Abstract
Cystic fibrosis (CF) is a monogenic autosomal recessive disorder that affects about 70 000 people worldwide. The clinical manifestations of the disease are caused by defects in the cystic fibrosis transmembrane conductance regulator (CFTR) protein. The discovery of the CFTR gene in 1989 has led to a sophisticated understanding of how thousands of mutations in the CFTR gene affect the structure and function of the CFTR protein. Much progress has been made over the past decade with the development of orally bioavailable small molecule drugs that target defective CFTR proteins caused by specific mutations. Furthermore, there is considerable optimism about the prospect of gene replacement or editing therapies to correct all mutations in cystic fibrosis. The recent approvals of ivacaftor and lumacaftor represent the genesis of a new era of precision medicine in the treatment of this condition. These drugs are having a positive impact on the lives of people with cystic fibrosis and are potentially disease modifying. This review provides an update on advances in our understanding of the structure and function of the CFTR, with a focus on state of the art targeted drugs that are in development.
Introduction
About 70 000 people have cystic fibrosis worldwide,1 with prevalence varying by location and ethnic background. It is most common in white people of Northern European descent and therefore its prevalence is highest in Europe, North America, and Australia (~1/3000 births). The prevalence is much lower in the Middle East (Bahrain 1/6000), South America (Brazil 1/7000), Africa (South Africa 1/12 000), and Asia (Japan 1/350 000).2 Cystic fibrosis is a multi-organ disease with an autosomal recessive pattern of inheritance.
Mutations in the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR) protein lead to the clinical manifestations of the disease. The CFTR is a cAMP regulated anion channel expressed on the apical surface of epithelial cells that line the airways, pancreatic ducts, and other tissues.3 When it is absent or its activity is reduced, chloride and bicarbonate transport are both reduced,4 which predisposes to inspissated secretions, obstruction, and eventual end organ damage.5
Until recently, medical treatments for cystic fibrosis exclusively targeted organ specific sequelae of the underlying disease. In the airways, inhaled dornase alfa,6 hypertonic saline,7 and mannitol8 9 10 are used to enhance airway mucociliary clearance; inhaled antibiotics are used to reduce bacterial infection11 12; and oral macrolide antibiotics and high dose ibuprofen are used to reduce inflammation.13 14 15 Over the past decade, advanced technologies have enabled high throughput screening (HTS) approaches to drug discovery that have yielded orally bioavailable small molecule compounds capable of targeting the underlying defect. This review will summarize the advances in our understanding of the structure and function of the CFTR with a focus on CFTR targeted drugs in development (fig 1).
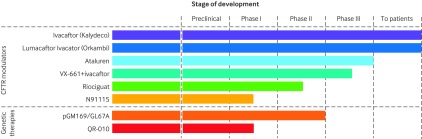
Fig 1 Cystic fibrosis transmembrane conductance regulator (CFTR) modulators and genetic therapies in development. Adapted, with permission, from the CF Foundation drug development pipeline
Sources and selection criteria
We conducted a PubMed search from 1 January 2005 to 15 July 2015. For comprehensiveness, we used the search terms “cystic fibrosis” and “CFTR” with the filters “therapy” and “year”. We initially screened more than 1140 articles but focused on original articles (preclinical, randomized controlled trials, and observational studies) investigating treatments that targeted the CFTR. Emphasis was placed on drugs that are currently available or in late phase clinical development. We also screened reference lists of clinical trials and included relevant articles.
Molecular basis of CFTR dysfunction
About 2000 mutations have been identified in the CFTR gene since its discovery in 1989,16 and about 242 mutations have currently been confirmed to cause cystic fibrosis.17 Despite the allelic diversity in this disease causing gene, 85-90% of white people with cystic fibrosis carry at least one copy of the F508del (c.1521_1523delCTT) mutation.18 Previously, people with cystic fibrosis were genotyped only to confirm the diagnosis or to predict disease severity,19 but with the recent approval of mutation specific treatments, genotypic information is considered essential.20 To understand new and emerging molecular therapies, a basic knowledge of CFTR structure and function and how the various mutational classes lead to ion channel dysfunction is necessary.
Normal CFTR structure and function
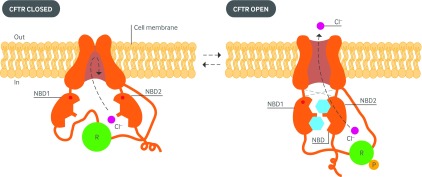
CFTR is a member of the ATP binding cassette (ABC) family of transporter proteins, characterized by two membrane spanning domains that form the channel pore and two nucleotide binding domains that bind and hydrolyze ATP.21 Unlike other ABC transporters, CFTR has an additional regulatory domain that regulates channel opening and closing.22 Phosphorylation of the regulatory domain by protein kinase A, followed by binding of ATP and its hydrolysis by the nucleotide binding domains23 leads to dimerization of the nucleotide binding domains and structural realignment of the membrane spanning domains to allow opening (or gating) of the CFTR channel pore (fig 2).25
Fig 2 Diagram of the proposed structure of the cystic fibrosis transmembrane conductance regulator (CFTR) in its closed (left) and open (right) configurations. The two transmembrane spanning domains form the channel pore. Gating of the channel is controlled by the two intracytoplasmic nucleotide binding domains (NBD1 and NBD2) as they bind and hydrolyze ATP, in addition to a regulatory domain (R), which contains numerous sites of phosphorylation (P). Normal activation of the protein requires phosphorylation of the R domain. The NBDs bind and hydrolyze ATP, inducing channel gating by conferring opening of the pore through interfaces with the transmembrane domains via their extracellular loops, which also function to stabilize the protein. Cl−=chloride ion. Adapted from Murray and Nadel’s Textbook of Respiratory Medicine24
The primary role of the CFTR protein is to transport anions (such as chloride and bicarbonate) through the apical membrane of epithelial cells, thereby creating an osmotic gradient for fluid secretion.26 The CFTR also has an absorptive role in some epithelial structures, such as the sweat gland. Absence or dysfunction of the CFTR results in dehydrated, thickened secretions that obstruct epithelium lined ducts (such as airways and biliary and pancreatic ducts) resulting in tissue damage.
Specific to the airways, reduced airway surface liquid (ASL) volume causes impaired mucociliary clearance and obstruction of small and medium sized airways with inspissated secretions.27 Inherent abnormalities of cystic fibrosis mucus that increase its viscosity and adhesion to the epithelial surface also affect the lungs and other organs. This creates a vicious cycle of mucus retention, infection, and inflammation, which further perpetuates the airway damage. In addition to impairments in mucociliary clearance, there is strong evidence that CFTR dysfunction itself leads to innate and adaptive immune defects that result in compromised bacterial clearance and dysregulated inflammation.28 29 30 31
Molecular consequences of variants in CFTR
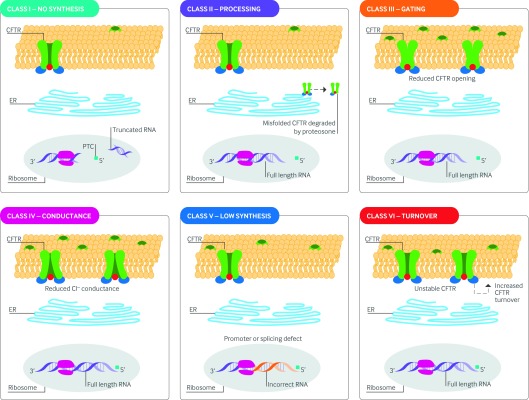
CFTR mutations can be broadly classified into six categories on the basis of the mechanisms that cause aberrant CFTR synthesis or function (fig 3).32 33 34 Class I mutations include in-frame UAA, UAG, or UGA stop codons in the mRNA preceding the native stop codon at the 3′ end of mRNA, and are thus referred to as premature termination codons (PTCs). The transcribed mRNA is truncated and unstable resulting in absent protein production.35 Other major insertions and deletions that disrupt normal translation are also included in this category.
Fig 3 CFTR mutational classes and molecular consequences. Class I mutations result in unstable truncated RNA and no synthesis of the CFTR protein. Class II mutations cause CFTR processing defects owing to misfolding of CFTR and degradation by the proteasome. Class III mutations cause reduced CFTR channel opening owing to defective channel gating or regulation. Class IV mutations cause reduced chloride conductance owing to defects within the CFTR channel. Class V mutations lead to reduced synthesis of CFTR owing to splicing defects. Class VI mutations result in reduced CFTR stability at the cell surface and hence increased CFTR turnover. The size of the inner dark circle of the CFTR channel at the apical surface reflects the extent of channel opening or conductance. CFTR=cystic fibrosis transmembrane conductance regulator; ER=endoplasmic reticulum; PTC=premature termination codon
Class II mutations cause abnormal CFTR processing or trafficking, which results in reduced amounts of CFTR at the cell surface. For example, the F508del-CFTR mutation is a three base pair deletion that leads to an amino acid deletion with subsequent misfolding of the CFTR protein. The misfolded protein fails to transport to the cell surface owing to premature degradation by the proteasome (see Glossary).36
Class III mutations are often referred to as “gating” mutations because they lead to disordered activation of the CFTR channel. Class IV mutations exhibit normal gating but changes in conductivity of the channel pore cause abnormal chloride permeability.
Class V mutations are located within promoter or splice sites in the gene; they lead to fewer CFTR transcripts and reduced protein production. Class VI mutations are the most recently discovered and result in reduced stability of CFTR at the cell surface, leading to increased turnover.34 Several mutations exhibit features of more than one class. For example, although the primary abnormality of F508del is aberrant cellular processing, it also exhibits defective gating and a reduced surface half life.37 38 39 40 R117H is often described as a partially active conductance mutation (see Glossary), but it also exhibits partially disrupted gating and is located in cis (see Glossary) with mutations that affect its expression.41 42
Impact of mutational class on CFTR activity
Each individual patient’s disease phenotype is partly determined by overall CFTR activity, which in turn is determined by the net impact of both of the disease causing alleles on the quantity and function of the CFTR. In general, people with two loss of function alleles (classes I-III) have low levels of CFTR activity (<10% of normal) and more serious lung disease and pancreatic insufficiency consistent with classic cystic fibrosis. By contrast, those with at least one residual functional allele (missense and splice mutations; classes II-VI; see Glossary) are expected to have residual CFTR activity (>10% of normal) and milder lung disease and pancreatic sufficiency consistent with non-classic or mild cystic fibrosis.19 Moreover, substantial phenotypic variation exists even for patients with the same combination of CFTR mutations owing to environmental influences and additional genetic variation that contributes to the final phenotype (genetic “modifiers” of disease).43 For example, polymorphisms in the gene encoding transforming growth factor β (TGF-β) modify the severity of lung disease in F508del homozygous patients.44
Molecular basis of new and emerging treatments
CFTR modulators
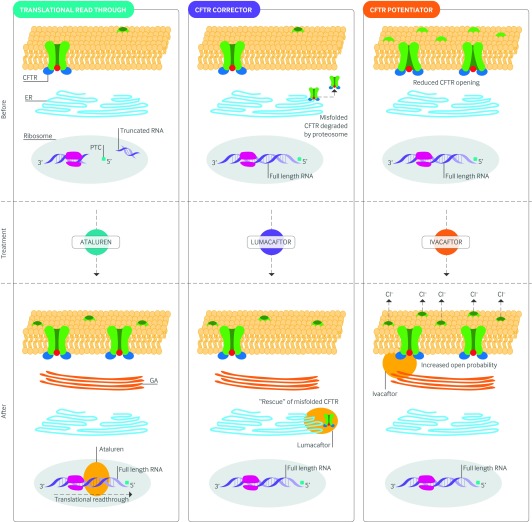
CFTR modulators are designed to treat the underlying cause of cystic fibrosis by targeting the CFTR protein defect. Small molecule pharmacologic agents that target defects in CFTR gating, processing, and synthesis have undergone rigorous preclinical evaluation over the past decade and include CFTR potentiators, correctors, and translational read-through (see Glossary) agents, respectively (fig 4).
