Abstract
Biological ion channels are nanoscale transmembrane pores. When water and ions are enclosed within the narrow confines of a sub-nanometer hydrophobic pore, they exhibit behavior not evident from macroscopic descriptions. At this nanoscopic level, the unfavorable interaction between the lining of a hydrophobic pore and water may lead to liquid-vapor oscillations. The resultant transient vapor state is ‘dewetted’ i.e. effectively devoid of water molecules within all, or part of the pore, thus leading to an energetic barrier to ion conduction. This process, termed ‘hydrophobic gating’, was first observed in molecular dynamics simulations of model nanopores, where the principles underlying hydrophobic gating (i.e. changes in diameter, polarity, or transmembrane voltage) have now been extensively validated. Computational, structural and functional studies now indicate that biological ion channels may also exploit hydrophobic gating to regulate ion flow within their pores. Here we review the evidence for this process, and propose that this unusual behavior of water represents an increasingly important element in understanding the relationship between ion channel structure and function.
Keywords: Hydrophobic gating, nanopore, ion channel, potassium channel, K2P channel
Introduction
The unusual behavior of water in narrow hydrophobic pores, as opposed to bulk, macroscopic solution, can be described as an energetic balance between wetting and de-wetting (i.e. drying). The first observations of these transitions were made from molecular dynamics (MD) simulations of explicit water in carbon nanotubes [1] as well as simple model nanopores [2] and led to the concept now referred to as ‘hydrophobic gating’. At a simple level, the diameter of one water molecule is ~ 3 Å, yet at a diameter below ~14 Å a hydrophobic pore can begin to exhibit liquid-vapor oscillations switching stochastically between both wet and dry states. The most dynamic range for these oscillations is between 9-12 Å, and below this range the pore will be largely de-wetted. Therefore the hydrophobicity of the pore can result in a highly effective barrier to ion permeation (Figure 1).
Figure 1. Principles of hydrophobic gating.
(a) Cartoon representation of a cross-section through a model hydrophobic nanopore. Hydrophobic surfaces are shown in yellow, the membrane in green. In solution, these nanopores can switch stochastically between both wet and dry states via liquid-vapor oscillations within the pore. The dewetted vapor state presents an effective barrier to water and ion permeation. (b) These oscillations occur on the nanosecond timescale, and the stability of the wetted state is highly dependent upon pore diameter. (c) The probability of the pore being in the liquid or wetted state is not only dependent upon diameter, but also the hydrophobicity of atoms lining the pore. This was shown by progressively adding hydrophilic atoms to a model nanopore [3]. A fully hydrophilic pore remains fully occupied by water. However, a hydrophobic pore starts dewetting below 14 Å and becomes completely dewetted below ~8-10 Å. Semi-hydrophobic pores also exhibit similar dewetting below ~ 10 Å (dotted vertical line). (d) The process of hydrophobic gating has now been shown to be influenced by pore diameter, hydrophobicity and also changes in transmembrane voltage. This figure is adapted from results within references [2,3].
Ion channels are specialized membrane proteins which act as pores to enable ion movement across the cell membrane. In addition to their ability to be selective between different types of ions they can also be switched or gated between an open (i.e. ion conducting) and closed (non-conductive) state by external signals such as changes in transmembrane voltage, binding of ligands, and mechanical stress. Interestingly, the pores of many ion channels also have internal dimensions within the range where hydrophobic gating is observed in model nanopores. It was therefore anticipated that some ion channels might also exhibit hydrophobic gating and that this property might be tunable by local changes in the diameter and/or hydrophilicity of the channel pore. Over the last decade these ideas have gained momentum driven both by advances in computational techniques, as well as by the increasing availability of crystal structures for many different classes of ion channels. In this review we examine the evidence for hydrophobic gating in ion channels and highlight recent studies of both channels and model nanopores which indicate that this unusual behavior of water may play a critical role in our understanding of ion channel permeation and gating.
Behavior of water in model hydrophobic pores
The concept of hydrophobic gating and its possible influence on the flow of ions through protein ion channels was first elaborated in a series of simulation studies of simple model nanopores with a hydrophobic central region. These narrow pores were not physically occluded, but could be shown to form a hydrophobic gate due to liquid-vapor oscillation of water within the pore [3,4]. In particular, it was shown that a functionally closed (i.e. de-wetted; vapor state) pore could be opened yielding a wetted, liquid state either by a small increase in radius and/or a small increase in polarity (e.g. via the introduction of molecular dipoles) in the narrowest region of the pore [3] (Figure 1).
Subsequent simulation and theoretical studies confirmed that a narrow hydrophobic nanopore presents a significant energetic barrier (i.e. a gate) not only to water but also to ions [5]. Recent experimental studies on (non-biological) nanopores have also provided further direct experimental evidence for hydrophobic gating. In particular, these studies have demonstrated experimentally that wetting of functionally closed hydrophobic nanopores can also be achieved by application of a voltage across the pore [6]. This is a key functional property of a hydrophobic gate that was originally predicted in simulation studies of simple model nanopores [7]. Other studies have even shown that an asymmetric flow of ions (i.e. rectification) can be introduced by simply altering the relative shape of the nanopore [8].
