Abstract
Background
Electrodiagnosis can reveal the nerve and muscle changes following surgical placement of an extracellular matrix (ECM) bioscaffold for treatment of volumetric muscle loss (VML).
Objective
The purpose of this study was to characterize nerve conduction study (NCS) and electromyography (EMG) changes following ECM bioscaffold placement in individuals with VML. The ability of presurgical NCS and EMG to be used as a tool to help identify candidates who are likely to display improvements postsurgically also was explored.
Design
A longitudinal case series design was used.
Methods
The study was conducted at the McGowan Institute for Regenerative Medicine at the University of Pittsburgh. Eight individuals with a history of chronic VML participated. The intervention was surgical placement of an ECM bioscaffold at the site of VML. The strength of the affected region was measured using a handheld dynamometer, and electrophysiologic evaluation was conducted on the affected limb with standard method of NCS and EMG. All measurements were obtained the day before surgery and repeated 6 months after surgery.
Results
Seven of the 8 participants had a preoperative electrodiagnosis of incomplete mononeuropathy within the site of VML. After ECM treatment, 5 of the 8 participants showed improvements in NCS amplitude or needle EMG parameters. The presence of electrical activity within the scaffold remodeling site was concomitant with clinical improvement in muscle strength.
Limitations
This study had a small sample size, and participants served as their own controls. The electromyographers and physical therapists performing the evaluation were not blinded.
Conclusions
Electrodiagnostic data provide objective evidence of physiological improvements in muscle function following ECM placement at sites of VML. Future studies are warranted to further investigate the potential of needle EMG as a predictor of successful outcomes following ECM treatment for VML.
Volumetric muscle loss (VML) may be defined as an injury that results in a loss of more than 25% of muscle mass, often resulting in significant functional deficits.1 It is generally caused by trauma (including battlefield injuries2–4), degenerative disease, or tissue resection for removal of neoplasms.5 Volumetric muscle loss is associated with the deposition of scar tissue and denervation of the muscle distal to the defect.6 Unfortunately, treatment options for VML are limited and typically include scar tissue debridement, autologous tissue transfer, muscle transposition, free muscle flap creation, and, in the most severe cases, amputation. In addition, the outcome scores following these procedures are often low and reflect persistent functional impairment and the presence of abnormal sensation in a high percentage of individuals.7 The unfavorable outcomes, as well as the potential for donor site morbidity,7–9 underscore the need for the development of novel, more effective interventions to promote functional recovery after severe injuries.
Recently, the use of biologic scaffolds composed of extracellular matrix (ECM) has been evaluated as a treatment option for VML. The ECM is formed by the secreted products of resident cells in every tissue and organ. The ECM provides both a mechanical framework for structural support and an inductive substrate for modulating cellular responses.10 Preclinical studies have shown that ECM bioscaffolds facilitate constructive, site-appropriate tissue remodeling in musculotendinous,11,12 abdominal wall,13–15 urinary bladder,16 esophageal,17–19 and cardiac tissue20–22 applications.
A recent report described positive outcomes in 5 patients treated with an ECM scaffold for VML.1 In that study, actively regenerating myofibers were observed at the scaffold placement site at 6 months following surgery. In addition, there was an average improvement of 25% in muscle strength and 220% improvement in functional outcome measures. However, there was variability in the functional improvements among the 5 patients. Although some patients demonstrated marked improvements in both their strength and function, others showed no improvement in either measure. A better understanding of the relationship among people's baseline characteristics may aid in the identification of the most suitable candidates for this approach.
The restoration of nerves and the formation of new motor endplates within the ECM bioscaffold placement site are likely crucial to the success of this approach for skeletal muscle applications23–26 and may explain the variability among patients following ECM treatment for VML. Indeed, immunolabeling findings have demonstrated the presence of reinnervating tissue in ECM-treated patients in multiple body systems.1,27,28 However, it is not yet clear in humans whether any new nerve tissue is functional and how this finding may relate to functional outcomes.
Electrophysiologic evaluation is widely used to evaluate the function of the motor units29 by recording nerve action potentials and motor unit action potentials (MUAPs) using either surface or intramuscular techniques.30,31 Such techniques are commonly used to evaluate muscle activity in healthy people,32–34 patients with muscle dysfunction,31,35 and patients with muscle injuries.36–38 Recently, 2 preclinical studies have used electrophysiologic evaluation to evaluate reinnervation and muscle activation in murine and dog models of ECM treatment for VML. Six months after surgery, remodeling at the ECM placement site showed more rapid recruitment and a profile similar to the muscle activation observed in uninjured muscle in mice.1 In addition, when compared with untreated VML defects, recruited muscle fibers were faster and had greater peak-to-peak voltages.1 In the dog model, electrical response and twitches were observed in the ECM-treated site in one animal 3 months after surgery, suggesting the formation of contractile myofibers.6
Given that nerve conduction study (NCS) and needle electromyography (EMG) are recognized standards for diagnosis of neurophysiologic abnormalities,30 a combination of NCS and EMG was used for the electrophysiologic evaluation of VML sites treated with ECM. In addition, we performed a descriptive analysis to explore the potential of presurgical NCS and EMG to serve as a predictor of postsurgical outcomes. This study tested our hypotheses that NCS and EMG improvements would be observed following ECM implantation and that baseline NCS and EMG findings would be concomitant with changes of strength postoperatively.
Method
Setting and Participants
A total of 8 participants (including 5 individuals who were included in a previous report1) were included. Participant recruitment, inclusion and exclusion criteria, and the study protocol have been previously described.1 This study was conducted with informed patient consent and approvals from the Institutional Review Board of the University of Pittsburgh and the US Department of Defense Human Research Protection Office (ClinicalTrials.gov identifier: NCT01292876).
Three participants had muscle loss and functional deficit of the anterior tibial compartment of the leg, 4 had VML of the quadriceps muscle compartment, and 1 had sustained VML of the biceps brachii muscle. The extent of muscle loss was determined by magnetic resolution image or computed tomography. Strength of target muscles was assessed by a licensed physical therapist using a handheld dynamometer on the day immediately prior to surgery and again 6 months postoperatively. The strength was recorded as the average of 3 trials. Successful improvement in strength was operationally defined a priori as an increase of ≥20% compared with maximum preoperative values.
