Abstract
OBJECTIVE
Paraoxonase 1 (PON1) is synthesized in the liver and bound to high density lipoprotein (HDL) particles in blood. PON1 protects against the development of atherosclerosis by metabolizing pro-atherogenic oxidized lipids. The Southeastern United States (excluding Florida) has the country's highest age-adjusted mortality rate from cardiovascular disease. The current study determines the association of PON1 status with atherosclerosis (ATH) in individuals from the Southeastern United States.
METHODS
Eighty African Americans (40 male, 40 female) and 120 Caucasians (60 male, 60 female) were enrolled from a cardiology practice in northeastern Mississippi. Serum PON1 activities were determined using diazoxon, paraoxon, and phenyl acetate (PhAc) as substrates. The PON1192 genotype of each individual was also determined. A multivariable logistic regression model was developed to identify the associations of clinical characteristics, serum PON1 activity, and PON1192 genotype of the study population with ATH.
RESULTS
A core model consisting of age, gender, history of smoking, hypertension, and LDL-cholesterol group was constructed. The maximum-rescaled generalized r2 value for the core model was 0.35. Addition of PON1 activity assessed by PhAc hydrolysis was the only measure of PON1 enzymatic activity to add significant information to the core model (p= 0.0317) with the maximum-rescaled generalized r2 value increasing to 0.37. Increasing PON1 activity was associated with decreased odds of ATH. The PON1192 genotype was not significantly associated with ATH.
CONCLUSIONS
Increasing PON1 activity assessed by the hydrolysis of PhAc is associated with decreased odds of ATH in a group of African American and Caucasian Southerners.
Keywords: paraoxonase, atherosclerosis, coronary artery disease, cardiovascular disease, health disparities
INTRODUCTION
The paraoxonase (PON) gene family has been extensively investigated recently. One member of the PON family, paraoxonase-1 (PON1) has been implicated in a wide variety of human illnesses including cardiovascular disease (CVD), diabetes mellitus, and obesity [1-3]. PON1 performs multiple functions. In humans, PON1 is mainly expressed in the liver, then transported to the blood where it is tightly bound to high density lipoprotein (HDL) particles (KD <1 nM) [4]. One physiological function is the prevention of low density lipoprotein (LDL) and HDL oxidation by hydrolyzing lipid peroxides in lipoprotein particles; therefore it may protect against the development of atherosclerosis (ATH) [3, 5]. PON1 also protects against the acute toxicity of some organophosphorus anticholinesterase insecticides through hydrolysis of the compounds or their active metabolites [6]. In fact, paraoxonase received its name for its ability to hydrolyze paraoxon, the active metabolite of the insecticide parathion.
Studies using mouse models of ATH (ApoE−/− and LDL-R−/−) have consistently shown that the over expression of PON1 reduces ATH by reducing oxidative stress, hydrolyzing lipid peroxides, promoting cholesterol efflux from macrophages, and increasing reverse cholesterol transport [7-9]. Conversely, studies have shown that PON1-deficient mice have increased susceptibility to LDL oxidation, increased measures of macrophage oxidative stress, and larger atherosclerotic lesions than controls [10, 11].
Differences in PON1 activity have been linked to common polymorphisms in both coding and promoter regions [12, 13]. Two common polymorphisms in the coding region of PON1 are PON1 L55M and PON1 Q192R. The PON1 Q192R polymorphism has the greater influence on enzyme activity measured in vitro [12, 14]. This polymorphism involves a glutamine (Q) to arginine (R) substitution at position 192 [14, 15]. Several promoter region polymorphisms have been described with the C-108T polymorphism influencing expression the most [13]. The recognition that PON1 prevents lipid peroxide accumulation and that the PON1 Q192R polymorphism affects the rate of hydrolysis of several pesticide metabolites led to investigation of the possible association of the PON1 Q192R polymorphism with atherosclerosis.
The relationship, if any, of the PON1 Q192R polymorphism with the development of atherosclerosis is unclear. Early research suggested that the PON1192Q alloform was more efficient at metabolizing lipid peroxides in human atherosclerotic plaques than the PON1192R alloform [16], and thus was believed to be more effective at preventing ATH. In addition several early studies (reviewed in Costa et al. [17]) as well as two more recent reports [18, 19] have suggested an association of the PON1192R allele with atherosclerosis. Other studies have indicated that the PON1192R alloform binds to HDL with greater affinity than the PON1192Q alloform and therefore demonstrates increased stability and greater lipolactonase activity [20]. Also the prevalence of ATH was greatest in subjects with the PON1192QQ genotype [21]. A recent meta-analysis found only a weak association of PON1192R with vascular disease and pointed out the need for more definitive studies [22]. Richter and Furlong [23] have argued that to accurately assess PON1's role in atherosclerosis that both PON1 genotype and phenotype (i.e. activity level), together known as the PON1 status, must be determined.
An individual's functional PON1192 genotype can be determined using a 2-substrate assay to generate a 2-dimensional enzyme activity plot [23]. Without actual genotyping, the plot of the rates of diazoxon hydrolysis in the presence of 2 M NaCl (diazoxonase; DZOaseNa) vs. paraoxon hydrolysis in the presence of 2 M NaCl (paraoxonase; POaseNa) separates individuals into 3 functional genotypes, PON1192QQ, PON1192QR and PON1192RR. PON1 activity determined using PhAc as a substrate in the absence of NaCl does not distinguish between the PON1192Q and the PON1192R alloforms and thus serves as a measure of PON1 activity independent of genotype [24].