Hydrophobic gating in biological ion channels
These early descriptions of hydrophobic gating in model nanopores, combined with some of the first high resolution channel structures quite naturally suggested that a similar mechanism may also exist in biological ion channels such as bacterial mechanosensitive channels, pentameric ligand-gated ion channels, and even members of the superfamily of tetrameric P-loop cation channels [9]. The concept of hydrophobic gating in ion channels has therefore attracted significant interest over the last decade and there are now several examples where multiple layers of experimental evidence exist to support this idea.
Prokaryotic Mechanosensitive channels
The bacterial mechanosensitive channels open in response to membrane tension to allow survival of bacteria under hypo-osmotic shock (for detailed review see [10]). The first structure of the heptameric small conductance channel, (MscS) was initially thought to be open because its central pore had a diameter of ~5 Å [11] (Figure 2a). Yet the pore is highly hydrophobic with branched hydrophobic side-chains Leu109 and Leu105 pointing into the pore lumen. First evidence for hydrophobic gating in these channels was reported in MD simulation studies where a vapor lock was observed within the pore [12,13]. Furthermore, a hydrophilic mutation of Leu109, which had been reported to have a gain-of-function phenotype [14], disrupted this hydrophobic gate in silico. Therefore, this initial structure was subsequently considered to be in a closed, non-conductive state [12]. A later structure of an open form of MscS revealed an iris like rotation of Leu105 and Leu109 away from the pore, causing a change in diameter of >8 Å and opening of its hydrophobic gate [15]. Thus the concept of hydrophobic gating in a biological ion channel is now experimentally well established. Further details of the MscS gating mechanism are reviewed extensively elsewhere [16,17].
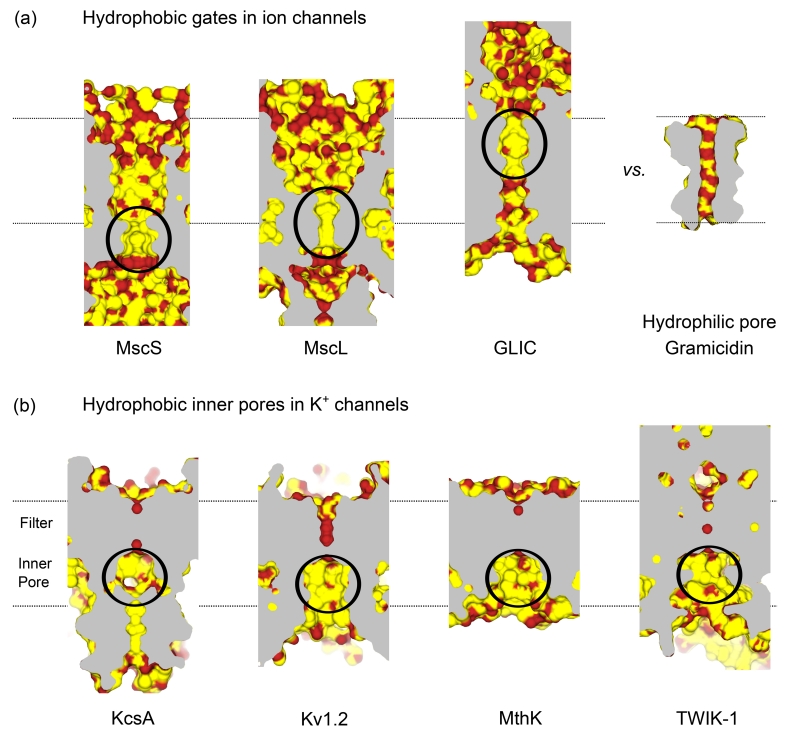
Figure 2. Hydrophobic gates and pores in biological ion channels.
(a) Longitudinal sections through the centre of the pore lumen for several different ion channels. Carbon and sulphur atoms are colored yellow, and hydrophilic atoms red. The approximate position of the channels within the membrane is marked by dotted lines. The channels shown are: the closed pores of MscS (2OAU), MscL (2OAR) and GLIC (4NPQ). The positions of the hydrophobic gates are circled; in MscS this gate contains Leu105 and Leu109, in MscL Gly22 (Ala20 in 2OAR), and Ile-9’-Ile-16’ in GLIC. These pores are in marked contrast to gramicidin (1MAG) which is hydrophilic throughout the pore. (b) The inner pore of many K+ channels is also hydrophobic (circled). Shown are sections of KcsA (1K4C), Kv1.2 (2A79), MthK (3LDC) and TWIK-1/K2P1 (3UKM). The circled region of MthK contains Ala88 [49] whilst TWIK-1 contains Leu146 and Leu261 [61] (see also Fig. 3). Structures are colored and positioned as in (a).
Simulation studies have now extended this idea to other bacterial mechanosensitive channels (e.g. MscL) [18] and are supported by a range of experimental observations such as the clustering of (hydrophilic) gain-of-function mutations onto the pore-lining face of the M1 helix [19,20], as well as a direct correlation between residue hydrophilicity and channel function at Gly22 in TM1 [21]. Cysteine modification of this same site (Gly22) has also been used to probe the mechanism of hydrophobic gating by demonstrating that that modification of even a single subunit by a hydrophilic MTS reagent was sufficient to gate the channel open [22].