All participants were entered into a physical therapy program preoperatively. They participated in physical therapy until they reached a plateau in strength and functional capacity, defined as an improvement of <2% in the primary outcome variables over the course of 2 weeks. Following plateau, participants were then surgically implanted with an ECM bioscaffold.
Surgical Procedure
Through an open incision, debridement of scar tissue with selective tenolysis was performed, as previously described.1 For the reconstruction of injured muscle compartment, the ECM scaffold was implanted within the VML injury site. At times, the implanted scaffold spanned multiple muscles, such as in the case of quadriceps injuries (participants 4–7). The ECM device (MatriStem, ACell Inc, Columbia, Maryland; Biodesign, Cook Medical, Bloomington, Indiana) consisted of specific layers of decellularized porcine urinary bladder or small intestinal submucosa. Within 48 hours postoperatively, a physical therapy program was initiated. The program was based on the preoperative physical therapy program and progressed according to participants' pain limitations. Strength was measured on the day immediately prior to surgery and again 6 months postoperatively.
NCS and EMG Recording
Nerve conduction study and EMG recording were performed at the site of injury at both baseline and 6 months after ECM placement using a Synergy EMG machine (Cardinal Health, Dublin, Ohio). One of two electromyographers completed all studies. Both electromyographers have more than 15 years of experience and are certified by the American Board of Electrodiagnostic Medicine. Standardized NCSs were performed with monitoring of limb temperature. For motor studies, latencies were measured at 5.5 cm from the distal stimulation to recording site in the hand and 8 cm in the foot. For proximal motor studies, standard distances were used based on laboratory protocol. For needle EMG, concentric needle electrodes were used. The needle was placed in the standard muscle belly according to the anatomical guide.39 Needle EMG was performed repeatedly at the proximal and distal sites of the injured muscle if the standardized muscle belly site did not show evidence of electrical activity. After the conventional needle EMG, motor unit potential traces were obtained at the target muscle in one participant to explore the characteristics of new tissue, and a decomposition-based quantitative EMG (DQEMG) algorithm was applied to calculate jiggle, near fiber dispersion, and maximal near fiber interval.
Nerve conduction study improvement was defined as an increase in motor nerve conduction amplitude greater than 20%. For EMG analysis, positive change was defined as either (1) evidence of an increased firing in volitional recruitment of target muscles or (2) a decrease in abnormal spontaneous activity (ASA) compared with prior surgery.
Data Analysis
Frequency counts were calculated and summarized for nominal variables, and measures of central tendency (means, medians) and dispersion (standard deviations, interquartile ranges) were calculated for continuous variables showing demographic information of participants.
Role of the Funding Source
The US Department of Defense's Limb Salvage and Regenerative Medicine Initiative and the Muscle Tendon Tissue Unit Repair and Reinforcement Reconstructive Surgery Research study is collaboratively managed by the Office of the Secretary of Defense. The Initiative is focused on rapidly and safely transitioning advanced medical technology in commercially viable capabilities to provide our wounded warriors the safest and most advanced care possible today. Dr Han was supported by a grant (#20140100) from Inje University.
Results
Participant Demographics
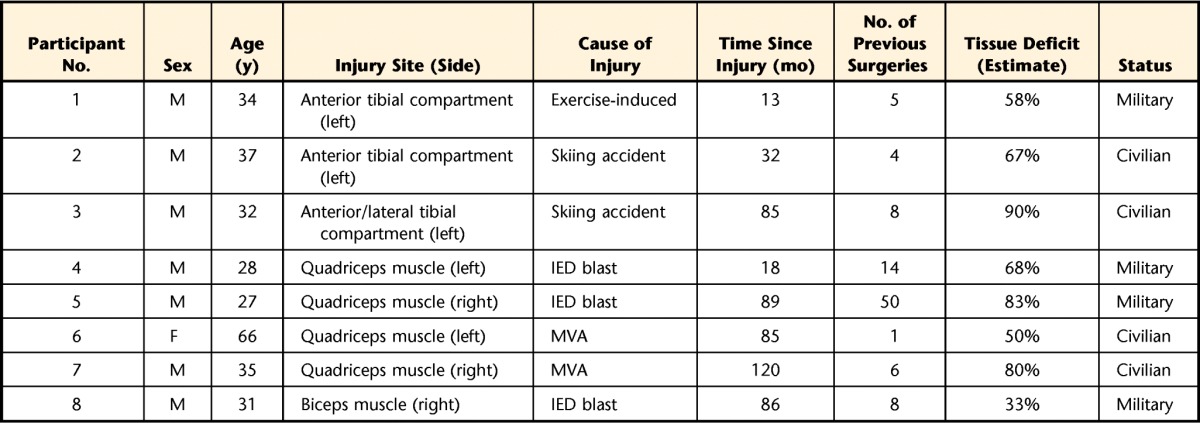
The mean age of the 8 participants was 32 years. The average time between injury to surgery was 5.5 years (SD=3.3), and the average percentage of estimated tissue loss (determined by magnetic resolution image or computed tomography scan) was 66.1% (SD=18.8%) (Tab. 1).
Table 1.
Participant Demographic Informationa
M=male, F=female, IED=improvised explosive device, MVA=motor vehicle accident.
Preoperative NCS and EMG
Seven of the 8 participants showed patterns of mononeuropathies in their involved limbs at baseline testing. Three participants with anterior compartment injuries in the lower leg showed deep peroneal mononeuropathy. Three of 4 participants with quadriceps muscle injury showed femoral mononeuropathy, whereas 1 individual with a quadriceps muscle injury displayed no abnormal finding. The individual with a biceps brachii injury showed musculocutaneous mononeuropathy. The mononeuropathies observed in 7 participants were incomplete patterns. The distributions of abnormalities were limited to the area of the muscle injury site and did not extend distally along the nerve distribution as in typical nerve injury. A few involved muscles were totally denervated (ie, tibialis anterior [TA] muscle of participants 1 and 2, vastus medialis [VM] muscle of participants 4 and 5, and vastus intermedius muscle of participant 5; Tab. 2). However, the majority of the muscles were only partially denervated. Some muscles were preserved at the proximal portion, but the distal portion showed no motor unit activity.