Numerous epidemiological studies have identified factors that increase the risk of ATH. Important risk factors include: dyslipidemias (e.g., low HDL cholesterol and high LDL cholesterol), smoking, diabetes mellitus, and hypertension. States in the Southeast (except Florida) have higher age-adjusted annual mortality rates from CVD than any other geographic region in the US [25]. The cause for this disparity is unknown. Low socioeconomic status, exposure to environmental toxicants, and the increasing prevalence of hypertension have been proposed to explain the higher stroke mortality rate in the South [26]. African American men and women have higher age-adjusted mortality rates from CVD than Caucasian men and women [25]. Little information is available on the frequency distribution of PON1192 genotypes of US Southern populations, which have among the worst health statistics in America. Early studies reported that African Americans have a weighted PON1192Q allele frequency of 0.37 and a weighted PON1192R allele frequency of 0.63, while Caucasians have a weighted PON1192Q allele frequency of 0.73 and a weighted PON1192R allele frequency of 0.27 [27]. A study from our laboratory [28] showed a similar distribution with African Americans having a higher PON1192R allele frequency, 0.66, and Caucasians having a higher PON1192Q allele frequency, 0.77.
In the present study, the PON1192 genotype and the PON1 enzymatic activity (phenotype) were determined for African American and Caucasian male and female Southerners for whom demographic and clinical information was available. The enzymatic activity was assessed with three substrates, paraoxon, diazoxon and PhAc. Statistically significant associations of clinical characteristics and PON1192 genotype and phenotype with ATH were determined using logistic regression. A model was developed to test the association of PON1 status with ATH while accounting for a core group of clinical and demographic variables.
MATERIALS AND METHODS
Study Population
Subjects were enrolled in the study from the clinical practice of Cardiology Associates of North Mississippi, LLC, a cardiology group located in Tupelo, Mississippi, which draws the vast majority of its patients from the 26 counties comprising northeast Mississippi and northwest Alabama. The research division of this clinic, Cardiology Associates Research, LLC (CARe), collected a total of 200 serum samples from Caucasians (60 male, 60 female) and African Americans (40 male, 40 female), ages 45 and older. A 60/40 split was chosen to reflect the racial composition of Mississippi.
Subjects were excluded if they were being evaluated for acute coronary syndrome (recent myocardial infarction or unstable angina), were known or suspected to be infected with human immunodeficiency virus or hepatitis B, or did not self-declare as either Caucasian or African American. Participants underwent the diagnostic tests recommended by their cardiologist. Study records that identified the subject were kept confidential as required by Federal Privacy Regulations. The protocol was approved by both the Institutional Review Board (IRB) of Mississippi State University and the IRB of North Mississippi Medical Center; the latter provides oversight for studies performed at CARe.
After obtaining informed written consent, blood samples were collected when vascular access was established for clinical testing. Serum was isolated by allowing blood to clot, then centrifuging at 2500-3000 × g at 4°C for 10 to 20 minutes. The serum was frozen on dry ice and stored at −70°C until analysis. An aliquot of whole blood anti-coagulated with K2EDTA was stored at −70°C for PON1 genotypic analysis.
Age, gender, race, diabetes, history of tobacco use, family history of coronary artery disease or stroke, presence of hypertension, the use of statins, height, weight, waist circumference in inches, blood pressure, and pulse were also obtained for each individual. A fasting lipid panel was obtained on the serum collected. All clinical information was de-identified before release from the clinic. Subjects were classified as having ATH if they had either a history of a clinical event resulting from or a mechanical revascularization procedure for treating coronary artery disease, cerebrovascular disease, abdomino-aortic disease, or peripheral vascular disease. Subjects were also considered to have ATH if they had reversible perfusion defects interpreted as myocardial ischemia on a nuclear stress test. Subjects who did not meet the criteria for ATH were considered not to have ATH. All diagnostic tests were interpreted by a CARe physician who had no knowledge of the results of the PON1 assays.
Statin usage of a subject was designated as ‘yes’ if the subject was currently on a statin drug or designated as ‘no’ if the subject was not taking a statin. Smoking status of an individual was assigned an ‘ever’ if the subject was still smoking or had ever smoked or ‘never’ if the subject had never smoked.
Because of the large number of participants on statins, LDL cholesterol levels and statin usage were used to place subjects into one of two categories. Subjects were considered normal for LDL cholesterol if their levels were ≤ 160 mg/dL, they did not meet criteria for pharmacologic treatment [29], and they were not currently taking a statin. Subjects’ LDL cholesterol levels were considered elevated if their levels were >160 mg/dL, they were currently taking a statin, or they met the criteria for pharmacological treatment of elevated LDL cholesterol [29]. Subjects were considered normal for HDL cholesterol if their levels were ≥ 40 mg/dL and low if their levels were < 40 mg/dL.
Subjects were considered normal for triglycerides if their levels were <200 mg/dL and they were not taking a fibrate. Subjects were considered abnormal for triglycerides if their levels were ≥200 mg/dL or they were taking a fibrate. Subjects who were on statin therapy and had triglycerides <200 mg/dL were considered indeterminate as it was assumed statin therapy was prescribed for elevated LDL cholesterol but would influence triglyceride levels and prevent an accurate designation of baseline triglyceride status (i.e., off statin therapy).
Subjects were considered normal for total cholesterol if their levels were <200 mg/dL and they were not taking statins. Subjects were considered abnormal for total cholesterol if their levels were ≥200 mg/dL. Subjects who were on statin therapy and had a total cholesterol <200 mg/dL were considered indeterminate as it was assumed statin therapy was prescribed for elevated LDL cholesterol but would influence total cholesterol levels and prevent an accurate designation of baseline total cholesterol status (i.e., off statin therapy).
Chemicals and Samples
Paraoxon was synthesized as described previously [30]. Diazoxon was purchased from ChemService (West Chester, PA). All other chemicals were purchased from Sigma Chemical Company (St. Louis, MO).
Paraoxonase and Diazoxonase Assays
Paraoxonase and diazoxonase activities were determined by the hydrolysis of paraoxon and diazoxon, respectively, as described by Richter and Furlong [23] with minor modifications [28]. The paraoxonase assay was conducted in the presence of 2 M NaCl (POaseNa) and the absence of NaCl (POase). The diazoxonase assay was conducted in the presence of 2 M NaCl (DZOaseNa) and the absence of NaCl (DZOase). Samples were run in triplicate. Data were expressed as units per liter of serum (U/L) where 1 unit = 1 micromole of paraoxon or diazoxon hydrolyzed per minute.
