ABSTRACT
The gut microbiota is found to be strongly associated with atherosclerosis (AS). Resveratrol (RSV) is a natural phytoalexin with anti-AS effects; however, its mechanisms of action remain unclear. Therefore, we sought to determine whether the anti-AS effects of RSV were related to changes in the gut microbiota. We found that RSV attenuated trimethylamine-N-oxide (TMAO)-induced AS in ApoE−/− mice. Meanwhile, RSV decreased TMAO levels by inhibiting commensal microbial trimethylamine (TMA) production via gut microbiota remodeling in mice. Moreover, RSV increased levels of the genera Lactobacillus and Bifidobacterium, which increased the bile salt hydrolase activity, thereby enhancing bile acid (BA) deconjugation and fecal excretion in C57BL/6J and ApoE−/− mice. This was associated with a decrease in ileal BA content, repression of the enterohepatic farnesoid X receptor (FXR)-fibroblast growth factor 15 (FGF15) axis, and increased cholesterol 7a-hydroxylase (CYP7A1) expression and hepatic BA neosynthesis. An FXR antagonist had the same effect on FGF15 and CYP7A1 expression as RSV, while an FXR agonist abolished RSV-induced alterations in FGF15 and CYP7A1 expression. In mice treated with antibiotics, RSV neither decreased TMAO levels nor increased hepatic BA synthesis. Additionally, RSV-induced inhibition of TMAO-caused AS was also markedly abolished by antibiotics. In conclusion, RSV attenuated TMAO-induced AS by decreasing TMAO levels and increasing hepatic BA neosynthesis via gut microbiota remodeling, and the BA neosynthesis was partially mediated through the enterohepatic FXR-FGF15 axis.
IMPORTANCE
Recently, trimethylamine-N-oxide (TMAO) has been identified as a novel and independent risk factor for promoting atherosclerosis (AS) partially through inhibiting hepatic bile acid (BA) synthesis. The gut microbiota plays a key role in the pathophysiology of TMAO-induced AS. Resveratrol (RSV) is a natural phytoalexin with prebiotic benefits. A growing body of evidence supports the hypothesis that phenolic phytochemicals with poor bioavailability are possibly acting primarily through remodeling of the gut microbiota. The current study showed that RSV attenuated TMAO-induced AS by decreasing TMAO levels and increasing hepatic BA neosynthesis via gut microbiota remodeling. And RSV-induced hepatic BA neosynthesis was partially mediated through downregulating the enterohepatic farnesoid X receptor-fibroblast growth factor 15 axis. These results offer new insights into the mechanisms responsible for RSV’s anti-AS effects and indicate that the gut microbiota may become an interesting target for pharmacological or dietary interventions to decrease the risk of developing cardiovascular diseases.
INTRODUCTION
The incidence of cardiovascular diseases (CVDs), such as atherosclerosis (AS), is increasing globally and has become an expensive public health issue (1). A recent metabolomics approach identified that plasma trimethylamine-N-oxide (TMAO), a choline metabolite, is a novel and independent risk factor for promoting AS (2, 3). TMAO generation is dependent on the gut microbiota, which first metabolizes dietary choline to trimethylamine (TMA). Thereafter, the TMA is metabolized to TMAO by enzymes of the flavin monooxygenase (FMO) family in the liver (4). Thus, the gut microbiota has been put forward as a key player in the pathogenesis of TMAO-induced AS, which might be a new potential therapeutic target for the prevention and treatment of CVD. It was found that antibiotics (Abs) could attenuate TMAO-caused AS by reducing TMAO synthesis via blocking the pathway of l-carnitine to TMA governed by the gut microbiota (4). However, the side effects and resistance potential of Abs limit their utility. Therefore, the identification of natural products with excellent antimicrobial effects to inhibit gut microbial TMA production might be useful for the prevention and treatment of TMAO-induced AS.
TMAO has been shown to induce AS by affecting cholesterol metabolism through inhibiting hepatic bile acid (BA) synthesis (2–4). Recently, the gut microbiota has been found to play an important role in hepatic BA synthesis (5). Primary BAs are synthesized from cholesterol in the liver and further metabolized by the gut microbiota into secondary BAs via deconjugation, dehydrogenation, and dehydroxylation in the gut (6). This process is controlled by a negative-feedback loop through the activation of the nuclear farnesoid X receptor (FXR) in the ileum and liver (7, 8). Furthermore, many studies have shown that the gut microbiota regulates secondary BA metabolism and BA synthesis in the liver by alleviating the enterohepatic FXR-fibroblast growth factor 15 (FGF15) axis (6, 9). VSL#3 probiotics can induce hepatic BA neosynthesis via downregulation of the gut-liver FXR-FGF15 axis (10). Sayin et al. found that the gut microbiota can reduce the levels of tauro-beta-muricholic acid (T-βMCA), a naturally occurring FXR antagonist. This subsequently inhibits cholesterol 7a-hydroxylase (CYP7A1), the rate-limiting enzyme in BA synthesis, by activating FXR-FGF15 signaling and ultimately regulating BA metabolism in the liver (5, 11). Additionally, tempol, an antioxidant and protective agent against radiation, can alter the gut microbiome, leading to the accumulation of intestinal T-βMCA, thereby inhibiting intestinal FXR signaling and decreasing obesity (12). These results indicate that microbiota-targeted therapies could be effective in preventing gut-related diseases, including AS, by regulating BA metabolism via the enterohepatic FXR-FGF15 axis.
Resveratrol (RSV) is a natural polyphenol that mainly occurs in grapes, berries, and other dietary constituents and is beneficial for treatment of many metabolic diseases, including AS (13). Previous investigations have shown that the physiological effects of dietary RSV are in striking contrast to its poor bioavailability, which is a major concern for the development of this class of compounds into therapeutic agents (14, 15). However, a growing body of evidence supports the hypothesis that phenolic phytochemicals with poor bioavailability are possibly acting primarily through remodeling the gut microbiota. Researchers have confirmed that a polyphenol-rich cranberry extract and metformin attenuate diet-induced metabolic syndrome in mice in a gut microbiota-dependent manner (16, 17). Moreover, it has been found that RSV consumption can significantly modulate the growth of certain gut microbiota in vivo, including increasing the Bacteroidetes-to-Firmicutes ratios, and the growth of Bacteroides, Lactobacillus, and Bifidobacterium (18–22). These results suggested that RSV could be a good candidate for prebiotics and could be used to promote the growth of beneficial commensals and thus to confer health benefits to the host.
Given the close association among TMAO levels, gut microbiota, BA metabolism, and AS, we hypothesized that RSV could attenuate TMAO-induced AS by regulating TMAO synthesis and BA metabolism via the modulation of the gut microbiota. To verify this hypothesis, we examined the effects of RSV on TMAO-induced AS, gut microbiota, TMAO synthesis, and BA metabolism in C57BL/6J and ApoE−/− mice. Furthermore, the potential involvement of the enterohepatic FXR-FGF15 axis was also investigated. We showed, for the first time, that RSV attenuated TMAO-induced AS by decreasing TMAO levels and increasing hepatic BA neosynthesis via gut microbiota remodeling and that RSV-induced BA neosynthesis was partially mediated through the enterohepatic FXR-FGF15 axis.
RESULTS
RSV inhibited TMAO synthesis in C57BL/6J mice.
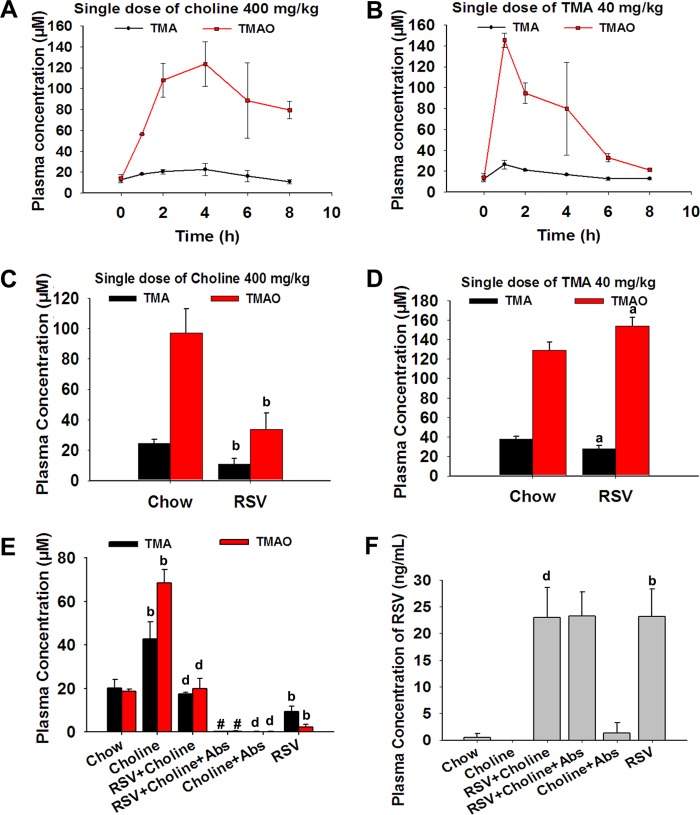
In order to investigate the effect of RSV on TMAO synthesis in C57BL/6J mice, TMA and TMAO contents in serum were measured at 0, 1, 2, 4, 6 and 8 h after administration of a single dose of choline (400 mg/kg of body weight) or TMA (40 mg/kg) in mice by intragastric gavage. Via liquid chromatography-tandem mass spectrometry (LC/MS), we found that peak concentrations of TMA and TMAO occurred 4 h after choline administration and 1 h after TMA administration (Fig. 1A and B). Thus, in subsequent experiments we used serum samples taken at 4 h or 1 h after the administration of choline or TMA, respectively. Serum TMA and TMAO levels were much lower in RSV-treated mice given a single dose of choline than in those in the control group (Fig. 1C). For RSV-treated mice given TMA, TMAO levels were higher and TMA levels were lower than those in the control group (Fig. 1D), indicating that RSV may induce the metabolism of TMA to TMAO in the liver. Moreover, the addition of 1% choline over 30 days significantly increased the levels of TMA and TMAO in mice; however, this was reversed by RSV administration (Fig. 1E). Additionally, plasma RSV content was higher in mice fed RSV than in mice fed a standard diet (Fig. 1F). Our results suggested that RSV reduced TMAO synthesis levels in C57BL/6J mice.
