Abstract
Background
The study aimed to determine the effect of preference-based tailored navigation on colorectal cancer (CRC) screening adherence and related outcomes among African Americans (AAs).
Methods
We conducted a randomized controlled trial that included 764 AA patients who were age 50 to 75 years, were eligible for CRC screening, and had received care through primary care practices in Philadelphia. Consented patients completed a baseline telephone survey and were randomized to either a Standard Intervention (SI) group (n = 380) or a Tailored Navigation Intervention (TNI) group (n = 384). The SI group received a mailed stool blood test kit plus colonoscopy instructions, and a reminder. The TNI group received tailored navigation (a mailed stool blood test kit or colonoscopy instructions based on preference, plus telephone navigation) and a reminder. A six-month survey and a 12-month medical records review were completed to assess screening adherence, change in overall screening preference, and perceptions about screening. Multivariable analyses were performed to assess intervention impact on outcomes.
Results
At six months, adherence in the TNI group was statistically significantly higher than in the SI group (OR = 2.1, 95% CI = 1.5 to 2.9). Positive change in overall screening preference was also statistically significantly greater in the TNI group compared with the SI group (OR = 1.5, 95% CI = 1.0 to 2.3). There were no statistically significant differences in perceptions about screening between the study groups.
Conclusions
Tailored navigation in primary care is a promising approach for increasing CRC screening among AAs. Research is needed to determine how to maximize intervention effects and to test intervention impact on race-related disparities in mortality and survival.
Recently, it was estimated there will be 6850 deaths from colorectal cancer (CRC) among African Americans, and late diagnosis will account for many of these deaths (1,2). It is well known that CRC screening can detect colorectal adenomas, before they progress to CRC, and can cure early-stage CRC. “Healthy People 2020” has called for CRC screening rates of at least 70% (3). CRC screening adherence among African Americans (AAs), however, is below this goal, and is lower than among whites (1,4–7). Low screening rates fuel disparities in mortality and survival among AAs (8). Effective methods are needed to raise CRC screening rates among AAs.
Patient-oriented behavioral interventions such as reminders, small media, and one-on-one education are recommended strategies to increase CRC screening. Telephone contact delivered by a patient navigator has also been advanced as a promising strategy to increase screening adherence (9). Studies that have used this approach in conjunction with mailed contacts delivered outside the context of a primary care office visit have increased screening adherence from 27% to 41% (10–12). Navigation that is tailored according to patient preference for recommended CRC screening tests may serve to further boost adherence (10,13).
This paper presents findings from an American Cancer Society–funded randomized controlled trial (clinical trials identifier NCT00893295) of CRC screening interventions conducted among AA primary care patients. The study was designed to test the impact of a preference-based tailored navigation intervention strategy vs a standard mailed intervention strategy on screening adherence. Outcomes included patient screening adherence, change in overall screening preference, and change in perceptions about screening. To our knowledge, no other study has tested the impact of tailored navigation on these outcomes in an AA primary care patient population.
Methods
Study Design and Participants
The study was conducted between 2008 and 2012. Study sites included three primary care practices of Thomas Jefferson University’s Jefferson Family Medicine Associates (JFMA) and 10 primary care practices affiliated with the Albert Einstein Health Care Network (AEHN) in Philadelphia. Following procedures approved by the institutional review boards of Thomas Jefferson University and AEHN, the research team reviewed administrative records to identify patients who were African Americans 50 to 75 years of age, had no prior diagnosis of colorectal neoplasia or inflammatory bowel disease, had visited a participating practice within the previous two years, had complete contact information, and were not compliant with American Cancer Society (ACS) CRC screening guidelines.
Baseline Telephone Survey
Patients identified in 14 successive randomly selected cohorts were mailed an introductory letter and were called to verify eligibility and obtain verbal consent. A baseline telephone survey was administered to collect data on participant sociodemographic background and Preventive Health Model (PHM) perceptions related to CRC screening (14,15). PHM items were assessed on a five-point Likert-type scale. Five PHM subscales included: 1) perceived CRC risk/susceptibility (three items, α = 0.81), 2) perceived salience of screening (three items, α = 0.78), 3) perceived response efficacy of screening (two items, α = 0.59), 4) worries about potentially abnormal screening results (two items, α = 0.60), 5) and social support and influence regarding screening (four items, α = 0.64). A global PHM measure was computed using items from these subscales (14 items, α = 0.72). A religiosity/fatalism scale was also included (five items, α = 0.64).