The functional genotype was determined by plotting the DZOaseNa activity versus the POaseNa activity for each sample [23].
Arylesterase Assay
Arylesterase activity was determined using PhAc as a substrate. The phenol released from PhAc hydrolysis was measured spectrophotometrically as described previously [28] and the activity was expressed as U/L where 1 unit = 1 micromole of PhAc hydrolyzed per minute. Samples were run in triplicate.
PON1 Genotyping
Genomic DNA was isolated from whole blood using a Sigma-Aldrich® GenElute™ Blood Genomic DNA Kit and concentrated using a Qiagen QIAEX®II Gel Extraction Kit. Following the method received from the Medical Genetics Department of the University of Washington (Jane Ranchalis, personal communication), a region containing the PON1192 codon was amplified by PCR to yield a 99 bp product using the primer pair F 5' TATTGTTGCTGTGGGACCTGAG 3' and R 5' TGAAAGACTTAAACT 3'. Each PCR reaction contained ~200 ng of purified human DNA at ~100 ng/μl, 2 μl of 10X AmpliTaq Gold® 360 PCR Buffer (ABI), 2 μl of 25 mM MgCl2, 0.16 μl 25 mM dNTPs (Promega), 0.2 μl of each primer at 25 μM, 0.2 μl AmpliTaq Gold polymerase (ABI) at 5U/μl, 1 μl of dimethylsulfoxide and water sufficient to bring the final volume to 20 μl. Since there is a native Alw I restriction site at codon 192, 10 μl of the PCR product solution was digested with Alw I (NEB) overnight and visualized by ethidium bromide staining on a 3% agarose gel next to 10 μl of undigested product solution. The digestion resulted in 63- and 36-bp fragments for individuals with the PON1192RR genotype; 99-, 63-, and 36-bp fragments for individuals with the PON1192QR genotype; and a single, non-digested 99-bp fragment for individuals with the PON1192QQ genotype. Sequence data confirmed the results from the restriction digest analyses for 50 of the 200 participants sequenced.
Statistical Analysis and Model Development
SAS for Windows 9.1.3 (SAS Institute Inc., Cary, NC, USA) was used for statistical analysis. A chi-square (χ2) test (PROC FREQ) with Bonferroni correction for multiple comparisons when a significant χ2 was found (p ≤ 0.05), was used to determine differences among race-gender groups for PON1 enzymatic activities and genotype. One-way analysis of variance was used (PROC GLM) to determine if significant differences among race-gender groups (p ≤ 0.05) existed for continuous clinical variables. If the model indicated significant differences among groups, mean separation tests were conducted using the Tukey adjustment. Differences in mean POase activity, DZOase activty, and PhAc hydrolysis between subjects with or without atherosclerosis were assessed by t-test using PROC TTEST.
The strength of association between the occurrence of ATH and each of the explanatory variables listed in Table 3 was assessed by odds ratios using logistic regression (PROC LOGISTIC, SAS for Windows v. 9.1.3, SAS Institute, Cary, NC). Clinical variables that were found to be associated with ATH with a p-value ≤ 0.25 were used as candidates in a multivariable model [31]. After the model was fit, the variable with the highest p-value was removed manually and the remaining explanatory variables reassessed. This process was continued until only clinical variables with p-value ≤ 0.05 remained in the model. This multivariable model was designated the core factor model. The experimental variables for PON1 activity (i.e., activities for hydrolysis of paraoxon, diazoxon, and PhAc) were then added individually to the core factor model to test the association between ATH and the experimental variables for PON1 activity while accounting for the core clinical variables.
Table 3.
Odds ratios of atherosclerosis associated with clinical and serum factors.
| Factor1 | Comparison | Units | n | Odds Ratio | 95% CI | P-Value |
|---|---|---|---|---|---|---|
| Age | 5 years | 200 | 1.22 | (1.04,1.43) | 0.0138 | |
| Gender | Male vs Female | 200 | 4.13 | (2.29,7.46) | <0.0001 | |
| Smoker | Ever vs Never | 200 | 2.45 | (1.38,4.31) | 0.0023 | |
| LDLGroup | Elevated vs Normal | 197 | 10.08 | (3.75,27.08) | <0.0001 | |
| HDLGroup | Low vs Normal | 196 | 2.66 | (1.47,4.82) | 0.0012 | |
| Triglyceride | Abnormal vs Normal | 97 | 6.57 | (2.70,15.97) | 0.0267 | |
| BMI | 5 kg/m2 | 198 | 0.75 | (0.59,0.95) | 0.018 | |
| POaseNa2 | 150 U/L | 199 | 0.78 | (0.62,0.98) | 0.0326 | |
| DZOaseNa2 | 1000 U/L | 199 | 0.89 | (0.80,0.99) | 0.0346 | |
| POase2 | 15 U/L | 199 | 0.92 | (0.85,0.99) | 0.0334 | |
| DZOase2 | 1000 U/L | 199 | 0.85 | (0.75,0.97) | 0.0169 | |
| PhAc2 | 1000 U/L | 199 | 0.89 | (0.82,0.98) | 0.0128 |
Factors that were included in study but not found significant at p≤ 0.05 included race, hypertension, diabetes, family history, waist circumference, total cholesterol group, LDL/HDL ratio, genotype, DZOaseNa/POaseNa ratios, and DZOase/POase ratios
Activities of POaseNa, DZOaseNa, POase, DZOase, and hydrolysis of PhAc are expressed as micromoles substrate hydrolyzed per minute per liter of serum (U/L), means ± SD.
RESULTS
Characterization of the Study Population
Clinical variables were expressed as mean ± SD for continuous data and ratios for categorical data for each race-gender group (Table 1). Height and weight were used to determine body mass index (BMI). The categorical variables LDL group, triglyceride (trig) group, and TotalChol group were determined using the appropriate values from the lipid panel and information of statin use as described in detail in the Methods section.
Table 1.