Fig 4 Molecular basis of CFTR modulators: fate of CFTR before and after CFTR modulator treatment. Ataluren permits selective ribosomal read-through of the premature termination codon allowing the production of full length transcript and CFTR protein. Lumacaftor is capable of interacting directly with the CFTR to facilitate its correct folding or by modulating components of the cellular quality control machinery to allow proper trafficking of CFTR to the cell surface. Ivacaftor stabilizes the open state of the CFTR, thus increasing channel opening time. Areas shaded in red are the presumed targeted sites of action. The size of the inner dark circle of the CFTR channel at the apical surface reflects the extent of channel opening before and after CFTR potentiator therapy. CFTR=cystic fibrosis transmembrane conductance regulator; GA=Golgi apparatus; ER=endoplasmic reticulum; PTC=premature termination codon
CFTR potentiators
CFTR potentiators increase the flow of ions through surface localized, activated CFTR channels.45 People with class III mutations such as G551D (c.1652G>A) have normal amounts of CFTR protein at the cell surface but have primary defects in CFTR channel gating, making them ideal targets for potentiator therapy.46
In preclinical studies, ivacaftor (formerly VX-770) was identified as a promising CFTR potentiator after HTS of more than 200 000 chemically diverse compounds and medicinal chemistry optimization.47 Using G551D recombinant cell lines (see Glossary), ivacaftor increased CFTR channel opening time sufficiently to increase CFTR activity from about 5% to about 50% of wild-type levels as assessed by electrophysiologic measurements.45 Ivacaftor also approximately doubled wild-type CFTR chloride transport.45 Although the precise mechanism of its action remains incompletely understood, evidence suggests that ivacaftor stabilizes the open state of CFTR, thus increasing channel opening time.48 The discovery of ivacaftor has provided proof of the concept that CFTR related chloride secretion can be potentiated, and other oral compounds such as QBW251 have entered phase II investigation (NCT02190604; EudraCT 2011-005085-37); several other agents such as GLPG1837 are also in development.
CFTR correctors
CFTR correctors repair defective CFTR processing by facilitating proper maturation and delivery of protein to the plasma membrane. Correctors act by interacting directly with CFTR to facilitate its correct folding or by modulating components of the cellular quality control machinery.49 Patients with class II mutations (such as F508del) are primary targets for CFTR corrector therapy because the misfolded protein is retained within the endoplasmic reticulum and prematurely degraded. The potential impact of developing efficacious CFTR corrector therapy is profound, because 85-90% of patients with cystic fibrosis have at least one copy of the F508del allele.
Alongside the discovery of ivacaftor, HTS identified small molecules capable of augmenting F508del-CFTR activity in recombinant cell based assays.50 Using human bronchial epithelial cell lines from the lungs of F508del homozygotes, lumacaftor (formerly known as VX-809) improved CFTR maturation eightfold and enhanced F508del-CFTR mediated chloride transport fourfold. However, lumacaftor only partially rescued the F508del-CFTR processing defect, because the maximum chloride transport achieved was estimated to be about 15% of wild-type levels. Mechanistic studies have shown that lumacaftor probably increases the conformational stability of F508del-CFTR, thus reducing cellular misprocessing and allowing at least some of the CFTR to move from the endoplasmic reticulum to the cell surface.50 51 52 53
CFTR read-through agents
CFTR read-through agents promote the ribosomal “read-through” of PTCs in CFTR mRNA. The first read-through agents examined in cystic fibrosis were aminoglycoside antibiotics,54 which are commonly used in cystic fibrosis to combat Gram negative bacteria such as Pseudomonas aeruginosa. Aminoglycoside antibiotics such as gentamicin are capable of inhibiting ribosomal “proofreading” by binding to the decoding site of rRNA. This reduces the fidelity of the codon-anticodon pairing and permits the erroneous addition of an amino acid to the polypeptide chain at the site of the PTC, allowing translation to continue to the end of the gene.
Read-through seems to be selective to PTCs because in vitro studies have found no detectable elongation beyond the native stop codon located at the 3′ end of mRNA owing to mechanistic differences between premature and normal termination codons.55 Unfortunately, high systemic levels of gentamicin, which can cause serious renal toxicity and ototoxicity, are needed to induce translational read-through.56
Efforts have been made to modify the aminoglycoside chemical structure to provide higher read-through activity with less toxicity.57 58 59 For example, NB124 is a novel aminoglycoside derivative that was rationally designed to provide 2.5-fold greater read-through activity than gentamicin, restoring CFTR function to roughly 7% of wild-type levels. This compound was also less cytotoxic than gentamicin when evaluated in a tissue based model of ototoxicity.59
To identify more potent, orally bioavailable, non-toxic alternatives to aminoglycosides that are capable of selective read-through of PTCs in cystic fibrosis and other genetic disorders characterized by nonsense mutations (such as Duchenne muscular dystrophy and Hurler’s disease), HTS was performed on about 800 000 compounds.56 Ataluren (formerly PTC124) was identified as a lead candidate after medicinal chemistry optimization. The compound has no structural similarity to aminoglycosides or other clinically developed drugs. PTC124 promoted dystrophin production in primary muscle cells from humans with Duchenne muscular dystrophy and a mouse model expressing dystrophin nonsense alleles.56 Subsequent in vivo experiments found that PTC124 could suppress the G542X nonsense mutation in a cystic fibrosis mouse model expressing the human CFTR-G542X transgene, restoring CFTR expression and function.60
CFTR combination therapies
It has been estimated that 10-35% of normal CFTR function can result in a milder cystic fibrosis phenotype, offering promise that CFTR function does not have to be completely restored to have therapeutic benefit.45 61 In vitro and early clinical studies have shown that neither correctors nor potentiators alone are sufficiently active to provide clinical benefit in patients who are homozygous for the F508del mutation.45 50 62 63 64 A pre-clinical study of lumacaftor and ivacaftor combined found that F508del-CFTR mediated chloride transport nearly doubled compared with lumacaftor alone, increasing chloride transport to about 25% of wild-type levels.50 Similar to the strategy used to treat HIV, a therapeutic cocktail consisting of multiple CFTR correctors, potentiators, and stabilizers might be needed to enhance CFTR function in challenging multidimensional genotypes such as F508del.65 Because many CFTR channels also exhibit abnormal gating, and even those with normal gating can be activated further, CFTR potentiators may also be useful to augment function when less than complete rescue has occurred with other agents.
There is evidence that wild-type CFTR counteracts the absorptive function of the epithelial sodium channel (ENaC), thus preventing excessive sodium and water intake.66 Unopposed ENaC function therefore exacerbates ASL desiccation, which is also caused by a lack of CFTR mediated fluid secretion. An investigational ENaC inhibitor to restore ASL known as P-1037 is being evaluated in a phase II placebo controlled trial of patients (aged ≥12 years) with mild to moderate lung disease (NCT02343445); there are plans to conduct a phase IIa trial combining it with lumacaftor and ivacaftor in patients who are homozygous for F508del because these pathways may be additive or synergistic.
Other CFTR modulators
Other CFTR modulators currently in clinical development include agents that can directly and indirectly modulate the nitric oxide pathway, including phosphodiesterase-5 inhibitors (PDE-5; sildenafil; see Glossary) and guanylate cyclase stimulators (riociguat; see Glossary), and a new class of small molecule inhibitors of S-nitrosoglutathione reductase (GSNOR; see Glossary) (see fig 1).
Preclinical studies have shown that PDE-5 inhibitors can act as both potentiators and correctors of mutant CFTR through cGMP dependent and independent mechanisms, respectively.67 68 69 70 Preclinical studies have also shown that soluble guanylate cyclase stimulators have CFTR corrector action and an ongoing phase II study is evaluating riociguat in F508del homozygous CF adults (NCT02170025).
N91115 is an orally bioavailable inhibitor of GSNOR. In preclinical studies, increased levels of S-nitrosoglutathione (GSNO) achieved through GSNOR inhibition modified CFTR chaperone proteins (see Glossary), stabilizing the CFTR both inside the cell and at the cell surface.71 72 N91115 thus represents a unique CFTR stabilizer under investigation. A phase Ib PCT is currently evaluating the safety and pharmacokinetics of N91115 as a sole CFTR modulator in F508del homozygous subjects (NCT02275936). Subsequent clinical trials will focus on combining N91115 with ivacaftor or lumacaftor (or both) as a combined CFTR potentiator-corrector-stabilizer treatment for F508del homozygotes.
Genetic therapeutic technologies
Gene replacement therapy
The cloning of the CFTR gene led to the hope that gene replacement therapy using a mutation independent approach could be used to cure this monogenic disease.16 21 Unfortunately, early progress was impeded by difficulties in finding a suitable gene transfer vector capable of overcoming the robust physical and immunologic defense mechanisms of the airways.73 The airway of people with cystic fibrosis is particularly inhospitable to the aerosolized delivery of gene therapy vectors owing to thickened secretions and airflow obstruction. Even if the vector does penetrate these barriers, it must then transduce (see Glossary) the apical surface of the airway epithelium and be transported to the nucleus for transcription.