Pentameric ligand-gated ion channels
Pentameric ligand-gated ion channels (pLGICs) mediate fast neurotransmission in the nervous system and were the subject of several groundbreaking structural studies that provided the first glimpse into the structure of a eukaryotic ion channel [23,24]. These structures suggested that branched aliphatic side-chains within the pore formed a ‘hydrophobic girdle’ with an internal diameter of ~6 Å. A detailed simulation study later demonstrated that this girdle created an energetic barrier to the movement of water and sodium ions through the pore [25].
Subsequent crystal structures of prokaryotic homologs of nAChR in different conformational states (GLIC and ELIC) have now significantly refined our understanding of gating in pLGIC channels (for detailed review see [26]). Initially, the architecture of the pore lining helix suggested that the ELIC channel represented a closed state, whilst the GLIC structure represented an open state [27,28,29,30]. Much like the nAChR, the GLIC channel contains a ring of branched hydrophobic residues within the inner pore, and MD simulations suggested a role for hydrophobic gating within this region (Ile 9’-Ile 16’) [31] (Figure 2a). Later studies reported drying transitions during steered MD simulations of the GLIC transmembrane domain from a putative open to closed state conformation [32], and also estimated the energetic cost of opening this hydrophobic gate [33]. This latterstudy found that the free energy cost of hydrating the gate was ~11 Kcal/mol, whilst the energy required for a solvated ion to subsequently move into this gate was only 4 Kcal/mol greater. This suggested that the largest energy barrier to ion movement was due to hydration of the pore itself and that drying of this hydrophobic constriction therefore represented the major determinant of ion conductance. Interestingly, more recent structures of GLIC in an apparently closed (or resting) state [34] now appear to confirm the hydrophobic gating mechanism proposed by Zhu & Hummer [32,33].
The hydrophobic gate region within the nAchR and GLIC structures also appears to be conserved in a related eukaryotic glutamate-gated chloride channel [35]. Thus although the precise details of the structural changes induced by ligand binding remain to be determined, the basic principle of hydrophobic gating within the pore may be more conserved than the mechanisms of ligand binding or ionic selectivity within the pLGIC superfamily.
Tetrameric cation channels
The superfamily of tetrameric ‘P-loop’ cation channels includes various potassium, sodium and calcium selective channels as well as the non-selective TRP and cyclic nucleotide gated channels. The ability of these channels to select between different cations and to be gated by a diverse range of biochemical and biophysical stimuli enables them to play fundamental roles in the control of nearly all forms of cellular electrical activity. It is therefore not surprising that they have been the subject of intense investigation over the last 50 years [36].
Crystal structures of prokaryotic homologs have now provided us with detailed insights into the mechanisms of cation selectivity whilst comparison of their transmembrane pore architecture has led to the classical ‘helix-bundle crossing’ gating model in which the pore-lining helices intersect at the cytoplasmic entrance to seal the permeation pathway shut, but then bend and splay outward to expose the inner cavity in the open state [37,38,39,40]. For many members of this superfamily there is now such a wealth of supporting experimental evidence for this model of activation gating that it has found its way into many text books. Indeed, the intuitive simplicity of this mechanism and the way it has been adapted into the modular design of this superfamily is one of its major attractions.
However, despite the structural conservation within the transmembrane/pore-modules of this superfamily, there now appear to be other structural and biophysical mechanisms which may also gate the pore. In particular, dynamic structural rearrangements within the selectivity filter are known to be important for gating and extensively reviewed elsewhere [41,42]. Instead we examine how hydrophobic gating may be important for the gating of K+ channels, especially those which appear to lack a classical helix bundle-crossing gate.
The hydrophobic inner-pore of the K channel
Potassium channels are one of the best characterized groups within this superfamily with functional studies stretching back over many decades; experiments from the 1960s first indicated that the inner pore of the voltage-gated K+ channel was relatively hydrophobic because of its relative affinity for tetraalkylammonium blockers such as TEA [43]. Other early studies also demonstrated that the open probability and conductance of these K+ channels were sensitive to the osmolarity of the bulk surroundings and may involve depletion of water from the channel [44]. The availability of crystal structures for so many different types of K+ channel now allows us to directly visualize these pores (Figure 2b). These reveal that the region where the TM-helices intersect at the bundle crossing is relatively hydrophobic, but perhaps more surprisingly, the lining of the whole inner pore in many K+ channels is also hydrophobic. The relative hydrophobicity the bundle-crossing gate is not unexpected because this permits tight packing of these helices in the closed state, but the hydrophobic nature of the rest of the inner cavity is of particular interest because ions clearly have to pass through this region to access the selectivity filter (Figure 2b).
Kv channels
A number of refined structural models now exist for the gating of voltage-dependent (Kv) potassium channels. In addition to the obvious fascination with the mechanisms of voltage-dependent gating which occurred when the first Kv structures were solved, advances in MD simulation methodologies have allowed extended timescale (μs-ms) simulations of the Kv channel pore structure itself. These simulations demonstrated that the hydrophobic nature of the inner pore appeared to promote dehydration of the cavity which then underwent a hydrophobic collapse during transition from the open to closed states of the channel [45]. Further simulations with the voltage sensors intact also reported that when the channel was open under depolarizing conditions, the inner pore remained fully hydrated, but when subjected to hyperpolarizing potentials, the channel exhibited a transient inward current followed by dewetting of the cavity, thereby halting ion conduction [46]. This dewetting step was concurrent with pore closure and occurred before the voltage sensor moved to the down position. Together these results suggest that hydrophobic gating may contribute to even the standard textbook models of channel gating.
