mice begins at approximately postnatal day (P)10, abnormalities in brain development are observed as early as P5 (49). These observations suggest that structural alterations, aberrant cell adhesive interactions, and abnormal excitability early in development may be causative factors in epileptogenesis in Scn1b null animals. A knock-in mouse model of p.C121W-mediated GEFS+ displays hyperexcitability in specific subpopulations of central neurons, reduced dendritic arborisation of subicular pyramidal neurons, and increased susceptibility to febrile seizures (31; 61). This is consistent with in vitro studies demonstrating that effects of p.C121W on VGSC gating and kinetics are worsened at higher temperatures, leading to hyperexcitabilty, increased peak current, and increased channel availability (62). Interestingly, p.C121W abrogates normal β1-mediated adhesion without altering the ability of β1 to modulate VGSC function in vitro (63).
Lastly, although Scn2b null mice express only 50% of normal cell surface VGSCs in brain and are more susceptible to pharmacologically induced seizures (27). In contrast, patients with cardiac arrhythmia do have SCN2B mutations (64; 65), suggesting that altered β2 function may have a larger impact in heart or in cardiac innervation compared to brain.
2. SUDEP and Sudden Death
Epilepsy patients are at increased risk of sudden death compared to the general population. SUDEP is defined as sudden death that is not a result of trauma or drowning, does not result from status epilepticus, and in which post-mortem examination does not uncover a toxicological or anatomical cause (66). SUDEP is estimated to occur in 7.5–17% of all epilepsy patients (67). The risk of SUDEP increases with the severity of the epilepsy: across all epilepsy patients the annual risk is 0.1%, whereas in DS patients, this risk increases to a mean annual rate of 0.6% (54). Across childhood epilepsies, the risk of sudden death is increased 24-fold compared to the general population (67). These drastic increases in risk of sudden death point to the need for a clearer understanding of the mechanisms that underlie SUDEP as well as effective therapeutic approaches for prevention and intervention.
While some risk factors for SUDEP have been proposed, there are currently no reliable biomarkers. The age and severity of seizure onset correlate with a higher SUDEP risk (68). While a limited number of retrospective studies have linked SUDEP to generalized convulsive seizure episodes, it is likely that death is triggered by seizure-related or associated alterations in multiple organ systems. These include autonomic dysfunction, cardiac arrhythmia, central or obstructive apnea, pulmonary edema, and hypoventilation (69). Consequently, it is critical to establish effective control of seizures, whether through pharmacological or surgical intervention; this is supported by observations that SUDEP risk increases with pharmacoresistance of seizures and non-compliance with anti-epileptic drug therapy (68).
Animal models of DS and other epilepsy disorders, spanning multiple genetic mutations, are also models of SUDEP. The Scn1b null mouse is a model of DS and SUDEP, displaying severe spontaneous seizures, ataxia, developmental delay, and premature death by ~P21 (28). Scn1a+/− mice model the haploinsufficiency typically seen in human DS patients with de novo SCN1A mutations and exhibit a severe seizure phenotype with SUDEP (70). Other channelopathy models of epilepsy display SUDEP, including the VGKC Kcna1 (Kv1.1) null mouse, with severe seizures and sudden death observed in ~25% of pups by P21 (71). The mechanism of SUDEP has been proposed to be “arrhythmia of brain and heart,” leading to not only seizures but also cardiac dysfunction, dysregulation of respiratory centers in the brain, and autonomic dysregulation (72). During or following a seizure, patients may experience cardiac events including arrhythmia, tachycardia, bradycardia, T-wave alteration, asystole, or atrial fibrillation (73). The autonomic nervous system is a key regulator of cardiac function and autonomic alterations occur in both focal and generalized seizures and in both ictal and interictal periods (74). Epileptic discharges may propagate to the autonomic nervous system, altering vagal tone and producing downstream discharges onto the heart that can lead to arrhythmias and other potentially fatal cardiac events (69; 73). In animal models, cardiac and autonomic pathologies are frequently observed. Interestingly, these appear to vary between models, such that animals presenting with similar seizure and SUDEP phenotypes display mechanistic differences that may be informative in the elucidation of the etiology of sudden death. Scn1b null mice have increased cardiac INa and prolonged QT and RR intervals on the electrocardiogram (ECG) of anesthetised animals (75). Kcna1 null mice display atrioventricular (AV) block, bradycardia, premature ventricular contractions and altered HRV (71). Scn1a+/− mice have increased cardiac INa, similar to Scn1b null mice, and display bradycardia, focal discharges, R-R variability, and bundle branch block but not AV block on ECGs obtained by telemetry of conscious animals (76). AV block in both Kcna1 null mice (71) and Scn1a+/− (77) mice is removed with atropine, pointing to excessive parasympathetic tone, whereas Scn1b null mice do not display changes in QT interval following treatment with either atropine or propranolol (75), suggesting that the Scn1b cardiac phenotype may not be caused by autonomic changes.
Sudden Infant Death Syndrome (SIDS) describes sudden unexpected death in an infant between the ages of one week and one year, where post-mortem analysis does not uncover a clear cause of death (78). The etiology of SIDS, as with SUDEP, remains unclear. Public education efforts in recent years to prompt changes in sleep position in infants have led to a significant reduction in SIDS incidence. However, after this decrease, the number of SIDS cases has remained static, suggesting the influence of non-environmental risk factors (79).
An estimated 10% of SIDS cases are associated with mutations in ion channel genes; within this population, approximately 50% are linked to mutations in SCN5A (79). Other ion channel genes mutated in SIDS include KCNQ1, KCNE1, KCNH2, and RYR2 (80), all of which carry mutations identified in SUDEP (81). This overlap in implicated genes draws a clear parallel to genetic causes of SUDEP as well as cardiac channelopathies. Mutations in VGSC β subunit genes have been linked to SIDS, including SCN3B (p.V36M, p.V54G) and SCN4B (p.S206L) (82). One of the two identified mutations in SCN3B, p.V54G, has also been linked to idiopathic ventricular fibrillation (83). The SCN4B mutation p.S206L leads to increased persistent INa and AP duration (APD) prolongation, a phenotype similar to a separate SCN4B mutation (p.L179F) associated with cardiac dysfunction (82; 84). The dual expression patterns of SCN3B and SCN4B in brain and heart strengthen the proposed involvement of both systems in this disease.
The involvement of various brain regions in SIDS pathophysiology has been extensively investigated, including hippocampus, cerebellum, medulla, and brainstem (reviewed in (85)) and suggest some similarities to Scn1b-linked disease models. Thickening of the cerebellar external granule layer (EGL) and abnormal persistence of the granule cell layer into later stages of development has been reported in SIDS, suggesting deficits in granule cell migration in cerebellar development (86), similar to effects seen in Scn1b null mouse brain (49). Multiple reports have shown astrogliosis in SIDS brainstem (85). β1 protein is known to be upregulated in reactive astrocytes in human brain in both epileptic and non-epileptic patients (87; 88). Lastly, diffusely delayed myelination in cerebellum and brainstem is observed in SIDS brain (86; 89). Scn1b null mice have deficiencies in central nervous system (CNS) myelination in optic nerve, where the thickness of the myelin sheath and formation of normal tight adhesive junctions at the paranodal axo-glial interface are both abnormal (28). While these observations in SIDS patients have not yet been associated with mutations in genes encoding any of the VGSC β subunits, they may inform new lines of investigation, including possible links between SIDS and SUDEP.
SIDS, sudden death, and cardiac arrhythmia may be linked via mutations in the cardiac VGSC complex (90). A connection between SIDS and Brugada Syndrome (BrS) is supported by a mutation in β1B (p.R214Q) present in two adult BrS patients as well as one incident of SIDS (91). This mutation affects two channel types in distinct manners. When co-expressed with Nav1.5, critical in cardiac excitability, β1B p.R214Q results in Nav1.5 loss-of-function. When co-expressed with Kv4.3, normally involved in generation of Ito, β1B p.R214Q leads instead to gain-of-function (91). This dual modulation is consistent with previous in vitro and in vivo studies demonstrating functional effects of Scn1b on both VGKCs and VGSCs (6; 7; 92). In non-epileptic adults, Sudden Unexpected Death Syndrome (SUDS) and Sudden Unexpected Nocturnal Death Syndrome (SUNDS) are also linked to mutations in SCN5A and, less frequently, to SCN1A (93). Two mutations in SCN1B (p.V138I, p.T189M) and one in SCN3B (p.A195T) were identified in a SUNDS patient cohort from China; however, the clinical relevance of these mutations has not yet been established (94).
Taken together, these observations offer the potential for common mechanisms linking various forms of sudden death across all ages from neonate to adult. The presence of VGSC β subunit gene mutations in a subset of these cases suggests these proteins may play key roles in pathophysiology and etiology of sudden death.
3. Cardiac Arrhythmia
In normal cardiac function, VGSCs are responsible for rapid influx of Na+ during the AP upstroke. In addition, they contribute to conduction velocity throughout the myocardium and participate in excitation-contraction coupling within the myocyte (95; 96) VGSC β subunits are expressed throughout the heart as well as in the conduction system. In human heart, SCN1B expression is higher in atria and endocardium than in ventricles and epicardium, whereas SCN2B and SCN3B expression patterns are found in all regions of the heart (97). At the intercalated disc of ventricular myocytes, the predominant cardiac VGSC, TTX-R channel Nav1.5, has been shown by immunofluorescence to be expressed with β2, β4, and tyrosine-phosphorylated β1 (35; 98–100). At the t-tubules of ventricular myocytes, the TTX-sensitive (TTX-S) channels Nav1.1, Nav1.3 and Nav1.6 are co-expressed with non-phosphorylated β1, β2, and β3 (35; 98; 100; 101). As a result, β subunits are positioned to contribute to all aspects of normal VGSC-linked cardiac function. This suggests that mutant VGSC β subunit proteins and/or alterations in expression levels are likely to contribute to the pathophysiology of cardiac channelopathies.