Table 2.
Clinical NCS and EMG and Muscle Strength of Treated Musclesa
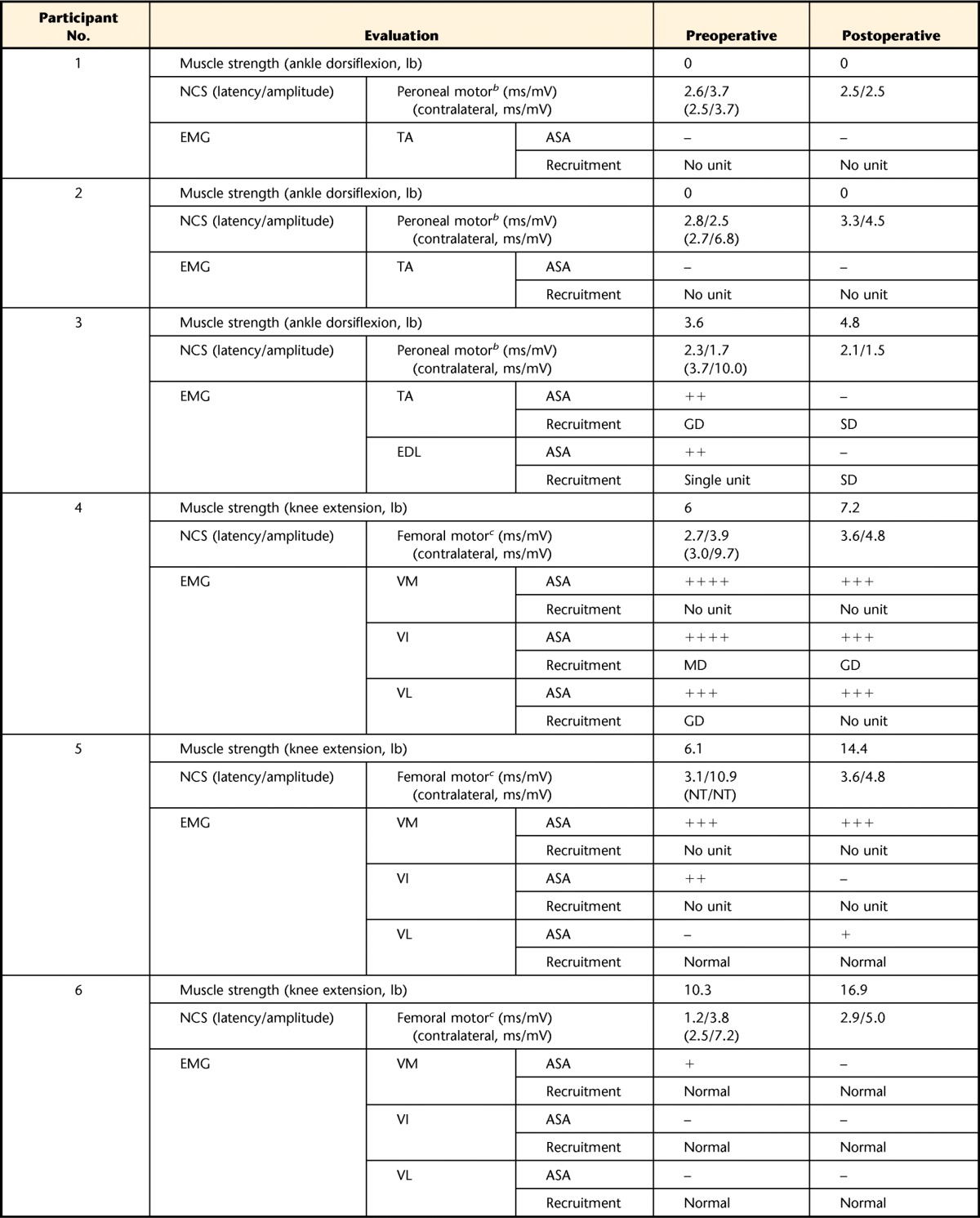
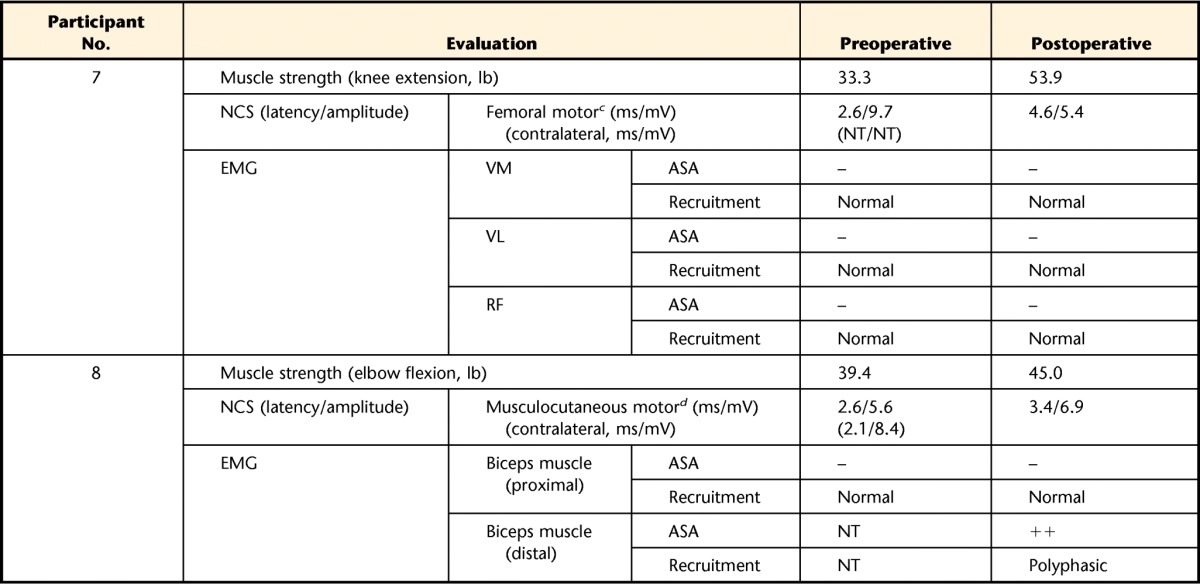
NCS=nerve conduction study, EMG=electromyography, ASA=abnormal spontaneous activity, –=not observed, +=persistent single trains in at least 2 muscle regions,++=moderate numbers in 3 or more muscle areas, +++=many in all muscle regions, ++++=baseline obliterated with fibrillation potentials in all areas of muscle examined, GD=greatly decreased, MD=moderately decreased, SD=slightly decreased, TA=tibialis anterior muscle, EDL=extensor digitorum longus muscle, VM=vastus medialis muscle, VI=vastus intermedius muscle, VL=vastus lateralis muscle, RF=rectus femoris muscle, NT=not tested.