Characterization of study population by race-gender group.
| Variables1 | Units | TotalPop | AAF2 | AAM2 | CF2 | CM2 |
|---|---|---|---|---|---|---|
| n=200 | n=40 | n=40 | n=60 | n=60 | ||
| Age | Years | 63 ± 9 | 60 ± 11 | 60 ± 7 | 65 ± 9 | 64 ± 9 |
| Smoker (Ever/Never) | 113/87 | 13/27 | 27/13 | 31/29 | 42/18 | |
| Diabetes (Yes/No) | 70/130 | 18/22 | 15/25 | 19/41 | 18/42 | |
| Hypertension (Yes/No) | 154/46 | 35/5 | 34/6 | 44/16 | 41/19 | |
| Height | Inches | 67 ± 4 | 65 ± 3 | 69 ± 3 | 65 ± 3 | 70 ± 3 |
| Weight | pounds | 198 ± 44 | 197 ± 54 | 201 ± 36 | 183 ± 43 | 214 ± 38 |
| BMI | kg/m2 | 31 ± 6 | 33 ± 8 | 30 ± 5 | 31 ± 7 | 30 ± 5 |
| HDL | mg/dL | 48 ± 16 | 56 ±13 | 43 ±12 | 51 ± 16 | 40 ± 13 |
| LDL | mg/dL | 107 ± 36 | 123 ± 37 | 98 ± 30 | 116 ± 40 | 95 ± 29 |
| Triglycerides | mg/dL | 149 ± 97 | 119 ± 53 | 126 ± 113 | 162 ± 73 | 171 ± 122 |
| TotalChol | mg/dL | 185 ± 45 | 203 ± 43 | 165 ± 34 | 202 ± 48 | 168 ± 39 |
| Statin Use (Yes/No) | 121/79 | 19/21 | 29/11 | 30/30 | 43/17 | |
| HDL group (Low/Normal) | 76/120 | 5/34 | 18/22 | 14/44 | 39/20 | |
| LDL group (Elevated/Normal) | 156/41 | 25/14 | 35/5 | 43/15 | 53/7 | |
| Trig group (Abnormal/Normal)3 | 43/63 | 3/19 | 5/9 | 19/20 | 16/15 | |
| TotalChol group (Abnormal/Normal)3 | 69/37 | 21/9 | 7/8 | 29/10 | 12/10 | |
| Atherosclerosis (Yes/No) | 96/104 | 10/30 | 25/15 | 21/39 | 40/20 |
Variables: Age, Height, Weight, Body Mass Index (BMI), Total Cholesterol, HDL cholesterol, and LDL cholesterol are expressed as means ± SD. Variables: Smoker, Diabetes, Hypertension, Statin Use, HDL group, LDL group, Trig group, and TotalChol group were expressed as ratios.
AAF=African American Female, AAM=African American Male, CF=Caucasian Female, and CM=Caucasian Male.
Subjects receiving statin therapy were not included in the summary statistics for these variables
PON1 Genotypes
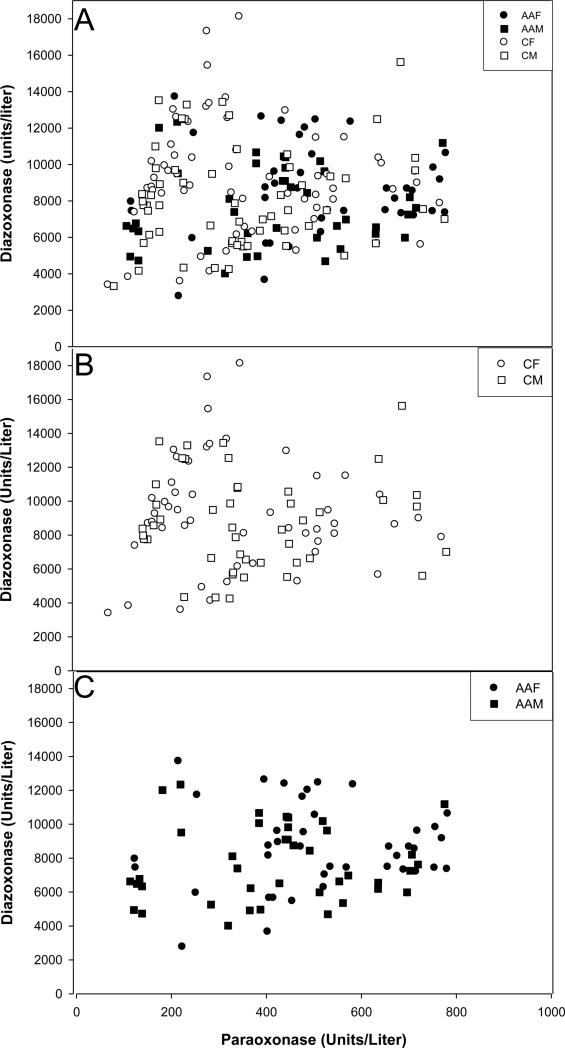
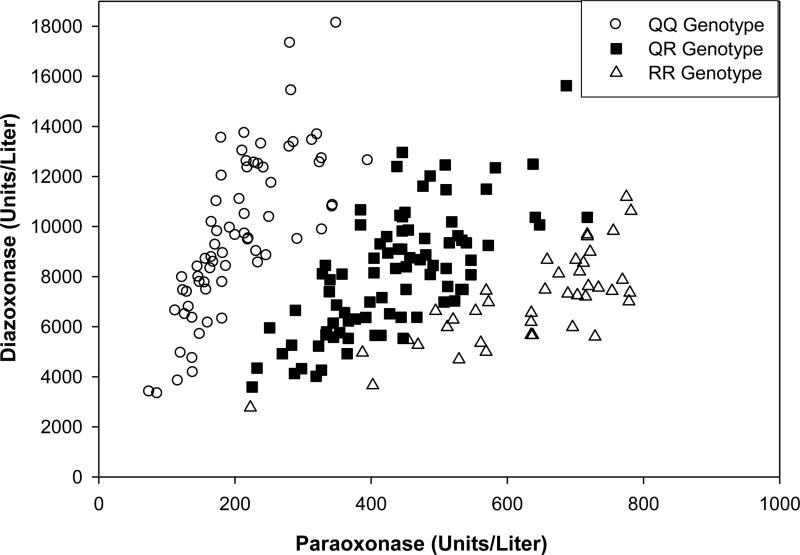
Separation of the 200 individuals of this study into the three functional PON1192 genotypes through the 2-dimensional plot of POaseNa vs. DZOaseNa was not as clear as previously reported [23, 28] (Figure 1a). In fact, the DZOaseNa vs POaseNa plot alone was unable to unambiguously assign a functional genotype to a number of individuals. Therefore, the PON1192 genotype was determined using a restriction fragment length polymorphism assay. Assignment of genotypes to the data points on the DZOaseNa vs POaseNa plot showed that individuals with each of the three PON1192 genotypes were located in the expected region of the plot (Figure 2). A greater portion of Caucasians had the PON1192QQ genotype (p≤ 0.0001) than did the African Americans, while the reverse was true for the PON1192RR genotype (p≤ 0.0001) (Figure 1b, c and Table 2). No significant differences were noted in the population distribution of the PON1192QR genotype (Table 2).