We also included items on the survey that described SBT and colonoscopy screening (tests most commonly recommended for screening in primary care), and asked respondents to report their Precaution Adoption Process Model (PAPM) screening decision stage for each test (1 = decided against, 2 = never heard of, 3 = not considering, 4 = undecided, or 5 = decided to do) (10,16). Responses to these items were analyzed to determine the individual’s proximity to screening with any test (“overall screening preference”) and proximity to performance of a given test (“screening test preference”). For example, if a participant reported that s/he had decided to do colonoscopy screening and was undecided about doing SBT, we assigned the individual’s overall screening preference as decided to do screening; and colonoscopy was assigned as the screening test that was preferred.
Randomization and Intervention
Baseline survey respondents were randomly assigned at the participant level, using electronic allocation files, to one of two study groups: a Standard Intervention (SI) group or a Tailored Navigation Intervention (TNI) group. The SI group received a mailed CRC screening informational booklet, a personalized letter that included a contact telephone number to schedule a colonoscopy appointment, and an SBT kit (InSure® FIT™). A reminder letter was mailed at 45 days postrandomization to those who had not returned the SBT kit.
TNI group participants were mailed a CRC screening informational booklet, along with a personalized message page that noted PHM barriers to screening the individual identified on the baseline survey (eg, low social support for screening) and a barrier-specific response (eg, “Screening is an important preventive health behavior that is endorsed by your primary care physician”). The page also identified the individual’s screening test preference. For patients who preferred colonoscopy screening, a toll-free telephone number was included for use in arranging a colonoscopy appointment. Alternatively, an SBT kit was included for those who preferred at-home SBT screening. In accordance with JFMA and AEHN leadership recommendations, colonoscopy instructions were sent to participants had an equal preference for colonoscopy and SBT screening. After this initial mailing, a trained navigator called each participant to: 1) review the mailed materials, 2) reassess screening test preference, 3) discuss concerns or barriers to test performance, 4) help to develop a plan to complete the preferred screening test, and 5) arrange a follow-up call. If the navigator found that the participant’s screening test preference had changed from baseline, new screening materials related to the current preferred test were sent. Finally, a reminder was sent at 45 days.
Endpoint Survey and Medical Records Review
Six months after randomization, an endpoint survey was administered to participants. This survey collected data on baseline PHM items (PHM scales) and PAPM variables, as well as self-reported screening adherence (any CRC screening test performed and corresponding date). Beginning at 12 months, a medical records review was completed to obtain additional information on screening adherence.
Data Analysis
The study’s primary outcome was CRC screening adherence within six months after randomization. As a secondary outcome, we also analyzed screening at 12 months. For both outcomes, adherence was defined as performance of any CRC screening test recommended in ACS and United States Preventive Services Task Force (USPSTF) guidelines that applied when the study was initiated. Any screening test that was reported on the endpoint survey or was found in laboratory and medical records reviews was counted, as long as the report was accompanied by a date within the observation period (10,16,17). Another secondary outcome was change in overall screening preference measured using data from the baseline and six-month surveys. Changes in participant perceptions about CRC screening constituted a third set of outcome measures. Finally, we inspected open-ended responses to an item on the endpoint survey that asked nonadherers to provide a primary reason for not screening. Using standard content analysis procedures, study personnel reviewed those responses, defined response categories, classified responses separately, and compared assignments to arrive at consensus.
Statistical Analyses
All main analyses followed the intent-to-treat principle, with participants being analyzed in their study group. Analyses of adherence were based on logistic regression and controlled for selection cohort and practice, as well as participant age and sex. A Generalized Estimating Equations approach that accounted for potential within-practice clustering yielded results that were almost identical to those using ordinary logistic regression, and therefore, results of the latter are presented. Participants who did not complete an endpoint survey were excluded from analyses of change in overall screening preference and PHM measures. We used logistic regression for the analysis of change in overall screening preference (any forward change vs no change or backward change) and linear regression for the analyses of change in PHM variables. These analyses controlled for all participant baseline characteristics. All statistical tests were two-sided, and a P value of less than .05 was considered statistically significant.