Initial gene therapy efforts focused on adenovirus and adeno-associated virus vectors (see Glossary), and although CFTR mRNA has been expressed using this approach, the effect wanes after repeated administration owing to an immune response against the vector.74 75 76 More recently, the focus has been on non-viral vectors because of the reduced risk of immunogenicity.77 Liposomal carriers (such as GL67; see Glossary) complexed with plasmid DNA encoding the CFTR gene were administered intranasally to people with cystic fibrosis in a double blind placebo controlled trial; no immune response or reduced efficacy was detected after weekly administration for four weeks.78
A later double blind placebo controlled trial comparing the GL67-DNA complex with GL67 alone aerosolized to the lungs of 16 patients found a 25% restoration of chloride channel function in the treatment group but no change in the placebo group (P<0.05), on the basis of lower airway potential difference testing, although flu-like symptoms did occur.79 Subsequent studies have established that unmethylated CG dinucleotide motifs (CpG) present in foreign bacterial or recombinant viral plasmid DNA are immunogenic and that inflammation is reduced and pulmonary gene expression is more sustained if CpG-free plasmids (such as pGM169) are used (see below for a more detailed discussion).80
To overcome the harsh physiological environment of the airways in cystic fibrosis,81 synthetic nanoparticle (see Glossary) based gene delivery systems are also being studied.82 83 A drawback of cationic (positively charged) liposomal carriers is that they can interact with negatively charged mucus constituents, thereby reducing penetration.84 Biodegradable polymers consisting of poly(β amino esters) (PBAE) are stable in physiological fluids and can penetrate the highly adhesive human mucus gel layer to reach the underlying epithelium.85 In a very recent preclinical study, PBAE based DNA nanoparticles were able to penetrate freshly expectorated mucus from patients with cystic fibrosis in vitro and provided sustained transgene expression in mouse lungs in vivo, demonstrating superior performance to several gold standard gene delivery systems.86
Genome editing
DNA editing—Correction of genetic defects in situ, rather than through transgene complementation (see Glossary), is an emerging therapeutic strategy in cystic fibrosis.87 DNA editing involves the insertion, replacement, or removal of DNA from a genome using “molecular scissors” such as nucleases. The use of artificially engineered nucleases that introduce precise breaks in DNA to remove mutated gene segments followed by homologous recombination with the wild-type gene represents a potential therapeutic strategy in cystic fibrosis. Cas9, a programmable DNA nuclease from the microbial adaptive immune system CRISPR (clustered regularly interspaced short palindromic repeats), is one of the most promising nucleases for genome editing88 CRISPR-Cas9 systems use an RNA guide with a region complementary to the target DNA, which ensures site specific cleavage and precise discrimination of mutant alleles from normal alleles. This technology has been used in vitro to repair the CFTR locus in human intestinal stem cells and robustly restored CFTR function.89
RNA editing—RNA editing is potentially a more readily applicable genome editing strategy because mRNA is more accessible and the editing effects are more transient, thus reducing the impact of any unintended off-target effects. Antisense oligonucleotides are currently being studied to replace deleted segments of mRNA.90 A phase IB randomized, placebo controlled trial is currently investigating an aerosolized synthetic RNA oligonucleotide known as QR-010 in people who are homozygous for the F508del mutation. Whole strand mRNA transmission of codon optimized CFTR is an alternative approach that is in preclinical development.
Late phase clinical trials of treatments targeting the underlying CFTR defect
CFTR potentiators
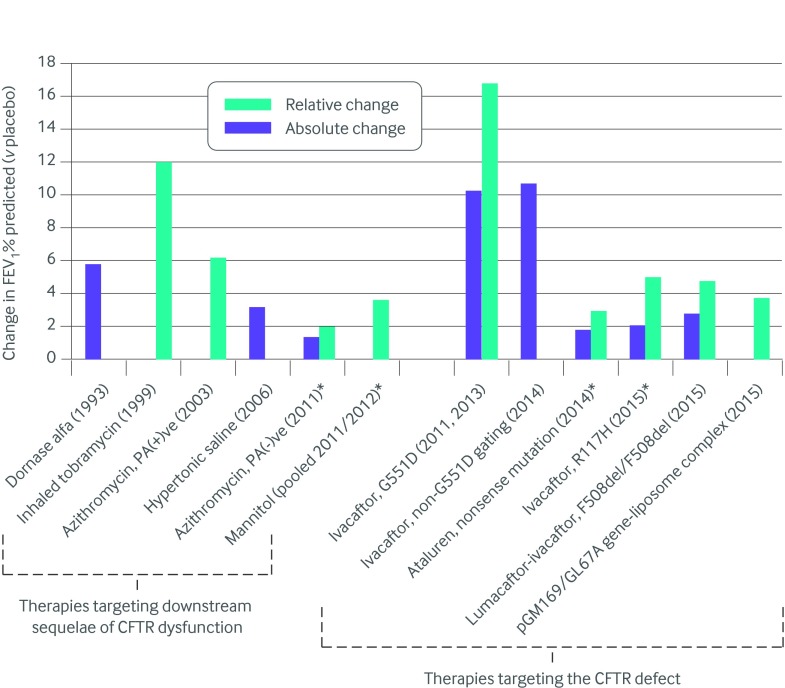
Ivacaftor, an orally bioavailable CFTR potentiator, was the first CFTR modulator approved for clinical use in cystic fibrosis. The randomized controlled trial (RCT) supporting its use involved people (≥12 years’ old) with cystic fibrosis and a class III G551D mutation on at least one allele.20 The forced expiratory volume in one second (FEV1) at baseline ranged from 40% to 90% predicted, and participants were permitted to continue standard therapies except for hypertonic saline. A 10.6% absolute improvement in FEV1% predicted was achieved by week 24 of treatment compared with placebo (10.4% v −0.2%; P<0.001), with much of the benefit achieved by the second week of treatment; benefit was also sustained until the end of the 48 week trial. Although cross study comparisons must be performed with caution owing to differences in the underlying study populations and trial designs, as well as changes in standard treatment over time, the absolute improvement in FEV1% predicted for ivacaftor relative to placebo exceeds that seen in all other placebo controlled trials of treatments for cystic fibrosis (fig 5). Furthermore, the risk of pulmonary exacerbations was significantly reduced and symptoms and weight improved with no serious adverse effects. Mean sweat chloride (see Glossary), a marker of CFTR function, also decreased by about 50 mmol/L within two weeks of starting therapy, with a mean level below the typical cystic fibrosis diagnostic threshold (<60 mmol/L) being achieved.
Fig 5 Impact of cystic fibrosis therapies on FEV1% predicted relative to placebo based on published clinical trial data. *Change in FEV1% predicted not statistically significant. Note: Treatment effects are not directly comparable owing to differences in study populations and changes in standard therapy over time. CFTR=cystic fibrosis transmembrane conductance regulator; FEV1=forced expiratory volume in one second; PA=Pseudomonas aeruginosa
Similar results were seen in an RCT of ivacaftor in children aged 6-11 years with at least one G551D mutation and milder lung disease (baseline FEV1 40-105% predicted).91 Ivacaftor has subsequently been studied in people 6 years or more (mean age 22.8) with nine non-G551D gating mutations (G178R, S549N, S549R, G551S, G970R, G1244E, S1251N, S1255P, G1349D), which are found in about 1% of all patients with cystic fibrosis worldwide. With the exception of people with the G970R mutation, who were under-represented in the study, improvements in FEV1 and sweat chloride were seen after eight weeks of treatment compared with placebo, and the size of the benefit was similar to that seen for the use of ivacaftor in people with G551D gating mutations.92
As a result, regulatory agencies around the world extended the approval of ivacaftor to people with cystic fibrosis aged 6 years or more with at least one CFTR gating mutation (G551D and eight or nine other gating mutations, depending on the country). The results of an open label, single armed 24 week safety and pharmacokinetic study of ivacaftor in patients aged 2-5 years weighing 8 kg or more with a CFTR gating mutation has just been reported.93 With the exception of abnormalities in liver function tests (5/34; 15%), ivacaftor at doses of 50 mg and 75 mg every 12 hours was considered safe, and the pharmacokinetics of ivacaftor in this young age group were similar to those seen in older age groups. There were also significant improvements in some of the secondary endpoints examined including sweat chloride, weight, and BMI relative to baseline (all P<0.0001). As a result, ivacaftor is now approved for patients aged 2 years or more with selected gating mutations in the US, Europe, and Canada (table 1).
Table 1.
CFTR modulators licensed for use in cystic fibrosis with a summary of the indications and jurisdictions with licensed approval
| CFTR modulator | Mutation | Age group approved | Licensed approval |
| Ivacaftor | G551D* | ≥2 years | US, Europe, Canada |
| ≥6 years | Australia, New Zealand | ||
| R117H* | ≥2 years | US | |
| ≥18 years | Europe, Canada | ||
| G1244E, G1349D, G178R, G551S, S1251N, S1255P, S549N, S549R* | ≥2 years | US, Europe, Canada | |
| ≥6 years | Australia | ||
| G970R* | ≥2 years | Canada | |
| ≥6 years | Australia | ||
| Lumacaftor-ivacaftor | F508del homozygous | ≥12 years | US, Europe, Canada |
*At least one copy of the mutation.
Safety
Although ivacaftor was well tolerated, with no major safety concerns, in phase III RCTs, a five year safety surveillance study is ongoing in the US and Europe to better understand long term safety. The manufacturer recommends that liver transaminases are monitored before starting therapy, every three months for the first year of treatment, and annually thereafter because increased levels have been reported in patients with cystic fibrosis who are taking ivacaftor (http://pi.vrtx.com/files/uspi_ivacaftor.pdf). However, the association with ivacaftor therapy is uncertain because such rises are more common in patients with a history of increased transaminases.
Because cataracts have also been reported in children with cystic fibrosis, baseline and follow-up ophthalmological examinations are recommended in those taking ivacaftor. Ivacaftor is a CYP3A (cytochrome P450, family 3, subfamily A) substrate, so serious drug-drug interactions are possible in the presence of CYP3A inhibitors and inducers.94 Dose reduction is recommended by the manufacturer in the presence of strong CYP3A inhibitors (such as ketoconazole and other azoles, clarithromycin) and the concomitant use of strong CYP3A inducers is not recommended (for example, rifampin, carbamazepine, phenytoin) (http://pi.vrtx.com/files/uspi_ivacaftor.pdf).