Non-standard models of K+ channel gating
Although comparison of the KcsA vs MthK structures has been extremely valuable in terms of understanding the classical K+ channel ‘bundle-crossing’ gating mechanism, there is now clear evidence that some channels within this superfamily do not utilize a bundle-crossing gate. In some cases this may be explained by the presence of a filter-gating mechanism, but in other channels, additional mechanisms have been proposed [47,48,49,50,51,52]. As a more general channel gating mechanism which also obviates the requirement of a bundle-crossing gate, Eisenberg and colleagues have suggested that liquid-vapor oscillations within the pore may not only gate ion flow, but also underlie the on-off transitions of single-channel currents [53]. Although this remains an appealing hypothesis consistent with the general principles of hydrophobic gating, it is technically challenging to relate such nanoscopic properties to experimentally observed single-channel gating events.
Both the small conductance (SK) and large conductance (BK) Ca2+-activated channels appear to lack a bundle-crossing gate, and in addition to a filter gate they are also thought to possess a gating mechanism involving hydrophobic residues deep within the inner cavity [47,49,50,51]. Unfortunately there are no crystal structures available for these specific channels and so our understanding of their precise inner pore structure is limited. However, several high resolution structures are available for the prokaryotic MthK channel which is highly homologous to the BK channel [38].
MthK channel
MthK is considered to be the archetypal “open state” structure and it was originally proposed that ligand-induced movement of the intracellular domains controlled opening and closing of a helix-bundle crossing gate [38,54]. However, several studies now indicate that MthK does not gate at the bundle-crossing gate and instead the selectivity filter plays a major role in the control of channel gating [51]. Nevertheless, MthK possesses a hydrophobic inner cavity and residues within this region have also been shown to directly affect channel gating [49]. Recent high-resolution crystal structures have now defined the position of both K+ ions and water molecules within the pore and highlight a hydrophobic gap in the middle of the inner cavity [55]. This gap is defined by a ring of alanine side-chains such that the pore diameter at this position (Ala88) is ~9 Å (Figure 2). Water and ion movement through this constriction would therefore be highly dependent upon the relative hydrophobicity of this region. Mutation of this alanine (Ala88) to valine or leucine not only results in a progressive decrease in channel conductance, but also a decrease in open probability [49]. By marked contrast to leucine, mutation to similar sized branched hydrophilic sidechains, asparagine or aspartate cause both an increased conductance and open probability. Such observations are therefore consistent with the existence of a hydrophobic barrier within the pore, but whether dewetting actually occurs at this position and the extent to which this would influence ion permeation requires further investigation.
K2P channels
Another group of potassium channels which lack a classical bundle crossing gate are the subfamily of two-pore (K2P) channels. Although these channels share some structural similarity with classical tetrameric K+ channels they assemble as dimers with two pore domains per subunit [56]. This pseudo-fourfold symmetry has recently been directly confirmed by crystal structures of the TWIK-1 and TRAAK channels [57,58]. Despite these insights, the unusual transmembrane architecture of K2P channels poses a number of important questions about how they gate. Studies which examined the state-dependent access of high-affinity blockers to the inner pore concluded that K2P channels also do not utilize a lower bundle-crossing gate and suggested that gating occurs primarily within the selectivity filter [52,59]. External stimuli such as extracellular pH are thought to gate the filter in a process similar to C-type inactivation, whilst internal stimuli are thought to induce subtle movements of the TM-helices to modulate the filter gate [60].
TWIK-1 has a hydrophobic inner cavity
In an attempt to address how K2P channels gate, a recent MD simulation study examined the TWIK-1 crystal structure embedded in a phospholipid bilayer [61]. Interestingly, stochastic wetting and dewetting events were observed deep within inner pore. Upon examination of the residues lining the pore (Figure 2b), it was hypothesized that the hydrophobicity of this region might create an energetic barrier to ion permeation.
Within this hydrophobic region of TWIK-1, two leucine residues (Leu146 on TM2 and Leu261 on TM4) line the pore forming a ‘hydrophobic cuff’ with a diameter of 8.5 Å. Mutagenesis of these two leucine residues to isosteric but polar side chains (aspargine) not only led to the retention of water in silico, but also robust whole cell currents when expressed in vivo (Figure 3) [61]. This suggested a hydrophobic barrier within the inner pore might also contribute to the low levels of functional activity generally observed for TWIK-1.
Figure 3. Hydrophobic barrier in a K2P channel pore.
(a) MD simulations of the TWIK-1 K2P potassium channel structure (3UKM) demonstrate that dewetting occurs deep within the inner pore thus creating an energetic barrier to ion permeation [61]. Shown are the average water densities within the inner pore during simulations of a wild-type and L146N mutant pore which disrupts this hydrophobic barrier. The transparent cyan surface is contoured at 0.50 of bulk water density, overlaid on a snapshot of the inner-pore at 100 ns. The side chains at position 146 are highlighted. The K+ ions at the S4 position are shown as purple spheres. (b) Averaged whole-cell currents for WT TWIK-1*, and L146N TWIK-1* mutant channels. Disruption of the hydrophobic barrier produces a large increase in channel activity. Hydrophobic gating may therefore contribute to the regulation of channels which do not possess a classical cytoplasmic bundle-crossing gate. This figure is adapted from results within reference [61].