VGSC β subunit gene mutations are linked to multiple forms of arrhythmia, as recently reviewed (102). The first cardiac-associated mutations identified in VGSC β subunits were SCN1B mutations linked to BrS (103), which carries a high risk of sudden cardiac death due to ventricular fibrillation (VF). Multiple BrS-associated mutations have now been identified in SCN1B (p.E87Q, β1B p.H162P, p.W179X, p.R214Q) (91; 103; 104), SCN2B (p.D211G) (64), and SCN3B (p.L10P, V110I) (105; 106). These mutations are linked to reductions in TTX-R INa density and may include hyperpolarized inactivation kinetics or alterations in rate of recovery from inactivation, exacerbating the severity of the Nav1.5 loss-of-function phenotype. This is similar to SCN5A-linked BrS mutations, which result in Nav1.5 loss-of-function (107). One identified SCN1B BrS mutation also leads to increased Ito by modulation of Kv4.3 channels, providing the first human in vivo evidence for a functional link between VGSC β1/β1 subunits and VGKCs (91). Atrial fibrillation (AF) also occurs in patients with β subunit gene mutations, including SCN1B (p.R85H, p.D153N, β1B p.R214Q) (65; 108), SCN2B (p.R28W, p.R28Q) (65), SCN3B (p.R6K, p.L10P, p.M161T, p.A130V) (105; 108; 109), and SCN4B (p.V162G, p.I166L) (110). One case of idiopathic ventricular fibrillation has also been identified with a mutation in SCN3B (p.V54G) (83). In general, these mutations lead to decreased Nav1.5-generated INa and altered channel kinetics. In several of the SCN3B mutations, this loss of function is postulated to result from the failure of Nav1.5 cell surface trafficking (82; 83; 105; 106). Interestingly, one SCN1B AF-linked mutation has also been described in an epilepsy patient (60; 65), further supporting a phenotypic link between brain and heart disease resulting from VGSC β subunit gene mutations.
Long QT syndrome (LQTS) is characterized by delayed cardiac AP repolarization that can be measured by prolongation of the QT interval on the ECG, leading to increased risk of ventricular fibrillation that can result in sudden cardiac death (111). At least 15 forms of LQTS have been identified, each with specific associated genes, variations in penetrance and allele dominance, and comorbidities. Genes with identified LQTS mutations frequently encode ion channel subunits, including several VGKCs as well as Nav1.5. Two gain-of-function mutations in VGSC β subunit genes have also been identified. The SCN4B missense mutation p.L179F has been linked to LQT10, in which a positive shift in INa inactivation leads to increased window INa and increased late INa, consistent with aberrant AP repolarization (84; 112), and similar to the phenotype of the SCN4B p.S206L mutation linked to SIDS (82). A recent report also identified a LQTS-associated mutation in β1B, p.P213T (113). Interestingly, although β1B and β4 do not share similar binding mechanisms to VGSC α subunits or have a high degree of sequence homology, the SCN1B mutation leads to a similar phenotype to that of SCN4B, resulting in altered window INa, increased channel availability, and increased late INa (84; 113). Thus, these effects appear to converge despite the structural differences between β1B and β4, perhaps suggesting functional interactions between these two subunits.
The importance of VGSC β subunits in heart has also been demonstrated in null mouse models. Scn1b null hearts exhibit increased APD and prolonged QT intervals, consistent with LQTS, that persist after pharmacological autonomic blockade (75). Scn1b null ventricular myocytes also display increased transient and persistent peak INa, with increases in both Nav1.5 expression and 3H-saxitoxin binding, suggesting increased TTX-R and TTX-S VGSC expression (75). Scn3b null mice display multiple cardiac abnormalities in both atria and ventricles, including increased susceptibility to arrhythmia and conduction abnormalities that may constitute a BrS model. Loss of β3 in these animals leads to reduced peak INa, bradycardia, AV block, and alterations in SA node recovery (114; 115). In contrast to Scn1b null animals, however, Scn3b null nice do not display an overt neurological phenotype, suggesting that the primary functional role of Scn3b in vivo may be cardiac.
4. Pain
VGSC α and β subunits are key components of nociceptive and neuropathic pain pathways. Nociceptive pain occurs in response to noxious stimuli in the absence of inflammation or nerve injury, and is mediated by sensory neurons extending from the dorsal root ganglia (DRG), including both small nonmyelinated C fibres and thinly myelinated Aδ fibres (116). DRG neurons express all five VGSC β subunit genes, with mRNA for Scn1b being the most abundant (117–119). There is heterogeneity of β expression between the three classes of DRG neurons: small C fibers contain high levels of SCN3B mRNA and low levels of SCN1B, SCN2B, and SCN4B, while medium and large A fibers express high levels of SCN1B and SCN4B but low levels of SCN2B and SCN3B (118) (Table 1). DRG neurons also express both TTX-S (Nav1.1, Nav1.2, Nav1.6, and Nav1.7) and TTX-R (Nav1.8 and Nav1.9) VGSCs, with Nav1.7, Nav1.8, and Nav1.9 as the highest among these (118).
Whereas nociceptive pain permits the appropriate detection of noxious stimuli, neuropathic pain represents a dysregulation of normal sensory and pain pathways, resulting in allodynia. This may be associated with damage or disease, and lead to changes in the structure or molecular composition of pain pathway components, including those in the sensory neurons and spinal cord, to produce persistent pain sensation (116). There are a variety of animal models available for the study of pain, including acute and chronic pain, inflammatory pain and neuropathic pain. Alterations in VGSC subunit expression occur in multiple models, demonstrating the importance of these proteins in the normal regulation of excitability in sensory neurons and tissues receiving input from peripheral nerve.
SCN1B loss-of-function is implicated in pain. Scn1b null DRG neurons are hyperexcitable, consistent with observations in central neurons, exhibiting a complex phenotype that includes decreased persistent INa, a hyperpolarizing shift in inactivation and slowed recovery from inactivation, and decreased surface expression of Nav1.9 (120). Taken together, these observations suggest that global loss of Scn1b leads to allodynia. In contrast, in a model of chronic constrictive nerve injury, Scn1b mRNA was increased in the laminae of the spinal cord dorsal horn (121). Thus, the phenotypic outputs of genetic deletion of Scn1b vs. nerve injury in the presence of Scn1b expression appear to be different, perhaps implicating roles for other genes that are up- or down-regulated in the different models.
Evidence for the role of SCN2B in pain has provided contrasting results, offering the intriguing possibility for differential VGSC regulation by β2 between specific pain pathways and cell types. Scn2b null mice demonstrate reduced sensitivity to inflammatory and neuropathic pain paradigms (117; 122). β2 protein is rapidly increased without concurrent increases in mRNA in both injured and, to a lesser extent, adjacent non-injured neurons in both a spared nerve injury and a spinal nerve ligation model in rat (122). However, Scn2b mRNA expression is decreased in cervical sensory ganglia following avulsion injury (123), and decreased in spinal cord dorsal horn in chronic constrictive nerve injury in a rat model (121). Lastly, Scn2b deletion reduces TTX-S INa, particularly Nav1.7, in small DRG neurons as well as modulates kinetics of TTX-S INa activation and inactivation, without significant effects on TTX-R channels (117).
Upregulation of Scn3b mRNA has been observed in multiple pain models. Changes in expression are fiber-type specific and model- dependent. In a chronic constrictive injury model in rat, Scn3b mRNA was increased in small C-fibers, in which Scn3b is normally highly expressed (26). In contrast, in the streptozotocin model of diabetic neuropathy in rat, Scn3b mRNA was increased instead in normally low-expressing medium Aδ fibers and lumbar spinal cord, but not in C fibers (124). Scn3b mRNA is also increased in the spared nerve injury model of neuropathic pain in rat as well as in small and medium fibers in the sciatic nerve transection model of axotomy (125).
Little data exist to support a role for SCN4B in pain. However, a study of the inherited pain syndrome Paroxysmal Extreme Pain Disorder (PEPD), in which SCN9A mutations lead to increased excitability, showed a synergistic effect between disease-associated mutations and β4 in heterologous systems (126). The C-terminal domain of β4 contributes to the generation of resurgent INa in both cerebellar Purkinje neurons and DRG neurons following peptide cleavage and subsequent generation of a short peptide to induce channel blockade (127). PEPD-linked SCN9A mutations result in significant increases in Nav1.7-generated resurgent INa in the presence of the β4 pore-blocking peptide, implicating β4 as a modulator of neuronal hyperexcitability in this pain syndrome (126; 128).
Overall, the involvement of β subunits in pain pathways position them as potential candidates for therapeutic intervention that may offer more subtle regulation than drugs targeting VGSC α subunits.
5. Demyelinating and Neurodegenerative Disorders
Neurodegenerative disorders involve progressive neuronal loss or damage, and may be associated with other phenomena including pathological protein deposition and lesion development in distinct brain regions, potentially leading to atrophy and functional deficits (129). Loss of myelin or deficiencies in remyelination can confound attempts to restore normal conduction even if the neuron is preserved, leading to aberrant electrical signalling (130). Several lines of evidence implicate VGSC β subunits in demyelinating and neurodegenerative disorders. Scn1b null mice display defects in central myelination, with decreased numbers of optic nerve nodes of Ranvier, increased axonal degeneration, spinal cord dysmyelination, and disruption of nodal ultrastructure in both CNS and peripheral nervous system (PNS). At nodes of Ranvier, loss of β1-mediated adhesion appears to be sufficient to disrupt proper formation of paranodal junctions; eversion of the last paranodal loop adjacent to the nodal gap is observed, resulting from loss of the tight septate-like junctions formed by adhesive interactions involving β1, contactin-1, Caspr, and Nf-155 that maintain proper axo-glial contact at the paranodes (28). In complementary evidence for the importance of these nodal protein-protein interactions, Cntn1 null mice display decreased β1 protein expression (37). Taken together, these data suggest that maintenance and/or establishment of myelination are mediated in part by β1 cell adhesive interactions. Axonal electrical activity is also a key factor in normal myelination (131); it is not yet known, however, if the channel modulation function of β1 contributes to these mechanisms.