Related therapeutic effects
After approval of ivacaftor for use in patients with a G551D mutation, a six month observational study was conducted to provide mechanistic insights into the therapeutic benefits.95 In the subset of patients evaluated, mucociliary clearance, as measured by gamma scintigraphy, significantly improved, supporting the concept that improved CFTR function leads to improved mucociliary clearance. Gastrointestinal pH, based on capsule endoscopy measurement, also increased, suggesting that alkalinization of the gastrointestinal tract through CFTR potentiation of chloride and bicarbonate transport may improve nutrient absorption and account for the robust improvements in weight. Rates of colonization with P aeruginosa also decreased, particularly in people with mild lung disease or intermittent growth of the organism, suggesting that improved CFTR function leads to eradication of these bacteria.96
An open label extension study examining ivacaftor in people with at least one G551D mutation enrolled in the two RCTs20 91 has shown a durable treatment response up to 144 weeks, with no new safety concerns.97 An observational follow-up study of these patients using CF Foundation registry data up to three years showed that ivacaftor can reduce the rate of FEV1 decline by about 50% relative to propensity score matched F508del homozygous controls, suggesting that ivacaftor has a disease modifying effect.98
Although these clinical trials excluded patients with advanced lung disease (FEV1 <40% predicted), improvements in FEV1 (absolute 4-6%), weight gain (2-4 kg), and reduced intravenous antibiotic requirements have been seen in those with advanced disease.99 100 101 Other beneficial effects reported with ivacaftor in patients with G551D mutations include improvements in exhaled nitric oxide levels102; reduced streptococcus sputum bacterial load103; reduced mucus plugging, bronchiectasis,104 105 106 107 and sinus disease on computed tomography108 109 110; and positive extrapulmonary effects (improved insulin secretion,111 112 resolution of cystic fibrosis related diabetes,113 and reduced hepatic steatosis).114
In addition to targeting class III gating mutations, ivacaftor has been evaluated in patients over 6 years who have at least one class IV R117H mutation, a mutation associated with residual CFTR function.115 This mutation is present in about 2% of patients with cystic fibrosis worldwide and the clinical consequences are variable because an associated splice mutation alters gene expression.116 In a 24 week RCT, symptoms and sweat chloride improved in the ivacaftor group, but no significant improvement in the primary outcome of FEV1% predicted was seen, except in a prespecified subgroup analysis of adults.117 In another subgroup analysis, there was a non-significant trend towards ivacaftor having more benefit in people with more advanced disease (lower FEV1% predicted, P aeruginosa infection, and the 5T intron 8 variant). Despite failing to meet its primary end point in the entire study population, ivacaftor has been approved by the Food and Drug Administration for use in patients aged 6 or more with a R117H mutation, and it is probably most beneficial in those with more established lung disease.
CFTR correctors
As a follow-up to preclinical studies, a phase IIa study evaluating the CFTR corrector lumacaftor alone in F508del homozygous patients found no clinical benefit, despite a dose dependent reduction in sweat chloride.64 A phase II study of the combination of lumacaftor and ivacaftor in F508del homozygous people was therefore undertaken on the premise that both the correction of cellular misprocessing to increase localization of F508del CFTR to the cell surface and subsequent potentiation to increase channel opening would be needed to restore chloride transport in these patients.63 No clinical benefit was seen after 28 days of lumacaftor alone when evaluated at four different doses. In fact, there was a dose dependent trend towards a decline in FEV1% predicted for the groups assigned to higher lumacaftor doses, which was associated with increased dyspnea and chest tightness; this finding was later determined to be an off target adverse event because it was replicated in healthy volunteers. When ivacaftor was added to the two highest doses of lumacaftor for an extra 28 days, FEV1% predicted improved compared with placebo, with no further increase in the reporting of dyspnea and chest tightness; the absolute change in FEV1 was 3-4% across the 56 day treatment period.
The same phase II study included a small cohort of F508del heterozygous people who showed no clinical benefit when taking the lumacaftor-ivacaftor combination. This represents a gene-dose effect and suggests that combination therapy does not provide benefit in F508del heterozygotes unless the second mutation is responsive to ivacaftor alone (for example, class III mutations or R117H).
The phase II studies set the stage for two identical phase III studies evaluating lumacaftor-ivacaftor in patients homozygous for the F508del mutation, which were recently completed.118 Two doses of lumacaftor (600 mg daily or 400 mg twice daily) with ivacaftor (250 mg twice daily) were evaluated. Unlike the phase III ivacaftor study in G551D,20 the use of inhaled hypertonic saline was permitted during these trials. In the pooled analysis, a significant albeit modest (~3%) improvement in FEV1% predicted was seen for the two treatment doses compared with placebo (3.0% and 2.5% v −0.3%). The effects of two doses of lumacaftor were equivalent except for fewer pulmonary exacerbation related events in the higher dose group. Both doses were generally well tolerated, with the important exception of increased dyspnea and chest tightness in the active treatment groups, as was seen with the phase II trials. Although uncommon, there was also increased reporting of serious adverse events related to abnormal liver function in the active treatment groups (7/371 people; 0.9%) compared with placebo groups (0/371; 0%). In all seven cases, liver function tests improved or returned to baseline after discontinuation or interruption of treatment. On the basis of the results of these two pivotal phase III studies, lumacaftor-ivacaftor was the first CFTR corrector approved for use in F508del homozygous people.
A phase III development program is currently evaluating the use of an investigational small molecule known as VX-661 (a CFTR corrector structurally distinct from lumacaftor) combined with ivacaftor. VX-661 has a more favorable pharmacokinetic profile than lumacaftor, which permits once daily dosing; it also exhibits less drug-drug interaction with ivacaftor,64 enabling the use of lower doses of ivacaftor. Furthermore, unlike lumacaftor, it was not associated with bronchospasm in healthy volunteers.
Two phase II studies have evaluated VX-661 combined with ivacaftor in F508del homozygous people. The first four week placebo controlled trial found a 4.8% absolute improvement in FEV1% predicted for the once daily 100 mg dose of VX-661 combined with ivacaftor twice daily relative to placebo (4.4% v −0.4%; P=0.01).119 All patients from the four week trial rolled over into an eight week extension (total 12 week trial) and the within-group improvement in FEV1% predicted for the treatment group remained significant relative to baseline (3%; P=0.03) but the improvement was modest when compared with placebo (3% v 1%).120
Four cohorts of more than 1000 patients will be evaluated in the phase III VX-661 program. The study will comprise a group of patients with two copies of the F508del mutation and groups of heterozygous patients with one copy of the F508del plus a mutation on the second allele that results in:
Minimal CFTR function (for example, class I, II)
A gating defect in the CFTR protein (for example, class III mutations or R117H), and
Residual CFTR function.
CFTR read-through agents
Open label pilot studies have shown that both the topical and systemic administration of the aminoglycoside gentamicin can augment chloride transport (based on measurement of nasal potential difference; NPD) in people with cystic fibrosis and at least one nonsense mutation in the CFTR gene.121 122 123 A placebo controlled crossover trial investigating the topical administration of gentamicin to the nasal epithelium for 14 days was conducted in patients who had one or two stop mutations or were homozygous for the F508del mutation.124 Similar to the findings of the open pilot studies, NPD was reduced and surface expression of CFTR was increased in nasal epithelial cells in patients with stop mutations but not in those who were homozygous for F508del.
Open label phase II clinical trials of ataluren in patients with at least one nonsense mutation showed improved CFTR activity, as assessed by transepithelial NPD measurements.125 126 127 However, another study did not demonstrate efficacy. Although short term studies found no significant change in FEV1% predicted,125 127 a longer term 12 week study showed a trend towards an effect, albeit in an open label design.126
A subsequent phase III RCT of oral ataluren in 232 patients found no significant difference between treatment and placebo groups in the primary outcome of relative change in FEV1% predicted after 48 weeks.128 Furthermore, there were no differences in other clinical outcomes of interest, including weight, frequency of exacerbations, and symptoms, or in biomarkers of CFTR activity (sweat chloride and NPD testing). Safety profiles were similar for ataluren and placebo groups except for a significant increase in the reporting of acute kidney injury in the ataluren group (15% v <1%). Most of these events were mild but 4% of patients had to stop treatment because of increases in serum creatinine. A pre-defined subgroup analysis found a significant improvement in FEV1% predicted and a significant reduction in the pulmonary exacerbation rate for patients not receiving chronic inhaled tobramycin maintenance therapy (37% of the study population). A post hoc luciferase assay (see Glossary) demonstrated reduced ataluren induced read-through when cells were co-incubated with aminoglycosides but not with colisitin or aztreonam. As a result, a phase III clinical trial is currently evaluating ataluren in people with nonsense mutations who are not receiving chronic inhaled tobramycin therapy (NCT02139306).
Gene replacement therapy
An initial RCT evaluating nebulized adeno-associated virus vectors containing CFTR cDNA showed a positive trend with respect to lung function compared with placebo,129 but this was not reproduced in a larger phase IIB trial.130 The UK CF Gene Therapy Consortium has focused primarily on non-viral vectors, and through a series of phase I and phase IIa studies it has optimized the formulation and delivery of an aerosolized plasmid DNA-liposome complex referred to as pGM169/GL67A.80 131 132 133 134
A phase IIB placebo controlled trial of nebulized pGM169/GL67A versus normal saline at 28 day intervals over 12 months in 140 patients with any combination of CFTR mutations was recently completed in the United Kingdom.135 This represents the largest cystic fibrosis gene therapy trial performed to date and the first to be powered to detect clinically relevant pulmonary changes. Routine treatments were permitted with the exception of DNase, which was withheld for 24 hours before and after dosing. The study met its primary endpoint, with a significant difference in relative change in FEV1% predicted of 3.7% favoring the treatment group compared with placebo (relative −0.4% v −4.0%; P=0.046). However, the treatment effect was mainly due to stabilization of lung function rather than an improvement on treatment, as there was a 4% relative decrease in FEV1% predicted for the placebo group by the end of the one year study. Apart from improved forced vital capacity (FVC) and reduced measures of gas trapping on computed tomography with treatment, there were no significant between-group differences for other secondary outcomes of interest, including other measures of lung function, imaging, and quality of life.
In a subgroup analysis, there was a trend for the group with more severe lung disease (FEV1 <70% predicted) to derive the most benefit from treatment, suggesting the potential impact of differences in airway deposition. In a bronchoscopy substudy, there was a bronchial potential difference response in favor of active treatment, although this was driven by changes in the placebo group rather than improvements in the treatment group. Although the treatment effects were modest, the treatment was safe, with no treatment related serious adverse events other than transient fever, which could be managed with paracetamol (acetaminophen). This study has provided hope that gene therapy can be effective in cystic fibrosis, but the delivery vectors and conditions need to be optimized to provide clinically meaningful benefit.
Guidelines
The European Cystic Fibrosis Society best practice guidelines have recommended that ivacaftor be part of standard of care for patients with the G551D mutation136 and the US CF Foundation pulmonary clinical practice guidelines committee has assigned it a grade A recommendation (high certainty of substantial net benefit) for patients 6 years and older with at least one G551D mutation using the US Preventive Services Task Force Scheme.137 Although ivacaftor has been approved by some regulatory agencies around the world for use in patients with non-G551D gating mutations, and lumacaftor-ivacaftor by the FDA in the US for patients homozygous for the F508del mutation, international cystic fibrosis guidelines have not yet been updated to reflect these recent approvals. Better understanding of the cost effectiveness of these drugs is needed, especially in people in whom therapeutic benefit is difficult to ascertain, particularly given their high cost (ivacaftor, for example, costs £182 000 ;$262 400; €240 400/year).138
Precision medicine: evaluating individual response to CFTR modulators
People with cystic fibrosis show variable responses to CFTR modulator therapy owing to undefined mechanisms.20 118 128 Although sweat chloride is considered a reliable biomarker or readout of CFTR function and is responsive to CFTR modulation with potentiators and correctors as single agents, individual response is variable and does not correlate with established clinical endpoints such as FEV1% predicted.139 140 Because most confirmed disease causing mutations are rare it will not be feasible to perform randomized placebo controlled trials to guide the use of CFTR modulators in all patients with the disease. There is therefore an urgent need for innovative CFTR biomarkers and study designs to predict or evaluate individual response to CFTR modulator therapy.