b Stimulated at fibular head and recorded on TA.
c Stimulated above inguinal ligament and recorded on VM.
d Stimulated at axilla and recorded on biceps brachii muscle.
Postoperative Findings
Strength.
Among the total of 8 participants, including 5 individuals for whom data have been previously reported,1 6 participants showed some increase in strength of the treated muscles between preoperative and postoperative time points. However, 2 of the 6 participants demonstrated an increase in strength of less than 0.91 kg (2 lb), within the range of measurement error. Two participants showed no change. The range of strength gain was between 0% and 136.1%, and the average was a 41.2% increase in strength.
NCS and EMG.
Four participants showed increased amplitudes of compound muscle action potential (CMAP) recorded in target muscles: 1 in the TA (participant 2), 2 in the VM (participants 4 and 6), and 1 in the biceps brachii muscle (participant 8). The remaining participants showed either no change (participants 1 and 3) or a decreased amplitude (participants 5 and 7) in NCS findings. For the needle EMG study, improvements were detected in 2 categories. The first was the disappearance of ASA after the surgery, as shown in the TA and extensor digitorum longus (EDL) muscle of participant 3 and the VM of participant 6. Other participants showed no remarkable change in ASA appearance. The second improvement was an enhanced recruitment pattern of the involved muscle. The TA and EDL of participant 3 showed an improved recruitment pattern after surgery compared with the baseline findings of greatly decreased recruitment pattern and single motor unit firing (Tab. 2). In contrast, participant 4 presented decreased recruitment pattern in the vastus lateralis muscle, and other participants showed no change in recruitment pattern of the treated muscle.
Strength and electrodiagnostic alterations for each subject are presented in Table 2. Among nerves and muscles examined during clinical NCS and EMG, the target muscles and the most involved nerves innervating target muscles are included in the table.
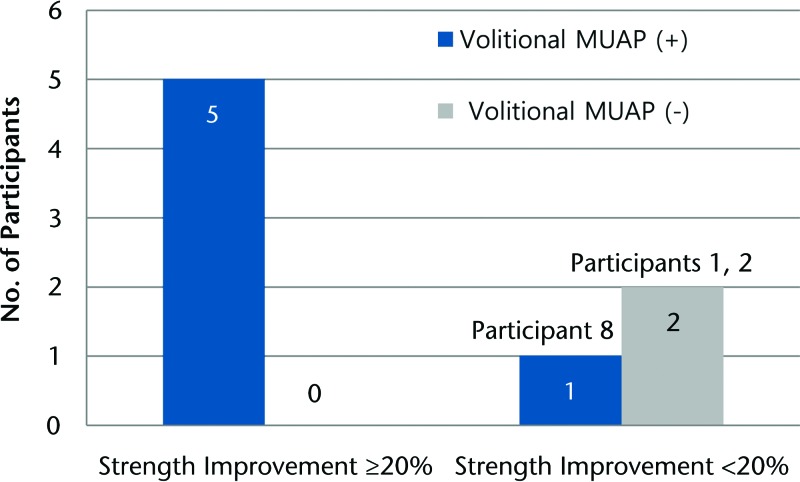
The mean CMAP amplitude of baseline NCS was 5.225 mV, and that of postoperative NCS was 4.425 mV. Five of 6 participants who showed EMG activity at baseline showed successful improvement in strength (20% or more improvement) at an average of approximately 6 months after ECM implantation surgery (Figure). These findings suggest that muscles judged to have no electrical activity at baseline are unlikely to display improved strength following ECM implantation.
Figure.
Volitional motor unit action potential (MUAP) at baseline electromyography testing and improvement of strength.
Discussion
Extracellular matrix bioscaffolds have been proposed as a viable treatment option for VML. However, there is considerable variability associated with the individual impairment and downstream functional responses to such an intervention. The reason for this variability is still unknown, but it is likely attributable, at least in part, to differences in the degree and type of initial injury. To our knowledge, the change in physiologic tissue function following ECM implantation has never previously been measured in humans using electrodiagnostic tools. The present study characterized alterations in motor activity in 8 individuals with chronic VML who had failed all attempts at traditional treatment approaches and who subsequently were treated by implantation of an ECM bioscaffold.
Five of the 8 participants showed improved electrophysiological function. In participants 4 and 6, the amplitudes of femoral CMAP increased more than 20% between preoperative and postoperative time points, even though the amplitude remained relatively low compared with that of the uninjured contralateral limbs. Participant 8 also exhibited greater than 20% improvement of CMAP amplitude of the musculocutaneous nerve, resulting in an amplitude that is considered to be “normal,” compared with the uninjured contralateral side. The strength of these 3 individuals was improved concomitantly with their CMAP improvement. However, not all electrophysiological improvements coincided with a detectable increase in strength. In participant 2, the amplitude of the deep peroneal CMAP recorded in the TA increased by 80%, but the ankle dorsiflexor strength showed no change. This finding suggests that the primary defect in force-producing capacity of this individual was not solely attributed to decreased motor activity. Participants 1 and 3 showed no improvement of CMAP amplitude, even though participant 3 showed a 33.3% improvement in strength. Unexpectedly, the CMAP of 2 participants (participants 5 and 7) decreased after ECM placement, despite the fact that they both demonstrated a dramatic increase in strength 6 months postoperatively. In these cases, it is possible that the increased strength was a result of a restoration of mechanical integrity, rather than electrical conductance, of the muscle after ECM implantation. The effect of ECM implantation on the structural characteristics of muscles with VML and how this effect may contribute to muscle function would be an interesting area for future investigations. Taken together, the changes in NCS observed in this study did not show a linear correlation with strength.