In contrast to Scn1b, Scn2b null mice appear to have normal myelination (27; 29). However, Scn2b deletion is neuroprotective in a mouse model of Multiple Sclerosis (MS), with decreased axonal degeneration, fewer demyelinated and dysmyelinated axons, increased survival, and reduced symptom severity (29). In MS and other neurodegenerative disorders, aberrant VGSC α subunit expression is observed, including redistribution of channels from normal cellular subcompartments and altered levels of channel expression. These changes are proposed to contribute to neurodegeneration via abnormal influx of Na+ into damaged axons, particularly via increased Nav1.6 expression, which displays higher levels of persistent INa compared to other neuronal VGSCs. This is then postulated to lead to reverse activation of the sodium-calcium exchanger (NCX) and increased local intracellular calcium concentrations that activate downstream injury cascades, axonal degeneration, and neuronal loss (130). Interestingly, cerebrospinal fluid (CSF) from MS patients exhibits decreased BACE1 activity, suggesting decreased β2 cleavage and consequent alterations in normal VGSC α expression and localization. Low BACE1 activity in MS is linked to worsened disease severity and longer disease duration, and BACE1 expression continues to decrease throughout disease progression (132).
Altered VGSC β subunit expression and protein modification have been observed in studies of human neurodegenerative disease, including amyotrophic lateral sclerosis (ALS), Parkinson’s disease (PD), and Huntington’s disease (HD). ALS is characterized by degeneration of motoneurons in the spinal cord, motor cortex, and brainstem, and ALS neurons are hyperexcitable, potentially implicating VGSCs in disease pathology. At P120 in the Sod1 mouse model of ALS, an age consistent with the adult onset typical of human ALS, the level of Scn8a mRNA is decreased in spinal cord, concurrent with decreased Scn1b mRNA and increased Scn3b mRNA and protein in ventral dorsal horn. These changes provide a potential molecular basis for hyperexcitability (133). In a PD mouse model, Scn4b mRNA and protein were increased, including higher expression of a more heavily glycosylated form of β4 that displayed developmental regulation during disease progression (134). Lastly, SCN4B mRNA and protein are downregulated in the striatum of HD patients and mice before the onset of motor symptoms, a hallmark brain region in HD that typically exhibits progressive neuronal loss. Decreases in β2 protein levels also occur in this mouse model, but significantly later than changes in β4 (135). The importance of β4 in HD pathology may be a consequence of neuritic degeneration secondary to β4 downregulation, as overexpression of β4 in vitro leads to neurite outgrowth and branching, dendrite thickening and increased numbers of dendritic spines (135).
VGSC β subunits are targets for sequential proteolytic cleavage by BACE1 and γ-secretase, offering potential links to the pathophysiology of Alzheimer’s disease (AD) and neurodegeneration in addition to normal development. BACE1 is ubiquitously expressed in brain, and its expression increases with age as well as in AD patient cortex. In AD pathology, BACE1 and γ-secretase cleave APP to release the Aβ peptide, which then accumulates to form aggregates in brain (136). Interestingly, BACE1 cleavage of β2 reverses normal β2 effects on VGSC α subunit modulation, and in BACE1 null mice, decreased cleavage of β2 in brain may contribute to increased neuronal excitability (137). AD patients are at increased risk of seizure (138), which may be partly predicated on VGSC-mediated alterations in brain excitability following cell surface downregulation, and may affect both excitatory and inhibitory neurons (41). Finally, SCN3B mRNA is decreased in AD brains that contain deleterious neurofibrillary tangles (NFT), a key pathological feature of AD alongside Aβ-containing plaques, as compared to both non-NFT patient brain and control brain tissue (139).
6. Cancer
VGSCs are implicated in multiple types of cancer, particularly in the development of metastatic potential, or the ability of the tumour cell to decrease adhesive interactions with neighbouring cells and become motile (140). The identity of the predominant VGSC α subunit expressed varies between cancer types; e.g., Nav1.5 has been implicated in breast and ovarian cancer, whereas the critical channel subtype in prostate cancer is Nav1.7 (141) and Nav1.6 in cervical cancer (142). Increased VGSC α expression correlates with increased metastasis, and pharmacological blockade of INa with TTX abrogates tumour cell invasive capability in vitro (140)
Consistent with its known functions in cell adhesion, SCN1B has been implicated in cancer, particularly breast cancer, and may play an important role in metastasis. In breast cancer, expression of β1 is high in weakly metastatic and low in highly metastatic cell lines in vitro (143). In contrast, in prostate cancer, SCN1B mRNA is increased in highly metastatic compared to weakly metastatic epithelial cell lines (141). In an implanted tumour model in mouse, β1-expressing tumours displayed increased growth rate and size with corresponding decreases in survival rates. Cells from these tumours showed decreased levels of apoptosis, enhanced angiogenesis, and metastasis to both lung and liver (36). In cervical cancer biopsy tissue, SCN1B mRNA expression is decreased (142), whereas in human breast, SCN1B mRNA is increased in cancerous compared to non-cancerous tissue (36). Reminiscent of SCN1B function in neurite outgrowth, alterations in the morphology of β1-expressing breast cancer cells are observed in vitro, including process extension that requires expression of the β1 Ig domain as well as activation of fyn kinase (36). Thus, similar to brain, in which the functional roles of SCN1B appear to vary with neuronal cell type (140), the roles of SCN1B in cancer may be tissue specific.
Of the four β subunit genes, SCN1B expression predominates in many cancers. Other β subunits may also be important, although these observations have not yet been assessed for pathogenicity or clinical relevance. A high-throughput screen of post-metastasis samples from late-stage colorectal cancer patients identified two separate heterozygous missense mutations in SCN3B, p.Q89L and p.A195T (144). SCN3B is also upregulated in human cancer cell lines after DNA damage (145). In prostate cancer cell lines, mRNA for all four β subunit genes is present, although, again, expression of SCN1B predominates. In highly metastatic cell lines in vitro, SCN4B mRNA expression is decreased (141). In cervical cancer biopsy tissue, SCN3B mRNA levels are increased while both SCN2B and SCN4B are decreased. Interestingly, for SCN2B and SCN4B, differing results were observed between primary culture and biopsy samples, indicating the importance of evaluating expression in native contexts (142).
Taken together, these data suggest VGSC β subunit genes may be biomarkers in cancer as well as future therapeutic targets for the treatment of metastatic cancer. The differences in β subunit expression among cancer types, however, point to the importance of understanding the molecular mechanisms of β subunit function in order to tailor therapies appropriately.
7. Autism Spectrum and Mood Disorders
Autism spectrum disorders (ASDs) are comorbidities of DS (54) and some cases of GEFS+ (146). Thus, a promising avenue of investigation is ion channel expression in ASDs and other neuropsychiatric disease including mood disorders and psychiatric disorders, as many of these diseases share common genetic foundations. ASDs are phenotypically diverse, but also have ~90% heritability, indicating the significance of genetic components (147). There is a high degree of overlap between ASD and epilepsy: an estimated 15–35% of pediatric epilepsy patients are autistic, and conversely, 7–46% of autistic patients display some form of epilepsy (148). VGSCs, VGKCs, and voltage-gated calcium channels (VGCCs) have all been associated with ASD and mood disorders, as well as other genes important in neural plasticity and synaptic function (147). Scn1a+/− mice, which exhibit impaired GABAergic transmission and behavioral defects similar to those seen in ASD, including stereotypy and impaired social interaction, had ameliorations in these behaviors after treatment with the GABAA-R modulator clonazepam (149). No cases of VGSC β subunit mutations have yet been reported with direct association to ASD; however, given both the importance of β subunits on VGSC function as well as the emergence of SCN1B mutations in DS, it is likely that such a linkage may be found in future patient cohorts.
β1-interacting proteins are also associated with neuropsychiatric disorders. Multiple mutations have been found in ANK3, encoding ankyrin-G, which interacts with the C-termini of β1 and β2 in multiple cellular subcompartments including the AIS and nodes of Ranvier in neurons (150). A gain-of-function mutation has been identified in KCND2, encoding the β1-interacting VGKC Kv4.2, in autistic twins who also exhibited seizures (148). Lastly, genetic variation including copy number variation and deletion has been reported in three members of the contactin gene family of CAMs, Cntn4, Cntn5, and Cntn6 (151). ASD-linked mutations have not yet been reported in Cntn1, a known heterophilic binding partner of β1; however, Cntn5 null mice show decreased β1 protein expression in brain and, conversely, Scn1b null mice show increased Cntn5 protein expression, suggesting reciprocal modulation (H. O’Malley, unpublished observations).
IV. Concluding Remarks
To conclude, VGSC β subunits are more than auxiliary. At the molecular level, these multifunctional proteins play roles in VGSC and VGKC modulation, trans and cis adhesion, and modulation of gene expression. At the tissue level, they are critical in neuronal development, including neuronal proliferation, migration, and pathfinding, neurite extension, axonal fasciculation, and myelination, and thus the regulation of excitability. In heart, β subunits are proposed to play similar roles in cell-cell communication at the intercalated disc, where multi-protein complexes of VGSCs and VGKCs co-exist, as well as modulation of channel complexes linked to calcium handling at the t-tubules. Mutations in the genes encoding β subunits are linked to a myriad of channelopathies, including epilepsy, sudden death syndromes like SUDEP and SIDS, and cardiac arrhythmia. Furthermore, changes in β subunit expression may modulate pain, demyelinating and neurodegenerative disorders, cancer, and autism spectrum and mood disorders. Clearly, the VGSC β subunits are an untapped reservoir of novel therapeutic potential.