This hypothesis was validated computationally with free-energy calculations which showed an energetic barrier to ion movement through the hydrophobic wild-type, but not the L146N mutant pore. Likewise, functional studies demonstrated that a series of hydrophilic, but not hydrophobic substitutions, within the cuff produced robust currents by disrupting this hydrophobic barrier. Furthermore, increased voltages were required to drive currents through the hydrophobic wild-type channel pore compared to the L146N mutant [61], thereby reflecting similar results obtained for the voltage-dependent hydration of nanopores [6].
Interestingly, both sequence and structural alignments suggest that the hydrophobic cuff in TWIK-1 is equivalent to the hydrophobic constriction formed by residue Ala88 in MthK (see above) [49]. In other K2P channels the nature of the side chains at this position varies considerably, but THIK1 channels, which also exhibit low basal currents, have an isoleucine at this position on TM2 and changing this to a more polar side chain also leads to a gain-of-function [62]. Furthermore, mutation of the equivalent position in the Drosophila KCNKØ channel also demonstrates a correlation between channel activity and side chain polarity [63]. However, the physiological and structural mechanisms which might modulate this hydrophobic cuff within TWIK-1 remain to be determined, as does the importance of equivalent hydrophobic barriers in other K2P channels.
Further experimental validation
In addition to the channels described above, hydrophobic pores have also recently been described in several other types of ion channels and transporters thereby adding further experimental systems in which these principles can now be tested and validated. For example, the behavior of water within the pores of the calcium release-activated calcium channel (CRAC) [64] as well as the CorA family of Mg2+ transporters [65] have also recently been suggested to be important for their structural and functional properties.
Although the computational and theoretical studies which have highlighted the unusual behavior of water in model pores and ion channels are now being supported by a range of structural and functional data, more systematic methods are clearly required to assess the role of hydrophobic pore in channels and transporters. Computationally, improved water-water and water-protein interaction parameters are needed to describe the relative wettability of transmembrane pores (see discussion in [18]). Polarizable force fields and better descriptions of transmembrane voltage are also needed [66,67]. Furthermore, methods to define the relationship between dewetting on the nanosecond timescale with millisecond timescale single-channel biophysical properties are clearly required.
Crystallographic studies of water in ion channel pores are challenging due to the resolution required, but indirect measurements of hydrophobicity can be achieved by examination of densities for non-polar gases, such as xenon, or lipids. Such density has been observed in the hydrophobic gate of ELIC [27] and GLIC [29], and TWIK-1 [57]. Furthermore, next generation prediction and visualization software are needed to combine and display both the radius and hydrophobicity of the pore when reporting new structures.
Functionally, comparison of the effects of hydrophilic and hydrophobic pore mutations on channel pore properties represents one of the more obvious approaches. Indeed, this has been done for several types of channels, but more extensive comparison of series of different substitutions, or even unnatural amino acids and other forms of synthetic biology could be useful additional studies. Similarly, as shown for the MscL channels, dynamic alteration of the hydrophobic gate by reaction of hydrophilic MTS-reagents to engineered cysteine mutations could also be considered [22]. Electric field induced wetting of ion channel pores might also be used as a test for hydrophobic gating. Finally, the role of water could also be tested by altering the relative osmolarity, and it may even be possible to modify other methods which detect water-protein interactions, such as X-ray radioloysis or ‘footprinting’ to monitor the dynamic accessibility of waters to channel pores in response to different gating signals [68].
Conclusions
In summary, the behavior of water in confined hydrophobic pores appears to contribute to the biophysical and functional properties of a range of different ion channels. However, a combination of structural, functional and computational approaches will be required to address the role of hydrophobic gating in biological ion channels. For example, it remains intriguing that several K+ channels which do not utilize a classical bundle-crossing gate all seem to possess a highly hydrophobic inner pore which can function as an effective barrier to ion permeation. In particular, it will be important to understand how physiological stimuli may affect these gates and whether this occurs through subtle structural changes to the relative hydrophobicity of the pore, or through larger conformational changes in pore diameter. In reality, such effects may be inextricably linked and difficult to dissect. However, this unusual property of water appears to represent an emerging theme in our understanding of ion channel permeation and the rapidly expanding number of high resolution channel structures will inevitably help us to address this challenge.
Acknowledgements
We would like thank members of the SBCB (Dept Biochemistry, Oxford), for their helpful discussions; in particular Caroline Dahl and Dr Oliver Beckstein (Arizona State University). This work was supported by grants from the Wellcome Trust and the BBSRC. P.A. is a Wellcome Trust OXION Training Fellow.