The study’s original target enrollment was 896 subjects. This sample size was computed to give the study approximately 95% power to detect a difference between the two study groups of 15% in overall CRC screening (50% in TNI vs 35% in SI) and a difference of about 10% to 15% in overall screening preference. The sample size calculations assumed 20% missing outcome data and an inflation factor of 10% for potential within-practice clustering. In the course of the study, it became apparent that medical record reviews would be available for virtually all participants. Therefore, with essentially no missing data on the CRC screening outcome, the target enrollment was recomputed to be 750 participants, preserving the original 95% power for the screening adherence outcome. Because there were about 30% missing data on the endpoint surveys, this revised sample size reduced power for the overall screening preference and screening perceptions outcomes to about 80%. “As treated analyses” were also completed for TNI group participants who were and were not navigated. Secondary analyses were also conducted to determine the frequency with which SBT and colonoscopy screening tests were performed by adherers who preferred SBT screening, had an equal preference for SBT and colonoscopy screening, and preferred colonoscopy.
Results
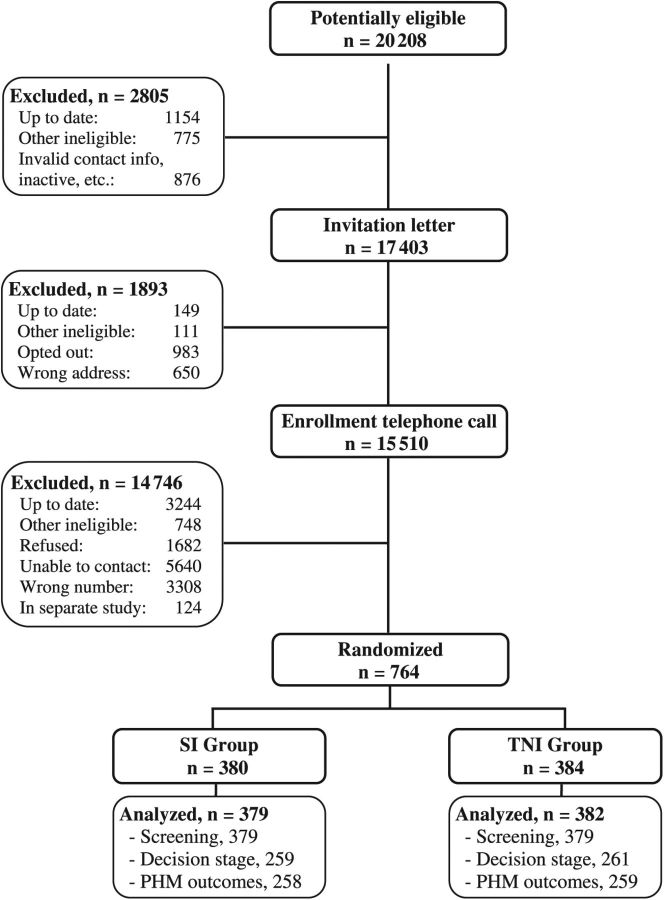
A total of 20202 potential participants were identified between December 2008 and October 2011, and 764 patients were randomized either to the SI group (n = 380) or the TNI group (n = 384) (Figure 1). During the study, three participants withdrew consent and were excluded from all analyses. Final analyses included 761 participants. Participant baseline characteristics are summarized in Table 1. Despite randomization, the TNI group included more participants who were female and were less than 60 years of age than the SI group.
Figure 1.
Trial schema. PHM = Preventive Health Model; SI = standard intervention; TNI = tailored navigation intervention.
Table 1.