FIG 1 .
RSV inhibited TMAO synthesis in C57BL/6J mice. (A and B) Eight-week-old female C57BL/6J mice were administered choline (400 mg/kg of body weight, n = 10) (A) or TMA (40 mg/kg, n = 10) (B). Blood samples were collected at the indicated times. Serum TMA and TMAO levels were determined by LC/MS. (C and D) Eight-week-old female C57BL/6J mice were fed a chow diet with or without RSV (0.4%) in the presence or absence of choline (1%) or Abs. After 30 days, several mice of the chow-and-RSV-fed group were administered choline (400 mg/kg, n = 10) (C) or TMA (40 mg/kg, n = 10) (D). At 4 h after choline was given, or 1 h after TMA was given, the mice were euthanized and blood was collected. Serum TMA and TMAO levels were determined by LC/MS. (E and F) The other mice were also euthanized, and blood was collected. Serum TMA and TMAO levels (E) and RSV levels (F) were determined by LC/MS. Values are presented as means ± SD (n = 10). a, P < 0.05; b, P < 0.01 (versus vehicle-treated control group); d, P < 0.01 (versus choline-treated group); #, P < 0.05 (versus group cotreated with choline and RSV).
RSV inhibited TMAO synthesis by decreasing TMA generation via remodeling microbiota.
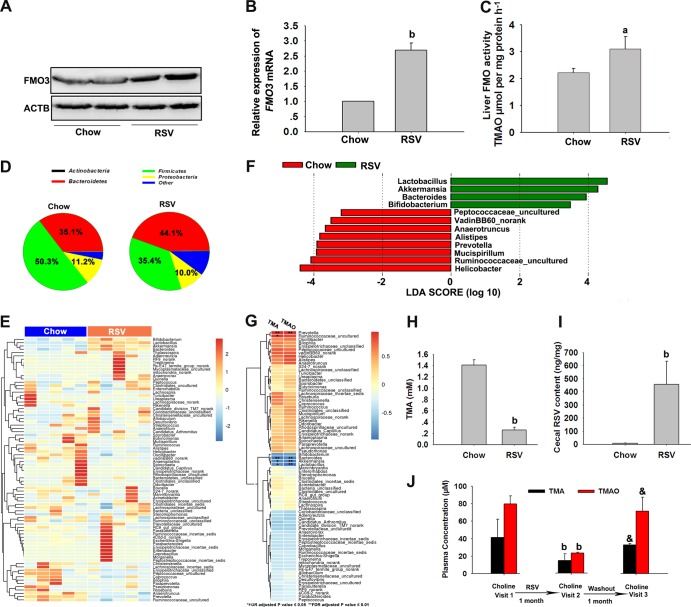
TMAO generation is dependent on the gut microbiota, which can metabolize dietary choline to TMA. The TMA is then metabolized by enzymes of the FMO family in the liver, among which flavin monooxygenase 3 (FMO3) is the most active (23). Thus, we first examined the effects of RSV on FMO3 expression and activity in the liver. Treatment with RSV resulted in a significant increase in protein and mRNA levels of FMO3 (Fig. 2A and B). RSV also markedly increased FMO activity in the liver (Fig. 2C). These findings corresponded with our earlier results showing that RSV could increase TMAO levels in mice treated with a single dose of TMA (Fig. 1D). These results indicated that the RSV-induced decrease in TMAO levels was not due to its regulation of FMO3 in the liver.
FIG 2 .
RSV inhibited TMAO synthesis by decreasing TMA generation via remodeling microbiota in C57BL/6J mice. Eight-week-old female C57BL/6J mice (n = 5 per group) were fed chow with or without RSV (0.4%) for 30 days. ACTB, β-actin. (A) Western blotting detection of FMO3 in the liver. (B) Expression levels of FMO3 gene mRNAs were quantified using qPCR assays. (C) Liver FMO activity was assessed as described in Materials and Methods. (D) 16S rRNA gene sequencing analysis of cecal content at the phylum level. (E) Heat map of 16S rRNA gene sequencing analysis of cecal content at the genus level. The scale reflects the data as follows: red indicates high values whereas blue indicates low values for the percentages of reads that were classified at that rank. (F) Linear discriminant analysis (LDA) coupled with effect size measurements identifies the taxons most differentially abundant between the chow and RSV diets at the genus level. RSV-diet-enriched taxa are indicated with a positive LDA score (green), and taxa enriched in the normal chow diet have a negative score (red). Only taxa meeting an LDA significant threshold value of >2 are shown. (G) Correlation heat map demonstrating the association between the indicated microbiota taxonomic genera and TMA and TMAO concentrations (all reported as means ± SD in micromoles) from mice grouped by dietary status (chow and RSV). Red denotes a positive association, blue a negative association, and white no association. A single asterisk indicates a significant FDR-adjusted association at P values of ≤0.05, and a double asterisk indicates a significant FDR-adjusted association at P values of ≤0.01. (H) The production of TMA from choline by cecal content in vitro. (I) Cecal RSV content. (J) The same 8-week-old female C57BL/6J mice (n = 5 per group) were administered choline (400 mg/kg) at visit 1 (baseline), visit 2 (RSV treatment for 1 month), and visit 3 (washout for 1 month). Four hours after choline was given at each visit, blood samples were obtained from the tail veins. Serum TMA and TMAO levels were determined by LC/MS. Values are presented as means ± SD (n = 5). a, P < 0.05; b, P < 0.01 (versus vehicle-treated control group); &, P < 0.01 (versus RSV-treated group).
To determine the role of the gut microbiota in the RSV-induced decrease of TMAO levels, we explored bacterial populations in the gut at the phylum and genus levels. Analysis at the phylum level revealed that the bacterial population of vehicle-treated mice was dominated by Bacteroidetes (35.1%) and Firmicutes (50.3%), with a low level of Proteobacteria (11.2%) (Fig. 2D). The proportion of sequences assigned to Bacteroidetes was significantly increased in metagenomes of RSV-fed animals at the expense of Firmicutes (Fig. 2D). Genus-level analysis showed that RSV induced an increase in the relative abundances of Bacteroides, Lactobacillus, Bifidobacterium, and Akkermansia in mice (Fig. 2E and F). RSV administration resulted in a decrease in the relative abundances of Prevotella, uncultured Ruminococcaceae (Ruminococcaceae_uncultured), Anaerotruncus, Alistipes, Helicobacter, and uncultured Peptococcaceae (Peptococcaceae_uncultured) (Fig. 2E and F). Using Pearson’s correlation tests, we found that, in general, taxa that were present at a relatively high proportion coincident with high plasma TMA concentrations also tended to be present at a relatively high proportion coincident with high plasma TMAO concentrations. Species of several bacterial taxa (Ruminococcaceae_uncultured, Prevotella, Bacteroides, Akkermansia, and Lactobacillus) remained significantly associated with plasma TMA and/or TMAO levels after false discovery rate (FDR) adjustment for multiple comparisons (Fig. 2G). Via a direct ex vivo assay, we found that the production of TMA from choline was lower in cecal content from RSV-treated mice and that the concentration of RSV in the cecal content was much higher than that in the plasma (Fig. 2H and I). Moreover, we found that the same mice fed with RSV could show markedly decreased TMA and TMAO levels after choline treatment; however, after washout with a chow diet for 1 month, they failed to reduce TMA and TMAO contents after choline administration (Fig. 2J). Additionally, real-time quantitative PCR (qPCR) analysis of suspected cecal microbes from mice treated with RSV for 2 months revealed that changes in the gut flora caused by RSV were similar to those observed in mice fed with RSV for 1 month (see Fig. S2A and B in the supplemental material). These results indicated that RSV remodeled the gut microbiome in mice, which might contribute to decreased gut microbial TMA production, thereby inhibiting TMAO synthesis.
RSV enhanced BA deconjugation and fecal excretion through microbiota modulation.