Footnotes
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Hummer G, Rasaiah JC, Noworyta JP. Water conduction through the hydrophobic channel of a carbon nanotube. Nature. 2001;414:188–90. doi: 10.1038/35102535. [DOI] [PubMed] [Google Scholar]
- 2.Beckstein O, Biggin PC, Sansom MSP. A hydrophobic gating mechanism for nanopores. J Phys Chem B. 2001;105:12902–12905. [Google Scholar]
- 3.Beckstein O, Sansom MS. Liquid-vapor oscillations of water in hydrophobic nanopores. Proc Natl Acad Sci U S A. 2003;100:7063–8. doi: 10.1073/pnas.1136844100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen R, Hansen JP, Melchionna S. Molecular dynamics investigation of water permeation through nanopores. Journal of Chemical Physics. 2003;119:3905–3919. [Google Scholar]
- 5.Beckstein O, Tai K, Sansom MSP. Not Ions Alone: Barriers to Ion Permeation in Nanopores and Channels. Journal of the American Chemical Society. 2004;126:14694–14695. doi: 10.1021/ja045271e. [DOI] [PubMed] [Google Scholar]
- 6.Powell MR, Cleary L, Davenport M, Shea KJ, Siwy ZS. Electric-field-induced wetting and dewetting in single hydrophobic nanopores. Nat Nanotechnol. 2011;6:798–802. doi: 10.1038/nnano.2011.189. [DOI] [PubMed] [Google Scholar]
- 7.Dzubiella J, Allen RJ, Hansen JP. Electric field-controlled water permeation coupled to ion transport through a nanopore. Journal of Chemical Physics. 2004;120:5001–5004. doi: 10.1063/1.1665656. [DOI] [PubMed] [Google Scholar]
- 8.Guo W, Tian Y, Jiang L. Asymmetric ion transport through ion-channel-mimetic solid-state nanopores. Acc Chem Res. 2013;46:2834–46. doi: 10.1021/ar400024p. [DOI] [PubMed] [Google Scholar]
- 9.Beckstein O, Biggin PC, Bond P, Bright JN, Domene C, Grottesi A, Holyoake J, Sansom MSP. Ion channel gating: insights via molecular simulations. Febs Letters. 2003;555:85–90. doi: 10.1016/s0014-5793(03)01151-7. [DOI] [PubMed] [Google Scholar]
- 10.Kung C, Martinac B, Sukharev S. Mechanosensitive channels in microbes. Annu Rev Microbiol. 2010;64:313–29. doi: 10.1146/annurev.micro.112408.134106. [DOI] [PubMed] [Google Scholar]
- 11.Bass RB, Strop P, Barclay M, Rees DC. Crystal structure of Escherichia coli MscS, a voltage-modulated and mechanosensitive channel. Science. 2002;298:1582–7. doi: 10.1126/science.1077945. [DOI] [PubMed] [Google Scholar]
- 12.Anishkin A, Sukharev S. Water dynamics and dewetting transitions in the small mechanosensitive channel MscS. Biophys J. 2004;86:2883–95. doi: 10.1016/S0006-3495(04)74340-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sotomayor M, Schulten K. Molecular dynamics study of Gating in the mechanosensitive channel of small conductance MscS. Biophys J. 2004;87:3050–3065. doi: 10.1529/biophysj.104.046045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller S, Bartlett W, Chandrasekaran S, Simpson S, Edwards M, Booth IR. Domain organization of the MscS mechanosensitive channel of Escherichia coli. EMBO J. 2003;22:36–46. doi: 10.1093/emboj/cdg011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang W, Black SS, Edwards MD, Miller S, Morrison EL, Bartlett W, Dong C, Naismith JH, Booth IR. The structure of an open form of an E. coli mechanosensitive channel at 3.45 A resolution. Science. 2008;321:1179–83. doi: 10.1126/science.1159262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haswell ES, Phillips R, Rees DC. Mechanosensitive channels: what can they do and how do they do it? Structure. 2011;19:1356–69. doi: 10.1016/j.str.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson ME, Maksaev G, Haswell ES. MscS-like mechanosensitive channels in plants and microbes. Biochemistry. 2013;52:5708–22. doi: 10.1021/bi400804z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anishkin A, Akitake B, Kamaraju K, Chiang CS, Sukharev S. Hydration properties of mechanosensitive channel pores define the energetics of gating. J Phys Condens Matter. 2010;22:454120. doi: 10.1088/0953-8984/22/45/454120. [DOI] [PubMed] [Google Scholar]
- 19.Ou XR, Blount P, Hoffman RJ, Kung C. One face of a transmembrane helix is crucial in mechanosensitive channel gating. Proc Natl Acad Sci (USA) 1998;95:11471–11475. doi: 10.1073/pnas.95.19.11471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blount P, Moe PC. Bacterial mechanosensitive channels: integrating physiology, structure and function. Trends in Microbiology. 1999;7:420–424. doi: 10.1016/s0966-842x(99)01594-2. [DOI] [PubMed] [Google Scholar]
- 21.Yoshimura K, Batiza A, Schroeder M, Blount P, Kung C. Hydrophilicity of a single residue within MscL correlates with increased channel mechanosensitivity. Biophysical Journal. 1999;77:1960–1972. doi: 10.1016/S0006-3495(99)77037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Birkner JP, Poolman B, Kocer A. Hydrophobic gating of mechanosensitive channel of large conductance evidenced by single-subunit resolution. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:12944–12949. doi: 10.1073/pnas.1205270109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Unwin N. Acetylcholine-Receptor Channel Imaged in the Open State. Nature. 1995;373:37–43. doi: 10.1038/373037a0. [DOI] [PubMed] [Google Scholar]