Summary of participant baseline characteristics by study group (n = 761)*
| Characteristic | SI | TNI |
|---|---|---|
| No. (%) | No. (%) | |
| (n = 379) | (n = 382) | |
| Study site | ||
| Jefferson | 236 (62.3) | 235 (61.5) |
| Einstein | 143 (37.7) | 147 (38.5) |
| Age, y | ||
| 50–59 | 255 (67.3) | 287 (75.1) |
| 60+ | 124 (32.7) | 95 (24.9) |
| Sex | ||
| Female | 243 (64.1) | 278 (72.7) |
| Male | 136 (35.9) | 104 (27.3) |
| Education | ||
| High school or less | 227 (59.9) | 224 (58.6) |
| Greater than high school | 151 (40.1) | 157 (41.4) |
| Marital status | ||
| Married (or living as married) | 110 (29.0) | 124 (32.5) |
| Single/divorced/widowed | 269 (71.0) | 257 (67.5) |
| Global PHM scale | ||
| Low (1.0–3.0) | 33 (8.7) | 25 (6.5) |
| High (3.1–5.0) | 346 (91.3) | 356 (93.5) |
| Perceived susceptibility | ||
| Low (1.0–3.0) | 277 (74.6) | 286 (75.9) |
| High (3.1–5.0) | 94 (25.4) | 91 (24.1) |
| Salience and coherence | ||
| Low (1.0–3.0) | 11 (2.9) | 10 (2.6) |
| High (3.1–5.0) | 367 (97.1) | 370 (97.4) |
| Response efficacy | ||
| Low (1.0–3.0) | 49 (13.6) | 45 (12.3) |
| High (3.1–5.0) | 311 (86.4) | 322 (87.7) |
| Worries and concerns | ||
| Low (1.0–3.0) | 205 (55.5) | 226 (60.8) |
| High (3.1–5.0) | 164 (44.5) | 146 (39.1) |
| Social support and influence | ||
| Low (1.0–3.0) | 46 (12.4) | 42 (11.1) |
| High (3.1–5.0) | 326 (87.6) | 332 (89.9) |
| Religiosity/fatalism | ||
| Low (1.0–3.0) | 249 (69.0) | 259 (72.8) |
| High (3.1–5.0) | 112 (31.0) | 97 (27.2) |
| Overall screening preference | ||
| Decided against/never heard of | 7 (1.8) | 5 (1.3) |
| Not considering/undecided | 36 (9.5) | 34 (8.9) |
| Decided to do | 336 (88.7) | 343 (89.8) |
| Screening test preference | ||
| Stool blood test | 66 (17.4) | 70 (18.3) |
| Equal preference | 220 (58.1) | 214 (56.0) |
| Colonoscopy | 93 (24.5) | 98 (25.7) |
* Counts may not sum to each group’s total because of occasional missing data. SI = standard intervention; TNI = tailored navigation intervention.
Telephone navigation contacts were delivered to 293 (76.7%) TNI group participants. The endpoint survey completion rate was 67.9% in both the SI and TNI groups (P = 1.00), and medical records were reviewed for all participants.
CRC Screening Adherence
Table 2 summarizes results regarding CRC screening within six months after randomization and also includes 12-month adherence. The TNI group exhibited a statistically significantly higher level of six-month screening adherence than the SI group (38.0% vs 23.7%, OR = 2.1, CI = 1.5 to 2.9, P = .001). At 12 months, adherence was also statistically significantly higher in the TNI group than the SI group (43.5% vs 32.2%, OR = 1.7, CI = 1.2 to 2.3, P = .001). Findings from “as treated analyses” showed that screening adherence at six months in the TNI group among those who were navigated was substantially higher than those who were not navigated (45.7% vs 12.4%, respectively). We observed an adherence difference of similar magnitude at 12 months (50.9% vs 19.1%, respectively).
Table 2.
Colorectal cancer screening adherence by study group (n = 761)
| SI | TNI | TNI vs SI | ||
|---|---|---|---|---|
| No. (%) | No. (%) | |||
| Screening adherence | (n = 379) | (n = 382) | OR* (95% CI) | P† |
| Any screening within 6 months | 90 (23.7) | 145 (38.0) | 2.1 (1.5 to 2.9) | .001 |
| SBT within 6 months | 58 (15.3) | 82 (21.5) | ||
| CX within 6 months | 32 (8.4) | 63 (16.5) | ||
| Any screening within 12 months | 122 (32.2) | 166 (43.4) | 1.7 (1.2 to 2.3) | .001 |
| SBT within 12 months | 70 (18.5) | 88 (23.0) | ||
| CX within 12 months | 52 (13.7) | 78 (20.4) |
* Odds ratios were adjusted for study wave and practice, and participant age and sex. Referent: SI group. CI = confidence interval; CX = colonoscopy; OR = odds ratio; SBT = stool blood test; SI = standard intervention; TNI = tailored navigation intervention.
† Logistic regression analyses; two-sided P values from Wald Chi-square tests.