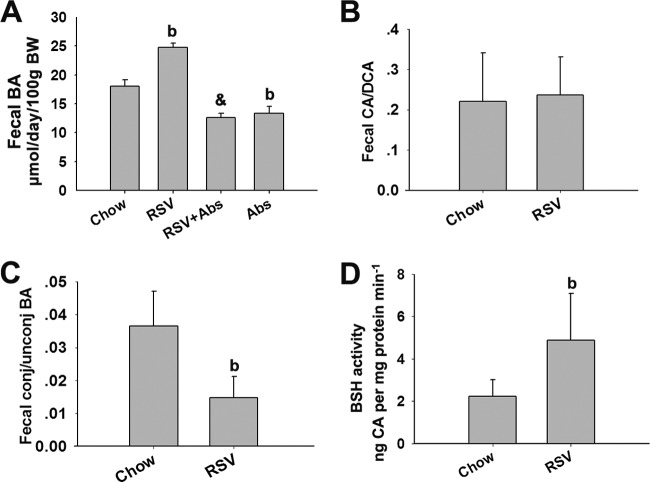
The gut microbiota plays an important role in BA metabolism, by mediating deconjugation and 7-α-dehydroxylation of primary BAs (24). To assess the extent of BA biotransformation upon prebiotic colonization, we measured the effects of RSV on fecal BA excretion and composition. RSV significantly enhanced fecal BA loss, which was inhibited by Abs (Fig. 3A). It has been demonstrated that major secondary BAs are deoxycholic acid (DCA) and murideoxy cholic acid (MDCA), which are the 7-α-dehydroxylation products of cholic acid (CA) and beta-muricholic acid (β-MCA), respectively (25). Here, we found that the fecal CA/DCA ratio was unchanged by RSV administration, indicating that RSV had no effect on 7-α-dehydroxylation activity in the gut (Fig. 3B; see also Table S4 in the supplemental material). In contrast, there was a significant decrease in the fecal conjugated/unconjugated BA ratio, along with an increase in bile salt hydrolase (BSH) enzyme activity, in the feces of RSV-treated mice (Fig. 3C and D; see also Table S4). Moreover, we found that RSV treatment resulted in an increase in the proportions of Lactobacillus and Bifidobacterium that were present in the gut (Fig. 2E and F), likely contributing to its effects on BSH activity. Of note, administration of RSV resulted in a significant reduction in small-intestine (SI) BA content, including taurocholic acid (TCA), TβMCA, CA, chenodeoxycholic acid (CDCA), and DCA, in which the decrease in levels of TCA, CA, CDCA, and DCA might explain nearly 90% of the lower SI BA content (see Fig. S2C and Table S4). It has been demonstrated that the apical sodium bile acid transporter (ASBT) is responsible for ileal BA uptake, with a preference for conjugated over unconjugated BAs (26). We assessed mRNA and protein levels of ASBT and found that RSV had no significant influence on ASBT mRNA or protein levels (see Fig. S2D and E), suggesting that the RSV-mediated increase in fecal BA loss was independent of the presence of ASBT. Finally, we observed a reduction in the mRNA levels of the BA transporters organic solute transporter alpha (OSTα) and beta (OSTβ) in RSV-treated mice, which might contribute to the RSV-induced decrease in ileal BA content (see Fig. S2F). These results suggested that RSV-induced modulation of the gut microbiome contributed to increased BSH activity and subsequently promoted the generation of unconjugated BAs from conjugated BAs, thereby enhancing fecal BA loss.
FIG 3 .
RSV enhanced BA deconjugation and fecal excretion in C57BL/6J mice. Eight-week-old female C57BL/6J mice (n = 10 per group) were fed chow with or without RSV (0.4%) in the presence or absence of Abs for 30 days. Fecal samples were collected. (A) Fecal BA excretion was measured by a total BA test (Wako). Fecal BA composition was determined by LC/MS as described in Materials and Methods. (B) CA/DCA ratio. (C) Conjugated/unconjugated (conj/unconj) BA ratio in fecal samples. (D) BSH activity. Values are expressed as means ± SD (n = 10). b, P < 0.01 (versus vehicle-treated control group); &, P < 0.01 (versus RSV-treated group).
RSV induced hepatic BA synthesis in C57BL/6J mice.
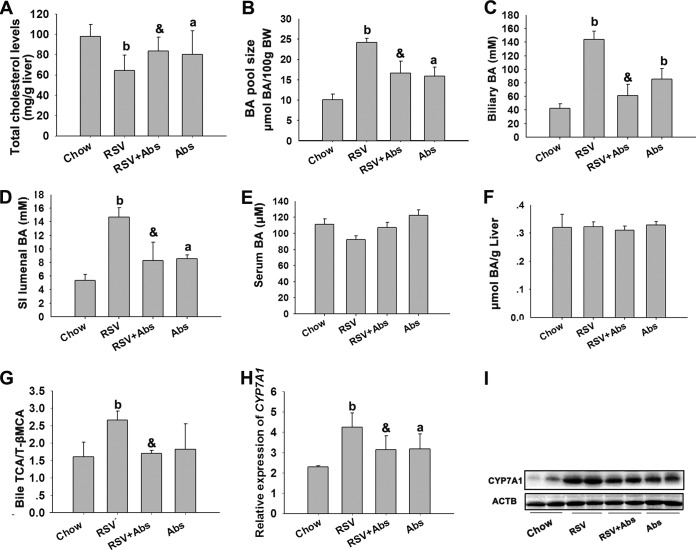
It was reported previously that enhanced fecal BA loss is accompanied by enhanced hepatic BA neosynthesis (27, 28). We found that RSV significantly decreased the liver cholesterol level and increased the BA pool size and the gallbladder and SI luminal BA content; these effects were reversed by treatment with Abs (Fig. 4A to D). No differences were observed with respect to serum and hepatic BA content (Fig. 4E and F). In addition, the expression of CYP7A1 mRNA and protein, which is the rate-limiting enzyme in hepatic BA synthesis, was also investigated (29). As shown in Fig. 4G to I and in Table S4 in the supplemental material, RSV increased the bile TCA/TβMCA ratio and induced CYP7A1 mRNA and protein expression and the effects were inhibited by Abs treatment. These results suggested that RSV possibly increased hepatic BA synthesis by inducing CYP7A1 expression in a gut microbiota-dependent manner.
FIG 4 .
RSV induced hepatic BA synthesis in C57BL/6J mice. Eight-week-old female C57BL/6J mice (n = 10 per group) were fed chow with or without RSV (0.4%) in the presence or absence of Abs for 30 days. (A) Liver cholesterol content. (B) The BA pool size was determined for the total BA content of gallbladder bile, liver, and the SI lumenal. (C to F) Total biliary BA content (C) and total BA content in SI lumenal (D), serum (E), and liver (F) were detected. (G) Gallbladder bile samples were subjected to LC/MS, and TCA/TβMCA ratios were calculated. (H) Relative expression levels of the indicated mRNAs in the liver were determined by qPCR. (I) Expression of CYP7A1 was analyzed by Western blotting. Values are expressed as means ± SD (n = 10). a, P < 0.05; b, P < 0.01 (versus vehicle-treated control group); &, P < 0.01 (versus RSV-treated group).
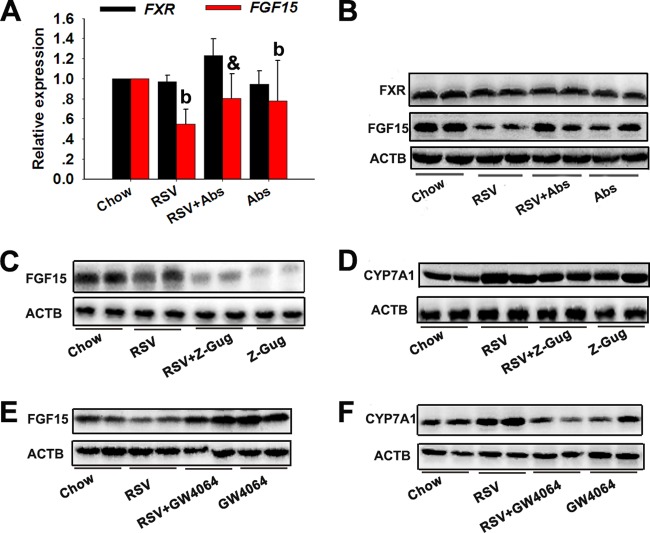
The enterohepatic FXR-FGF15 axis played a key role in RSV-induced BA synthesis.
The feedback suppression of CYP7A1 gene transcription by FXR is one of the most important mechanisms in the maintenance of BA homeostasis (30). Previous studies have shown that FXR activation is a major mechanism in the suppression of BA synthesis and induces small heterodimer partner (SHP) and FGF15, which work together to suppress CYP7A1 (31, 32). Thus, we sought to determine the possible roles of the gut-liver FXR signaling in RSV-induced BA synthesis. RSV treatment resulted in a significant decrease in ileal FGF15 mRNA and protein levels; however, ileal FXR mRNA and protein levels were unchanged (Fig. 5A and B). These observations suggested that RSV downregulated FXR transcriptional activity, as demonstrated by the reduction in mRNA levels of its target genes. In the liver, RSV treatment appeared to have no significant effect on the mRNA level of the SHP gene and the mRNA and protein levels of FXR (see Fig. S3A and B in the supplemental material). These findings highlighted that RSV downregulated the ileal FXR-FGF15 axis, which might result from RSV-induced significant reduction in FXR agonists (TCA, DCA, CA, and CDCA) in SI tissues (see Table S4). Furthermore, we used Z-Guggulsterone (Z-Gug), a natural antagonist of FXR, to suppress FXR in mice as outlined previously (33). Treatment with Z-Gug markedly inhibited the expression of FGF15, thereby inducing CYP7A1 expression in mice (Fig. 5C and D). Additionally, similar results were seen in mice cotreated with RSV and Z-Gug (Fig. 5C and D). The presence of synthetic FXR agonist GW4064 was able to reverse RSV-induced alterations in the expression of FGF15 and CYP7A1 in mice (Fig. 5E and F). Taken together, these observations indicated that the gut-liver FXR-FGF15 axis played a key role in RSV-induced BA synthesis.
FIG 5 .
The enterohepatic FXR-FGF15 axis played a key role in RSV-induced BA synthesis. Eight-week-old female C57BL/6J mice (n = 10 per group) were fed chow with or without RSV (0.4%) for 30 days. Z-Gug (100 mg/kg body weight) or GW4064 (75 mg/kg body weight) was given 7 days prior to surgical procedures. (A) Relative expression levels of ileal FXR and FGF15 gene mRNAs. (B) Western blotting was used to detect ileal FXR and FGF15 expression. (C) Expression of the indicated proteins in ileal tissues. (D) Liver CYP7A1 expression was detected by Western blotting. (E) FGF15 expression in ileal tissues. (F) CYP7A1 expression in liver samples. Values are expressed as means ± SD (n = 10). b, P < 0.01 (versus vehicle-treated control group); &, P < 0.01 (versus RSV-treated group).