We cannot exclude the possibility that muscle strength improvements may be the result of other aspects of the surgical procedure, such as fibrosis debridement or tenolysis. However, the fact that many of the participants had extensive past medical histories of surgical procedures that were likely to have included debridement or tenolysis suggests that the improvements observed postoperatively were likely a result of ECM implantation and not solely other aspects of the surgery.
The improvement of CMAP amplitude observed in 4 of the 8 participants in this study is encouraging, as changes in CMAP amplitude are known to be the result of changes intrinsic to muscle tissue, as opposed to alterations in the conduction velocity or distal motor latency.40 The amplitude of CMAP relies on the amount of action potential detected at the recording site. An increased amplitude indicates that the action potential generated by the muscle fiber is increased, a result that may be interpreted as an increased number of muscle fibers at the location of the recording area. These findings are consistent with previous findings by Sicari et al, which showed histologic evidence of actively regenerating skeletal muscle cells present at the site of remodeling ECM bioscaffolds implanted for the treatment of VML.1 Other studies dealing with chronic muscle atrophy caused by poliomyelitis or spinal cord injury showed that chronic atrophied muscles rarely experience hyperplasia.41,42 Considering the fact that the participants were also in a state of chronic muscle atrophy as a result of VML, the change of CMAP amplitude observed in this study was most likely associated with ECM-induced effects.
Analysis of needle EMG findings showed that 2 participants had some improvements in target muscles. In participant 3, the ASA observed in the EDL and TA was resolved and recruitment patterns of both muscles were improved. In participant 6, ASA disappeared and the recruitment pattern was normalized without polyphonic potentials in the vastus lateralis muscle. These 2 participants showed increased motor strength of treated muscle after surgery. Among the 4 remaining participants with a baseline EMG deficit who did not show improvement in needle EMG at follow-up, 2 (participants 1 and 2) demonstrated no improvement in strength following ECM placement.
Needle EMG generally reveals 2 aspects of electrophysiology related to muscle fibers: (1) instability of muscle fibers, expressed as ASA, and (2) recruitment pattern, expressed as MUAPs. In 2 of 4 participants displaying ASA in baseline EMG activity, ASA was decreased or diminished at the follow-up study, and the change was accompanied with an improved recruitment. Similarly, although the improvements of MUAPs observed were generally associated with increased strength, this was not the case for all participants. This mismatch is consistent with previous studies demonstrating that EMG does not correlate linearly with strength.43 Indeed, the EMG signal can be influenced by various factors other than the muscle fibers, including nerve conduction properties and neuromuscular junction activity. As such, when considering functional outcomes, clinical EMG should be considered to be a complementary tool.
One remarkable finding was that polyphasic MUAPs and ASA were observed in the site of matrix implantation. Abnormal spontaneous activity is a marker of membrane instability and is generally considered to be an abnormal sign. It typically decreases over the course of reinnervation and disappears with complete healing.44,45 Aside from successful somatic reinnervation, ASA also can disappear due to muscle atrophy.45 In the case of participant 8, ASA was detected at the distal site of the biceps muscle postoperatively. Of note, for this individual, preoperative EMG was performed only in the proximal biceps muscle because the mid-to-distal belly of the biceps muscle was deemed to consist only of fibrotic tissue on musculoskeletal ultrasonography. As such, the baseline needle EMG finding at the proximal biceps muscle was qualified as “normal.” However, for the postoperative evaluation, the needle EMG was performed at the site of matrix placement (mid-to-distal belly) in order to explore new tissue. Although a direct comparison with preoperative values is not possible, the activities observed postoperatively suggest that the area is in the process of reinnervation. As the implanted matrix is completely devoid of cells, the fact that activity was detected in the distal biceps muscle at 6 months after implantation suggests that the recruitment of endogenous cells at the ECM placement site may be responsible for the observed increased activity.
Polyphasic MUAPs are also well known to result from newly reinnervated motor fibers.46 To identify the character of needle EMG findings of the lesion in participant 8, decomposition-based quantitative EMG (DQEMG) was performed with particular focus on the region of ECM placement. There were many polyphonic potentials with decreased recruitment and rapid firing rates at 20 to 25 Hz. Using quantitative EMG, near fiber jiggle was greatly elevated at 83.5%, compared with 33.8% (SD=3.7%), which is the reported normal value.47 Jiggle is used to express the variability of the shape of the MUAPs based on the consecutive amplitude and shape difference, and it is the result of highly immature neuromuscular connections.48 Postoperatively, participant 8 displayed near fiber dispersion and the maximal near fiber interval, as calculated by DQEMG, of 11.0 and 5.6 milliseconds, respectively. Near fiber dispersion is the time interval between the first and the last detected fibers' contribution to the near fiber motor unit potential, and the normal value has been reported to be 1.7 milliseconds (SD=0.4).47 Maximal near fiber interval is the maximum time between consecutive detected near fiber distributions, reflecting the length of axonal sprout, and the value observed in the “normal” population is reported to be 1.0 milliseconds (SD=0.2).47 Therefore, the DQEMG data observed in participant 8 are consistent with immature, recent innervation. This additional evaluation at the site of ECM placement provides interesting electrophysiologic evidence that suggests ongoing motor unit regeneration.