Among SI group participants, those who preferred SBT at baseline were somewhat more likely to complete the SBT kit than colonoscopy (12.1% and 7.6%), as were those who had an equal preference for SBT and colonoscopy (18.2% and 6.8%), while those who preferred colonoscopy had similar screening performance for SBT and colonoscopy (10.8% and 12.9%). Among TNI group participants, however, those who preferred SBT were much more likely to complete SBT than colonoscopy (41.1% and 7.1%), those who had an equal preference for the two screening methods displayed equal performance of the tests (17.8% for both), and those who preferred colonoscopy were somewhat more likely to complete colonoscopy screening than the SBT (20.4% and 15.3%).
Overall Screening Preference
Table 3 summarizes the baseline-to-endpoint forward change in overall screening preference. Because of small numbers, the stages “decided against screening” and “never heard of” were combined, as were the stages “not considering” and “undecided.” A statistically significant change in overall screening preference was observed in 35.0% of TNI group participants as compared with 27.8% of SI group participants (OR = 1.5, 95% CI = 1.0 to 2.3, P = .04). Screening adherence accounted for a larger percentage of the change in overall screening preference in the TNI group (89.0%) than in the SI group (70.8%).
Table 3.
Baseline-to-endpoint change in overall screening preference by study group (n = 519*)
| SI | TNI | TNI vs SI | ||
|---|---|---|---|---|
| No. (%) | No. (%) | |||
| Overall screening preference | (n = 259) | (n = 260) | OR* (95% CI) | P† |
| Any forward change | 72 (27.8) | 91 (35.0) | 1.5 (1.0 to 2.3) | .04 |
| “Undecided” to “Decided to do” | 17 (6.6) | 8 (3.1) | ||
| “Undecided” to “Screened” | 0 (0) | 6 (2.3) | ||
| “Decided to do” to “Screened” | 51 (19.7) | 75 (28.8) | ||
| Other forward changes | 4 (1.5) | 2 (0.8) |
* Odds ratios were adjusted for study cohort and practice, and participant age, sex, education, marital status, baseline global Preventive Health Model score, baseline decision stage, and baseline preferred test. Referent: SI group. CI = confidence interval; OR = odds ratio; SI = standard intervention; TNI = tailored navigation intervention.
† Logistic regression analyses; 2-sided p-values from Wald Chi-square tests
Perceptions Related to CRC Screening
Table 4 presents participant baseline and six-month endpoint survey PHM measures, as well as the baseline-to-endpoint change in these measures. We found no statistically significant changes between the study groups in participant perceptions about CRC screening. There was a small but not statistically significant difference between the two study groups in terms of positive change in screening salience and coherence (P = .09).
Table 4.
Baseline-to-endpoint change in Preventive Health Model variables by study group (n = 517*)
| Variables | SI | TNI | TNI vs SI | |
|---|---|---|---|---|
| mean (SD) | mean (SD) | mean diff (95% CI)† | P‡ | |
| Global PHM scale | ||||
| Baseline | 3.9 (0.6) | 3.9 (0.5) | ||
| Endpoint | 3.8 (0.6) | 3.8 (0.6) | ||
| Baseline-to-endpoint change | -0.1 (0.6) | 0.0 (0.5) | 0.0 (-0.1 to 0.1) | .49 |
| Perceived susceptibility | ||||
| Baseline | 2.6 (1.1) | 2.5 (1.1) | ||
| Endpoint | 2.5 (1.2) | 2.5 (1.2) | ||
| Baseline-to-endpoint change | -0.1 (1.3) | 0.0 (1.2) | 0.1 (-0.1 to 0.2) | .58 |
| Salience and coherence | ||||
| Baseline | 4.8 (0.5) | 4.8 (0.5) | ||
| Endpoint | 4.8 (0.5) | 4.9 (0.4) | ||
| Baseline-to-endpoint change | 0.0 (0.5) | 0.0 (0.6) | 0.1 (0.0 to 0.1) | .09 |
| Response efficacy | ||||
| Baseline | 4.4 (0.8) | 4.5 (0.7) | ||
| Endpoint | 4.6 (0.8) | 4.5 (0.8) | ||
| Baseline-to-endpoint change | 0.1 (0.9) | 0.0 (0.8) | 0.0 (-0.1 to 0.1) | .94 |
| Worries and concerns | ||||
| Baseline | 3.0 (1.4) | 2.8 (1.4) | ||
| Endpoint | 2.7 (1.4) | 2.6 (1.4) | ||
| Baseline-to-endpoint change | -0.3 (1.4) | -0.2 (1.4) | -0.1 (-0.3 to 0.2) | .60 |
| Social support and influence | ||||
| Baseline | 4.2 (0.9) | 4.3 (0.8) | ||
| Endpoint | 4.2 (0.8) | 4.2 (0.8) | ||
| Baseline-to-endpoint change | -0.1 (0.9) | -0.1 (0.9) | 0.0 (-0.1 to 0.2) | .47 |
| Religiosity/fatalism | ||||
| Baseline | 2.5 (1.1) | 2.5 (1.0) | ||
| Endpoint | 2.5 (1.1) | 2.5 (1.1) | ||