RSV protected ApoE−/− mice from TMAO-induced AS in a gut microbiota-dependent manner.
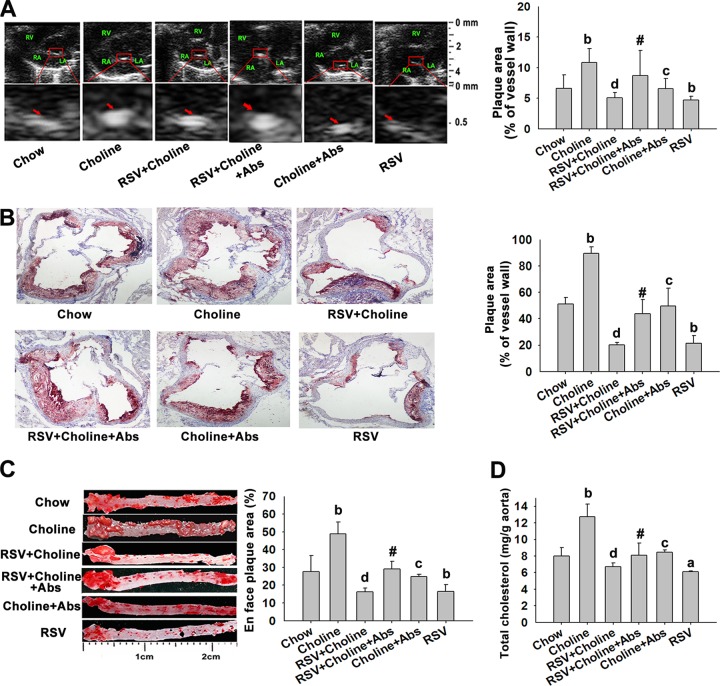
Plasma TMAO is strongly associated with AS (2). Therefore, we investigated whether changes in TMAO induced by RSV through microbiota remodeling were associated with AS regression in ApoE−/− mice. The atherosclerotic lesion area and the cholesterol content in the whole aorta, as indexes of AS severity, were much higher in mice fed with choline than in chow-fed mice, and the effects were markedly reversed by RSV or Abs administration (Fig. 6). Moreover, when the gut microbiota was suppressed by Abs, RSV-induced inhibition of TMAO-caused AS was significantly abolished (Fig. 6). Additionally, RSV single treatment could also attenuate the pathogenesis of AS in ApoE−/− mice (Fig. 6). These results suggested that RSV attenuated TMAO-induced AS in a gut microbiota-dependent manner.
FIG 6 .
RSV protected ApoE−/− mice from TMAO-induced AS. Eight-week-old female ApoE−/− mice (n = 10 per group) were given RSV (0.4%) with or without choline (1%) in the absence or presence of Abs for 4 months. The control group was fed with a chow diet. (A) Ultrasound B-mode images of the aortic sinus and quantification. Arrows indicate the regions of interest. RV, right ventricle; RA, right atrium; LA, left atrium. (B) Oil red O-stained aortic roots (counterstained with hematoxylin) and quantification. (C) Oil red O staining of whole aortas, including the aortic arch, thoracic, and abdominal regions, and their quantitation. (D) Total cholesterol content in the thoracic and abdominal aorta. Values are expressed as means ± SD (n = 10). a, P < 0.05; b, P < 0.01 (versus vehicle-treated control group); c, P < 0.05; d, P < 0.01 (versus choline-treated group); #, P < 0.05 (versus group cotreated with RSV and choline).
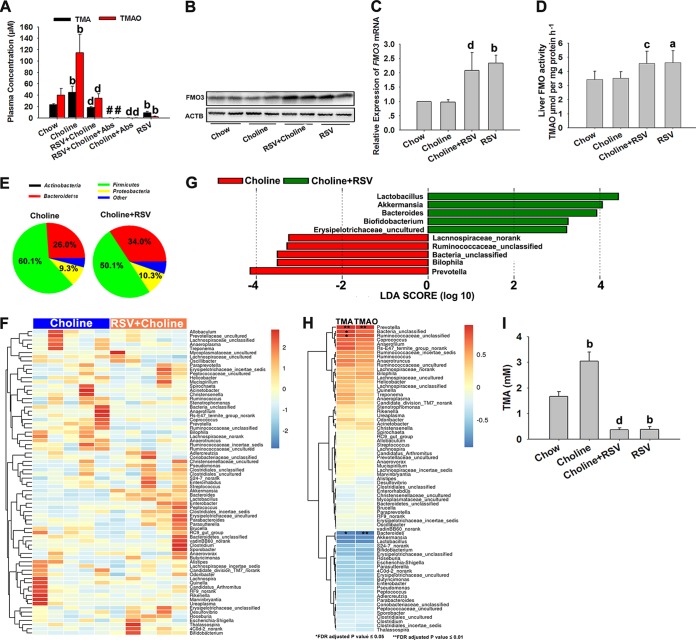
RSV reduced TMAO levels by decreasing TMA generation via remodeling microbiota in ApoE−/− mice.
As shown in Fig. 7A to D, RSV administration markedly decreased TMA and TMAO contents and increased the expression levels of liver FMO3 and FMO activity. These results were consistent with our findings in C57BL/6J mice, suggesting the important role of the gut microbiota in the RSV-induced decrease of TMAO levels.
FIG 7 .
RSV reduced TMAO levels by decreasing TMA generation via remodeling microbiota in ApoE−/− mice. Eight-week-old female ApoE−/− mice (n = 5 per group) were fed chow, chow with RSV (0.4%), chow with choline (1%), or chow with choline (1%) plus RSV (0.4%) in the absence or presence of Abs for 4 months. (A) Serum TMA and TMAO levels were measured by LC/MS. (B) Western blotting detection of FMO3 expression in the liver. (C) Expression levels of FMO3 gene mRNAs were quantified using qPCR assays. (D) Liver FMO activity was assessed as described in Materials and Methods. (E) 16S rRNA gene sequencing analysis of cecal content at the phylum level. (F) Heat map of 16S rRNA gene sequencing analysis of cecal content at the genus level. The scale reflects the data as follows: red indicates high values whereas blue indicates low values for the spercentage of reads that were classified at that rank. (G) Linear discriminant analysis (LDA) coupled with effect size measurements identifies the most differentially abundant taxons at the genus level between the choline diet and the RSV-plus-choline diet. RSV-plus-choline-diet-enriched taxa are indicated with a positive LDA score (green), and taxa enriched in the choline diet have a negative score (red). Only taxa meeting an LDA significance threshold of >2 are shown. (H) Correlation heat map demonstrating the association between the indicated microbiota taxonomic genera and TMA and TMAO concentrations (all reported as means ± SD in micromoles) from mice grouped by dietary status (choline and RSV plus choline). Red denotes a positive association, blue a negative association, and white no association. A single asterisk indicates a significant FDR-adjusted association of P values of ≤0.05, and a double asterisk indicates a significant FDR-adjusted association of P values of ≤0.01. (I) Production of TMA from choline by cecal content in vitro. Values are expressed as means ± SD (n = 5). a, P < 0.05; b, P < 0.01 (versus vehicle-treated control group); c, P < 0.05; d, P < 0.01 (versus choline-treated group); #, P < 0.05 (versus group cotreated with RSV and choline).
The composition of the gut microbiota in cecal content was then determined. Via qPCR and sequencing 16S rRNA assays, we found that choline administration markedly increased the bacterial concentrations of Bacteroidetes phyla and decreased the concentrations of Firmicutes phyla compared to the control group (see Fig. S4A in the supplemental material). The Proteobacteria and Actinobacteria phyla showed no significant changes after choline treatment in ApoE−/− mice (see Fig. S4A). Moreover, compared to chow-fed ApoE−/− mice, choline significantly increased the number of Prevotella and decreased the concentrations of Bacteroides, Lactobacillus, and Bifidobacterium (see Fig. S4B). These results indicated that chronic choline supplementation might alter cecal microbial composition, thereby enhancing the synthesis of TMA and TMAO and ultimately increasing AS. As expected, treatment with RSV resulted in an increase in Bacteroidetes abundance (from 20.6 to 34.0%) at the expense of Firmicutes (from 60.1 to 50.1%) in choline-treated ApoE−/− mice (Fig. 7E). RSV also increased the relative abundances of Bacteroides, Lactobacillus, Bifidobacterium, and Akkermansia and decreased the relative abundances of Prevotella, Ruminococcaceae_unclassified, and Biophila in choline-fed ApoE−/− mice (Fig. 7F and G). After FDR adjustment for multiple comparisons, the abundance of genera Prevotella and Bacteroides was still significantly associated with TMAO and TMA levels in choline-treated ApoE−/− mice (Fig. 7H). Additionally, via a direct ex vivo assay, we found that the production of TMA from choline was higher in cecal content from choline-treated mice and that the effect was reversed by RSV administration (Fig. 7I). Combined with the results seen in C57BL/6J mice, we postulated that RSV attenuated TMAO-induced AS by reducing TMAO synthesis via inhibition of the gut microbial TMA production.