Figure 1. Plot of serum DZOaseNa vs. POaseNa enzymatic activities.
The sample population (n=200) consisted of 120 Caucasians (60 male, 60 female) and 80 African Americans (40 male, 40 female). (A) Plot of DZOaseNa activities vs. POaseNa activities in the sera of male and female African American and Caucasian Southerners. (B) Plot of DZOaseNa activities vs. POaseNa activities in the sera of male and female Caucasian Southerners. (C) Plot of DZOaseNa activities vs. POaseNa activities in the sera of male and female African American Southerners
Abbreviations: AAF, African American Female (●); AAM, African American Male (■); CF, Caucasian Female (○); CM, Caucasian Male (□)
Figure 2. Plot of DZOaseNa vs. POaseNa enzymatic activities in the sera of male and female African American and Caucasian Southerners.
Genotypes are PON1192QQ (○); PON1192QR (■); and PON1192RR (Δ). The sample population (n=200) consisted of 120 Caucasians (60 male, 60 female) and 80 African Americans (40 male, 40 female)
Table 2.
Serum PON1 parameters of Caucasian and African American males and females.
| Variables | Units | TotalPop | AAF | AAM | CF | CM |
|---|---|---|---|---|---|---|
| n = 200 | n = 40 | n = 40 | n = 60 | n = 60 | ||
| POaseNa1 | U/L | 401 ± 188 | 509 ± 183A | 421 ± 189AB | 363 ± 173B | 354 ± 178B |
| DZOaseNa1 | U/L | 8460 ± 2757 | 8649 ± 2566AB | 7699 ± 2231B | 9191 ± 3110A | 8130 ± 2741AB |
| POase1 | U/L | 98 ± 54 | 129 ± 56A | 104 ± 53AB | 85 ± 50B | 85 ± 50B |
| DZOase1 | U/L | 6437 ± 2249 | 6141 ± 1814A | 6033 ± 1858A | 7052 ± 2472A | 6300 ± 2445A |
| PhAc1 | U/L | 15404 ± 3351 | 16919 ± 3165A | 15685 ± 3542AB | 14984 ± 3386B | 14656 ± 3041B |
| Genotype2 | ||||||
| 68 | 5A | 9A | 29B | 25B | ||
| QR | 87 | 17A | 18A | 25A | 27A | |
| RR | 40 | 16A | 13A | 5B | 6B |
Activities of POaseNa, DZOaseNa, POase, DZOase, and hydrolysis of PhAc are expressed as micromoles substrate hydrolyzed per minute per liter of serum (U/L), means ± SD.
Number of individuals in each race-gender group with the indicated genotype.
Values within a row with the same superscript letter are not significantly different (p≤ 0.05) as determined by analysis of variance with Tukey adjustment or by analysis of chi-square (χ2) with Bonferroni adjustment.
Values within a row with the same superscript letter are not significantly different (p≤ 0.05) as determined by analysis of variance with Tukey adjustment or by analysis of chi-square (χ2) with Bonferroni adjustment.
Differences in Serum Enzyme Activities
Serum variables were expressed as mean ± SD for each race-gender group (Table 2). African American females had significantly higher POaseNa activities than Caucasian females (p= 0.0005) and Caucasian males (p= 0.0002) but no differences between the other groups were observed (p> 0.1171). Caucasian females had significantly higher DZOaseNa activities than African American males (p= 0.0321) but no differences between the other groups were observed (p> 0.1470). African American females had significantly higher POase activities than Caucasian females (p= 0.0004) and Caucasian males (p= 0.0003) but no differences between the other groups were observed (p> 0.1382). No significant differences (p> 0.1054) were detected with DZOase activity levels among the race-gender groups. African American females had significantly higher PhAc activities than Caucasian females (p= 0.0216) and Caucasian males (p= 0.0049) but no difference between the other groups were observed (p> 0.2898).
Associations of ATH with Clinical and Serum Factors
Statistically significant associations (p≤ 0.05) between the presence of ATH and each of the clinical and serum factors individually were determined by logistic regression (Table 3). In our study population, increasing age, gender, smoking status, and classification in the elevated LDL, the low HDL, and the abnormal triglyceride groups were all associated with ATH. Classification in the elevated LDL cholesterol group had the strongest association with ATH of all the explanatory variables. Surprisingly, increasing body mass index (BMI) was associated with decreased odds of ATH. Increased levels of PON1 activity (expressed as POaseNa, DZOaseNa, POase, DZOase or PON1 activity determined by the hydrolysis of PhAc) were associated with decreased odds of ATH. Not surprisingly, individuals with ATH also had significantly lower PON1 activities than individuals without ATH using either paraoxon (89 ± 51 U/L vs. 105 ± 57 U/L, p = 0.0316) diazoxon (6033 ± 2031 U/L vs. 6806 ± 2382 U/L, p = 0.0151), or PhAc (14778 ± 3163 vs. 15976 ± 3430, p = 0.0114) as the substrate.