- 24.Miyazawa A, Fujiyoshi Y, Unwin N. Structure and gating mechanism of the acetylcholine receptor pore. Nature. 2003;423:949–955. doi: 10.1038/nature01748. [DOI] [PubMed] [Google Scholar]
- 25.Beckstein O, Sansom MSP. A hydrophobic gate in an ion channel: the closed state of the nicotinic acetylcholine receptor. Physical Biology. 2006;3:147–159. doi: 10.1088/1478-3975/3/2/007. [DOI] [PubMed] [Google Scholar]
- 26.Sauguet L, Shahsavar A, Delarue M. Crystallographic studies of pharmacological sites in pentameric ligand-gated ion channels. Biochim Biophys Acta. 2014 doi: 10.1016/j.bbagen.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Hilf RJC, Dutzler R. X-ray structure of a prokaryotic pentameric ligand-gated ion channel. Nature. 2008;452:375–U12. doi: 10.1038/nature06717. [DOI] [PubMed] [Google Scholar]
- 28.Zimmermann I, Dutzler R. Ligand Activation of the Prokaryotic Pentameric Ligand-Gated Ion Channel ELIC. PLOS Biol. 2011;9 doi: 10.1371/journal.pbio.1001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bocquet N, Nury H, Baaden M, Le Poupon C, Changeux JP, Delarue M, Corringer PJ. X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature. 2009;457:111–114. doi: 10.1038/nature07462. [DOI] [PubMed] [Google Scholar]
- 30.Hilf RJC, Dutzler R. Structure of a potentially open state of a proton-activated pentameric ligand-gated ion channel. Nature. 2009;457:115–U122. doi: 10.1038/nature07461. [DOI] [PubMed] [Google Scholar]
- 31.Nury H, Poitevin F, Van Renterghem C, Changeux JP, Corringer PJ, Delarue M, Baaden M. One-microsecond molecular dynamics simulation of channel gating in a nicotinic receptor homologue. Proc Natl Acad Sci (USA) 2010;107:6275–6280. doi: 10.1073/pnas.1001832107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu F, Hummer G. Pore opening and closing of a pentameric ligand-gated ion channel. Proc Natl Acad Sci U S A. 2010;107:19814–9. doi: 10.1073/pnas.1009313107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu F, Hummer G. Drying transition in the hydrophobic gate of the GLIC channel blocks ion conduction. Biophys J. 2012;103:219–27. doi: 10.1016/j.bpj.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sauguet L, Shahsavar A, Poitevin F, Huon C, Menny A, Nemecz A, Haouz A, Changeux JP, Corringer PJ, Delarue M. Crystal structures of a pentameric ligand-gated ion channel provide a mechanism for activation. Proc Natl Acad Sci U S A. 2014;111:966–71. doi: 10.1073/pnas.1314997111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hibbs RE, Gouaux E. Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature. 2011;474:54–U80. doi: 10.1038/nature10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Catterall WA, Raman IM, Robinson HP, Sejnowski TJ, Paulsen O. The Hodgkin-Huxley heritage: from channels to circuits. J Neurosci. 2012;32:14064–73. doi: 10.1523/JNEUROSCI.3403-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 38.Jiang Y, Lee A, Chen J, Cadene M, Chait BT, MacKinnon R. The open pore conformation of potassium channels. Nature. 2002;417:523–6. doi: 10.1038/417523a. [DOI] [PubMed] [Google Scholar]
- 39.Payandeh J, Scheuer T, Zheng N, Catterall WA. The crystal structure of a voltage-gated sodium channel. Nature. 2011;475:353–8. doi: 10.1038/nature10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang L, Gamal El-Din TM, Payandeh J, Martinez GQ, Heard TM, Scheuer T, Zheng N, Catterall WA. Structural basis for Ca2+ selectivity of a voltage-gated calcium channel. Nature. 2014;505:56–61. doi: 10.1038/nature12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berneche S, Roux B. A gate in the selectivity filter of potassium channels. Structure. 2005;13:591–600. doi: 10.1016/j.str.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 42.McCoy JG, Nimigean CM. Structural correlates of selectivity and inactivation in potassium channels. Biochim Biophys Acta. 2012;1818:272–85. doi: 10.1016/j.bbamem.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Armstrong CM, Binstock L. Anomalous Rectification in the Squid Giant Axon Injected with Tetraethylammonium Chloride. J Gen Physiol. 1965;48:859–72. doi: 10.1085/jgp.48.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zimmerberg J, Parsegian VA. Polymer inaccessible volume changes during opening and closing of a voltage-dependent ionic channel. Nature. 1986;323:36–9. doi: 10.1038/323036a0. [DOI] [PubMed] [Google Scholar]
- 45.Jensen MO, Borhani DW, Lindorff-Larsen K, Maragakis P, Jogini V, Eastwood MP, Dror RO, Shaw DE. Principles of conduction and hydrophobic gating in K+ channels. Proc Natl Acad Sci U S A. 2010;107:5833–8. doi: 10.1073/pnas.0911691107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jensen MO, Jogini V, Borhani DW, Leffler AE, Dror RO, Shaw DE. Mechanism of voltage gating in potassium channels. Science. 2012;336:229–33. doi: 10.1126/science.1216533. [DOI] [PubMed] [Google Scholar]