| Baseline-to-endpoint change | 0.0 (1.1) | 0.0 (1.0) | 0.0 (-0.2 to 0.2) | .90 |
* n = 517 for global Preventive Health Model scale, 499 for susceptibility, 516 for salience, 485 for response efficacy, 499 for worries and concerns, 504 for social support and influence, and 488 for religiosity/fatalism. CI = confidence interval; PHM = Preventive Health Model; SI = standard intervention; TNI = tailored navigation intervention.
† Mean differences were adjusted for study wave and practice, participant age, sex, education, marital status, baseline decision stage, baseline preferred test, and the baseline value of each outcome).
‡ Linear regression analyses; two-sided P values from Wald Chi-square tests.
Reasons for Nonadherence
Of 519 endpoint survey respondents, 388 did not screen (208 in the SI group and 180 in the TNI group). A total of 243 (62.6%) nonadherers reported a major reason for not completing a screening test. Reasons for nonadherence were: low perceived importance of CRC screening (33.3%), lack of transportation or time needed to attend a screening appointment (14.0%), fear of screening procedures or results (13.2%), dislike of screening-related procedures (9.9%), lack of insurance coverage or limited capacity to pay for screening (9.5%), confusion about the screening process (7.4%), existence of a health condition that precluded screening (8.2%), and other reasons (4.5%). The distribution of these reasons was comparable in both study groups.
Discussion
This study is the first trial designed to test the impact of a preference-based tailored navigation intervention on CRC screening adherence among AAs. Study interventions were applied to patients who were nonadherent to CRC screening guidelines outside the context of a primary care office visit, and did not involve direct physician contact or referral for screening. In this setting, the TNI strategy produced a CRC screening rate that was statistically significantly higher that observed in the SI approach.
Elsewhere, the research team has reported results of a trial of tailored navigation that was conducted in a predominantly white primary care patient population (10). In that study, tailored navigation also produced higher CRC screening adherence than a standard mailed intervention, but the difference was not statistically significant. Secondary analyses showed that the impact of tailored navigation was greater among nonwhites than whites, but nonwhites were underrepresented in the study population. The current study was conducted with AA primary care patients who were not up to date with CRC screening guidelines. In this segment of the patient population, mailed interventions can boost screening adherence, and a preference-based TNI produced even greater gains. Further research is needed to refine the TNI approach for use in settings where CRC screening rates are low and where disparities in screening use are pronounced. Pragmatic trials would be an important next step to determine the public health impact of streamlined TNI methods.
Other recently published studies have demonstrated the benefit of multicomponent intervention strategies in general primary care patient populations. Green et al. (18) reported on the use multicomponent interventions in a predominantly white primary care patient population. Participants received different combinations of mailed stool blood tests and instructions for arranging a screening appointment and multiple proactive telephone contacts by research staff to encourage screening and assist in scheduling screening colonoscopy. Gupta et al. (19) reported an intervention trial conducted with a largely white patient population enrolled in a hospital-based primary care clinic medical assistance program. Study participants were randomized to receive usual care, a mailed stool blood test kit with nurse telephone follow-up contact, or mailed instructions for scheduling colonoscopy with nurse telephone follow-up. Both intervention groups also received intensive follow-up contacts via automated telephone contacts and direct contact by study personnel to encourage screening. Interventions in both studies statistically significantly increased CRC screening adherence. Interestingly, intervention increases largely reflected high SBT use.