Summary Points.
Sodium channel β subunits are multifunctional molecules that signal through multiple signaling pathways on multiple time scales, with and without the pore-forming α subunits.
Sodium channel β subunits are Ig superfamily cell adhesion molecules.
Sodium channel β subunits play roles in sodium channel modulation, sodium channel trafficking and localization, and cellular migration, proliferation, and process outgrowth.
Sodium channel β subunits associate with and modulate potassium channels.
Sodium channel β subunits are substrates for sequential cleavage by β- and γ-secretases and, through this mechanism, modulate gene expression.
Mutations in the genes encoding β subunits are linked to a myriad of channelopathies, including epilepsy, sudden death syndromes like SUDEP and SIDS, and cardiac arrhythmia. Furthermore, changes in β subunit expression may modulate pain, demyelinating and neurodegenerative disorders, cancer, and autism spectrum and mood disorders.
VGSC β subunits are an untapped reservoir of novel therapeutic potential.
Future Issues.
Are all VGSCs heterotrimers? Which α and β subunits associate in specific tissues in vivo?
Does the α/β subunit composition of VGSCs change in pathology?
How is the splicing of SCN1B regulated?
How is the sequential cleavage of β subunits by BACE and γ-secretase regulated?
Are β subunits expressed in the absence of α subunits in vivo and if so, in which cell types?
What is the most effective way to target β subunits for the development of novel therapeutics?
Acknowledgments
The authors would like to acknowledge Dr. James Offord for critical reading of the manuscript.
Acronyms and Definitions
- AD
Alzheimer’s disease
- AIS
axon initial segment
- ALS
amyotrophic lateral sclerosis
- ASD
autism spectrum disease
- BACE
beta-site APP cleaving enzyme
- BrS
Brugada Syndrome
- DRG
dorsal root ganglion
- DS
Dravet Syndrome
- GEFS+
Generalized Epilepsy with Febrile Seizures Plus
- HD
Huntington’s disease
- IgSF
Immunoglobulin superfamily
- MS
Multiple Sclerosis
- NCX
sodium-calcium exchanger
- PD
Parkinson’s disease
- PEPD
Paroxysmal Extreme Pain Disorder
- SIDS
Sudden Infant Death Syndrome
- SUDEP
Sudden Unexpected Death in Epilepsy
- SUDS
Sudden Unexpected Death Syndrome
- SUNDS
Sudden Unexpected Nocturnal Death Syndrome
- TTX
tetrodotoxin
Literature Cited
- 1.Catterall WA. Voltage-Gated Sodium Channels at 60:Structure, Function, and Pathophysiology. J Physiol. 2012 doi: 10.1113/jphysiol.2011.224204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Messner DJ, Catterall WA. The sodium channel from rat brain. Separation and characterization of subunits. J Biol Chem. 1985;260:10597–604. Demonstration that sodium channel α and β subunits are separate proteins. [PubMed] [Google Scholar]
- 3.Brackenbury WJ, Isom LL. Na Channel beta Subunits: Overachievers of the Ion Channel Family. Front Pharmacol. 2011;2:53. doi: 10.3389/fphar.2011.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calhoun JD, Isom LL. The Role of Non-pore-Forming beta Subunits in Physiology and Pathophysiology of Voltage-Gated Sodium Channels. Handbook of experimental pharmacology. 2014;221:51–89. doi: 10.1007/978-3-642-41588-3_4. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen HM, Miyazaki H, Hoshi N, Smith BJ, Nukina N, et al. Modulation of voltage-gated K+ channels by the sodium channel beta1 subunit. Proc Natl Acad Sci U S A. 2012;109:18577–82. doi: 10.1073/pnas.1209142109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marionneau C, Carrasquillo Y, Norris AJ, Townsend RR, Isom LL, et al. The sodium channel accessory subunit Navbeta1 regulates neuronal excitability through modulation of repolarizing voltage-gated K(+) channels. J Neurosci. 2012;32:5716–27. doi: 10.1523/JNEUROSCI.6450-11.2012. Sodium channel β subunits also modulate potassium channels in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deschenes I, Armoundas AA, Jones SP, Tomaselli GF. Post-transcriptional gene silencing of KChIP2 and Navbeta1 in neonatal rat cardiac myocytes reveals a functional association between Na and Ito currents. J Mol Cell Cardiol. 2008;45:336–46. doi: 10.1016/j.yjmcc.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong HK, Sakurai T, Oyama F, Kaneko K, Wada K, et al. beta subunits of voltage-gated sodium channels are novel substrates of BACE1 and gamma -secretase. J Biol Chem. 2005 doi: 10.1074/jbc.M414648200. [DOI] [PubMed] [Google Scholar]
- 9.Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev. 2005;57:397–409. doi: 10.1124/pr.57.4.4. [DOI] [PubMed] [Google Scholar]
- 10.Isom LL, De Jongh KS, Patton DE, Reber BFX, Offord J, et al. Primary structure and functional expression of the β1 subunit of the rat brain sodium channel. Science. 1992;256:839–42. doi: 10.1126/science.1375395. This paper was the first description of the cloning and expression of a sodium channel β subunit cDNA. [DOI] [PubMed] [Google Scholar]
- 11.Kazen-Gillespie KA, Ragsdale DS, D’Andrea MR, Mattei LN, Rogers KE, Isom LL. Cloning, localization, and functional expression of sodium channel β1A subunits. J Biol Chem. 2000;275:1079–88. doi: 10.1074/jbc.275.2.1079. This manuscript describes the cloning of β1A (later called β1B), a secreted splice variant of Scn1b generated by in-frame retention of intron 3. [DOI] [PubMed] [Google Scholar]
- 12.Patino GA, Brackenbury WJ, Bao Y, Lopez-Santiago LF, O’Malley HA, et al. Voltage-gated Na+ channel beta1B: a secreted cell adhesion molecule involved in human epilepsy. J Neurosci. 2011;31:14577–91. doi: 10.1523/JNEUROSCI.0361-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qin N, D’Andrea MR, Lubin ML, Shafaee N, Codd EE, Correa AM. Molecular cloning and functional expression of the human sodium channel beta1B subunit, a novel splicing variant of the beta1 subunit. Eur J Biochem. 2003;270:4762–70. doi: 10.1046/j.1432-1033.2003.03878.x. [DOI] [PubMed] [Google Scholar]
- 14.Isom LL, Ragsdale DS, De Jongh KS, Westenbroek RE, Reber BF, et al. Structure and function of the β2 subunit of brain sodium channels, a transmembrane glycoprotein with a CAM motif. Cell. 1995;83:433–42. doi: 10.1016/0092-8674(95)90121-3. [DOI] [PubMed] [Google Scholar]
- 15.Morgan K, Stevens EB, Shah B, Cox PJ, Dixon AK, et al. β3: An additional auxiliary subunit of the voltage-sensitive sodium channel that modulates channel gating with distinct kinetics. Proc Natl Acad Sci USA. 2000;97:2308–13. doi: 10.1073/pnas.030362197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu FH, Westenbroek RE, Silos-Santiago I, McCormick KA, Lawson D, et al. Sodium channel beta4, a new disulfide-linked auxiliary subunit with similarity to beta2. J Neurosci. 2003;23:7577–85. doi: 10.1523/JNEUROSCI.23-20-07577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isom LL, Catterall WA. Na+ channel subunits and Ig domains. Nature. 1996;383:307–8. doi: 10.1038/383307b0. Sodium channel β subunits are shown to be homologous to the Ig superfamily of cell adhesion molecules. [DOI] [PubMed] [Google Scholar]
- 18.McCormick KA, Isom LL, Ragsdale D, Smith D, Scheuer T, Catterall WA. Molecular determinants of Na+ channel function in the extracellular domain of the β 1 subunit. J Biol Chem. 1998;273:3954–62. doi: 10.1074/jbc.273.7.3954. [DOI] [PubMed] [Google Scholar]
- 19.Spampanato J, Kearney JA, de Haan G, McEwen DP, Escayg A, et al. A novel epilepsy mutation in the sodium channel SCN1A identifies a cytoplasmic domain for beta subunit interaction. J Neurosci. 2004;24:10022–34. doi: 10.1523/JNEUROSCI.2034-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meadows L, Malhotra JD, Stetzer A, Isom LL, Ragsdale DS. The intracellular segment of the sodium channel β1 subunit is required for its efficient association with the channel α subunit. J Neurochem. 2001;76:1871–8. doi: 10.1046/j.1471-4159.2001.00192.x. [DOI] [PubMed] [Google Scholar]
- 21.Buffington SA, Rasband MN. Na+ channel-dependent recruitment of Navbeta4 to axon initial segments and nodes of Ranvier. J Neurosci. 2013;33:6191–202. doi: 10.1523/JNEUROSCI.4051-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen C, Calhoun JD, Zhang Y, Lopez-Santiago L, Zhou N, et al. Identification of the cysteine residue responsible for disulfide linkage of Na+ channel alpha and beta2 subunits. J Biol Chem. 2012 doi: 10.1074/jbc.M112.397646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilchrist J, Das S, Van Petegem F, Bosmans F. Crystallographic insights into sodium-channel modulation by the beta4 subunit. Proc Natl Acad Sci U S A. 2013;110:E5016–24. doi: 10.1073/pnas.1314557110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Namadurai S, Balasuriya D, Rajappa R, Wiemhofer M, Stott K, et al. Crystal Structure and Molecular Imaging of the Nav Channel beta3 Subunit Indicates a Trimeric Assembly. J Biol Chem. 2014;289:10797–811. doi: 10.1074/jbc.M113.527994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng G, Deak P, Chopra M, Hall LM. Cloning and functional analysis of TipE, a novel membrane protein that enhances Drosophila para sodium channel function. Cell. 1995;82:1001–11. doi: 10.1016/0092-8674(95)90279-1. [DOI] [PubMed] [Google Scholar]