In a previous study, EMG detected the improvement of muscle activity after ECM treatment in a murine model of VML.1 The maximal EMG amplitude was increased, and the recruitment rate also was increased in ECM-treated animals. Direct comparison with the current study is not possible given that the electrodes used in the mice were insulated and because different parameters, such as recruitment rate and peak-to-peak voltage of MUAP, were applied for the animal model. In addition, the present results revealed considerable variability in participants' responses. For example, 3 participants (participants 4, 5, and 7) showed no improvement in EMG or NCS findings, despite the fact that an improvement in muscle strength was observed (Tab. 3).
Table 3.
Relationship of Muscle Strength Change (≥20% Improvement From Baseline) and Clinical NCS and EMG Dataa
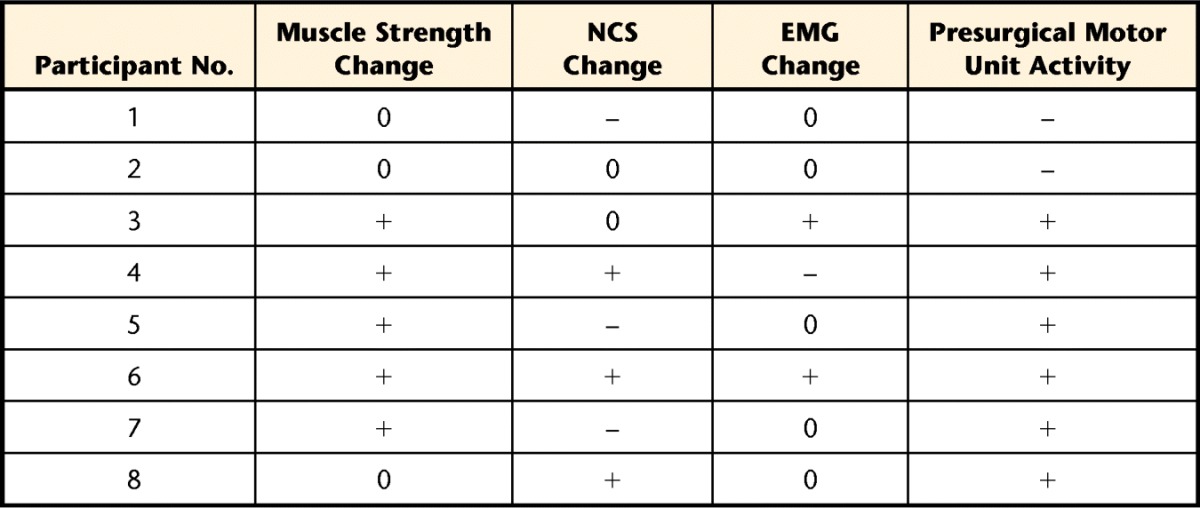
NCS=nerve conduction study, EMG=electromyography, 0=no change, –=absent activity or negative change, +=present activity or positive change.
We also explored the potential utility of preoperative EMG as a predictor of outcome following surgery. For this investigation, participants were dichotomized according to whether they met our a priori criterion for success (ie, ≥20% improvement). Among parameters of needle EMG, the presence of MUAPs in preoperative evaluation appeared to be associated with an increased likelihood for strength improvement. Neither of the electrically silent muscles at baseline (participants 1 and 2) demonstrated gains in electrical activity after ECM implantation (Tab. 3). In contrast, some muscles that had greatly decreased motor unit activity, such as the EDL of participant 3, presented greatly enriched activity postoperatively. Participant 8 was the only individual who did display electrical muscle activity preoperatively but did not meet the criterion for success. However, he did demonstrate a 14% increase in strength postoperatively. These findings suggest that the absence of MUAP in target muscle preoperatively may be indicative of an unfavorable outcome after ECM treatment for VML. Future studies with an increased sample size are warranted.
The presence of ASA, especially fibrillation potentials, has been suggested to be a key prognostic factor of successful recovery after nerve repair surgery.45 Among key changes in prolonged denervated muscles, fibrillation potentials have the tendency to persist as long as denervated muscles retain a blood supply.49 Likewise, resolution of fibrillation potentials indicates total dystrophic change of muscle.50 However, the ASA in our study did not consistently relate to the improvement in strength because the absence of ASA also can be indicative of electrical stability, especially when accompanied by MUAPs with a normal recruitment pattern.
The fact that this is a case series and the small sample size are limitations of the study. In addition, the inclusion of evaluation measures for chronic VML at longer time points may reveal important information regarding the constructive muscle remodeling following ECM implantation. Finally, methods for standardized electrodiagnostic evaluation at the site of injury and ECM placement should be developed. As presented in Table 3, not all data changed from baseline to postoperatively in a synchronized way. Because of the massive loss of muscle and surrounding tissue, traditional methods may not allow for sufficient information at the site of active remodeling. When the injury changes the muscle tissue structure anatomically or volumetrically, NCS and EMG examination should be conducted at both a standard anatomic location and at the modified location according to the patient's postoperative anatomy. Finally, the electromyographers and physical therapists were not blinded to the treatment time point, which may have affected results.
In conclusion, this case series study provides preliminary findings that electrodiagnostic testing provides objective evidence of physiological improvements in muscle function following ECM placement at sites of VML. These findings have relevance to physical therapist practice, as an improved understanding of physiological changes following ECM implantation into regions of severe muscle injuries may help support the rational design of regenerative rehabilitation protocols following this tissue engineering approach. Finally, the data provide insight as to the importance of baseline innervation status when selecting the most suitable candidate for ECM implantation in the treatment of VML.
Footnotes
Dr Han, Mr Yabroudi, Dr Stearns-Reider, Dr Sicari, Dr Rubin, Dr Badylak, Dr Boninger, and Dr Ambrosio provided concept/idea/research design and writing. Dr Stearns-Reider, Dr Helkowski, Dr Badylak, and Dr Ambrosio provided data collection. Dr Han, Mr Yabroudi, Dr Sicari, Dr Rubin, Dr Badylak, Dr Boninger, and Dr Ambrosio provided data analysis. Dr Boninger, Dr Badylak, Dr Rubin, and Dr Ambrosio provided project management. Dr Rubin and Dr Badylak provided fund procurement. Dr Rubin, Dr Badylak, and Dr Boninger provided participants. Dr Badylak provided facilities/equipment and administrative support. Dr Stearns-Reider, Dr Helkowski, Dr Sicari, Dr Rubin, Dr Badylak, and Dr Boninger provided consultation (including review of manuscript before submission).