Findings from secondary analyses related to the type of screening test performed by screening test preference at baseline indicate that when provided with access to both SBT and colonoscopy screening via mail (SI group), adherers tended to perform SBT screening, irrespective to expressed test preference. Screening adherers who reported that they preferred SBT screening were sent an SBT kit and received navigation (TNI group) and were much more likely to complete an SBT than a colonoscopy; adherers who preferred colonoscopy were sent colonoscopy instructions and received tailored navigation and were somewhat more likely to have a colonoscopy than an SBT; and those who had an equal preference for SBT and colonoscopy screening had comparable SBT and colonoscopy screening rates. Thus, when participants had a preference for a given test and were navigated toward performance of that test, use of the preferred test tended to be higher than use the test that was not preferred. Furthermore, overall screening adherence tended to be higher in among those who had a test preference and were navigated through the screening process than those who did not express a test preference. These findings are consistent with a report by Inadomi et al., who found that CRC screening was higher among patients who expressed a screening test preference and were given assistance in completing that test (13). Research is needed to better understand the relative effects of screening test preference, access, and navigation on overall and test-specific screening adherence (20).
Regarding change in overall screening preference, finding that tailored navigation produced a greater impact than a mailed intervention was largely driven by participant movement from being in a “decided to do” stage to actual screening. Similar findings were reported in an earlier study conducted in a general patient population (10). We also determined that exposure to tailored navigation did not make a measurable difference in PHM variables relative to the standard intervention; this is consistent with results we have reported elsewhere (10). Further research is needed to gain insights into how the tailored navigation intervention operated on participant psychosocial factors in order to catalyze screening performance. It is important to determine if current PHM metrics lack the precision needed to reflect intervention effects or if additional concepts should be developed for inclusion in the PHM.
The current study included AA patients from primary care practices in one large city. Thus, results may not be generalizable to other settings. Generalizability may also be limited because participants volunteered to participate in the study. Intervention impact may be underestimated because patient navigators were unable to contact all participants in the TNI group. Moreover, patient navigators were not authorized to schedule colonoscopy appointments.
It is important to note that there is no commonly accepted method for measuring overall screening preference and screening test preference. We based our assessments of overall screening preference and screening test preference on PAPM decision staging and interpreted screening decision stage as a measure of an individual’s proximity to action. Further conceptual work is needed to clarify how preference may be consistently defined, measured, and used in screening programs, especially when more than one test is made available, and in research studies that aim to assess the impact of preference on overall and test-specific adherence.
Finally, many screening nonadherers stated that being given an opportunity to screen alone did not spur the individual to act. Medina et al. (21) found similar results among patients in a large urban city. Other reasons for nonadherence may include financial concerns and lack of insurance, worry about screening results, and reticence related to having specific screening tests (22,23). Future research on increasing CRC screening among AAs must address the need to explain the importance of adherence and address salient test access barriers.
Funding
Ronald E. Myers, Principal Investigator, received funding from the American Cancer Society (RSGT-08-017-01-CPPB) to support the study.
The funder had no role in the design of the study, the collection, analysis, or interpretation of the data, the writing of the manuscript, nor the decision to submit the manuscript for publication.
We wish to acknowledge Edith Mitchell, MD, Barbara Powe, PhD, Claudia Parvanta, PhD, and Mark Capkin, MD.
References
- 1. American Cancer Society. Cancer Facts & Figures for African Americans 2013–2014. Available at: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-036921.pdf Accessed August 20, 2015.
- 2.U.S. Department of Health and Human Services, Healthy People 2020 – Topics and Objectives: Cancer Available at: http://healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicid=5 Accessed August 20, 2015.
- 3. Center for Disease Control and Prevention. Colorectal Cancer Screening Rates. Available at: http://www.cdc.gov/cancer/colorectal/statistics/screening_rates.htm Accessed August 20, 2015.