At the preclinical level, primary bronchial epithelial cell lines45 47 50 and airway epithelial cells derived from pluripotent stem cells141 from patients with cystic fibrosis have been used in vitro to analyze the drug responses of people with specific mutations. More recently, innovative in vitro model systems using intestinal stem cells derived from patients’ rectal biopsies have been created to replicate in vivo tissue architecture.142
When intestinal organoids (see Glossary) from healthy people are activated by the cAMP agonist forskolin, the organoid rapidly swells. This swelling is fully dependent on CFTR mediated chloride secretion, which occurs on the apical membrane facing the organoid lumen. By contrast, such swelling is not seen in organoids from patients with cystic fibrosis. Forskolin induced swelling can be induced by addition of CFTR correctors to the model system.142 Intestinal organoids thus represent a promising functional CFTR assay whereby therapeutic choices might be driven by patient specific biomarker responses to enable precision medicine. Other approaches, such as analysis of human nasal airway cells, may also be possible, including measures of ion transport and mucus clearance in vitro with optical coherence tomography.143
At a clinical level, novel clinical trial designs are being used to permit the evaluation of CFTR modulators in individual patients. In “N-of-1” studies, investigational treatments are evaluated in single patients to assess the treatment response, with patients serving as their own control using multiple on-off treatment cycles to ensure reproducibility.144 This strategy was used successfully to evaluate the effects of ivacaftor in patients with residual function CFTR mutations.145
Future research questions
The success of CFTR modulator treatment has spurred a number of new and important clinical research questions:
How early should treatment be started? Although the benefits of ivacaftor in patients with the G551D and other gating mutations are compelling, it is unclear how early treatment should be started. Short term clinical trial data show that ivacaftor is safe and effective as early as 2 years of age, but whether this can be extended to the newborn period requires further study. Data from the AREST cystic fibrosis newborn screening cohort show that bronchiectasis is present in the first few months of life,146 so it would make sense to start treatment as early as possible to prevent the development of structural lung disease.
What are the long term effects of CFTR modulators? Longitudinal data suggest that ivacaftor can modify disease progression in patients with the G551D mutation,98 but longer term data on benefit and safety are needed. Similarly, although the short term benefits of lumacaftor-ivacaftor appear modest, disease stability is a commendable goal, especially if treatment is started early in the disease, but such effects will only be apparent with longer term follow-up data.
What are the extra-pulmonary benefits of CFTR modulator therapy? There is some evidence to suggest that ivacaftor improves pancreatic endocrine function and sinus disease.108 109 111 113 However, it is unclear whether CFTR modulator therapy will permit some chronic maintenance therapies to be safely discontinued to reduce treatment burden, which is currently overwhelming and untenable for many patients.147
What is the potential of combination strategies in F508del homozygous individuals? A multifaceted strategy will probably be needed to restore robust CFTR function in these patients, so future studies will focus on combining different modulators with complementary mechanisms to boost CFTR activity. Although some therapies may be synergistic, the potential for antagonistic effects also exists. For example, in vitro studies utilizing primary cells and cell lines have suggested that prolonged ivacaftor administration may reduce the stability and expression of lumacaftor corrected F508del.148 149 150
Conclusions
Patients with cystic fibrosis and their care providers are entering a new era filled with hope and optimism, unprecedented since the disease was first recognized in 1938.151 The discovery of the CFTR gene in 1989 has enabled a sophisticated understanding of CFTR structure and function, providing novel targets for CFTR modulation. The recent approvals of ivacaftor and lumacaftor represent the dawn of a new era of precision medicine in this area. These drugs have a positive impact on the lives of people with cystic fibrosis and are potentially disease modifying. Although much still needs to be accomplished to restore robust CFTR function in all patients, the cystic fibrosis therapeutic field is moving forward at an accelerated pace and can serve as a model for the successful implementation of precision medicine in other genetic disorders.
Glossary
Adeno-associated vector (AAV): A small virus that infects humans without causing disease. AAVs mostly remain episomal, performing long and stable expression, increasing their applicability for gene therapy
Adenovirus vector: Medium sized (90-100 nm) non-enveloped virus with a double stranded DNA genome that does not integrate into the host genome and is not replicated during cell division
Chaperone proteins: Proteins that assist the covalent folding or unfolding and the assembly or disassembly of other macromolecular structures such as DNA
cis mutations: Mutations occurring on two genes on the same chromosome of a homologous pair
Conductance mutation: Mutations in the CFTR channel that alter ion channel conductance (flux through the channel pore)
Forced expiratory volume in one second (FEV1): The volume of air a person can exhale forcibly in one second, after full inspiration
Gene replacement therapy: A genetic engineering based therapy in which a defective gene is replaced with a normal allele at the DNA level
Guanylate cyclase: Alyase enzyme that synthesizes cGMP in response to low calcium levels. cGMP keeps cGMP gated channels open, allowing calcium to enter the cell
High throughput screening (HTS): An automated drug discovery process that rapidly assays the biological or biochemical activity of a large number of drug-like compounds
Liposomal carrier: A spherical vesicle with at least one lipid bilayer that can be used as a vehicle for administration of nutrients and drugs
Luciferase assay: Luciferase is commonly used as a reporter to assess the transcriptional activity in cells that are transfected with a genetic construct containing the luciferase gene under the control of a promoter of interest. Luciferase is a generic term for the class of oxidative enzymes that produce bioluminescence
Missense mutation: A point mutation in which a single nucleotide change results in a codon that encodes a different amino acid
Nanoparticle: Particle between 1 nm and 100 nm in size
Optical coherence tomography (OCT): A non-invasive medical imaging technique that uses near infrared light to capture micrometer resolution, three dimensional images from within biological tissue
Organoids: Three dimensional organ buds grown in vitro
Phosphodiesterase-5: An enzyme of the phosphodiesterase class that specifically breaks down cGMP, which then acts as a second messenger, an intracellular signaling molecule released by the cell to trigger physiological changes such as proliferation, differentiation, migration, survival, and apoptosis in response to extracellular substances including peptide hormones such as epinephrine (adrenaline), growth factors, neurotransmitters such as serotonin, and steroid hormones
Precision medicine: An emerging approach for disease treatment and prevention that takes into account the individual’s variability in genes, environment, and lifestyle
Proteasome: Protein complexes inside all eukaryotes and archaea, and in some bacteria, which degrade unneeded or damaged proteins by a chemical reaction that breaks peptide bonds
Recombinant cell line: A cell line in which recombinant DNA has been introduced to produce high quantities of a protein of choice
S-nitrosoglutathione reductase: An enzyme that plays a crucial role in nitric oxide metabolism by reducing S-nitrosoglutathione (GSNO) to an unstable intermediate, S-hydroxylaminoglutathione, which then rearranges to form glutathione sulfonamide. Nitric oxide concentrations regulate respiratory function by modulating airway tone and proinflammatory and anti-inflammatory responses in the respiratory tract
Splice mutation: A genetic mutation that inserts, deletes, or changes several nucleotides in the specific site at which splicing takes place during the processing of precursor mRNA into mature mRNA
Sweat chloride: The sweat test measures the concentration of chloride that is excreted in sweat. It is used to test for cystic fibrosis. As a result of defective chloride channels (CFTRs), the concentration of chloride in sweat is raised in people with cystic fibrosis
Transduce: To introduce foreign DNA into another cell through a viral vector
Transgene complementation: The process of creating a genetically modified (or transgenic) mouse with a genetic mutation of interest to determine whether a therapeutic agent can overcome or correct the phenotype resulting from the mutation
Translational read-through: The continuation of translation beyond a stop codon through a pharmacologic approach
Contributors: Both authors contributed to the design of the work and drafting of the manuscript. BSQ created the first draft of the manuscript. Both authors approved the final version and are guarantors. The authors would like to thank Yeni Oh for her help with figure design and preparation and Richa Anand for her editing assistance.
Competing interests: We have read and understood BMJ policy on declaration of interests and declare the following: BSQ has had no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; SMR has received travel reimbursements to attend investigators’ meetings held by Vertex Pharmaceuticals, Novartis, Bayer Healthcare, PTC Therapeutics. SMR has an unlicensed patent held by the University of Alabama Birmingham on the use of CFTR activators for the treatment of respiratory diseases unaffected by acquired or genetic causes of CFTR dysfunction. SMR has an unlicensed patent held by the University of Alabama Birmingham for the use of optical coherence tomography as a diagnostic tool.
Provenance and peer review: Commissioned; externally peer reviewed.
No patients were asked for input in the creation of this article.
References
- 1.WHO. Genomic Resource Centre. Genes and human disease. http://www.who.int/genomics/public/geneticdiseases/en/index2.html.
- 2.Macneill SJ. Hodson and Geddes’ cystic fibrosis. Epidemiology of cystic fibrosis. 4th ed CRC Press, 2015. [Google Scholar]
- 3.Bear CE, Li CH, Kartner N, et al. Purification and functional reconstitution of the cystic fibrosis transmembrane conductance regulator (CFTR). Cell 1992;68:809-18. [DOI] [PubMed] [Google Scholar]
- 4.Rich DP, Anderson MP, Gregory RJ, et al. Expression of cystic fibrosis transmembrane conductance regulator corrects defective chloride channel regulation in cystic fibrosis airway epithelial cells. Nature 1990;347:358-63. [DOI] [PubMed] [Google Scholar]
- 5.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med 2005;352:1992-2001. [DOI] [PubMed] [Google Scholar]
- 6.Fuchs HJ, Borowitz DS, Christiansen DH, et al. The Pulmozyme Study Group. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. N Engl J Med 1994;331:637-42. [DOI] [PubMed] [Google Scholar]
- 7.Elkins MR, Robinson M, Rose BR, et al. National Hypertonic Saline in Cystic Fibrosis (NHSCF) Study Group. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med 2006;354:229-40. [DOI] [PubMed] [Google Scholar]
- 8.Aitken ML, Bellon G, De Boeck K, et al. CF302 Investigators. Long-term inhaled dry powder mannitol in cystic fibrosis: an international randomized study. Am J Respir Crit Care Med 2012;185:645-52. [DOI] [PubMed] [Google Scholar]
- 9.Bilton D, Robinson P, Cooper P, et al. CF301 Study Investigators. Inhaled dry powder mannitol in cystic fibrosis: an efficacy and safety study. Eur Respir J 2011;38:1071-80. [DOI] [PubMed] [Google Scholar]
- 10.Bilton D, Bellon G, Charlton B, et al. CF301 and CF302 Investigators. Pooled analysis of two large randomised phase III inhaled mannitol studies in cystic fibrosis. J Cyst Fibros 2013;12:367-76. [DOI] [PubMed] [Google Scholar]
- 11.Ramsey BW, Pepe MS, Quan JM, et al. Cystic Fibrosis Inhaled Tobramycin Study Group. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. N Engl J Med 1999;340:23-30. [DOI] [PubMed] [Google Scholar]
- 12.McCoy KS, Quittner AL, Oermann CM, Gibson RL, Retsch-Bogart GZ, Montgomery AB. Inhaled aztreonam lysine for chronic airway Pseudomonas aeruginosa in cystic fibrosis. Am J Respir Crit Care Med 2008;178:921-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saiman L, Marshall BC, Mayer-Hamblett N, et al. Macrolide Study Group. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA 2003;290:1749-56. [DOI] [PubMed] [Google Scholar]
- 14.Saiman L, Anstead M, Mayer-Hamblett N, et al. AZ0004 Azithromycin Study Group. Effect of azithromycin on pulmonary function in patients with cystic fibrosis uninfected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA 2010;303:1707-15. [DOI] [PubMed] [Google Scholar]
- 15.Konstan MW, Byard PJ, Hoppel CL, Davis PB. Effect of high-dose ibuprofen in patients with cystic fibrosis. N Engl J Med 1995;332:848-54. [DOI] [PubMed] [Google Scholar]
- 16.Kerem B, Rommens JM, Buchanan JA, et al. Identification of the cystic fibrosis gene: genetic analysis. Science 1989;245:1073-80. [DOI] [PubMed] [Google Scholar]
- 17.US CF Foundation, Johns Hopkins University, Hospital for Sick Children. CFTR2. Clinical and functional translation of CFTR. http://www.cftr2.org/files/CFTR2_13August2015.pdf.