- 47.Bruening-Wright A, Lee WS, Adelman JP, Maylie J. Evidence for a deep pore activation gate in small conductance Ca2+-activated K+ channels. J Gen Physiol. 2007;130:601–10. doi: 10.1085/jgp.200709828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Contreras JE, Srikumar D, Holmgren M. Gating at the selectivity filter in cyclic nucleotide-gated channels. Proc Natl Acad Sci U S A. 2008;105:3310–4. doi: 10.1073/pnas.0709809105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi N, Zeng W, Ye S, Li Y, Jiang Y. Crucial points within the pore as determinants of K(+) channel conductance and gating. J Mol Biol. 2011;411:27–35. doi: 10.1016/j.jmb.2011.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thompson J, Begenisich T. Selectivity filter gating in large-conductance Ca(2+)-activated K+ channels. J Gen Physiol. 2012;139:235–44. doi: 10.1085/jgp.201110748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomson AS, Rothberg BS. Voltage-dependent inactivation gating at the selectivity filter of the MthK K+ channel. J Gen Physiol. 2010;136:569–79. doi: 10.1085/jgp.201010507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Piechotta PL, Rapedius M, Stansfeld PJ, Bollepalli MK, Ehrlich G, Andres-Enguix I, Fritzenschaft H, Decher N, Sansom MS, Tucker SJ, Baukrowitz T. The pore structure and gating mechanism of K2P channels. EMBO J. 2011;30:3607–19. doi: 10.1038/emboj.2011.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roth R, Gillespie D, Nonner W, Eisenberg RE. Bubbles, gating, and anesthetics in ion channels. Biophys J. 2008;94:4282–98. doi: 10.1529/biophysj.107.120493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang Y, Lee A, Chen J, Cadene M, Chait BT, MacKinnon R. Crystal structure and mechanism of a calcium-gated potassium channel. Nature. 2002;417:515–22. doi: 10.1038/417515a. [DOI] [PubMed] [Google Scholar]
- 55.Ye S, Li Y, Jiang Y. Novel insights into K+ selectivity from high-resolution structures of an open K+ channel pore. Nat Struct Mol Biol. 2010;17:1019–23. doi: 10.1038/nsmb.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Enyedi P, Czirjak G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev. 2010;90:559–605. doi: 10.1152/physrev.00029.2009. [DOI] [PubMed] [Google Scholar]
- 57.Miller AN, Long SB. Crystal structure of the human two-pore domain potassium channel K2P1. Science. 2012;335:432–6. doi: 10.1126/science.1213274. [DOI] [PubMed] [Google Scholar]
- 58.Brohawn SG, del Marmol J, MacKinnon R. Crystal structure of the human K2P TRAAK, a lipid- and mechano-sensitive K+ ion channel. Science. 2012;335:436–41. doi: 10.1126/science.1213808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cohen A, Ben-Abu Y, Zilberberg N. Gating the pore of potassium leak channels. Eur Biophys J. 2009;39:61–73. doi: 10.1007/s00249-009-0457-6. [DOI] [PubMed] [Google Scholar]
- 60.Bagriantsev SN, Clark KA, Minor DL., Jr. Metabolic and thermal stimuli control K(2P)2.1 (TREK-1) through modular sensory and gating domains. EMBO J. 2012;31:3297–308. doi: 10.1038/emboj.2012.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aryal P, Abd-Wahab F, Bucci G, Sansom MS, Tucker SJ. A hydrophobic barrier deep within the pore of the TWIK-1 K2P potassium channel. Nat Comm. 2014 doi: 10.1038/ncomms5377. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chatelain FC, Bichet D, Feliciangeli S, Larroque MM, Braud VM, Douguet D, Lesage F. Silencing of the Tandem Pore Domain Halothane-inhibited K+ Channel 2 (THIK2) Relies on Combined Intracellular Retention and Low Intrinsic Activity at the Plasma Membrane. J Biol Chem. 2013;288:35081–92. doi: 10.1074/jbc.M113.503318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ben-Abu Y, Zhou Y, Zilberberg N, Yifrach O. Inverse coupling in leak and voltage-activated K+ channel gates underlies distinct roles in electrical signaling. Nat Struct Mol Biol. 2009;16:71–9. doi: 10.1038/nsmb.1525. [DOI] [PubMed] [Google Scholar]
- 64.Dong H, Fiorin G, Carnevale V, Treptow W, Klein ML. Pore waters regulate ion permeation in a calcium release-activated calcium channel. Proc Natl Acad Sci U S A. 2013;110:17332–7. doi: 10.1073/pnas.1316969110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nordin N, Guskov A, Phua T, Sahaf N, Xia Y, Lu S, Eshaghi H, Eshaghi S. Exploring the structure and function of Thermotoga maritima CorA reveals the mechanism of gating and ion selectivity in Co2+/Mg2+ transport. Biochem J. 2013;451:365–74. doi: 10.1042/BJ20121745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lopes PEM, Roux B, MacKerell AD. Molecular modeling and dynamics studies with explicit inclusion of electronic polarizability: theory and applications. Theoretical Chemistry Accounts. 2009;124:11–28. doi: 10.1007/s00214-009-0617-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kutzner C, Grubmuller H, de Groot BL, Zachariae U. Computational electrophysiology: the molecular dynamics of ion channel permeation and selectivity in atomistic detail. Biophys J. 2011;101:809–17. doi: 10.1016/j.bpj.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gupta S, Bavro VN, D’Mello R, Tucker SJ, Venien-Bryan C, Chance MR. Conformational changes during the gating of a potassium channel revealed by structural mass spectrometry. Structure. 2010;18:839–46. doi: 10.1016/j.str.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]