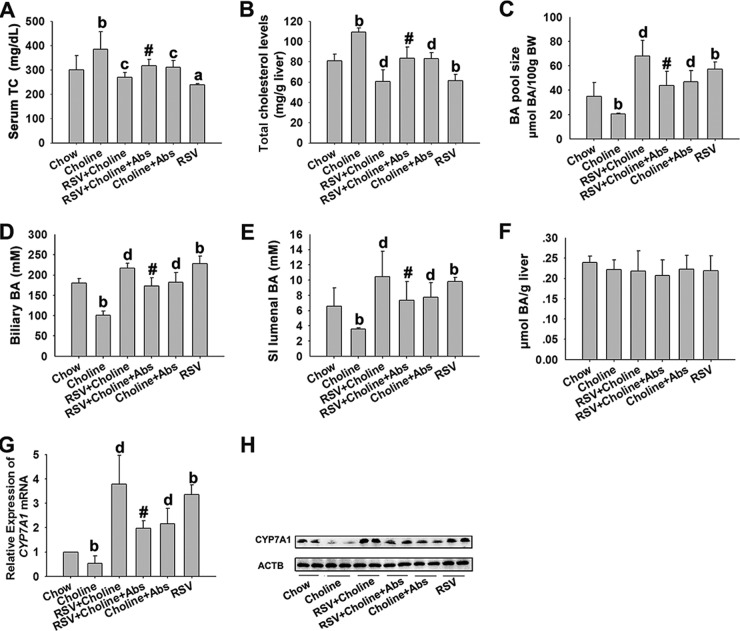
RSV induced hepatic BA synthesis in choline-treated ApoE−/− mice.
TMAO appears to contribute to the development of AS, in part by affecting cholesterol metabolism through the regulation of the BA synthesis pathway (4). Therefore, the influence of RSV on BA synthesis was investigated in choline-treated ApoE−/− mice. We found that the presence of choline significantly increased serum total cholesterol (TC) and liver cholesterol levels and decreased BA pool size and gallbladder and SI luminal BA content in ApoE−/− mice; these effects were reversed by treatment with RSV (Fig. 8A to E). And there were no significant differences in liver BA content among the groups (Fig. 8F). Meanwhile, RSV also increased the bile TCA/TβMCA ratio (see Fig. S5A and Table S4 in the supplemental material) and reversed the choline-induced decrease of CYP7A1 mRNA and protein expression in ApoE−/− mice (Fig. 8G and H). However, when the gut microbiota was suppressed by Abs, RSV-induced hepatic BA synthesis was significantly inhibited in choline-fed ApoE−/− mice (Fig. 8). Additionally, RSV single treatment could also induce hepatic BA synthesis in ApoE−/− mice (Fig. 8). These results suggested that RSV induced hepatic BA synthesis in a gut microbiota-dependent manner in choline-treated ApoE−/− mice.
FIG 8 .
RSV induced hepatic BA synthesis in choline-treated ApoE−/− mice. Eight-week-old female ApoE−/− mice (n = 10 per group) were fed chow, chow with RSV (0.4%), chow with choline (1%), or chow with choline (1%) plus RSV (0.4%) in the absence or presence of Abs for 4 months. (A) Serum TC levels were measured enzymatically using commercially available kits. (B) Liver cholesterol content. (C) The BA pool size was determined for the total BA content of gallbladder bile, liver, and the SI lumenal. BW, body weight. (D to F) Total biliary BA content (D) and total BA content in SI lumenal (E) and liver (F) were detected. (G) Relative expression levels of the indicated mRNAs in the liver were determined by qPCR. (H) Expression of CYP7A1 was analyzed by Western blotting. Values are expressed as means ± SD (n = 10). a, P < 0.05; b, P < 0.01 (versus vehicle-treated control group); c, P < 0.05; d, P < 0.01 (versus choline-treated group); #, P < 0.05 (versus choline and RSV-cotreated group).
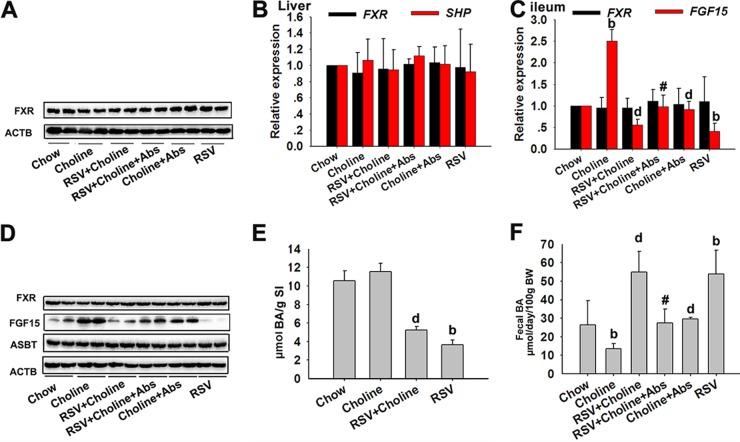
RSV downregulated the enterohepatic FXR-FGF15 axis in choline-treated ApoE−/− mice.
Finally, the effects of RSV on gut-liver FXR signaling were determined in choline-treated ApoE−/− mice. The mRNA level of the SHP gene and the mRNA and protein levels of FXR in the liver were unchanged by RSV (Fig. 9A and B). The ileal FGF15 mRNA and protein levels were markedly reduced by RSV; however, ileal FXR mRNA and protein levels were unchanged in choline-fed ApoE−/− mice (Fig. 9C and D). In addition, RSV had no effect on ASBT expression but still decreased the SI BA content, including TCA, CDCA, and TβMCA, in choline-fed ApoE−/− mice (Fig. 9D and E; see also Table S4 in the supplemental material). RSV also increased fecal BA loss and fecal conjugated/unconjugated BA levels and increased BSH activity, with fecal CA/DCA ratios unchanged in choline-fed ApoE−/− mice (Fig. 9F; see also Fig. S5B to D and Table S4). These results were similar to those found in C57BL/6J mice. Overall, we came to the conclusion that RSV attenuated TMAO-induced AS, partially by regulating BA synthesis through the enterohepatic FXR-FGF15 axis in a gut microbiota-dependent manner.
FIG 9 .
RSV downregulated the enterohepatic FXR-FGF15 axis in choline-treated ApoE−/− mice. Eight-week-old female ApoE−/− mice (n = 10 per group) were fed chow, chow with RSV (0.4%), chow with choline (1%), or chow with choline (1%) plus RSV (0.4%) in the absence or presence of Abs for 4 months. (A) Expression of CYP7A1 was analyzed by Western blotting. (B and C) Relative expression levels of the indicated mRNAs in the liver (B) and ileal tissue (C) were determined by qPCR. (D) Western blotting was used to detect ileal FXR and FGF15 expression. (E and F) BA content in SI tissue (E) and fecal samples (F). b, P < 0.01 (versus vehicle-treated control group); d, P < 0.01 (versus choline-treated group); #, P < 0.05 (versus group cotreated with choline and RSV).
DISCUSSION
In the current study, the role of the gut microbiota in the protective effects of RSV against AS was determined in mice. We found that RSV reduced TMAO levels by inhibiting gut microbial TMA formation via remodeling gut microbiota, thereby attenuating TMAO-induced AS. To the best of our knowledge, we are the first to demonstrate the role of the gut microbiota in RSV-induced protection against AS. Dietary choline is metabolized by the intestinal microbiota to TMA, which is further metabolized by FMO enzymes, in particular, FMO3, to produce TMAO in the liver (23). Recently, plasma TMAO was identified as a metabolite strongly associated with AS (2–4). Researchers confirmed that feeding AS-prone mice diets enriched in either choline or TMAO enhanced the development of AS. Enhanced AS, as observed by dietary choline supplementation, is entirely dependent on the gut microbiota. Treatment with Abs, or germ-free conditions, abolished dietary choline-driven TMAO generation and the development of AS (2, 4). We observed that RSV attenuated TMAO-induced AS and reduced TMA and TMAO levels in mice. Moreover, RSV increased the expression and activity of FMO3 in the liver, corresponding with previously published results (34). It has been demonstrated that the gene encoding FMO3 is a direct FXR target gene, and FXR has been found to be a target of sirtuin 1 (SIRT1) in metabolic regulation (23, 34, 35). Moreover, RSV is a well-known activator of SIRT1 and our previous study also found that RSV improved hepatic steatosis partially by inducing autophagy via the SIRT1 signaling pathway in vitro and in vivo (36–38). Therefore, RSV might increase liver FMO3 expression and activity via regulation of the SIRT1-FXR signaling pathway. However, the exact underlying mechanisms need to be further elucidated. Our findings suggested that RSV-induced TMAO reduction was not due to its regulation of FMO3 in the liver.
Previous results suggested possible prebiotic benefits associated with the inclusion of red wine polyphenols, especially RSV, in a diet (20). Qiao et al. (21) demonstrated that RSV improves the gut microbiota dysbiosis induced by a high-fat diet, including increasing the Bacteroidetes-to-Firmicutes ratios, significantly inhibiting the growth of Enterococcus faecalis, and increasing the growth of Lactobacillus and Bifidobacterium in mice. However, Etxeberria et al. (22) claimed that RSV supplementation alone or in combination with quercetin supplementation scarcely modified the profile of gut bacteria but acted at the intestinal level, altering the mRNA expression of tight-junction proteins and inflammation-associated genes in high-fat sucrose diet-fed rats. Here, we found that RSV inhibited TMAO synthesis by decreasing gut microbial TMA production through gut microbiota modulation, which subsequently attenuated TMAO-induced AS. Meanwhile, a recent report showed that 3,3-dimethyl-1-butanol can reduce TMAO levels by inhibiting the formation of TMA from microbes in mice, thereby attenuating choline diet-enhanced AS (39). These results indicated that targeting gut microbial production of TMA specifically and of nonlethal microbial inhibitors in general may serve as a potential therapeutic approach for the treatment of CVD. In addition, the physiological effects of dietary RSV appeared to be in striking contrast to its poor bioavailability, which has been a major concern in the development of this class of compounds into therapeutic agents (14, 15). Our findings suggested that RSV likely exerted its primary effects by remodeling gut microbiota. This might explain the paradox that RSV has low bioavailability in humans while exerting noticeable bioactivities. These findings provide a new insight into the potential mechanisms responsible for the cardiovascular protective effects of RSV and indicate that the gut microbiota may play an important role.