Factors that were included in the analysis but did not have statistically significant associations with ATH included race (p= 0.3264), hypertension (p= 0.0898), diabetes (p=0.6778), family history of ATH (p= 0.1242), waist circumference (p=0.8021), total cholesterol group (p= 0.8427), ratio LDL/HDL (p= 0.3466), and PON1192 genotype (p=0.9792).
Multivariable Logistic Regression Model
A multivariable logistic regression model was developed that showed the association of individual clinical and serum factors with the occurrence of ATH (Table 4). Triglyceride and total cholesterol groups were not included as variables in the model because over half the sample population was indeterminate for the variables due to statin therapy. Statin usage was not included as a variable in the model because it was used to define the LDL cholesterol groups. The factors age, gender, smoking status, hypertension, and LDL cholesterol group remained in the model to constitute the core model. Age showed a direct association with ATH (OR=1.04, p= 0.0251). Gender was associated with ATH (OR=3.13, p= 0.0011), with males being more likely than females to have ATH. Subjects who had a history of smoking (OR=1.98, p= 0.0560) or had hypertension (OR=2.05, p= 0.0888) were more likely to have ATH. History of smoking and hypertension were both left in the core model, even though their p-values were >0.05, because they are well-established risk factors for ATH.
Table 4.
Multivariable logistic regression model of clinical and serum factors associated with atherosclerosis occurrence
| Factor | Comparison | Units | Odds Ratio | 95% CI | P-Value |
|---|---|---|---|---|---|
| Age | 5 years | 1.04 | (1.01,1.08) | 0.0251 | |
| Gender | Male vs. Female | 3.13 | (1.58,6.23) | 0.0011 | |
| Smoker1 | Ever vs. Never | 1.98 | (0.98,3.99) | 0.0560 | |
| Hypertension1 | Yes vs. No | 2.05 | (0.90,4.70) | 0.0888 | |
| LDLGroup | High vs. Normal | 9.56 | (3.17, 28.79) | <0.0001 | |
| PhAc | 1000 U/L | 0.89 | (0.80,0.99) | 0.0317 |
Although the p-values for the association of smoking status and hypertension were greater than 0.05, the variables were retained in the model because each is an established risk factor for atherosclerosis
Following construction of the core factor model, the various PON1 activity measurements were added individually to the model to test the association of PON1 activity and ATH. PON1 activity as measured by PhAc hydrolysis was the only activity measurement that was significantly associated with ATH (p= 0.0317). The presence of hydrolysis of PhAc in the model increased the maximum-rescaled generalized r2 value from 0.35 to 0.37.
DISCUSSION
PON1 is now known to hydrolyze many substrates including organophosphorus insecticide metabolites and pharmaceuticals [6, 32]. PON1 has also been found to play an important role in the prevention of ATH by the metabolism of pro-atherogenic oxidized lipids [3]. To accurately assess the association of PON1 with ATH an individual's PON1 status (genotype and phenotype) must be determined. Even though individuals from the Deep South (except Floridians) have higher age-adjusted mortality rates of CVD than people in other regions of the United States, little is known about the PON1 status in the Southern population.
In the present study, each individual's PON1 status was determined. This analysis included determination of PON1 activity using the three substrates diazoxon, paraoxon, and PhAc and determination of each individual's PON1192 genotype. A core model consisting of risk factors known to be associated with ATH including age, gender, history of smoking, presence of hypertension, and LDL cholesterol group was developed. Family history was not associated with ATH in our study population possibly because many subjects were unable to provide enough information to accurately assign their family history. Diabetes also was not associated with ATH in our study population. The lack of association of diabetes with ATH may be the result of the over representation of diabetics in the group of individuals without ATH (35 of 104, more than would be expected in a random sampling of the population, 12.3 % prevalence of diabetes in MS). Most of the study subjects had been referred for diagnostic testing for coronary disease and, not surprisingly, had a much higher prevalence of diabetes than the general population. Increasing BMI was associated with decreased odds of ATH in our study population. The reason for this is not known though individuals with ATH may have undergone more intensive dietary counseling and been more motivated to follow the dietary recommendations than individuals without ATH.
The association of the various components of PON1 status with ATH was determined. Interestingly, all of the PON1 enzymatic measurements had an association with ATH when assessed individually. However, when assessed in the presence of the clinical factors comprising the core model, only PON1 activity determined by the hydrolysis of PhAc was associated with ATH with a p≤ 0.05. The maximum-rescaled generalized r2 value of the core model was 0.35. The addition of PON1 activity (assessed by the hydrolysis of PhAc) to the core model resulted in an increase of the maximum-rescaled generalized r2 value to 0.37. While this increase is not large, this small increase is of interest because only 5-10% of the risk of ATH is not accounted for by known risk factors in studies using very large numbers of patients [33]. Gupta et al. [18] have also reported an association of PON1 activity assessed by PhAc hydrolysis (as well as several other PON1 related characteristics) with atherosclerosis in Indian Punjabis.
The results of our study must be interpreted with a degree of caution. First, the study population was not randomly selected but consisted of individuals being treated or evaluated in a cardiology clinic. This selection bias resulted in an over representation of diabetes in individuals without ATH in the study population and prevented an adequate evaluation of diabetes in this study. Second, most patients with ATH in this study had a previous history of ATH and were already being treated (as evidenced by the extensive use of statins in the study population) thus allowing for the possible alteration of PON1 enzymatic activities. However, use of statins and fibrates has in general been associated with an increase in PON1 serum activity measures (reviewed in Paragh et al. [34]). If such an increase had occurred in the study population, it would obscure a sparing effect of PON1 activity on ATH occurrence. The ongoing statin treatment also resulted in the inability to include triglyceride levels and total cholesterol levels in the model. In addition, the only screening test for ATH was cardiac nuclear perfusion imaging for hemodynamically significant coronary artery disease. This may not have detected subclinical ATH as sensitively as some other imaging modalities used to diagnose atherosclerosis (e.g. carotid ultrasound) used in previously published studies [35, 36]. This would result in individuals with subclinical coronary artery disease being classified as normal i.e. no atherosclerosis. Despite these limitations, this study did demonstrate that increased PON1 activity, as measured by PhAc hydrolysis, is associated with decreased odds of ATH in this Southern population. Interestingly, in this population the PON1192 genotype was not significantly associated with ATH.