- 18.Cystic Fibrosis Canada. The Canadian cystic fibrosis registry. Annual Report. 2013. http://www.cysticfibrosis.ca/wp-content/uploads/2015/02/Canadian-CF-Registry-2013-FINAL.pdf.
- 19.McKone EF, Emerson SS, Edwards KL, Aitken ML. Effect of genotype on phenotype and mortality in cystic fibrosis: a retrospective cohort study. Lancet 2003;361:1671-6. [DOI] [PubMed] [Google Scholar]
- 20.Ramsey BW, Davies J, McElvaney NG, et al. VX08-770-102 Study Group. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 2011;365:1663-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riordan JR, Rommens JM, Kerem B, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 1989;245:1066-73. [DOI] [PubMed] [Google Scholar]
- 22.Riordan JR. CFTR function and prospects for therapy. Annu Rev Biochem 2008;77:701-26. [DOI] [PubMed] [Google Scholar]
- 23.Hwang TC, Sheppard DN. Gating of the CFTR Cl− channel by ATP-driven nucleotide-binding domain dimerisation. J Physiol 2009;587:2151-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Broaddus VC, Mason RJ, Ernst JD, et al. Murray and Nadel’s textbook of respiratory medicine. 6th ed Elsevier, 2015. [Google Scholar]
- 25.Kanelis V, Hudson RP, Thibodeau PH, Thomas PJ, Forman-Kay JD. NMR evidence for differential phosphorylation-dependent interactions in WT and DeltaF508 CFTR. EMBO J 2010;29:263-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quinton PM. Cystic fibrosis: a disease in electrolyte transport. FASEB J 1990;4:2709-17. [DOI] [PubMed] [Google Scholar]
- 27.Cantin AM, Hartl D, Konstan MW, Chmiel JF. Inflammation in cystic fibrosis lung disease: pathogenesis and therapy. J Cyst Fibros 2015;14:419-30. [DOI] [PubMed] [Google Scholar]
- 28.Birket SE, Chu KK, Liu L, et al. A functional anatomic defect of the cystic fibrosis airway. Am J Respir Crit Care Med 2014;190:421-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoegger MJ, Fischer AJ, McMenimen JD, et al. Impaired mucus detachment disrupts mucociliary transport in a piglet model of cystic fibrosis. Science 2014;345:818-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garcia MA, Yang N, Quinton PM. Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator-dependent bicarbonate secretion. J Clin Invest 2009;119:2613-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gustafsson JK, Ermund A, Ambort D, et al. Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J Exp Med 2012;209:1263-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zielenski J, Tsui LC. Cystic fibrosis: genotypic and phenotypic variations. Annu Rev Genet 1995;29:777-807. [DOI] [PubMed] [Google Scholar]
- 33.Welsh MJ, Smith AE. Molecular mechanisms of CFTR chloride channel dysfunction in cystic fibrosis. Cell 1993;73:1251-4. [DOI] [PubMed] [Google Scholar]
- 34.Haardt M, Benharouga M, Lechardeur D, Kartner N, Lukacs GL. C-terminal truncations destabilize the cystic fibrosis transmembrane conductance regulator without impairing its biogenesis. A novel class of mutation. J Biol Chem 1999;274:21873-7. [DOI] [PubMed] [Google Scholar]
- 35.Mendell JT, Dietz HC. When the message goes awry: disease-producing mutations that influence mRNA content and performance. Cell 2001;107:411-4. [DOI] [PubMed] [Google Scholar]
- 36.Welch WJ. Role of quality control pathways in human diseases involving protein misfolding. Semin Cell Dev Biol 2004;15:31-8. [DOI] [PubMed] [Google Scholar]
- 37.Denning GM, Anderson MP, Amara JF, Marshall J, Smith AE, Welsh MJ. Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature 1992;358:761-4. [DOI] [PubMed] [Google Scholar]
- 38.Denning GM, Ostedgaard LS, Welsh MJ. Abnormal localization of cystic fibrosis transmembrane conductance regulator in primary cultures of cystic fibrosis airway epithelia. J Cell Biol 1992;118:551-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lukacs GL, Chang XB, Bear C, et al. The delta F508 mutation decreases the stability of cystic fibrosis transmembrane conductance regulator in the plasma membrane. Determination of functional half-lives on transfected cells. J Biol Chem 1993;268:21592-8. [PubMed] [Google Scholar]
- 40.Dalemans W, Barbry P, Champigny G, et al. Altered chloride ion channel kinetics associated with the delta F508 cystic fibrosis mutation. Nature 1991;354:526-8. [DOI] [PubMed] [Google Scholar]
- 41.Castellani C, Cuppens H, Macek M Jr, et al. Consensus on the use and interpretation of cystic fibrosis mutation analysis in clinical practice. J Cyst Fibros 2008;7:179-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chu CS, Trapnell BC, Curristin S, Cutting GR, Crystal RG. Genetic basis of variable exon 9 skipping in cystic fibrosis transmembrane conductance regulator mRNA. Nat Genet 1993;3:151-6. [DOI] [PubMed] [Google Scholar]
- 43.Kerem E, Corey M, Kerem BS, et al. The relation between genotype and phenotype in cystic fibrosis--analysis of the most common mutation (delta F508). N Engl J Med 1990;323:1517-22. [DOI] [PubMed] [Google Scholar]
- 44.Drumm ML, Konstan MW, Schluchter MD, et al. Gene Modifier Study Group. Genetic modifiers of lung disease in cystic fibrosis. N Engl J Med 2005;353:1443-53. [DOI] [PubMed] [Google Scholar]
- 45.Van Goor F, Hadida S, Grootenhuis PD, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci U S A 2009;106:18825-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li C, Ramjeesingh M, Wang W, et al. ATPase activity of the cystic fibrosis transmembrane conductance regulator. J Biol Chem 1996;271:28463-8. [DOI] [PubMed] [Google Scholar]
- 47.Van Goor F, Straley KS, Cao D, et al. Rescue of DeltaF508-CFTR trafficking and gating in human cystic fibrosis airway primary cultures by small molecules. Am J Physiol Lung Cell Mol Physiol 2006;290:L1117-30. [DOI] [PubMed] [Google Scholar]
- 48.Jih KY, Hwang TC. Vx-770 potentiates CFTR function by promoting decoupling between the gating cycle and ATP hydrolysis cycle. Proc Natl Acad Sci U S A 2013;110:4404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rowe SM, Verkman AS. Cystic fibrosis transmembrane regulator correctors and potentiators. Cold Spring Harb Perspect Med 2013;3:a009761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Goor F, Hadida S, Grootenhuis PD, et al. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc Natl Acad Sci U S A 2011;108:18843-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mendoza JL, Schmidt A, Li Q, et al. Requirements for efficient correction of ΔF508 CFTR revealed by analyses of evolved sequences. Cell 2012;148:164-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rabeh WM, Bossard F, Xu H, et al. Correction of both NBD1 energetics and domain interface is required to restore ΔF508 CFTR folding and function. Cell 2012;148:150-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang C, Protasevich I, Yang Z, et al. Integrated biophysical studies implicate partial unfolding of NBD1 of CFTR in the molecular pathogenesis of F508del cystic fibrosis. Protein Sci 2010;19:1932-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Howard M, Frizzell RA, Bedwell DM. Aminoglycoside antibiotics restore CFTR function by overcoming premature stop mutations. Nat Med 1996;2:467-9. [DOI] [PubMed] [Google Scholar]
- 55.Amrani N, Ganesan R, Kervestin S, Mangus DA, Ghosh S, Jacobson A. A faux 3′-UTR promotes aberrant termination and triggers nonsense-mediated mRNA decay. Nature 2004;432:112-8. [DOI] [PubMed] [Google Scholar]
- 56.Welch EM, Barton ER, Zhuo J, et al. PTC124 targets genetic disorders caused by nonsense mutations. Nature 2007;447:87-91. [DOI] [PubMed] [Google Scholar]
- 57.Nudelman I, Rebibo-Sabbah A, Cherniavsky M, et al. Development of novel aminoglycoside (NB54) with reduced toxicity and enhanced suppression of disease-causing premature stop mutations. J Med Chem 2009;52:2836-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rowe SM, Sloane P, Tang LP, et al. Suppression of CFTR premature termination codons and rescue of CFTR protein and function by the synthetic aminoglycoside NB54. J Mol Med (Berl) 2011;89:1149-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xue X, Mutyam V, Tang L, et al. Synthetic aminoglycosides efficiently suppress cystic fibrosis transmembrane conductance regulator nonsense mutations and are enhanced by ivacaftor. Am J Respir Cell Mol Biol 2014;50:805-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Du M, Liu X, Welch EM, Hirawat S, Peltz SW, Bedwell DM. PTC124 is an orally bioavailable compound that promotes suppression of the human CFTR-G542X nonsense allele in a CF mouse model. Proc Natl Acad Sci U S A 2008;105:2064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang L, Button B, Gabriel SE, et al. CFTR delivery to 25% of surface epithelial cells restores normal rates of mucus transport to human cystic fibrosis airway epithelium. PLoS Biol 2009;7:e1000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Flume PA, Liou TG, Borowitz DS, et al. VX 08-770-104 Study Group. Ivacaftor in subjects with cystic fibrosis who are homozygous for the F508del-CFTR mutation. Chest 2012;142:718-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Boyle MP, Bell SC, Konstan MW, et al. VX09-809-102 study group. A CFTR corrector (lumacaftor) and a CFTR potentiator (ivacaftor) for treatment of patients with cystic fibrosis who have a phe508del CFTR mutation: a phase 2 randomised controlled trial. Lancet Respir Med 2014;2:527-38. [DOI] [PubMed] [Google Scholar]
- 64.Clancy JP, Rowe SM, Accurso FJ, et al. Results of a phase IIa study of VX-809, an investigational CFTR corrector compound, in subjects with cystic fibrosis homozygous for the F508del-CFTR mutation. Thorax 2012;67:12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Okiyoneda T, Veit G, Dekkers JF, et al. Mechanism-based corrector combination restores ΔF508-CFTR folding and function. Nat Chem Biol 2013;9:444-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boucher RC. Cystic fibrosis: a disease of vulnerability to airway surface dehydration. Trends Mol Med 2007;13:231-40. [DOI] [PubMed] [Google Scholar]