The US Department of Defense's Limb Salvage and Regenerative Medicine Initiative and the Muscle Tendon Tissue Unit Repair and Reinforcement Reconstructive Surgery Research study is collaboratively managed by the Office of the Secretary of Defense. The Initiative is focused on rapidly and safely transitioning advanced medical technology in commercially viable capabilities to provide our wounded warriors the safest and most advanced care possible today. Dr Han was supported by a grant (#20140100) from Inje University.
References
- 1. Sicari BM, Rubin JP, Dearth CL, et al. An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss. Sci Transl Med. 2014;6:234–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lew TA, Walker JA, Wenke JC, et al. Characterization of craniomaxillofacial battle injuries sustained by United States service members in the current conflicts of Iraq and Afghanistan. J Oral Maxillofac Surg. 2010;68:3–7. [DOI] [PubMed] [Google Scholar]
- 3. Mazurek MT, Ficke JR. The scope of wounds encountered in casualties from the global war on terrorism: from the battlefield to the tertiary treatment facility. J Am Acad Orthop Surg. 2006;14:S18–S23. [DOI] [PubMed] [Google Scholar]
- 4. Owens BD, Kragh JF, Jr, Wenke JC, et al. Combat wounds in operation Iraqi Freedom and operation Enduring Freedom. J Trauma. 2008;64:295–299. [DOI] [PubMed] [Google Scholar]
- 5. Sicari BM, Agrawal V, Siu BF, et al. A murine model of volumetric muscle loss and a regenerative medicine approach for tissue replacement. Tissue Eng Part A. 2012;18:1941–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Turner NJ, Badylak JS, Weber DJ, et al. Biologic scaffold remodeling in a dog model of complex musculoskeletal injury. J Surg Res. 2012;176:490–502. [DOI] [PubMed] [Google Scholar]
- 7. Klinkenberg M, Fischer S, Kremer T, et al. Comparison of anterolateral thigh, lateral arm, and parascapular free flaps with regard to donor-site morbidity and aesthetic and functional outcomes. Plast Reconstr Surg. 2013;131:293–302. [DOI] [PubMed] [Google Scholar]
- 8. Deutinger M, Kuzbari R, Paternostro-Sluga T, et al. Donor-site morbidity of the gracilis flap. Plast Reconstr Surg. 1995;95:1240–1244. [DOI] [PubMed] [Google Scholar]
- 9. Kimata Y, Uchiyama K, Ebihara S, et al. Anterolateral thigh flap donor-site complications and morbidity. Plast Reconstr Surg. 2000;106:584–589. [DOI] [PubMed] [Google Scholar]
- 10. Brown BN, Badylak SF. Extracellular matrix as an inductive scaffold for functional tissue reconstruction. Transl Res. 2014;163:268–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Turner NJ, Yates AJ, Jr, Weber DJ, et al. Xenogeneic extracellular matrix as an inductive scaffold for regeneration of a functioning musculotendinous junction. Tissue Eng Part A. 2010;16:3309–3317. [DOI] [PubMed] [Google Scholar]
- 12. Valentin JE, Turner NJ, Gilbert TW, et al. Functional skeletal muscle formation with a biologic scaffold. Biomaterials. 2010;31:7475–7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clarke KM, Lantz GC, Salisbury SK, et al. Intestine submucosa and polypropylene mesh for abdominal wall repair in dogs. J Surg Res. 1996;60:107–114. [DOI] [PubMed] [Google Scholar]
- 14. Badylak S, Kokini K, Tullius B, et al. Morphologic study of small intestinal submucosa as a body wall repair device. J Surg Res. 2002;103:190–202. [DOI] [PubMed] [Google Scholar]
- 15. Brown BN, Valentin JE, Stewart-Akers AM, et al. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials. 2009;30:1482–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kropp BP, Rippy MK, Badylak SF, et al. Regenerative urinary bladder augmentation using small intestinal submucosa: urodynamic and histopathologic assessment in long-term canine bladder augmentations. J Urol. 1996;155:2098–2104. [DOI] [PubMed] [Google Scholar]
- 17. Badylak SF, Vorp DA, Spievack AR, et al. Esophageal reconstruction with ECM and muscle tissue in a dog model. J Surg Res. 2005;128:87–97. [DOI] [PubMed] [Google Scholar]
- 18. Nieponice A, McGrath K, Qureshi I, et al. An extracellular matrix scaffold for esophageal stricture prevention after circumferential EMR. Gastrointest Endosc. 2009;69:289–296. [DOI] [PubMed] [Google Scholar]
- 19. Badylak SF, Hoppo T, Nieponice A, et al. Esophageal preservation in five male patients after endoscopic inner-layer circumferential resection in the setting of superficial cancer: a regenerative medicine approach with a biologic scaffold. Tissue Eng Part A. 2011;17:1643–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Badylak S, Obermiller J, Geddes L, et al. Extracellular matrix for myocardial repair. Heart Surg Forum. 2003;6:E20–E26. [DOI] [PubMed] [Google Scholar]
- 21. Kochupura PV, Azeloglu EU, Kelly DJ, et al. Tissue-engineered myocardial patch derived from extracellular matrix provides regional mechanical function. Circulation. 2005;112:I144–I149. [DOI] [PubMed] [Google Scholar]
- 22. Kelly DJ, Rosen AB, Schuldt AJ, et al. Increased myocyte content and mechanical function within a tissue-engineered myocardial patch following implantation. Tissue Eng Part A. 2009;15:2189–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grubic Z, Komel R, Walker WF, et al. Myoblast fusion and innervation with rat motor nerve alter distribution of acetylcholinesterase and its mRNA in cultures of human muscle. Neuron. 1995;14:317–327. [DOI] [PubMed] [Google Scholar]