CONCLUSIONS
This study documents a sparing effect of serum PON1 activity as assessed by the hydrolysis of PhAc on ATH occurrence adjusted for the effect of age, gender, history of smoking, hypertension, and elevated LDL-cholesterol. Diabetes, family history of ATH, total cholesterol levels and triglyceride levels are not well investigated in this model. The possibility of PON1 activity as an independent risk marker of ATH warrants further investigation to clarify these issues. This is the first study to document a relationship of PON1 activity with ATH in a population from the Deep South. In addition, the study has produced a model that will have utility for characterizing ATH risk factors utilizing larger data sets in the future.
Acknowledgments
FUNDING:
This research was supported in part by National Institutes of Health R21 ES015107. This is Center for Environmental Health Sciences publication 123.
ABBREVIATIONS AND DEFINITIONS
- ATH
Atherosclerosis
- CVD
cardiovascular disease
- DZOase
diazoxonase, i.e., enzymatic activity of paraoxonase assessed with diazoxon with no NaCl present
- DZOaseNa
diazoxonase, i.e., enzymatic activity of paraoxonase assessed with diazoxon with 2 M NaCl
- HDL
high-density lipoprotein
- LDL
low-density lipoprotein
- POase
paraoxonase, i.e., enzymatic activity of paraoxonase assessed with paraoxon with no NaCl present
- POaseNa
paraoxonase, i.e., enzymatic activity of paraoxonase assessed with paraoxon with 2 M NaCl
- PON1
paraoxonase-1
- PhAc
phenyl acetate
Footnotes
COMPETING INTERESTS DECLARATION:
The authors declare they have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Abbott CA, Mackness MI, Kumar S, Boulton AJ, Durrington PN. Serum paraoxonase activity, concentration, and phenotype distribution in diabetes mellitus and its relationship to serum lipids and lipoproteins. Arterioscler Thromb Vasc Biol. 1995;15:1812–1818. doi: 10.1161/01.atv.15.11.1812. [DOI] [PubMed] [Google Scholar]
- 2.Ferretti G, Bacchetti T, Moroni C, Savino S, Liuzzi A, Balzola F, et al. Paraoxonase activity in high-density lipoproteins: a comparison between healthy and obese females. J Clin Endocrinol Metab. 2005;90:1728–1733. doi: 10.1210/jc.2004-0486. [DOI] [PubMed] [Google Scholar]
- 3.Shih DM, Gu L, Xia YR, Navab M, Li WF, Hama S, et al. Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature. 1998;394:284–287. doi: 10.1038/28406. [DOI] [PubMed] [Google Scholar]
- 4.Gaidukov L, Tawfik DS. High affinity, stability, and lactonase activity of serum paraoxonase PON1 anchored on HDL with ApoA-I. Biochemistry. 2005;44:11843–11854. doi: 10.1021/bi050862i. [DOI] [PubMed] [Google Scholar]
- 5.Mackness MI, Arrol S, Durrington PN. Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Lett. 1991;286:152–154. doi: 10.1016/0014-5793(91)80962-3. [DOI] [PubMed] [Google Scholar]
- 6.Aldridge WN, Davison AN. The mechanism of inhibition of cholinesterases by organophosphorus compounds. Biochem J. 1953;55:763–766. doi: 10.1042/bj0550763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aviram M, Rosenblat M. Paraoxonases 1, 2, and 3, oxidative stress, and macrophage foam cell formation during atherosclerosis development. Free Radic Biol Med. 2004;37:1304–1316. doi: 10.1016/j.freeradbiomed.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 8.Rozenberg O, Shih DM, Aviram M. Paraoxonase 1 (PON1) attenuates macrophage oxidative status: studies in PON1 transfected cells and in PON1 transgenic mice. Atherosclerosis. 2005;181:9–18. doi: 10.1016/j.atherosclerosis.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 9.Tward A, Xia YR, Wang XP, Shi YS, Park C, Castellani LW, et al. Decreased atherosclerotic lesion formation in human serum paraoxonase transgenic mice. Circulation. 2002;106:484–490. doi: 10.1161/01.cir.0000023623.87083.4f. [DOI] [PubMed] [Google Scholar]
- 10.Rozenberg O, Rosenblat M, Coleman R, Shih DM, Aviram M. Paraoxonase (PON1) deficiency is associated with increased macrophage oxidative stress: studies in PON1-knockout mice. Free Radic Biol Med. 2003;34:774–784. doi: 10.1016/s0891-5849(02)01429-6. [DOI] [PubMed] [Google Scholar]
- 11.Shih DM, Xia YR, Wang XP, Miller E, Castellani LW, Subbanagounder G, et al. Combined serum paraoxonase knockout/apolipoprotein E knockout mice exhibit increased lipoprotein oxidation and atherosclerosis. J Biol Chem. 2000;275:17527–17535. doi: 10.1074/jbc.M910376199. [DOI] [PubMed] [Google Scholar]
- 12.Davies HG, Richter RJ, Keifer M, Broomfield CA, Sowalla J, Furlong CE. The effect of the human serum paraoxonase polymorphism is reversed with diazoxon, soman and sarin. Nat Genet. 1996;14:334–336. doi: 10.1038/ng1196-334. [DOI] [PubMed] [Google Scholar]
- 13.Leviev I, James RW. Promoter polymorphisms of human paraoxonase PON1 gene and serum paraoxonase activities and concentrations. Arterioscler Thromb Vasc Biol. 2000;20:516–521. doi: 10.1161/01.atv.20.2.516. [DOI] [PubMed] [Google Scholar]
- 14.Adkins S, Gan KN, Mody M, La Du BN. Molecular basis for the polymorphic forms of human serum paraoxonase/arylesterase: glutamine or arginine at position 191, for the respective A or B allozymes. Am J Hum Genet. 1993;52:598–608. [PMC free article] [PubMed] [Google Scholar]