Furthermore, it has been found that Abs could attenuate TMAO-caused AS by reducing TMAO synthesis via blocking the pathway of choline to TMA governed by the gut microbiota (4). Our results indicated that Abs treatment could markedly inhibit the RSV-induced decrease of AS in choline-fed ApoE−/− mice, which suggests that RSV’s protective effects on the proatherogenic phenotype might depend on other factors of the microbiota beside reducing gut microbial TMA production. Current evidence suggests that TMAO appears to contribute to the development of AS, in part by regulating cholesterol elimination and BA synthesis (2, 4). BAs are synthesized from cholesterol by CYP7A1 in the liver and play a central role in cholesterol homoeostasis (8). The gut microbiota is important for BA metabolism, as it mediates primary BA deconjugation and subsequent conversion to secondary BAs, which are responsible for BA synthesis in the liver (5). The gut microbiome produces potent ligands corresponding to BA receptors; probiotics or prebiotics could act as therapeutics of BA dysmetabolism, indicating that microbiota-targeted therapies could be effective in preventing and/or treating gut-related diseases, including AS (40). We observed that RSV significantly increased the expression of CYP7A1, subsequently inducing BA synthesis in the liver. RSV also increased the proportions of Lactobacillus and Bifidobacterium, which are considered BSH-active bacteria (41). Bacterial BSH activity affects systemic metabolic processes and adiposity in the host. It also represents a key mechanistic target for the control of obesity and hypercholesterolemia, acting by deconjugating BAs to generate unconjugated BAs (42). For the first time, we found that RSV induced hepatic BA synthesis by remodeling the gut microbiome. Dietary RSV has been shown to increase the expression of hepatic CYP7A1 and to ameliorate hypercholesterolemia in high-fat-fed C57BL/6J mice (43). Our findings were the first to indicate the crucial role of the gut microbiota in RSV-induced cholesterol metabolism in the liver, indicating that RSV could attenuate TMAO-caused AS, partially by inducing BA synthesis in a gut microbiota-dependent manner. These findings offer new insights into the protective effects of RSV against AS.
We also investigated the potential involvement of ASBT and the enterohepatic FXR-FGF15 axis in RSV-mediated BA synthesis. Recently, it was found that ASBT plays a key role in the enterohepatic recycling of BAs and indirectly contributes to cholesterol homoeostasis (26). RSV promotes the degradation of ASBT in vitro, which might have some clinical relevance with regard to the observed cholesterol-lowering effects of RSV (44). We found that RSV reduced ileal BA levels, with no effect on ASBT expression. Our results contradict those previously published, possibly because we were experimenting in vivo, which is a more complex environment than that in vitro. Moreover, we observed a reduction in the mRNA levels of the BA transporters encoded by the OSTα and OSTβ genes in RSV-treated mice; that reduction might contribute to the RSV-induced decrease in ileal BA content. Meanwhile, we found that RSV increased BSH activity, thereby enhancing BA deconjugation. This might also result in the decrease in ileal BA content, because the ileal BA transporters prefer conjugated BAs to unconjugated BAs (26). Interestingly, the SI lumenal BA content was higher upon RSV treatment, which was presumably a result of increased biliary output. However, the exact underlying mechanisms need to be further clarified. It has been reported that enhanced fecal BA loss, either driven by ASBT genetic deletion or induced by BA sequestrant administration, is accompanied by enhanced hepatic BA neosynthesis (27, 28). We showed that RSV induced BA synthesis in the liver, contributing to its protective effect on AS caused by TMAO. These findings provide new insights into the cardiovascular benefits of RSV.
FXR is highly expressed in the liver and intestine, where it functions as an intracellular sensor of BAs. FXR is required for the negative-feedback regulation of BA biosynthesis and the enterohepatic cycle (30). Previous studies have shown that FXR activation induces SHP and FGF15, thereby suppressing CYP7A1 expression and ultimately inhibiting BA synthesis (31, 32). BA synthesis and modulation of the enterohepatic FXR-FGF15 axis are seen in germ-free, gnotobiotic, and antibiotic-treated animals (5). Results from a recent study demonstrate that probiotic VSL#3 affects hepatic BA synthesis by downregulating the gut-liver FXR-FGF15 axis. This suggests that microbiota-targeted therapies affect cholesterol metabolism by inducing hepatic BA synthesis via the regulation of the gut-liver FXR-FGF15 axis (10). We also found that RSV decreased the activity of ileal FXR and the expression of FGF15 by decreasing the levels of FXR agonists (TCA and CDCA) in SI tissues (45, 46). When Z-Gug, a natural antagonist of FXR, was administered, RSV failed to reduce FGF15 expression and to induce the expression of CYP7A1 in mice. Additionally, the presence of GW4064, a synthetic FXR agonist, reversed RSV-induced alterations in FGF15 and CYP7A1 expression. These observations indicated that the gut-liver FXR-FGF15 axis was required for RSV to induce hepatic BA synthesis.
Finally, it is not known whether TMAO interacts directly with a specific receptor or whether it acts to alter signaling pathways indirectly by altering the protein conformation (that is, via allosteric effects), whereas TMA has been reported to influence signal transduction by direct interaction with a family of G protein-coupled receptors (47). TMAO, a small quaternary amine with an aliphatic character, is reportedly capable of directly inducing conformational changes in proteins and of stabilizing protein folding and acting as a small-molecule protein chaperone (48, 49). It is thus conceivable that TMAO may alter many signaling pathways without directly acting at a “TMAO receptor.” However, the exact mechanisms by which circulating TMAO promotes AS and whether RSV could also reverse TMAO-caused changes in other signaling pathways are still largely unknown and need to be further elucidated.
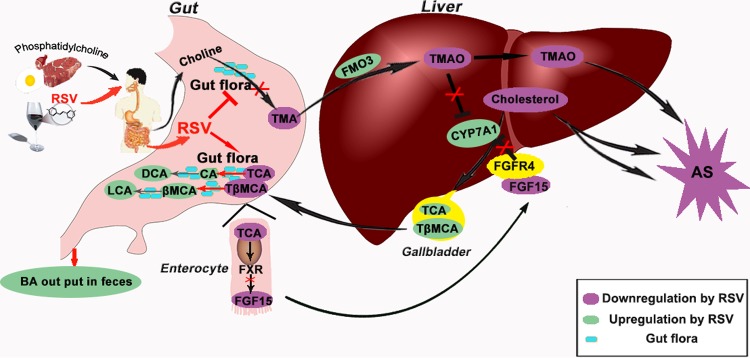
To the best of our knowledge, we have demonstrated for the first time that RSV attenuated TMAO-induced AS by regulating the synthesis of TMAO and BAs via remodeling of the gut microbiota and that RSV-mediated hepatic BA neosynthesis was partially modulated by downregulation of the gut-liver FXR-FGF15 axis (Fig. 10). Hence, these results open a new avenue of research regarding the potential cardiovascular protective effects of RSV and indicate that the gut microbiota may become an interesting target for pharmacological or dietary interventions to decrease the risk of developing CVD.
FIG 10 .
A model depicting use of RSV to attenuate AS by targeting gut microbiota. Dietary RSV can alter the composition of gut flora. On the one hand, this can result in reduced levels of gut microbial TMA production, subsequently leading to decreased TMAO synthesis in the liver and, ultimately, to inhibition of AS. On the other hand, RSV increases BSH activity by gut microbiota remodeling, which promotes the generation of unconjugated BAs from conjugated BAs and enhances fecal BA loss. RSV-induced fecal BA loss leads to a decrease in ileal BA content, thereby inhibiting the ileal FXR-FGF15 axis, and then increases the expression levels of CYP7A1 in the liver, subsequently inducing hepatic BA neosynthesis which contributes to cholesterol homeostasis, finally attenuating AS. Red indicates the effect of RSV, purple indicates downregulation by RSV, green indicates upregulation by RSV, and blue indicates the gut flora. LCA, lithocholic acid.
MATERIALS AND METHODS
Animals and treatments.
Female C57BL/6J mice and ApoE−/− mice with a C57BL/6 genetic background were purchased from Jackson Laboratory (Bar Harbor, ME, USA). Animals were maintained at a controlled temperature (22 ± 2°C), with a 12-h light/dark period. For all the experiments, mice were housed individually in cages and had ad libitum access to water. Mice of the control group were fed a standard chow diet (NIH31 modified mouse/rat diet; Harlan Teklad). Animal experiments were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health and were approved by the Animal Care and Use Committee of the Third Military Medical University (Chongqing, China; Approval SYXC-2014-00112). Pentobarbital sodium anesthesia (50 mg/kg of body weight) was administered prior to surgical procedures, with maximal effort exerted to minimize suffering. At the end of the experiments, mice were killed by cervical dislocation followed by decapitation.
Statistical analyses.
Quantitative data are presented as means ± standard deviations (SD) of results from three independent experiments. Statistical analysis was conducted with either Student’s t test or one-way analysis of variance using SPSS 13.0 (SPSS Inc., Chicago, IL). A P value less than 0.05 was considered statistically significant, and the Tukey-Kramer post hoc test was applied if the P value was less than 0.05. Correlations between host parameters and bacterial populations were assessed by Pearson’s correlation tests using GraphPad Prism version 6.00 (GraphPad Software, La Jolla, CA). FDR in the multiple comparisons were estimated for each taxon based on the P values that resulted from correlation estimates in R statistical software.