- 26.Shah BS, Stevens EB, Pinnock RD, Dixon AK, Lee K. Developmental expression of the novel voltage-gated sodium channel auxiliary subunit beta3, in rat CNS. J Physiol. 2001;534:763–76. doi: 10.1111/j.1469-7793.2001.t01-1-00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen C, Bharucha V, Chen Y, Westenbroek RE, Brown A, et al. Reduced sodium channel density, altered voltage dependence of inactivation, and increased susceptibility to seizures in mice lacking sodium channel beta 2-subunits. Proc Natl Acad Sci U S A. 2002;99:17072–7. doi: 10.1073/pnas.212638099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen C, Westenbroek RE, Xu X, Edwards CA, Sorenson DR, et al. Mice lacking sodium channel beta1 subunits display defects in neuronal excitability, sodium channel expression, and nodal architecture. J Neurosci. 2004;24:4030–42. doi: 10.1523/JNEUROSCI.4139-03.2004. This paper describes the first sodium channel β subunit transgenic mouse. The Scn1b null line was later shown to be a model of Dravet Syndrome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Malley HA, Shreiner AB, Chen GH, Huffnagle GB, Isom LL. Loss of Na+ channel beta2 subunits is neuroprotective in a mouse model of multiple sclerosis. Mol Cell Neurosci. 2009;40:143–55. doi: 10.1016/j.mcn.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brackenbury WJ, Calhoun JD, Chen C, Miyazaki H, Nukina N, et al. Functional reciprocity between Na+ channel Nav1.6 and beta1 subunits in the coordinated regulation of excitability and neurite outgrowth. Proc Natl Acad Sci U S A. 2010;107:2283–8. doi: 10.1073/pnas.0909434107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wimmer VC, Reid CA, Mitchell S, Richards KL, Scaf BB, et al. Axon initial segment dysfunction in a mouse model of genetic epilepsy with febrile seizures plus. J Clin Invest. 2010;120:2661–71. doi: 10.1172/JCI42219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malhotra JD, Koopmann MC, Kazen-Gillespie KA, Fettman N, Hortsch M, Isom LL. Structural requirements for interaction of sodium channel β1 subunits with ankyrin. J Biol Chem. 2002;277:26681–8. doi: 10.1074/jbc.M202354200. [DOI] [PubMed] [Google Scholar]
- 33.Kazarinova-Noyes K, Malhotra JD, McEwen DP, Mattei LN, Berglund EO, et al. Contactin associates with Na+ channels and increases their functional expression. J Neurosci. 2001;21:7517–25. doi: 10.1523/JNEUROSCI.21-19-07517.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McEwen DP, Meadows LS, Chen C, Thyagarajan V, Isom LL. Sodium channel β1 subunit-mediated modulation of Nav1.2 currents and cell surface density is dependent on interactions with contactin and ankyrin. J Biol Chem. 2004;279:16044–9. doi: 10.1074/jbc.M400856200. [DOI] [PubMed] [Google Scholar]
- 35.Malhotra JD, Thyagarajan V, Chen C, Isom LL. Tyrosine-phosphorylated and nonphosphorylated sodium channel beta1 subunits are differentially localized in cardiac myocytes. J Biol Chem. 2004;279:40748–54. doi: 10.1074/jbc.M407243200. [DOI] [PubMed] [Google Scholar]
- 36.Nelson M, Millican-Slater R, Forrest LC, Brackenbury WJ. The sodium channel beta1 subunit mediates outgrowth of neurite-like processes on breast cancer cells and promotes tumour growth and metastasis. Int J Cancer. 2014 doi: 10.1002/ijc.28890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brackenbury WJ, Davis TH, Chen C, Slat EA, Detrow MJ, et al. Voltage-gated Na+ channel beta1 subunit-mediated neurite outgrowth requires Fyn kinase and contributes to postnatal CNS development in vivo. J Neurosci. 2008;28:3246–56. doi: 10.1523/JNEUROSCI.5446-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson D, Montpetit ML, Stocker PJ, Bennett ES. The sialic acid component of the beta1 subunit modulates voltage-gated sodium channel function. J Biol Chem. 2004;279:44303–10. doi: 10.1074/jbc.M408900200. [DOI] [PubMed] [Google Scholar]
- 39.Davis TH, Chen C, Isom LL. Sodium Channel β1 Subunits Promote Neurite Outgrowth In Cerebellar Granule Neurons. J Biol Chem. 2004;279:51424–32. doi: 10.1074/jbc.M410830200. This was the first demonstration that β subunits are functional cell adhesion molecules in neurons and that trans homophilic adhesion results in neurite outgrowth. [DOI] [PubMed] [Google Scholar]
- 40.McEwen DP, Isom LL. Heterophilic interactions of sodium channel beta 1 subunits with axonal and glial cell adhesion molecules. J Biol Chem. 2004;279:52744–52. doi: 10.1074/jbc.M405990200. [DOI] [PubMed] [Google Scholar]
- 41.Kim DY, Carey BW, Wang H, Ingano LA, Binshtok AM, et al. BACE1 regulates voltage-gated sodium channels and neuronal activity. Nat Cell Biol. 2007;9:755–64. doi: 10.1038/ncb1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmidt JW, Catterall WA. Biosynthesis and processing of the alpha subunit of the voltage-sensitive sodium channel in rat brain neurons. Cell. 1986;46:437–45. doi: 10.1016/0092-8674(86)90664-1. [DOI] [PubMed] [Google Scholar]
- 43.Ratcliffe CF, Westenbroek RE, Curtis R, Catterall WA. Sodium channel beta1 and beta3 subunits associate with neurofascin through their extracellular immunoglobulin-like domain. J Cell Biol. 2001;154:427–34. doi: 10.1083/jcb.200102086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malhotra JD, Kazen-Gillespie K, Hortsch M, Isom LL. Sodium channel β subunits mediate homophilic cell adhesion and recruit ankyrin to points of cell-cell contact. J Biol Chem. 2000;275:11383–8. doi: 10.1074/jbc.275.15.11383. [DOI] [PubMed] [Google Scholar]
- 45.Xiao Z-C, Ragsdale DS, Malhorta JD, Mattei LN, Braun PE, et al. Tenascin-R is a functional modulator of sodium channel β subunits. J Biol Chem. 1999;274:26511–7. doi: 10.1074/jbc.274.37.26511. [DOI] [PubMed] [Google Scholar]
- 46.Srinivasan J, Schachner M, Catterall WA. Interaction of voltage-gated sodium channels with the extracellular matrix molecules tenascin-C and tenascin-R. Proc Natl Acad Sci U S A. 1998;95:15753–7. doi: 10.1073/pnas.95.26.15753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McEwen DP, Chen C, Meadows LS, Lopez-Santiago L, Isom LL. The voltage-gated Na+ channel beta3 subunit does not mediate trans homophilic cell adhesion or associate with the cell adhesion molecule contactin. Neurosci Lett. 2009;462:272–5. doi: 10.1016/j.neulet.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yereddi NR, Cusdin FS, Namadurai S, Packman LC, Monie TP, et al. The immunoglobulin domain of the sodium channel beta3 subunit contains a surface-localized disulfide bond that is required for homophilic binding. FASEB J. 2013;27:568–80. doi: 10.1096/fj.12-209445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brackenbury WJ, Yuan Y, O’Malley HA, Parent JM, Isom LL. Abnormal neuronal patterning occurs during early postnatal brain development of Scn1b-null mice and precedes hyperexcitability. Proc Natl Acad Sci U S A. 2013;110:1089–94. doi: 10.1073/pnas.1208767110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, et al. ILAE Official Report: A practical clinical definition of epilepsy. Epilepsia. 2014;55:475–82. doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- 51.Wallace RH, Wang DW, Singh R, Scheffer IE, George AL, Jr, et al. Febrile seizures and generalized epilepsy associated with a mutation in the Na+-channel beta1 subunit gene SCN1B. Nature Genetics. 1998;19:366–70. doi: 10.1038/1252. This was the first demonstration that mutations in sodium channel β subunit genes are linked to human disease. [DOI] [PubMed] [Google Scholar]
- 52.Scheffer IE, Zhang YH, Gecz J, Dibbens L. Genetics of the epilepsies: genetic twists in the channels and other tales. Epilepsia. 2010;51(Suppl 1):33–6. doi: 10.1111/j.1528-1167.2009.02440.x. [DOI] [PubMed] [Google Scholar]
- 53.Dravet C. Dravet syndrome history. Dev Med Child Neurol. 2011;53(Suppl 2):1–6. doi: 10.1111/j.1469-8749.2011.03964.x. [DOI] [PubMed] [Google Scholar]
- 54.Skluzacek JV, Watts KP, Parsy O, Wical B, Camfield P. Dravet syndrome and parent associations: the IDEA League experience with comorbid conditions, mortality, management, adaptation, and grief. Epilepsia. 2011;52(Suppl 2):95–101. doi: 10.1111/j.1528-1167.2011.03012.x. [DOI] [PubMed] [Google Scholar]
- 55.Takayama R, Fujiwara T, Shigematsu H, Imai K, Takahashi Y, et al. Long-term course of Dravet syndrome: A study from an epilepsy center in Japan. Epilepsia. 2014;55:528–38. doi: 10.1111/epi.12532. [DOI] [PubMed] [Google Scholar]