- 15.Humbert R, Adler DA, Disteche CM, Hassett C, Omiecinski CJ, Furlong CE. The molecular basis of the human serum paraoxonase activity polymorphism. Nat Genet. 1993;3:73–76. doi: 10.1038/ng0193-73. [DOI] [PubMed] [Google Scholar]
- 16.Aviram M, Hardak E, Vaya J, Mahmood S, Milo S, Hoffman A, et al. Human serum paraoxonases (PON1) Q and R selectively decrease lipid peroxides in human coronary and carotid atherosclerotic lesions: PON1 esterase and peroxidase-like activities. Circulation. 2000;101:2510–2517. doi: 10.1161/01.cir.101.21.2510. [DOI] [PubMed] [Google Scholar]
- 17.Costa LG, Cole TB, Jarvik GP, Furlong CE. Functional genomics of the paraoxonase (PON1) polymorphisms: Effects on pesticide sensitivity, cardiovascular disease, and drug metabolism. Annu Rev Med. 2003;54:371–392. doi: 10.1146/annurev.med.54.101601.152421. [DOI] [PubMed] [Google Scholar]
- 18.Gupta N, Singh S, Maturu VN, Sharma YP, Gill KD. Paraoxonase 1 (PON1) polymorphisms, haplotypes and activity in predicting cad risk in North-West Indian Punjabis. PLoS One. 2011;6:e17805. doi: 10.1371/journal.pone.0017805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohamed RH, Mohamed RH, Karam RA, Abd El-Aziz TA. The relationship between paraoxonase1-192 polymorphism and activity with coronary artery disease. Clinical Biochemistry. 2010;43:553–558. doi: 10.1016/j.clinbiochem.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 20.Gaidukov L, Rosenblat M, Aviram M, Tawfik DS. The 192R/Q polymorphs of serum paraoxonase PON1 differ in HDL binding, lipolactonase stimulation, and cholesterol efflux. J Lipid Res. 2006;47:2492–2502. doi: 10.1194/jlr.M600297-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Bhattacharyya T, Nicholls SJ, Topol EJ, Zhang R, Yang X, Schmitt D, et al. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA. 2008;299:1265–1276. doi: 10.1001/jama.299.11.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang M, Lang X, Zou L, Huang S, Xu Z. Four genetic polymorphisms of paraoxonase gene and risk of coronary heart disease: a meta-analysis based on 88 case-control studies. Atherosclerosis. 2011;214:377–385. doi: 10.1016/j.atherosclerosis.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 23.Richter RJ, Furlong CE. Determination of paraoxonase (PON1) status requires more than genotyping. Pharmacogenetics. 1999;9:745–753. [PubMed] [Google Scholar]
- 24.Richter RJ, Jarvik GP, Furlong CE. Determination of paraoxonase 1 status without the use of toxic organophosphate substrates. Circ Cardiovasc Genet. 2008;1:147–152. doi: 10.1161/CIRCGENETICS.108.811638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perry HM, Roccella EJ. Conference report on stroke mortality in the southeastern United States. Hypertension. 1998;31:1206–1215. doi: 10.1161/01.hyp.31.6.1206. [DOI] [PubMed] [Google Scholar]
- 27.Chen J, Kumar M, Chan W, Berkowitz G, Wetmur JG. Increased influence of genetic variation on PON1 activity in neonates. Environ Health Perspect. 2003;111:1403–1409. doi: 10.1289/ehp.6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis KA, Crow JA, Chambers HW, Meek EC, Chambers JE. Racial differences in paraoxonase-1 (PON1): a factor in the health of southerners? Environ Health Perspect. 2009;117:1226–1231. doi: 10.1289/ehp.0900569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKenney JM. Update on the National Cholesterol Education Program Adult Treatment Panel III guidelines: getting to goal. Pharmacotherapy. 2003;23:26S–33S. doi: 10.1592/phco.23.11.26s.32710. [DOI] [PubMed] [Google Scholar]
- 30.Chambers JE, Chambers HW. Time course of inhibition of acetylcholinesterase and aliesterases following parathion and paraoxon exposures in rats. Toxicol Appl Pharmacol. 1990;103:420–429. doi: 10.1016/0041-008x(90)90315-l. [DOI] [PubMed] [Google Scholar]
- 31.Hosmer DW, Lemeshow S. Applied logistic regression. Wiley; New York: 1989. [Google Scholar]
- 32.Draganov DI, Teiber JF, Speelman A, Osawa Y, Sunahara R, La Du BN. Human paraoxonases (PON1, PON2, and PON3) are lactonases with overlapping and distinct substrate specificities. J Lipid Res. 2005;46:1239–1247. doi: 10.1194/jlr.M400511-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.Yusuf PS, Hawken S, Ôunpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 34.Paragh G, Harangi M, Seres I. Effect of lipid lowering medications on PON1. In: Mackness B, Mackness M, Aviram M, Paragh G, editors. The paraoxonases: their role in diease development and xenobiotic metabolism. Springer; Dordrecht, The Netherlands: 2008. pp. 251–266. [Google Scholar]
- 35.Jarvik GP, Hatsukami TS, Carlson C, Richter RJ, Jampsa R, Brophy VH, et al. Paraoxonase activity, but not haplotype utilizing the linkage disequilibrium structure, predicts vascular disease. Arterioscler Thromb Vasc Biol. 2003;23:1465–1471. doi: 10.1161/01.ATV.0000081635.96290.D3. [DOI] [PubMed] [Google Scholar]
- 36.Jarvik GP, Rozek LS, Brophy VH, Hatsukami TS, Richter RJ, Schellenberg GD, et al. Paraoxonase (PON1) phenotype is a better predictor of vascular disease than is PON1(192) or PON1(55) genotype. Arterioscler Thromb Vasc Biol. 2000;20:2441–2447. doi: 10.1161/01.atv.20.11.2441. [DOI] [PubMed] [Google Scholar]