Full descriptions of additional materials and methods are given in Text S1 in the supplemental material.
SUPPLEMENTAL MATERIAL
Experimental design. The schematic depicts the overall design of the animal experiment to evaluate the effect of RSV on TMAO-induced AS in both C57BL/6J (A) and ApoE−/− (B) mice. Download
RSV remodeled gut microbiota and decreased ileal BA content without modifying ASBT function. Eight-week-old female C57BL/6J mice (n = 10 per group) were fed a chow diet with or without RSV (0.4%) for 1 or 2 months. (A and B) The relative abundances of the indicated bacterial strains at the phylum level (A) and the genus level (B) in the cecal content were assessed by qPCR assay. (C) Total BA content in ileal tissues. (D) Western blotting was used to detect the expression of ASBT. (E and F) Expression of ASBT gene mRNA (E) and OSTα and OSTβ gene mRNA (F) was determined by qPCR assays. Values are expressed as means ± SD (n = 10). a, P < 0.05; b, P < 0.01 (versus vehicle-treated control group). Download
RSV had no effect on liver FXR and SHP gene mRNA expression. Eight-week-old female C57BL/6J mice (n = 10 per group) were fed a chow diet with or without RSV (0.4%) in the presence or absence of Abs for 30 days. Liver tissues were collected. (A) Relative expression levels of the indicated mRNAs in the liver were determined by qPCR assays. (B) FXR expression was analyzed by Western blotting. Values are expressed as means ± SD (n = 10). Download
RSV remodeled gut microbiota in choline-fed ApoE−/− mice. Eight-week-old female ApoE−/− mice (n = 10 per group) were fed chow, chow with RSV (0.4%), chow with choline (1%), or chow with choline (1%) plus RSV (0.4%) for 4 months. The relative abundances of the indicated bacterial strains at the phylum level (A) and the genus level (B) in the cecal content were assessed by qPCR assay. a, P < 0.05; b, P < 0.01 (versus vehicle-treated control group); d, P < 0.01 (versus choline-treated group). Download
RSV altered the BA composition in gallbladder bile and feces and increased fecal BSH activity in choline-treated ApoE−/− mice. Eight-week-old female ApoE−/− mice (n = 10 per group) were fed chow, chow with choline (1%), or chow with choline (1%) plus RSV (0.4%) for 4 months. The BA composition of gallbladder bile and feces was analyzed by LC/MS. (A) TCA/TβMCA ratios in gallbladder bile. (B) Conjugated/unconjugated BA ratios in fecal samples. (C) Fecal BSH activity. (D) CA/DCA ratios in fecal samples. Values are expressed as means ± SD (n = 10). a, P < 0.05; b, P < 0.01 (versus vehicle-treated control group); d, P < 0.05 (versus choline-treated group). Download
Primers used for qPCR.
Composition of Mega medium.
Retention times of BAs.
Biliary, SI and fecal bile acid composition profile in mice (related to data shown in Fig. 3, 4, and 9 and in Fig. S2 and S5).
Supplemental materials and methods. Download
ACKNOWLEDGMENTS
We thank Zheng Liu and Wenhong Gao (Department of Ultrasound, Xinqiao Hospital, Third Military Medical University) for their assistance with aortic root lesion detection.
This work was supported by the National Natural Science Foundation of China (81470562).
Footnotes
Citation Chen M-L, Yi L, Zhang Y, Zhou X, Ran L, Yang J, Zhu J-D, Zhang Q-Y, Mi M-T. 2016. Resveratrol attenuates trimethylamine-N-oxide (TMAO)-induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. mBio 7(2):e02210-15. doi:10.1128/mBio.02210-15.
REFERENCES
- 1.Murray CJ, Lopez AD. 1997. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet 349:1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 2.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL. 2011. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. 2013. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH. 2013. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall HU, Bamberg K, Angelin B, Hyötyläinen T, Orešič M, Bäckhed F. 2013. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab 17:225–235. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Jones ML, Tomaro-Duchesneau C, Prakash S. 2014. The gut microbiome, probiotics, bile acids axis, and human health. Trends Microbiol 22:306–308. doi: 10.1016/j.tim.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Li-Hawkins J, Gåfvels M, Olin M, Lund EG, Andersson U, Schuster G, Björkhem I, Russell DW, Eggertsen G. 2002. Cholic acid mediates negative feedback regulation of bile acid synthesis in mice. J Clin Invest 110:1191–1200. doi: 10.1172/JCI16309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hylemon PB, Zhou H, Pandak WM, Ren S, Gil G, Dent P. 2009. Bile acids as regulatory molecules. J Lipid Res 50:1509–1520. doi: 10.1194/jlr.R900007-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyata M, Takamatsu Y, Kuribayashi H, Yamazoe Y. 2009. Administration of ampicillin elevates hepatic primary bile Acid synthesis through suppression of ileal fibroblast growth factor 15 expression. J Pharmacol Exp Ther 331:1079–1085. doi: 10.1124/jpet.109.160093. [DOI] [PubMed] [Google Scholar]
- 10.Degirolamo C, Rainaldi S, Bovenga F, Murzilli S, Moschetta A. 2014. Microbiota modification with probiotics induces hepatic bile acid synthesis via downregulation of the FXR-FGF15 axis in mice. Cell Rep 7:12–18. doi: 10.1016/j.celrep.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 11.Miyata M, Sakaida Y, Matsuzawa H, Yoshinari K, Yamazoe Y. 2011. Fibroblast growth factor 19 treatment ameliorates disruption of hepatic lipid metabolism in farnesoid X receptor (FXR)-null mice. Biol Pharm Bull 34:1885–1889. doi: 10.1248/bpb.34.1885. [DOI] [PubMed] [Google Scholar]
- 12.Li F, Jiang CT, Krausz KW, Li YF, Albert I, Hao HP, Fabre KM, Mitchell JB, Patterson AD, Gonzalez FJ. 2013. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat Commun 4:2384. doi: 10.1038/ncomms3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung JH, Manganiello V, Dyck JR. 2012. Resveratrol as a calorie restriction mimetic: therapeutic implications. Trends Cell Biol 22:546–554. doi: 10.1016/j.tcb.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Subramanian L, Youssef S, Bhattacharya S, Kenealey J, Polans AS, van Ginkel PR. 2010. Resveratrol: challenges in translation to the clinic—a critical discussion. Clin Cancer Res 16:5942–5948. doi: 10.1158/1078-0432.CCR-10-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asensi M, Medina I, Ortega A, Carretero J, Baño MC, Obrador E, Estrela JM. 2002. Inhibition of cancer growth by resveratrol is related to its low bioavailability. Free Radic Biol Med 33:387–398. doi: 10.1016/S0891-5849(02)00911-5. [DOI] [PubMed] [Google Scholar]
- 16.Shin NR, Lee JC, Lee HY, Kim MS, Whon TW, Lee MS, Bae JW. 2014. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 63:727–735. doi: 10.1136/gutjnl-2012-303839. [DOI] [PubMed] [Google Scholar]
- 17.Anhê FF, Roy D, Pilon G, Dudonné S, Matamoros S, Varin TV, Garofalo C, Moine Q, Desjardins Y, Levy E, Marette A. 2015. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 64:872–883 doi: 10.1136/gutjnl-2014-307142. [DOI] [PubMed] [Google Scholar]
- 18.Larrosa M, Yañéz-Gascón MJ, Selma MV, González-Sarrías A, Toti S, Cerón JJ, Tomás-Barberán F, Dolara P, Espín JC. 2009. Effect of a low dose of dietary resveratrol on colon microbiota, inflammation and tissue damage in a DSS-induced colitis rat model. J Agric Food Chem 57:2211–2220. doi: 10.1021/jf803638d. [DOI] [PubMed] [Google Scholar]
- 19.Jung CM, Heinze TM, Schnackenberg LK, Mullis LB, Elkins SA, Elkins CA, Steele RS, Sutherland JB. 2009. Interaction of dietary resveratrol with animal-associated bacteria. FEMS Microbiol Lett 297:266–273. doi: 10.1111/j.1574-6968.2009.01691.x. [DOI] [PubMed] [Google Scholar]
- 20.Queipo-Ortuño MI, Boto-Ordóñez M, Murri M, Gomez-Zumaquero JM, Clemente-Postigo M, Estruch R, Cardona Diaz F, Andrés-Lacueva C, Tinahones FJ. 2012. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am J Clin Nutr 95:1323–1334. doi: 10.3945/ajcn.111.027847. [DOI] [PubMed] [Google Scholar]
- 21.Qiao Y, Sun J, Xia S, Tang X, Shi Y, Le G. 2014. Effects of resveratrol on gut microbiota and fat storage in a mouse model with high-fat-induced obesity. Food Funct 5:1241–1249. doi: 10.1039/c3fo60630a. [DOI] [PubMed] [Google Scholar]
- 22.Etxeberria U, Arias N, Boqué N, Macarulla MT, Portillo MP, Martínez JA, Milagro FI. 2015. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J Nutr Biochem 26:651–660. doi: 10.1016/j.jnutbio.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Bennett BJ, de Aguiar Vallim TQ, Wang Z, Shih DM, Meng Y, Gregory J, Allayee H, Lee R, Graham M, Crooke R, Edwards PA, Hazen SL, Lusis AJ. 2013. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab 17:49–60. doi: 10.1016/j.cmet.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ridlon JM, Kang DJ, Hylemon PB. 2006. Bile salt biotransformations by human intestinal bacteria. J Lipid Res 47:241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Limaye PB, Lehman-McKeeman LD, Klaassen CD. 2012. Dysfunction of organic anion transporting polypeptide 1a1 alters intestinal bacteria and bile acid metabolism in mice. PLoS One 7:e34522. doi: 10.1371/journal.pone.0034522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alrefai WA, Gill RK. 2007. Bile acid transporters: structure, function, regulation and pathophysiological implications. Pharm Res 24:1803–1823. doi: 10.1007/s11095-007-9289-1. [DOI] [PubMed] [Google Scholar]