- 56.Patino GA, Claes LR, Lopez-Santiago LF, Slat EA, Dondeti RS, et al. A functional null mutation of SCN1B in a patient with Dravet syndrome. J Neurosci. 2009;29:10764–78. doi: 10.1523/JNEUROSCI.2475-09.2009. This was the first report of a human β subunit gene mutation linked to Dravet Syndrome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ogiwara I, Nakayama T, Yamagata T, Ohtani H, Mazaki E, et al. A homozygous mutation of voltage-gated sodium channel beta(I) gene SCN1B in a patient with Dravet syndrome. Epilepsia. 2012;53:e200–3. doi: 10.1111/epi.12040. [DOI] [PubMed] [Google Scholar]
- 58.Fendri-Kriaa N, Kammoun F, Salem IH, Kifagi C, Mkaouar-Rebai E, et al. New mutation c.374C>T and a putative disease-associated haplotype within SCN1B gene in Tunisian families with febrile seizures. Eur J Neurol. 2011;18:695–702. doi: 10.1111/j.1468-1331.2010.03216.x. [DOI] [PubMed] [Google Scholar]
- 59.Audenaert D, Claes L, Ceulemans B, Lofgren A, Van Broeckhoven C, De Jonghe P. A deletion in SCN1B is associated with febrile seizures and early-onset absence epilepsy. Neurology. 2003;61:854–6. doi: 10.1212/01.wnl.0000080362.55784.1c. [DOI] [PubMed] [Google Scholar]
- 60.Scheffer IE, Harkin LA, Grinton BE, Dibbens LM, Turner SJ, et al. Temporal lobe epilepsy and GEFS+ phenotypes associated with SCN1B mutations. Brain. 2007;130:100–9. doi: 10.1093/brain/awl272. [DOI] [PubMed] [Google Scholar]
- 61.Reid CA, Leaw B, Richards KL, Richardson R, Wimmer V, et al. Reduced dendritic arborization and hyperexcitability of pyramidal neurons in a Scn1b-based model of Dravet syndrome. Brain. 2014 doi: 10.1093/brain/awu077. [DOI] [PubMed] [Google Scholar]
- 62.Egri C, Vilin YY, Ruben PC. A thermoprotective role of the sodium channel beta1 subunit is lost with the beta1 (C121W) mutation. Epilepsia. 2012;53:494–505. doi: 10.1111/j.1528-1167.2011.03389.x. [DOI] [PubMed] [Google Scholar]
- 63.Meadows LS, Malhotra J, Loukas A, Thyagarajan V, Kazen-Gillespie KA, et al. Functional and biochemical analysis of a sodium channel β1 subunit mutation responsible for Generalized Epilepsy with Febrile Seizures Plus Type 1. J Neurosci. 2002;22:10699–709. doi: 10.1523/JNEUROSCI.22-24-10699.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Riuro H, Beltran-Alvarez P, Tarradas A, Selga E, Campuzano O, et al. A Missense Mutation in the Sodium Channel beta2 Subunit Reveals SCN2B as a New Candidate Gene for Brugada Syndrome. Hum Mutat. 2013 doi: 10.1002/humu.22328. [DOI] [PubMed] [Google Scholar]
- 65.Watanabe H, Darbar D, Kaiser DW, Jiramongkolchai K, Chopra S, et al. Mutations in Sodium Channel β1- and β2-Subunits Associated With Atrial Fibrillation. Circulation: Arrhythmia and Electrophysiology. 2009;2:268–75. doi: 10.1161/CIRCEP.108.779181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nashef L, So EL, Ryvlin P, Tomson T. Unifying the definitions of sudden unexpected death in epilepsy. Epilepsia. 2012;53:227–33. doi: 10.1111/j.1528-1167.2011.03358.x. [DOI] [PubMed] [Google Scholar]
- 67.Ficker DM, So EL, Shen WK, Annegers JF, O’Brien PC, et al. Population-based study of the incidence of sudden unexplained death in epilepsy. Neurology. 1998;51:1270–4. doi: 10.1212/wnl.51.5.1270. [DOI] [PubMed] [Google Scholar]
- 68.Hesdorffer DC, Tomson T, Benn E, Sander JW, Nilsson L, et al. Do antiepileptic drugs or generalized tonic-clonic seizure frequency increase SUDEP risk? A combined analysis. Epilepsia. 2012;53:249–52. doi: 10.1111/j.1528-1167.2011.03354.x. [DOI] [PubMed] [Google Scholar]
- 69.Surges R, Sander JW. Sudden unexpected death in epilepsy: mechanisms, prevalence, and prevention. Current opinion in neurology. 2012;25:201–7. doi: 10.1097/WCO.0b013e3283506714. [DOI] [PubMed] [Google Scholar]
- 70.Oakley JC, Kalume F, Catterall WA. Insights into pathophysiology and therapy from a mouse model of Dravet syndrome. Epilepsia. 2011;52(Suppl 2):59–61. doi: 10.1111/j.1528-1167.2011.03004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Glasscock E, Yoo JW, Chen TT, Klassen TL, Noebels JL. Kv1.1 potassium channel deficiency reveals brain-driven cardiac dysfunction as a candidate mechanism for sudden unexplained death in epilepsy. J Neurosci. 2010;30:5167–75. doi: 10.1523/JNEUROSCI.5591-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goldman AM, Glasscock E, Yoo J, Chen TT, Klassen TL, Noebels JL. Arrhythmia in heart and brain: KCNQ1 mutations link epilepsy and sudden unexplained death. Sci Transl Med. 2009;1:2ra6. doi: 10.1126/scitranslmed.3000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jansen K, Lagae L. Cardiac changes in epilepsy. Seizure. 2010;19:455–60. doi: 10.1016/j.seizure.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 74.Tolstykh GP, Cavazos JE. Potential mechanisms of sudden unexpected death in epilepsy. Epilepsy Behav. 2013;26:410–4. doi: 10.1016/j.yebeh.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 75.Lopez-Santiago LF, Meadows LS, Ernst SJ, Chen C, Malhotra JD, et al. Sodium channel Scn1b null mice exhibit prolonged QT and RR intervals. J Mol Cell Cardiol. 2007;43:636–47. doi: 10.1016/j.yjmcc.2007.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Auerbach DS, Jones J, Clawson BC, Offord J, Lenk GM, et al. Altered Cardiac Electrophysiology and SUDEP in a Model of Dravet Syndrome. PLoS One. 2013;8:e77843. doi: 10.1371/journal.pone.0077843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kalume F, Westenbroek RE, Cheah CS, Yu FH, Oakley JC, et al. Sudden unexpected death in a mouse model of Dravet syndrome. J Clin Invest. 2013;123:1798–808. doi: 10.1172/JCI66220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krous HF, Beckwith JB, Byard RW, Rognum TO, Bajanowski T, et al. Sudden infant death syndrome and unclassified sudden infant deaths: a definitional and diagnostic approach. Pediatrics. 2004;114:234–8. doi: 10.1542/peds.114.1.234. [DOI] [PubMed] [Google Scholar]
- 79.Van Norstrand DW, Ackerman MJ. Sudden infant death syndrome: do ion channels play a role? Heart Rhythm. 2009;6:272–8. doi: 10.1016/j.hrthm.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Klaver EC, Versluijs GM, Wilders R. Cardiac ion channel mutations in the sudden infant death syndrome. International journal of cardiology. 2011;152:162–70. doi: 10.1016/j.ijcard.2010.12.051. [DOI] [PubMed] [Google Scholar]
- 81.Massey CA, Sowers LP, Dlouhy BJ, Richerson GB. Mechanisms of sudden unexpected death in epilepsy: the pathway to prevention. Nat Rev Neurol. 2014 doi: 10.1038/nrneurol.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tan BH, Pundi KN, Van Norstrand DW, Valdivia CR, Tester DJ, et al. Sudden infant death syndrome-associated mutations in the sodium channel beta subunits. Heart Rhythm. 2010;7:771–8. doi: 10.1016/j.hrthm.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Valdivia CR, Medeiros-Domingo A, Ye B, Shen WK, Algiers TJ, et al. Loss-of-function mutation of the SCN3B-encoded sodium channel {beta}3 subunit associated with a case of idiopathic ventricular fibrillation. Cardiovasc Res. 2010;86:392–400. doi: 10.1093/cvr/cvp417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Medeiros-Domingo A, Kaku T, Tester DJ, Iturralde-Torres P, Itty A, et al. SCN4B-encoded sodium channel beta4 subunit in congenital long-QT syndrome. Circulation. 2007;116:134–42. doi: 10.1161/CIRCULATIONAHA.106.659086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Paine SM, Jacques TS, Sebire NJ. Review: Neuropathological features of unexplained sudden unexpected death in infancy: current evidence and controversies. Neuropathology and applied neurobiology. 2014;40:364–84. doi: 10.1111/nan.12095. [DOI] [PubMed] [Google Scholar]
- 86.Cruz-Sanchez FF, Lucena J, Ascaso C, Tolosa E, Quinto L, Rossi ML. Cerebellar cortex delayed maturation in sudden infant death syndrome. Journal of neuropathology and experimental neurology. 1997;56:340–6. doi: 10.1097/00005072-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 87.Aronica E, Troost D, Rozemuller AJ, Yankaya B, Jansen GH, et al. Expression and regulation of voltage-gated sodium channel beta1 subunit protein in human gliosis-associated pathologies. Acta Neuropathol (Berl) 2003;105:515–23. doi: 10.1007/s00401-003-0677-2. [DOI] [PubMed] [Google Scholar]
- 88.Gorter JA, van Vliet EA, Lopes da Silva FH, Isom LL, Aronica E. Sodium channel beta1-subunit expression is increased in reactive astrocytes in a rat model for mesial temporal lobe epilepsy. Eur J Neurosci. 2002;16:360–4. doi: 10.1046/j.1460-9568.2002.02078.x. [DOI] [PubMed] [Google Scholar]
- 89.Kinney HC, Brody BA, Finkelstein DM, Vawter GF, Mandell F, Gilles FH. Delayed central nervous system myelination in the sudden infant death syndrome. Journal of neuropathology and experimental neurology. 1991;50:29–48. doi: 10.1097/00005072-199101000-00003. [DOI] [PubMed] [Google Scholar]