- 24. Lefeuvre B, Crossin F, Fontaine-Perus J, et al. Innervation regulates myosin heavy chain isoform expression in developing skeletal muscle fibers. Mech Dev. 1996;58:115–127. [DOI] [PubMed] [Google Scholar]
- 25. Washabaugh CH, Ontell MP, Shan Z, et al. Role of the nerve in determining fetal skeletal muscle phenotype. Dev Dyn. 1998;211:177–190. [DOI] [PubMed] [Google Scholar]
- 26. Donghui C, Shicai C, Wei W, et al. Functional modulation of satellite cells in long-term denervated human laryngeal muscle. Laryngoscope. 2010;120:353–358. [DOI] [PubMed] [Google Scholar]
- 27. Caione P, Capozza N, Zavaglia D, et al. In vivo bladder regeneration using small intestinal submucosa: experimental study. Pediatr Surg Int. 2006;22:593–599. [DOI] [PubMed] [Google Scholar]
- 28. Agrawal V, Brown BN, Beattie AJ, et al. Evidence of innervation following extracellular matrix scaffold-mediated remodelling of muscular tissues. J Tissue Eng Regen Med. 2009;3:590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee DH, Claussen GC, Oh S. Clinical nerve conduction and needle electromyography studies. J Am Acad Orthop Surg. 2004;12:276–287. [DOI] [PubMed] [Google Scholar]
- 30. Robinson LR. Role of neurophysiologic evaluation in diagnosis. J Am Acad Orthop Surg. 2000;8:190–199. [DOI] [PubMed] [Google Scholar]
- 31. Suvinen TI, Kemppainen P. Review of clinical EMG studies related to muscle and occlusal factors in healthy and TMD subjects. J Oral Rehabil. 2007;34:631–644. [DOI] [PubMed] [Google Scholar]
- 32. Rilo B, Santana U, Mora MJ, et al. Myoelectrical activity of clinical rest position and jaw muscle activity in young adults. J Oral Rehabil. 1997;24:735–740. [DOI] [PubMed] [Google Scholar]
- 33. Reinold MM, Wilk KE, Fleisig GS, et al. Electromyographic analysis of the rotator cuff and deltoid musculature during common shoulder external rotation exercises. J Orthop Sports Phys Ther. 2004;34:385–394. [DOI] [PubMed] [Google Scholar]
- 34. Reinold MM, Macrina LC, Wilk KE, et al. Electromyographic analysis of the supraspinatus and deltoid muscles during 3 common rehabilitation exercises. J Athl Train. 2007;42:464–469. [PMC free article] [PubMed] [Google Scholar]
- 35. Corti M, Smith BK, Falk DJ, et al. Altered activation of the tibialis anterior in individuals with Pompe disease: implications for motor unit dysfunction. Muscle Nerve. 2015;51:877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fan C, Jiang P, Fu L, et al. Functional reconstruction of traumatic loss of flexors in forearm with gastrocnemius myocutaneous flap transfer. Microsurgery. 2008;28:71–75. [DOI] [PubMed] [Google Scholar]
- 37. Sole G, Milosavljevic S, Nicholson HD, et al. Selective strength loss and decreased muscle activity in hamstring injury. J Orthop Sports Phys Ther. 2011;41:354–363. [DOI] [PubMed] [Google Scholar]
- 38. Opar DA, Williams MD, Timmins RG, et al. Rate of torque and electromyographic development during anticipated eccentric contraction is lower in previously strained hamstrings. Am J Sports Med. 2013;41:116–125. [DOI] [PubMed] [Google Scholar]
- 39. Perotto A, Delagi EF. Anatomical Guide for the Electromyographer: The Limbs and Trunk. 3rd ed. Springfield, IL: Charles C Thomas; 1994. [Google Scholar]
- 40. Sims MH. Electrodiagnostic techniques in the evaluation of diseases affecting skeletal muscle. Vet Clin North Am Small Anim Pract. 1983;13:145–162. [DOI] [PubMed] [Google Scholar]
- 41. Kirshblum S, Lim S, Garstang S, et al. Electrodiagnostic changes of the lower limbs in subjects with chronic complete cervical spinal cord injury. Arch Phys Med Rehabil. 2001;82:604–607. [DOI] [PubMed] [Google Scholar]
- 42. McComas AJ, Quartly C, Griggs RC. Early and late losses of motor units after poliomyelitis. Brain. 1997;120(pt 8):1415–1421. [DOI] [PubMed] [Google Scholar]
- 43. Warren GL, Ingalls CP, Shah SJ, et al. Uncoupling of in vivo torque production from EMG in mouse muscles injured by eccentric contractions. J Physiol. 1999;515(pt 2):609–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Buchthal F. Spontaneous electrical activity: an overview. Muscle Nerve. 1982;5:S52–S59. [PubMed] [Google Scholar]
- 45. Wu P, Chawla A, Spinner RJ, et al. Key changes in denervated muscles and their impact on regeneration and reinnervation. Neural Regen Res. 2014;9:1796–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Borenstein S, Desmedt JE. Range of variations in motor unit potentials during reinnervation after traumatic nerve lesions in humans. Ann Neurol. 1980;8:460–467. [DOI] [PubMed] [Google Scholar]
- 47. Allen MD, Stashuk DW, Kimpinski K, et al. Increased neuromuscular transmission instability and motor unit remodelling with diabetic neuropathy as assessed using novel near fibre motor unit potential parameters. Clin Neurophysiol. 2015;126:794–802. [DOI] [PubMed] [Google Scholar]
- 48. Spitzer AR. Assessment of variability in the shape of the motor unit action potential, “the jiggle,” at consecutive discharges. Muscle Nerve. 1995;18:789. [PubMed] [Google Scholar]
- 49. Dumitru D, King JC. Fibrillation potential amplitude after denervation. Am J Phys Med Rehabil. 1998;77:483–489. [DOI] [PubMed] [Google Scholar]
- 50. Heaton JT, Kobler JB. Use of muscle fibrillation for tracking nerve regeneration. Muscle Nerve. 2005;31:235–241. [DOI] [PubMed] [Google Scholar]