- 4. Center for Disease Control and Prevention. Surveillance of demographic characteristics and health behaviors among adult cancer survivors-Behavioral Risk Factor Surveillance System, United States 2009. MMWR. 2012;61(3):41–45. Available at: http://www.cdc.gov/mmwr/pdf/ss/ss6101.pdf Accessed August 20, 2014. [PubMed] [Google Scholar]
- 5. Ananthakrishnan AN, Schellhase KG, Sparapani RA, Laud PW, Neuner JM. Disparities in colon cancer screening in the medicare population. Arch Intern Med. 2007;167(3):258–264. [DOI] [PubMed] [Google Scholar]
- 6. Fenton JJ, Reid RJ, Baldwin LM, et al. Influence of primary care use on population delivery of colorectal cancer screening. Cancer Epidemiol Biomarkers Prev. 2009;18(2):640–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Robbins AS, Seigel RL, Jemal A. Racial Disparities in Stage-specific Colorectal Cancer Mortality rates from 1985–2008. J Clin Oncol. 2011;30:401–405. [DOI] [PubMed] [Google Scholar]
- 8. Lansdorp-Vogelaar I, Kuntz KM, Knudsen AB, et al. Stool DNA Testing to Screen for Colorectal Cancer in the Medicare Population A Cost-Effectiveness Analysis. Ann Intern Med. 2010;153:368–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Centers for Disease Control and Prevention. Guide to Community Preventive Services. Cancer prevention and control, client-oriented screening interventions: client reminders. Available at: www.thecommunityguide.org/cancer/screening/client-oriented/reminders.html Updated January 27, 2014. Accessed August 20, 2014.
- 10. Myers RE, Bittner-Fagan H, Daskalakis C, et al. A randomized controlled trial of tailored navigation and standard intervention in colorectal cancer screening. Cancer Epidemiol Biomarkers Prev. 2013;22(1):109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Percac-Lima S, Grant RW, Green AR, et al. A culturally tailored navigator program for colorectal cancer screening in a community health center: A randomized, controlled trial. J Gen Intern Med. 2009;24(2):211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lasser KE, Murillo J, Medlin E, et al. A multilevel intervention to promote colorectal cancer screening among community health center patients: Results of a pilot study. BMC Fam Pract. 2009;10:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Inadomi JM, Vijan S, Janz NK, et al. Adherence to Colorectal Cancer Screening: A Randomized Clinical Trial of Competing Strategies. Archives of Internal Med. 2012;172(7):575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Myers RE, Jepson C, Ross E, et al. Modeling Adherence to Colorectal Cancer Screening. Prev Med. 1994;23(2):142–151. [DOI] [PubMed] [Google Scholar]
- 15. Vernon SW, Myers RE, Tilley BC. Development and Validation of an Instrument to Measure Factors Related to Colorectal Cancer Screening Adherence. Cancer Epidemiol Biomarkers Prev. 1997;6:825–832. [PubMed] [Google Scholar]
- 16. Myers RE, Sifri R, Hyslop T, et al. A randomized controlled trial of the impact of targeted and tailored interventions on colorectal cancer screening. Cancer. 2007;110(9):2083–2091. [DOI] [PubMed] [Google Scholar]
- 17. Myers RE, Hyslop T, Sifri R, et al. Tailored navigation in colorectal cancer screening. Medical Care. 2008;46(suppl 9):S123–S131. [DOI] [PubMed] [Google Scholar]
- 18. Green B, Wang CY, Anderson M, et al. An automated intervention with stepped increases in support to increase uptake of colorectal cancer screening: A randomized trial. Ann Intern Med. 2013;158(5):301–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gupta S, Halm EA, Rockey DC, et al. Comparative effectiveness of fecal immunochemical test outreach, colonoscopy outreach, and usual care for boosting colorectal cancer screening among the underserved: A randomized clinical trial. JAMA Intern Med. 2013;173(18):1725–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Daskalakis C, Vernon SW, Sifri R, et al. The effects of test preference, test access, and navigation on colorectal cancer screening. Cancer Epidemiol Biomarkers Prev. 2014;23(8):1521–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Medina GG, McQueen A, Greisinger AJ, Bartholomew LK, Vernon SK. What would make getting colorectal cancer screening easier? Perspectives from screeners and nonscreeners. Gastroenterol Res Pract. 2012;2012:895807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jones RM, Devers KJ, Kuzel AJ, Woolf SH. Patient-reported barriers to colorectal cancer screening: A mixed-methods analysis. Am J Prev Med. 2010;38(5):508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jones RM, Woolf SH, Cunningham TD, et al. The relative importance of patient-reported barriers to colorectal cancer screening. Am J Prev Med. 2010;38(5):499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]