- 90.Franco E, Dias A, Teresa D, Hebert K. EKG pattern of Brugada syndrome and sudden infant death syndrome--is it time to review the diagnostic criteria? Case report and review of literature. Annals of noninvasive electrocardiology : the official journal of the International Society for Holter and Noninvasive Electrocardiology, Inc. 2014;19:198–202. doi: 10.1111/anec.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hu D, Barajas-Martinez H, Medeiros-Domingo A, Crotti L, Veltmann C, et al. A novel rare variant in SCN1Bb linked to Brugada syndrome and SIDS by combined modulation of Na(v)1.5 and K(v)4.3 channel currents. Heart Rhythm. 2012;9:760–9. doi: 10.1016/j.hrthm.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Deschenes I, Tomaselli GF. Modulation of Kv4.3 current by accessory subunits. FEBS Lett. 2002;528:183–8. doi: 10.1016/s0014-5793(02)03296-9. [DOI] [PubMed] [Google Scholar]
- 93.Vatta M, Dumaine R, Varghese G, Richard TA, Shimizu W, et al. Genetic and biophysical basis of sudden unexplained nocturnal death syndrome (SUNDS), a disease allelic to Brugada syndrome. Hum Mol Genet. 2002;11:337–45. doi: 10.1093/hmg/11.3.337. [DOI] [PubMed] [Google Scholar]
- 94.Liu C, Tester DJ, Hou Y, Wang W, Lv G, et al. Is sudden unexplained nocturnal death syndrome in Southern China a cardiac sodium channel dysfunction disorder? Forensic science international. 2014;236:38–45. doi: 10.1016/j.forsciint.2013.12.033. [DOI] [PubMed] [Google Scholar]
- 95.Abriel H. Cardiac sodium channel Na(v)1.5 and interacting proteins: Physiology and pathophysiology. J Mol Cell Cardiol. 2010;48:2–11. doi: 10.1016/j.yjmcc.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 96.Maier SK, Westenbroek RE, Schenkman KA, Feigl EO, Scheuer T, Catterall WA. An unexpected role for brain-type sodium channels in coupling of cell surface depolarization to contraction in the heart. Proc Natl Acad Sci U S A. 2002;99:4073–8. doi: 10.1073/pnas.261705699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gaborit N, Le Bouter S, Szuts V, Varro A, Escande D, et al. Regional and tissue specific transcript signatures of ion channel genes in the non-diseased human heart. J Physiol. 2007;582:675–93. doi: 10.1113/jphysiol.2006.126714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kaufmann SG, Westenbroek RE, Maass AH, Lange V, Renner A, et al. Distribution and function of sodium channel subtypes in human atrial myocardium. J Mol Cell Cardiol. 2013;61:133–41. doi: 10.1016/j.yjmcc.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kaufmann SG, Westenbroek RE, Zechner C, Maass AH, Bischoff S, et al. Functional protein expression of multiple sodium channel alpha- and beta-subunit isoforms in neonatal cardiomyocytes. J Mol Cell Cardiol. 2010;48:261–9. doi: 10.1016/j.yjmcc.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 100.Maier SK, Westenbroek RE, McCormick KA, Curtis R, Scheuer T, Catterall WA. Distinct subcellular localization of different sodium channel alpha and beta subunits in single ventricular myocytes from mouse heart. Circulation. 2004;109:1421–7. doi: 10.1161/01.CIR.0000121421.61896.24. [DOI] [PubMed] [Google Scholar]
- 101.Malhotra J, Chen C, Rivolta I, Abriel H, Malhotra R, et al. Characterization of sodium channel alpha- and beta-subunits in rat and mouse cardiac myocytes. Circulation. 2001;103:1303–10. doi: 10.1161/01.cir.103.9.1303. [DOI] [PubMed] [Google Scholar]
- 102.Bao Y, Isom LL. NaV1.5 and regulatory β subunits in cardiac sodium channelopathies. Cardiac Electrophysiology Clinics. 2014 in press. [Google Scholar]
- 103.Watanabe H, Koopmann TT, Le Scouarnec S, Yang T, Ingram CR, et al. Sodium channel beta1 subunit mutations associated with Brugada syndrome and cardiac conduction disease in humans. J Clin Invest. 2008;118:2260–8. doi: 10.1172/JCI33891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Holst AG, Saber S, Houshmand M, Zaklyazminskaya EV, Wang Y, et al. Sodium current and potassium transient outward current genes in Brugada syndrome: screening and bioinformatics. Can J Cardiol. 2012;28:196–200. doi: 10.1016/j.cjca.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 105.Hu D, Barajas-Martinez H, Burashnikov E, Springer M, Wu Y, et al. A mutation in the beta 3 subunit of the cardiac sodium channel associated with Brugada ECG phenotype. Circ Cardiovasc Genet. 2009;2:270–8. doi: 10.1161/CIRCGENETICS.108.829192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ishikawa T, Takahashi N, Ohno S, Sakurada H, Nakamura K, et al. Novel SCN3B mutation associated with brugada syndrome affects intracellular trafficking and function of Nav1.5. Circ J. 2013;77:959–67. doi: 10.1253/circj.cj-12-0995. [DOI] [PubMed] [Google Scholar]
- 107.Adsit GS, Vaidyanathan R, Galler CM, Kyle JW, Makielski JC. Channelopathies from mutations in the cardiac sodium channel protein complex. J Mol Cell Cardiol. 2013;61:34–43. doi: 10.1016/j.yjmcc.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Olesen MS, Jespersen T, Nielsen JB, Liang B, Moller DV, et al. Mutations in sodium channel beta-subunit SCN3B are associated with early-onset lone atrial fibrillation. Cardiovasc Res. 2011;89:786–93. doi: 10.1093/cvr/cvq348. [DOI] [PubMed] [Google Scholar]
- 109.Wang P, Yang Q, Wu X, Yang Y, Shi L, et al. Functional dominant-negative mutation of sodium channel subunit gene SCN3B associated with atrial fibrillation in a Chinese GeneID population. Biochem Biophys Res Commun. 2010;398:98–104. doi: 10.1016/j.bbrc.2010.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li RG, Wang Q, Xu YJ, Zhang M, Qu XK, et al. Mutations of the SCN4B-encoded sodium channel beta4 subunit in familial atrial fibrillation. Int J Mol Med. 2013 doi: 10.3892/ijmm.2013.1355. [DOI] [PubMed] [Google Scholar]
- 111.Giudicessi JR, Ackerman MJ. Genotype- and phenotype-guided management of congenital long QT syndrome. Current problems in cardiology. 2013;38:417–55. doi: 10.1016/j.cpcardiol.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang DW, Yazawa K, George AL, Jr, Bennett PB. Characterization of human cardiac Na+ channel mutations in the congenital long QT syndrome. Proc Natl Acad Sci U S A. 1996;93:13200–5. doi: 10.1073/pnas.93.23.13200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Riuro H, Campuzano O, Arbelo E, Iglesias A, Batlle M, et al. A missense mutation in the sodium channel beta1b subunit reveals SCN1B as a susceptibility gene underlying LQT Syndrome. Heart Rhythm. 2014 doi: 10.1016/j.hrthm.2014.03.044. [DOI] [PubMed] [Google Scholar]
- 114.Hakim P, Thresher R, Grace AA, Huang CL. Effects of flecainide and quinidine on action potential and ventricular arrhythmogenic properties in Scn3b knockout mice. Clin Exp Pharmacol Physiol. 2010 doi: 10.1111/j.1440-1681.2010.05369.x. [DOI] [PubMed] [Google Scholar]
- 115.Hakim P, Gurung IS, Pedersen TH, Thresher R, Brice N, et al. Scn3b knockout mice exhibit abnormal ventricular electrophysiological properties. Prog Biophys Mol Biol. 2008;98:251–66. doi: 10.1016/j.pbiomolbio.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lopez-Santiago LF, Pertin M, Morisod X, Chen C, Hong S, et al. Sodium channel beta2 subunits regulate tetrodotoxin-sensitive sodium channels in small dorsal root ganglion neurons and modulate the response to pain. J Neurosci. 2006;26:7984–94. doi: 10.1523/JNEUROSCI.2211-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhao J, O’Leary ME, Chahine M. Regulation of Nav1.6 and Nav1.8 peripheral nerve Na+ channels by auxiliary beta-subunits. J Neurophysiol. 2011;106:608–19. doi: 10.1152/jn.00107.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Oh Y, Sashihara S, Black JA, Waxman SG. Na+ channel beta 1 subunit mRNA: differential expression in rat spinal sensory neurons. Brain Res Mol Brain Res. 1995;30:357–61. doi: 10.1016/0169-328x(95)00052-t. [DOI] [PubMed] [Google Scholar]
- 120.Lopez-Santiago LF, Brackenbury WJ, Chen C, Isom LL. Na+ channel SCN1B regulates dorsal root ganglion nociceptor excitability in vivo. J Biol Chem. 2011 doi: 10.1074/jbc.M111.242370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Blackburn-Munro G, Fleetwood-Walker SM. The sodium channel auxiliary subunits β1 and β2 are differentially expressed in the spinal cord of neuropathic rats. Neurosci. 1999;90:153–64. doi: 10.1016/s0306-4522(98)00415-1. [DOI] [PubMed] [Google Scholar]
- 122.Pertin M, Ji RR, Berta T, Powell AJ, Karchewski L, et al. Upregulation of the voltage-gated sodium channel beta2 subunit in neuropathic pain models: characterization of expression in injured and non-injured primary sensory neurons. J Neurosci. 2005;25:10970–80. doi: 10.1523/JNEUROSCI.3066-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Coward K, Jowett A, Plumpton C, Powell A, Birch R, et al. Sodium channel beta1 and beta2 subunits parallel SNS/PN3 alpha-subunit changes in injured human sensory neurons. Neuroreport. 2001;12:483–8. doi: 10.1097/00001756-200103050-00012. [DOI] [PubMed] [Google Scholar]