- 27.Herrema H, Meissner M, van Dijk TH, Brufau G, Boverhof R, Oosterveer MH, Reijngoud DJ, Müller M, Stellaard F, Groen AK, Kuipers F. 2010. Bile salt sequestration induces hepatic de novo lipogenesis through farnesoid X receptor- and liver X receptor alpha-controlled metabolic pathways in mice. Hepatology 51:806–816. doi: 10.1002/hep.23408. [DOI] [PubMed] [Google Scholar]
- 28.Out C, Hageman J, Bloks VW, Gerrits H, Sollewijn Gelpke MD, Bos T, Havinga R, Smit MJ, Kuipers F, Groen AK. 2011. Liver receptor homolog-1 is critical for adequate up-regulation of Cyp7a1 gene transcription and bile salt synthesis during bile salt sequestration. Hepatology 53:2075–2085. doi: 10.1002/hep.24286. [DOI] [PubMed] [Google Scholar]
- 29.Chiang JY. 2009. Bile acids: regulation of synthesis. J Lipid Res 50:1955–1966. doi: 10.1194/jlr.R900010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. 2000. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 102:731–744. doi: 10.1016/S0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 31.Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, Gerard RD, Repa JJ, Mangelsdorf DJ, Kliewer SA. 2005. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab 2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Lu TT, Makishima M, Repa JJ, Schoonjans K, Kerr TA, Auwerx J, Mangelsdorf DJ. 2000. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell 6:507–515. doi: 10.1016/S1097-2765(00)00050-2. [DOI] [PubMed] [Google Scholar]
- 33.Xiao D, Singh SV. 2008. z-Guggulsterone, a constituent of ayurvedic medicinal plant Commiphora mukul, inhibits angiogenesis in vitro and in vivo. Mol Cancer Ther 7:171–180. doi: 10.1158/1535-7163.MCT-07-0491. [DOI] [PubMed] [Google Scholar]
- 34.Kemper JK, Xiao Z, Ponugoti B, Miao J, Fang S, Kanamaluru D, Tsang S, Wu SY, Chiang CM, Veenstra TD. 2009. FXR acetylation is normally dynamically regulated by p300 and SIRT1 but constitutively elevated in metabolic disease states. Cell Metab 10:392–404. doi: 10.1016/j.cmet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Purushotham A, Xu Q, Lu J, Foley JF, Yan X, Kim DH, Kemper JK, Li X. 2012. Hepatic deletion of SIRT1 decreases hepatocyte nuclear factor 1 alpha/farnesoid X receptor signaling and induces formation of cholesterol gallstones in mice. Mol Cell Biol 32:1226–1236. doi: 10.1128/MCB.05988-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kulkarni SS, Cantó C. 2015. The molecular targets of resveratrol. Biochim Biophys Acta 1852:1114–1123. doi: 10.1016/j.bbadis.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 37.El-Mowafy AM, El-Mesery ME, Salem HA, Al-Gayyar MM, Darweish MM. 2010. Prominent chemopreventive and chemoenhancing effects for resveratrol: unraveling molecular targets and the role of C-reactive protein. Chemotherapy 56:60–65. doi: 10.1159/000298821. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Chen ML, Zhou Y, Yi L, Gao YX, Ran L, Chen SH, Zhang T, Zhou X, Zou D, Wu B, Wu Y, Chang H, Zhu JD, Zhang QY, Mi MT. 2015. Resveratrol improves hepatic steatosis by inducing autophagy through the cAMP signaling pathway. Mol Nutr Food Res 59:1443–1457. doi: 10.1002/mnfr.201500016. [DOI] [PubMed] [Google Scholar]
- 39.Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, Gu X, Huang Y, Zamanian-Daryoush M, Culley MK, DiDonato AJ, Fu X, Hazen JE, Krajcik D, DiDonato JA, Lusis AJ, Hazen SL. 2015. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell 163:1585–1595. doi: 10.1016/j.cell.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swann JR, Want EJ, Geier FM, Spagou K, Wilson ID, Sidaway JE, Nicholson JK, Holmes E. 2011. Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proc Natl Acad Sci U S A 108:4523–4530. doi: 10.1073/pnas.1006734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Begley M, Hill C, Gahan CGM. 2006. Bile salt hydrolase activity in probiotics. Appl Environ Microbiol 72:1729–1738. doi: 10.1128/AEM.72.3.1729-1738.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joyce SA, MacSharry J, Casey PG, Kinsella M, Murphy EF, Shanahan F, Hill C, Gahan CG. 2014. Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. Proc Natl Acad Sci U S A 111:7421–7426. doi: 10.1073/pnas.1323599111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Q, Wang E, Ma L, Zhai P. 2012. Dietary resveratrol increases the expression of hepatic 7alpha-hydroxylase and ameliorates hypercholesterolemia in high-fat fed C57BL/6J mice. Lipids Health Dis 11:56. doi: 10.1186/1476-511X-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chothe PP, Swaan PW. 2014. Resveratrol promotes degradation of the human bile acid transporter ASBT (SLC10A2). Biochem J 459:301–312. doi: 10.1042/BJ20131428. [DOI] [PubMed] [Google Scholar]
- 45.De Aguiar Vallim TQ, Tarling EJ, Edwards PA. 2013. Pleiotropic roles of bile acids in metabolism. Cell Metab 17:657–669. doi: 10.1016/j.cmet.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall HU, Bamberg K, Angelin B, Hyötyläinen T, Orešič M, Bäckhed F. 2013. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab 17:225–235. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Liberles SD, Buck LB. 2006. A second class of chemosensory receptors in the olfactory epithelium. Nature 442:645–650. doi: 10.1038/nature05066. [DOI] [PubMed] [Google Scholar]
- 48.Bai C, Biwersi J, Verkman AS, Matthay MA. 1998. A mouse model to test the in vivo efficacy of chemical chaperones. J Pharmacol Toxicol Methods 40:39–45. doi: 10.1016/S1056-8719(98)00034-3. [DOI] [PubMed] [Google Scholar]
- 49.Mello CC, Barrick D. 2003. Measuring the stability of partly folded proteins using TMAO. Protein Sci 12:1522–1529. doi: 10.1110/ps.0372903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental design. The schematic depicts the overall design of the animal experiment to evaluate the effect of RSV on TMAO-induced AS in both C57BL/6J (A) and ApoE−/− (B) mice. Download
RSV remodeled gut microbiota and decreased ileal BA content without modifying ASBT function. Eight-week-old female C57BL/6J mice (n = 10 per group) were fed a chow diet with or without RSV (0.4%) for 1 or 2 months. (A and B) The relative abundances of the indicated bacterial strains at the phylum level (A) and the genus level (B) in the cecal content were assessed by qPCR assay. (C) Total BA content in ileal tissues. (D) Western blotting was used to detect the expression of ASBT. (E and F) Expression of ASBT gene mRNA (E) and OSTα and OSTβ gene mRNA (F) was determined by qPCR assays. Values are expressed as means ± SD (n = 10). a, P < 0.05; b, P < 0.01 (versus vehicle-treated control group). Download
RSV had no effect on liver FXR and SHP gene mRNA expression. Eight-week-old female C57BL/6J mice (n = 10 per group) were fed a chow diet with or without RSV (0.4%) in the presence or absence of Abs for 30 days. Liver tissues were collected. (A) Relative expression levels of the indicated mRNAs in the liver were determined by qPCR assays. (B) FXR expression was analyzed by Western blotting. Values are expressed as means ± SD (n = 10). Download
RSV remodeled gut microbiota in choline-fed ApoE−/− mice. Eight-week-old female ApoE−/− mice (n = 10 per group) were fed chow, chow with RSV (0.4%), chow with choline (1%), or chow with choline (1%) plus RSV (0.4%) for 4 months. The relative abundances of the indicated bacterial strains at the phylum level (A) and the genus level (B) in the cecal content were assessed by qPCR assay. a, P < 0.05; b, P < 0.01 (versus vehicle-treated control group); d, P < 0.01 (versus choline-treated group). Download
RSV altered the BA composition in gallbladder bile and feces and increased fecal BSH activity in choline-treated ApoE−/− mice. Eight-week-old female ApoE−/− mice (n = 10 per group) were fed chow, chow with choline (1%), or chow with choline (1%) plus RSV (0.4%) for 4 months. The BA composition of gallbladder bile and feces was analyzed by LC/MS. (A) TCA/TβMCA ratios in gallbladder bile. (B) Conjugated/unconjugated BA ratios in fecal samples. (C) Fecal BSH activity. (D) CA/DCA ratios in fecal samples. Values are expressed as means ± SD (n = 10). a, P < 0.05; b, P < 0.01 (versus vehicle-treated control group); d, P < 0.05 (versus choline-treated group). Download
Primers used for qPCR.
Composition of Mega medium.
Retention times of BAs.
Biliary, SI and fecal bile acid composition profile in mice (related to data shown in Fig. 3, 4, and 9 and in Fig. S2 and S5).
Supplemental materials and methods. Download