- 67.Leier G, Bangel-Ruland N, Sobczak K, Knieper Y, Weber WM. Sildenafil acts as potentiator and corrector of CFTR but might be not suitable for the treatment of CF lung disease. Cell Physiol Biochem 2012;29:775-90. [DOI] [PubMed] [Google Scholar]
- 68.Lubamba B, Lebacq J, Reychler G, et al. Inhaled phosphodiesterase type 5 inhibitors restore chloride transport in cystic fibrosis mice. Eur Respir J 2011;37:72-8. [DOI] [PubMed] [Google Scholar]
- 69.Lubamba B, Lecourt H, Lebacq J, et al. Preclinical evidence that sildenafil and vardenafil activate chloride transport in cystic fibrosis. Am J Respir Crit Care Med 2008;177:506-15. [DOI] [PubMed] [Google Scholar]
- 70.Poschet JF, Timmins GS, Taylor-Cousar JL, et al. Pharmacological modulation of cGMP levels by phosphodiesterase 5 inhibitors as a therapeutic strategy for treatment of respiratory pathology in cystic fibrosis. Am J Physiol Lung Cell Mol Physiol 2007;293:L712-9. [DOI] [PubMed] [Google Scholar]
- 71.Marozkina NV, Yemen S, Borowitz M, et al. Hsp 70/Hsp 90 organizing protein as a nitrosylation target in cystic fibrosis therapy. Proc Natl Acad Sci U S A 2010;107:11393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zaman K, Bennett D, Fraser-Butler M, et al. S-Nitrosothiols increases cystic fibrosis transmembrane regulator expression and maturation in the cell surface. Biochem Biophys Res Commun 2014;443:1257-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Armstrong DK, Cunningham S, Davies JC, Alton EW. Gene therapy in cystic fibrosis. Arch Dis Child 2014;99:465-8. [DOI] [PubMed] [Google Scholar]
- 74.Zabner J, Ramsey BW, Meeker DP, et al. Repeat administration of an adenovirus vector encoding cystic fibrosis transmembrane conductance regulator to the nasal epithelium of patients with cystic fibrosis. J Clin Invest 1996;97:1504-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harvey BG, Leopold PL, Hackett NR, et al. Airway epithelial CFTR mRNA expression in cystic fibrosis patients after repetitive administration of a recombinant adenovirus. J Clin Invest 1999;104:1245-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Griesenbach U, Alton EW. Progress in gene and cell therapy for cystic fibrosis lung disease. Curr Pharm Des 2012;18:642-62. [DOI] [PubMed] [Google Scholar]
- 77.Mintzer MA, Simanek EE. Nonviral vectors for gene delivery. Chem Rev 2009;109:259-302. [DOI] [PubMed] [Google Scholar]
- 78.Hyde SC, Southern KW, Gileadi U, et al. Repeat administration of DNA/liposomes to the nasal epithelium of patients with cystic fibrosis. Gene Ther 2000;7:1156-65. [DOI] [PubMed] [Google Scholar]
- 79.Alton EW, Stern M, Farley R, et al. Cationic lipid-mediated CFTR gene transfer to the lungs and nose of patients with cystic fibrosis: a double-blind placebo-controlled trial. Lancet 1999;353:947-54. [DOI] [PubMed] [Google Scholar]
- 80.Hyde SC, Pringle IA, Abdullah S, et al. CpG-free plasmids confer reduced inflammation and sustained pulmonary gene expression. Nat Biotechnol 2008;26:549-51. [DOI] [PubMed] [Google Scholar]
- 81.Sanders NN, De Smedt SC, Van Rompaey E, Simoens P, De Baets F, Demeester J. Cystic fibrosis sputum: a barrier to the transport of nanospheres. Am J Respir Crit Care Med 2000;162:1905-11. [DOI] [PubMed] [Google Scholar]
- 82.Ensign LM, Schneider C, Suk JS, Cone R, Hanes J. Mucus penetrating nanoparticles: biophysical tool and method of drug and gene delivery. Adv Mater 2012;24:3887-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Suk JS, Kim AJ, Trehan K, et al. Lung gene therapy with highly compacted DNA nanoparticles that overcome the mucus barrier. J Control Release 2014;178:8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ferrari S, Kitson C, Farley R, et al. Mucus altering agents as adjuncts for nonviral gene transfer to airway epithelium. Gene Ther 2001;8:1380-6. [DOI] [PubMed] [Google Scholar]
- 85.Anderson DG, Akinc A, Hossain N, Langer R. Structure/property studies of polymeric gene delivery using a library of poly(beta-amino esters). Mol Ther 2005;11:426-34. [DOI] [PubMed] [Google Scholar]
- 86.Mastorakos P, da Silva AL, Chisholm J, et al. Highly compacted biodegradable DNA nanoparticles capable of overcoming the mucus barrier for inhaled lung gene therapy. Proc Natl Acad Sci U S A 2015;112:8720-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cox DB, Platt RJ, Zhang F. Therapeutic genome editing: prospects and challenges. Nat Med 2015;21:121-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell 2014;157:1262-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schwank G, Koo BK, Sasselli V, et al. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell 2013;13:653-8. [DOI] [PubMed] [Google Scholar]
- 90.Zamecnik PC, Raychowdhury MK, Tabatadze DR, Cantiello HF. Reversal of cystic fibrosis phenotype in a cultured Delta508 cystic fibrosis transmembrane conductance regulator cell line by oligonucleotide insertion. Proc Natl Acad Sci U S A 2004;101:8150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Davies JC, Wainwright CE, Canny GJ, et al. VX08-770-103 (ENVISION) Study Group. Efficacy and safety of ivacaftor in patients aged 6 to 11 years with cystic fibrosis with a G551D mutation. Am J Respir Crit Care Med 2013;187:1219-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.De Boeck K, Munck A, Walker S, et al. Efficacy and safety of ivacaftor in patients with cystic fibrosis and a non-G551D gating mutation. J Cyst Fibros 2014;13:674-80. [DOI] [PubMed] [Google Scholar]
- 93.Davies JC, Cunningham S, Harris WT, et al. KIWI Study Group. Safety, pharmacokinetics, and pharmacodynamics of ivacaftor in patients aged 2-5 years with cystic fibrosis and a CFTR gating mutation (KIWI): an open-label, single-arm study. Lancet Respir Med 2016;S2213-2600(15)00545-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Robertson SM, Luo X, Dubey N, et al. Clinical drug-drug interaction assessment of ivacaftor as a potential inhibitor of cytochrome P450 and P-glycoprotein. J Clin Pharmacol 2015;55:56-62. [DOI] [PubMed] [Google Scholar]
- 95.Rowe SM, Heltshe SL, Gonska T, et al. GOAL Investigators of the Cystic Fibrosis Foundation Therapeutics Development Network. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am J Respir Crit Care Med 2014;190:175-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Heltshe SL, Mayer-Hamblett N, Burns JL, et al. GOAL (the G551D Observation-AL) Investigators of the Cystic Fibrosis Foundation Therapeutics Development Network. Pseudomonas aeruginosa in cystic fibrosis patients with G551D-CFTR treated with ivacaftor. Clin Infect Dis 2015;60:703-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McKone EF, Borowitz D, Drevinek P, et al. VX08-770-105 (PERSIST) Study Group. Long-term safety and efficacy of ivacaftor in patients with cystic fibrosis who have the Gly551Asp-CFTR mutation: a phase 3, open-label extension study (PERSIST). Lancet Respir Med 2014;2:902-10. [DOI] [PubMed] [Google Scholar]
- 98.Sawicki GS, McKone EF, Pasta DJ, et al. Sustained benefit from ivacaftor demonstrated by combining clinical trial and cystic fibrosis patient registry data. Am J Respir Crit Care Med 2015;192:836-42. [DOI] [PubMed] [Google Scholar]
- 99.Taylor-Cousar J, Niknian M, Gilmartin G, Pilewski JM. VX11-770-901 investigators. Effect of ivacaftor in patients with advanced cystic fibrosis and a G551D-CFTR mutation: Safety and efficacy in an expanded access program in the United States. J Cyst Fibros 2016;15:116-22. [DOI] [PubMed] [Google Scholar]
- 100.Barry PJ, Plant BJ, Nair A, et al. Effects of ivacaftor in patients with cystic fibrosis who carry the G551D mutation and have severe lung disease. Chest 2014;146:152-8. [DOI] [PubMed] [Google Scholar]
- 101.Hebestreit H, Sauer-Heilborn A, Fischer R, Käding M, Mainz JG. Effects of ivacaftor on severely ill patients with cystic fibrosis carrying a G551D mutation. J Cyst Fibros 2013;12:599-603. [DOI] [PubMed] [Google Scholar]
- 102.Grasemann H, Gonska T, Avolio J, Klingel M, Tullis E, Ratjen F. Effect of ivacaftor therapy on exhaled nitric oxide in patients with cystic fibrosis. J Cyst Fibros 2015;14:727-32. [DOI] [PubMed] [Google Scholar]
- 103.Bernarde C, Keravec M, Mounier J, et al. Impact of the CFTR-potentiator ivacaftor on airway microbiota in cystic fibrosis patients carrying a G551D mutation. PLoS One 2015;10:e0124124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hayes D Jr, , Long FR, McCoy KS, Sheikh SI. Improvement in bronchiectasis on CT imaging in a pediatric patient with cystic fibrosis on ivacaftor therapy. Respiration 2014;88:345. [DOI] [PubMed] [Google Scholar]
- 105.Sheikh SI, Long FR, McCoy KS, Johnson T, Ryan-Wenger NA, Hayes D Jr. Computed tomography correlates with improvement with ivacaftor in cystic fibrosis patients with G551D mutation. J Cyst Fibros 2015;14:84-9. [DOI] [PubMed] [Google Scholar]
- 106.Hayes D Jr, , Long FR, McCoy KS, Sheikh SI. CT imaging of pediatric patients with cystic fibrosis on ivacaftor therapy. Lung 2014;192:823-4. [DOI] [PubMed] [Google Scholar]
- 107.Hoare S, McEvoy S, McCarthy CJ, et al. Ivacaftor imaging response in cystic fibrosis. Am J Respir Crit Care Med 2014;189:484. [DOI] [PubMed] [Google Scholar]
- 108.Chang EH, Tang XX, Shah VS, et al. Medical reversal of chronic sinusitis in a cystic fibrosis patient with ivacaftor. Int Forum Allergy Rhinol 2015;5:178-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hayes D Jr, , McCoy KS, Sheikh SI. Improvement of sinus disease in cystic fibrosis with ivacaftor therapy. Am J Respir Crit Care Med 2014;190:468. [DOI] [PubMed] [Google Scholar]