- 124.Shah BS, Stevens EB, Gonzalez MI, Bramwell S, Pinnock RD, et al. β3, a novel auxiliary subunit for the voltage-gated sodium channel, is expressed preferentially in sensory neurons and is upregulated in the chronic constriction injury model of neuropathic pain. Eur J Neurosci. 2000;12:3985–90. doi: 10.1046/j.1460-9568.2000.00294.x. [DOI] [PubMed] [Google Scholar]
- 125.Takahashi N, Kikuchi S, Dai Y, Kobayashi K, Fukuoka T, Noguchi K. Expression of auxiliary beta subunits of sodium channels in primary afferent neurons and the effect of nerve injury. Neuroscience. 2003;121:441–50. doi: 10.1016/s0306-4522(03)00432-9. [DOI] [PubMed] [Google Scholar]
- 126.Theile JW, Jarecki BW, Piekarz AD, Cummins TR. Nav1.7 mutations associated with paroxysmal extreme pain disorder, but not erythromelalgia, enhance Navbeta4 peptide-mediated resurgent sodium currents. J Physiol. 2011;589:597–608. doi: 10.1113/jphysiol.2010.200915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Grieco TM, Malhotra JD, Chen C, Isom LL, Raman IM. Open-channel block by the cytoplasmic tail of sodium channel β4 as a mechanism for resurgent sodium current. Neuron. 2005;45:233–44. doi: 10.1016/j.neuron.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 128.Theile JW, Cummins TR. Inhibition of Navbeta4 peptide-mediated resurgent sodium currents in Nav1.7 channels by carbamazepine, riluzole, and anandamide. Mol Pharmacol. 2011;80:724–34. doi: 10.1124/mol.111.072751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kovacs GG, Adle-Biassette H, Milenkovic I, Cipriani S, van Scheppingen J, Aronica E. Linking pathways in the developing and aging brain with neurodegeneration. Neuroscience. 2014;269C:152–72. doi: 10.1016/j.neuroscience.2014.03.045. [DOI] [PubMed] [Google Scholar]
- 130.Waxman SG. Axonal conduction and injury in multiple sclerosis: the role of sodium channels. Nat Rev Neurosci. 2006;7:932–41. doi: 10.1038/nrn2023. [DOI] [PubMed] [Google Scholar]
- 131.Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 2014;344:1252304. doi: 10.1126/science.1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mattsson N, Axelsson M, Haghighi S, Malmestrom C, Wu G, et al. Reduced cerebrospinal fluid BACE1 activity in multiple sclerosis. Multiple sclerosis. 2009;15:448–54. doi: 10.1177/1352458508100031. [DOI] [PubMed] [Google Scholar]
- 133.Nutini M, Spalloni A, Florenzano F, Westenbroek RE, Marini C, et al. Increased expression of the beta3 subunit of voltage-gated Na+ channels in the spinal cord of the SOD1G93A mouse. Mol Cell Neurosci. 2011;47:108–18. doi: 10.1016/j.mcn.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 134.Zhou TT, Zhang ZW, Liu J, Zhang JP, Jiao BH. Glycosylation of the sodium channel beta4 subunit is developmentally regulated and involves in neuritic degeneration. International journal of biological sciences. 2012;8:630–9. doi: 10.7150/ijbs.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Oyama F, Miyazaki H, Sakamoto N, Becquet C, Machida Y, et al. Sodium channel beta4 subunit: down-regulation and possible involvement in neuritic degeneration in Huntington’s disease transgenic mice. J Neurochem. 2006;98:518–29. doi: 10.1111/j.1471-4159.2006.03893.x. [DOI] [PubMed] [Google Scholar]
- 136.Evin G, Barakat A, Masters CL. BACE: Therapeutic target and potential biomarker for Alzheimer’s disease. Int J Biochem Cell Biol. 2010;42:1923–6. doi: 10.1016/j.biocel.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 137.Kim DY, Gersbacher MT, Inquimbert P, Kovacs DM. Reduced Sodium Channel Nav1.1 Levels in BACE1-null Mice. J Biol Chem. 2011;286:8106–16. doi: 10.1074/jbc.M110.134692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Amatniek JC, Hauser WA, DelCastillo-Castaneda C, Jacobs DM, Marder K, et al. Incidence and predictors of seizures in patients with Alzheimer’s disease. Epilepsia. 2006;47:867–72. doi: 10.1111/j.1528-1167.2006.00554.x. [DOI] [PubMed] [Google Scholar]
- 139.Dunckley T, Beach TG, Ramsey KE, Grover A, Mastroeni D, et al. Gene expression correlates of neurofibrillary tangles in Alzheimer’s disease. Neurobiology of aging. 2006;27:1359–71. doi: 10.1016/j.neurobiolaging.2005.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Brackenbury WJ. Voltage-gated sodium channels and metastatic disease. Channels. 2012;6:352–61. doi: 10.4161/chan.21910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Diss JK, Fraser SP, Walker MM, Patel A, Latchman DS, Djamgoz MB. Beta-subunits of voltage-gated sodium channels in human prostate cancer: quantitative in vitro and in vivo analyses of mRNA expression. Prostate Cancer Prostatic Dis. 2008;11:325–33. doi: 10.1038/sj.pcan.4501012. [DOI] [PubMed] [Google Scholar]
- 142.Hernandez-Plata E, Ortiz CS, Marquina-Castillo B, Medina-Martinez I, Alfaro A, et al. Overexpression of NaV 1.6 channels is associated with the invasion capacity of human cervical cancer. Int J Cancer. 2012;130:2013–23. doi: 10.1002/ijc.26210. [DOI] [PubMed] [Google Scholar]
- 143.Chioni AM, Brackenbury WJ, Calhoun JD, Isom LL, Djamgoz MB. A novel adhesion molecule in human breast cancer cells: voltage-gated Na+ channel beta1 subunit. Int J Biochem Cell Biol. 2009;41:1216–27. doi: 10.1016/j.biocel.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sjoblom T, Jones S, Wood LD, Parsons DW, Lin J, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–74. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 145.Adachi K, Toyota M, Sasaki Y, Yamashita T, Ishida S, et al. Identification of SCN3B as a novel p53-inducible proapoptotic gene. Oncogene. 2004;23:7791–8. doi: 10.1038/sj.onc.1208067. [DOI] [PubMed] [Google Scholar]
- 146.Dixon-Salazar TJ, Keeler LC, Trauner DA, Gleeson JG. Autism in several members of a family with generalized epilepsy with febrile seizures plus. J Child Neurol. 2004;19:597–603. doi: 10.1177/088307380401900806. [DOI] [PubMed] [Google Scholar]
- 147.Schmunk G, Gargus JJ. Channelopathy pathogenesis in autism spectrum disorders. Frontiers in genetics. 2013;4:222. doi: 10.3389/fgene.2013.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lee H, Lin MC, Kornblum HI, Papazian DM, Nelson SF. Exome sequencing identifies de novo gain of function missense mutation in KCND2 in identical twins with autism and seizures that slows potassium channel inactivation. Hum Mol Genet. 2014 doi: 10.1093/hmg/ddu056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Han S, Tai C, Westenbroek RE, Yu FH, Cheah CS, et al. Autistic-like behaviour in Scn1a+/− mice and rescue by enhanced GABA-mediated neurotransmission. Nature. 2012;489:385–90. doi: 10.1038/nature11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Iqbal Z, Vandeweyer G, van der Voet M, Waryah AM, Zahoor MY, et al. Homozygous and heterozygous disruptions of ANK3: at the crossroads of neurodevelopmental and psychiatric disorders. Hum Mol Genet. 2013;22:1960–70. doi: 10.1093/hmg/ddt043. [DOI] [PubMed] [Google Scholar]
- 151.Zuko A, Kleijer KT, Oguro-Ando A, Kas MJ, van Daalen E, et al. Contactins in the neurobiology of autism. Eur J Pharmacol. 2013;719:63–74. doi: 10.1016/j.ejphar.2013.07.016. [DOI] [PubMed] [Google Scholar]
- 152.Qu Y, Curtis R, Lawson D, Gilbride K, Ge P, et al. Differential modulation of sodium channel gating and persistent sodium currents by the beta1, beta2, and beta3 subunits. Mol Cell Neurosci. 2001;18:570–80. doi: 10.1006/mcne.2001.1039. [DOI] [PubMed] [Google Scholar]
- 153.Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–78. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Oh Y, Lee YJ, Waxman SG. Regulation of Na+ channel β1 and β2 subunit mRNA levels in cultured rat astrocytes. Neurosci Lett. 1997;234:107–10. doi: 10.1016/s0304-3940(97)00694-0. [DOI] [PubMed] [Google Scholar]
- 155.Maier SK, Westenbroek RE, Yamanushi TT, Dobrzynski H, Boyett MR, et al. An unexpected requirement for brain-type sodium channels for control of heart rate in the mouse sinoatrial node. Proc Natl Acad Sci U S A. 2003;100:3507–12. doi: 10.1073/pnas.2627986100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Canti C, Nieto-Rostro M, Foucault I, Heblich F, Wratten J, et al. The metal-ion-dependent adhesion site in the Von Willebrand factor-A domain of alpha2delta subunits is key to trafficking voltage-gated Ca2+ channels. Proc Natl Acad Sci U S A. 2005;102:11230–5. doi: 10.1073/pnas.0504183102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lin L, Sun W, Throesch B, Kung F, Decoster JT, et al. DPP6 regulation of dendritic morphogenesis impacts hippocampal synaptic development. Nat Commun. 2013;4:2270. doi: 10.1038/ncomms3270. [DOI] [PMC free article] [PubMed] [Google Scholar]