- 110.Sheikh SI, Long FR, McCoy KS, Johnson T, Ryan-Wenger NA, Hayes D Jr. Ivacaftor improves appearance of sinus disease on computerised tomography in cystic fibrosis patients with G551D mutation. Clin Otolaryngol 2015;40:16-21. [DOI] [PubMed] [Google Scholar]
- 111.Bellin MD, Laguna T, Leschyshyn J, et al. Insulin secretion improves in cystic fibrosis following ivacaftor correction of CFTR: a small pilot study. Pediatr Diabetes 2013;14:417-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tsabari R, Elyashar HI, Cymberknowh MC, et al. CFTR potentiator therapy ameliorates impaired insulin secretion in CF patients with a gating mutation. J Cyst Fibros 2015; online 4 Nov;S1569-1993(15)00255-6. [DOI] [PubMed] [Google Scholar]
- 113.Hayes D Jr, , McCoy KS, Sheikh SI. Resolution of cystic fibrosis-related diabetes with ivacaftor therapy. Am J Respir Crit Care Med 2014;190:590-1. [DOI] [PubMed] [Google Scholar]
- 114.Hayes D Jr, , Warren PS, McCoy KS, Sheikh SI. Improvement of hepatic steatosis in cystic fibrosis with ivacaftor therapy. J Pediatr Gastroenterol Nutr 2015;60:578-9. [DOI] [PubMed] [Google Scholar]
- 115.Sheppard DN, Rich DP, Ostedgaard LS, Gregory RJ, Smith AE, Welsh MJ. Mutations in CFTR associated with mild-disease-form Cl- channels with altered pore properties. Nature 1993;362:160-4. [DOI] [PubMed] [Google Scholar]
- 116.Boyle MP, De Boeck K. A new era in the treatment of cystic fibrosis: correction of the underlying CFTR defect. Lancet Respir Med 2013;1:158-63. [DOI] [PubMed] [Google Scholar]
- 117.Moss RB, Flume PA, Elborn JS, et al. VX11-770-110 (KONDUCT) Study Group. Efficacy and safety of ivacaftor in patients with cystic fibrosis who have an Arg117His-CFTR mutation: a double-blind, randomised controlled trial. Lancet Respir Med 2015;3:524-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wainwright CE, Elborn JS, Ramsey BW, et al. TRAFFIC Study Group TRANSPORT Study Group. Lumacaftor-ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med 2015;373:220-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pilewski JM, Donaldson SH, Cooke J, et al. Phase 2 studies reveal additive effects of VX-661, an investigational CFTR corrector, and ivacaftor, a CFTR potentiator, in patients who carry the F508del-CFTR mutation. Pediatr Pulmonol 2014;49(S38):157-8. [Google Scholar]
- 120.Vertex. Vertex announces data from 12-week phase 2 safety study of VX-661 in combination with ivacaftor in people with cystic fibrosis who have two copies of the F508del mutation. http://investors.vrtx.com/releasedetail.cfm?ReleaseID=902790.
- 121.Wilschanski M, Famini C, Blau H, et al. A pilot study of the effect of gentamicin on nasal potential difference measurements in cystic fibrosis patients carrying stop mutations. Am J Respir Crit Care Med 2000;161:860-5. [DOI] [PubMed] [Google Scholar]
- 122.Sermet-Gaudelus I, Renouil M, Fajac A, et al. In vitro prediction of stop-codon suppression by intravenous gentamicin in patients with cystic fibrosis: a pilot study. BMC Med 2007;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Clancy JP, Bebök Z, Ruiz F, et al. Evidence that systemic gentamicin suppresses premature stop mutations in patients with cystic fibrosis. Am J Respir Crit Care Med 2001;163:1683-92. [DOI] [PubMed] [Google Scholar]
- 124.Wilschanski M, Yahav Y, Yaacov Y, et al. Gentamicin-induced correction of CFTR function in patients with cystic fibrosis and CFTR stop mutations. N Engl J Med 2003;349:1433-41. [DOI] [PubMed] [Google Scholar]
- 125.Kerem E, Hirawat S, Armoni S, et al. Effectiveness of PTC124 treatment of cystic fibrosis caused by nonsense mutations: a prospective phase II trial. Lancet 2008;372:719-27. [DOI] [PubMed] [Google Scholar]
- 126.Wilschanski M, Miller LL, Shoseyov D, et al. Chronic ataluren (PTC124) treatment of nonsense mutation cystic fibrosis. Eur Respir J 2011;38:59-69. [DOI] [PubMed] [Google Scholar]
- 127.Sermet-Gaudelus I, Boeck KD, Casimir GJ, et al. Ataluren (PTC124) induces cystic fibrosis transmembrane conductance regulator protein expression and activity in children with nonsense mutation cystic fibrosis. Am J Respir Crit Care Med 2010;182:1262-72. [DOI] [PubMed] [Google Scholar]
- 128.Kerem E, Konstan MW, De Boeck K, et al. Cystic Fibrosis Ataluren Study Group. Ataluren for the treatment of nonsense-mutation cystic fibrosis: a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Respir Med 2014;2:539-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Moss RB, Rodman D, Spencer LT, et al. Repeated adeno-associated virus serotype 2 aerosol-mediated cystic fibrosis transmembrane regulator gene transfer to the lungs of patients with cystic fibrosis: a multicenter, double-blind, placebo-controlled trial. Chest 2004;125:509-21. [DOI] [PubMed] [Google Scholar]
- 130.Moss RB, Milla C, Colombo J, et al. Repeated aerosolized AAV-CFTR for treatment of cystic fibrosis: a randomized placebo-controlled phase 2B trial. Hum Gene Ther 2007;18:726-32. [DOI] [PubMed] [Google Scholar]
- 131.Davies LA, Nunez-Alonso GA, McLachlan G, Hyde SC, Gill DR. Aerosol delivery of DNA/liposomes to the lung for cystic fibrosis gene therapy. Hum Gene Ther Clin Dev 2014;25:97-107. [DOI] [PubMed] [Google Scholar]
- 132.McLachlan G, Davidson H, Holder E, et al. Pre-clinical evaluation of three non-viral gene transfer agents for cystic fibrosis after aerosol delivery to the ovine lung. Gene Ther 2011;18:996-1005. [DOI] [PubMed] [Google Scholar]
- 133.Alton EW, Baker A, Baker E, et al. The safety profile of a cationic lipid-mediated cystic fibrosis gene transfer agent following repeated monthly aerosol administration to sheep. Biomaterials 2013;34:10267-77. [DOI] [PubMed] [Google Scholar]
- 134.Alton EW, Boyd AC, Cheng SH, et al. Toxicology study assessing efficacy and safety of repeated administration of lipid/DNA complexes to mouse lung. Gene Ther 2014;21:89-95. [DOI] [PubMed] [Google Scholar]
- 135.Alton EW, Armstrong DK, Ashby D, et al. UK Cystic Fibrosis Gene Therapy Consortium. Repeated nebulisation of non-viral CFTR gene therapy in patients with cystic fibrosis: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir Med 2015;3:684-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Smyth AR, Bell SC, Bojcin S, et al. European Cystic Fibrosis Society. European Cystic Fibrosis Society standards of care: best practice guidelines. J Cyst Fibros 2014;13(Suppl 1):S23-42. [DOI] [PubMed] [Google Scholar]
- 137.Mogayzel PJ Jr, , Naureckas ET, Robinson KA, et al. Pulmonary Clinical Practice Guidelines Committee. Cystic fibrosis pulmonary guidelines. Chronic medications for maintenance of lung health. Am J Respir Crit Care Med 2013;187:680-9. [DOI] [PubMed] [Google Scholar]
- 138.Whiting P, Al M, Burgers L, et al. Ivacaftor for the treatment of patients with cystic fibrosis and the G551D mutation: a systematic review and cost-effectiveness analysis. Health Technol Assess 2014;18:1-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Durmowicz AG, Witzmann KA, Rosebraugh CJ, Chowdhury BA. Change in sweat chloride as a clinical end point in cystic fibrosis clinical trials: the ivacaftor experience. Chest 2013;143:14-8. [DOI] [PubMed] [Google Scholar]
- 140.Barry PJ, Jones AM, Webb AK, Horsley AR. Sweat chloride is not a useful marker of clinical response to ivacaftor. Thorax 2014;69:586-7. [DOI] [PubMed] [Google Scholar]
- 141.Wong AP, Bear CE, Chin S, et al. Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein. Nat Biotechnol 2012;30:876-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dekkers JF, Wiegerinck CL, de Jonge HR, et al. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med 2013;19:939-45. [DOI] [PubMed] [Google Scholar]
- 143.Liu L, Chu KK, Houser GH, et al. Method for quantitative study of airway functional microanatomy using micro-optical coherence tomography. PLoS One 2013;8:e54473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zucker DR, Ruthazer R, Schmid CH. Individual (N-of-1) trials can be combined to give population comparative treatment effect estimates: methodologic considerations. J Clin Epidemiol 2010;63:1312-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nick JA, Rodman D, St Clair C, et al. Utilization of an “N-of-1” study design to test the effect of ivacaftor in CF patients with residual CFTR function and FEV1 > 40% of Predicted. Pediatr Pulmonol 2014;49(S38):188-9. [Google Scholar]
- 146.Sly PD, Gangell CL, Chen L, et al. AREST CF Investigators. Risk factors for bronchiectasis in children with cystic fibrosis. N Engl J Med 2013;368:1963-70. [DOI] [PubMed] [Google Scholar]
- 147.Sawicki GS, Ren CL, Konstan MW, Millar SJ, Pasta DJ, Quittner AL. Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Treatment complexity in cystic fibrosis: trends over time and associations with site-specific outcomes. J Cyst Fibros 2013;12:461-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Veit G, Avramescu RG, Perdomo D, et al. Some gating potentiators, including VX-770, diminish ΔF508-CFTR functional expression. Sci Transl Med 2014;6:246ra97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cholon DM, Quinney NL, Fulcher ML, et al. Potentiator ivacaftor abrogates pharmacological correction of ΔF508 CFTR in cystic fibrosis. Sci Transl Med 2014;6:246ra96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liu X, Dawson DC. Cystic fibrosis transmembrane conductance regulator (CFTR) potentiators protect G551D but not ΔF508 CFTR from thermal instability. Biochemistry 2014;53:5613-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Andersen DH. Cystic fibrosis of the pancreas. J Chronic Dis 1958;7:58-90. [DOI] [PubMed] [Google Scholar]