Abstract
BACKGROUND:
Polycystic ovary syndrome (PCOS) is a metabolic syndrome, characterized by anovulation, hyperandrogenism, and polycystic ovary. With serological markers of autoimmunity found elevated in PCOS, there is a possible link between autoimmunity and PCOS.
AIM:
The study aimed to investigate the possible correlation between autoimmune markers of autoimmune thyroiditis (AIT) and PCOS.
SETTING AND DESIGN:
This case control study was conducted at the Department of Pathology of a tertiary care academic center during a 1-year period.
MATERIALS AND METHODS:
Fifty-five subjects with clinical PCOS and 51 age matched control non-PCOS subjects were recruited and subjected to clinical, biochemical, and endocrinal evaluation for AIT. All subjects underwent blood glucose and serum sampling for luteinizing hormone (LH), follicle stimulating hormone (FSH), testosterone, dehydroepi androsterone, thyroxine, thyroid stimulating hormone, anti-thyroid peroxidase, anti-thyroglobulin (Tg), and insulin.
STATISTICAL ANALYSIS:
Statistical analysis was performed using SPSS version 12 for Windows. The quantitative variables are described as mean ± standard deviation. To compare quantitative variables between two groups, unpaired t-test was used. The Chi-square/Fischer's exact test was used to compare qualitative variables. ANOVA was used to compare the PCOS and non-PCOS groups. P < 0.05 was considered significant.
RESULTS:
Significantly higher prevalence of AIT (anti-Tg antibodies) was noted in subjects with PCOS as compared to non-PCOS control subjects (P < 0.05). The PCOS subjects had higher insulin resistance index and also twice the level of LH: FSH ratio as compared to controls.
CONCLUSION:
Higher prevalence of AIT in PCOS subjects suggest possible role of autoimmune phenomenon in the etiopathogenesis of PCOS. More data from longitudinal follow-up studies is required to clearly establish this possible link.
Keywords: Anti-thyroglobulin antibody, autoimmune disorders, autoimmune thyroiditis, polycystic ovarian syndrome
INTRODUCTION
Polycystic ovary syndrome (PCOS) is a metabolic syndrome, characterized by anovulation, hyperandrogenism, and polycystic ovary. PCOS exists commonly among women at reproductive age with an incidence rate of 6–10%.[1] The clinical manifestation of PCOS includes oligomenorrhea, infertility, acne, hirsutism, fat, and acanthosis nigricans and so on. In addition, these patients may develop many other related endocrine and metabolic diseases, and have increased risk of suffering endometrial cancer, impaired glucose tolerance, diabetes, and cardiovascular disease.[2,3] Research about the pathogenesis of PCOS mainly focuses on two interrelated metabolic elements-insulin resistance (IR) and hyperandrogenism.[4] Nevertheless, pathogenesis of PCOS still remains unclear. Recently, several researchers suggested the relationship between PCOS and autoimmunity with controversial results, which showed that serologic markers of autoimmunity elevated in patients with PCOS.[5] Gleicher et al. hypothesized that functional autoantibodies could contribute to the development of PCOS, which represents hyperfunction of follicular recruitment in the ovaries, similar to hyperthyroidism in Graves' disease.[6] Hence, can we regard PCOS as an autoimmune disease?
Autoimmune thyroiditis (AIT), or named Hashimoto's thyroiditis, or chronic lymphocytic thyroiditis (CLT), is the most prevalent autoimmune state that affects up to 5–20% of women during the age of fertility[7] which is due to chronic inflammation of the thyroid and can lead to hypothyroidism finally. Most of the AIT patients show positivity for anti-thyroid peroxidase (TPO) and/or anti-Tg and atypical hypoechogenic pattern in ultrasound (USG) scan.[8,9] The link between AIT and PCOS has been reported in several studies, but its true pathogenesis is far from being elucidated. IR/hyperinsulinemia in PCOS being the main pathogenic defect is also supported by clinical, biochemical, and hormonal response to insulin sensitizers.[10,11] With adipose tissue being one of the important sources of pro-inflammatory cytokines, IR in obese women has been attributed to elevation of adipocytokines and other inflammatory markers. Women with PCOS have been demonstrated to have elevated cytokines such as tumor necrosis factor-alpha (TNF-a) and interleukin (IL)-6 concentrations independent of obesity, and these alterations in serum inflammatory markers in PCOS are unrelated to peroxisome proliferator-activated receptor-gamma variants.[12] It has been demonstrated that the therapy with metformin in PCOS leads to an increase in plasma adiponectin and TNF-α but not plasma IL-6 and C-reactive protein.[13] Some authors demonstrated a high prevalence of AIT in PCOS women suggesting a potential pathogenic link with autoimmunity/sub-inflammatory cascade.[14] Thus, the concept that inflammatory and immune markers may have a role in the pathogenesis of IR and hyperinsulinemia in PCOS has taken a front seat.
CLT is the most prevalent cause of hypothyroidism in areas with sufficient iodine intake, and is characterized by high levels of thyroid autoantibodies, lymphocytic infiltration prevalent cause of hypothyroidism in areas with of the thyroid gland, and a typical hypoechoic pattern on thyroid USG. High prevalence of CLT in Down's, and Turner's syndrome[15] and its association with the HLA DR3, DR5, and HLADRB1*1404 genes[16,17] suggests that CLT may have a genetic contribution. Similarly, the evidence of a strong familial association in the first-degree relatives of PCOS subjects suggests a role of genetics in its etiopathogenesis, and several genes have been identified to date.[18,19,20] Since both these disorders have a similar pathogenic mechanism comprising of familial aggregation, subinflammation/inflammation, and elevated adipocytokines, we hypothesize that there may be a potential cross-link between the two conditions.
MATERIALS AND METHODS
Every consecutive female patient in the age group of 13–45 years with hirsutism and menstrual abnormality (amenorrhea or oligomenorrhea) visiting the Outpatient Departments of Department of Gynaecology during a 1-year period in 2013–2014 was screened for this study. The Institutional Ethical Committee approved the study. Subjects were briefed about the study and informed consent was obtained. The Rotterdam classification was used to define PCOS in the event of: (1) Menstrual anomalies such as amenorrhea (no cycles in the past 6 months), oligomenorrhea (cycles lasting longer than 35 days), or long cycles, (2) clinical and/or biochemical hyperandrogenism, and (3) USG appearance of polycystic ovaries (multiple cysts > 12 in number of 2–9 mm size). The presence of two of these three criteria was required to diagnose PCOS, once all other diagnosis, like congenital adrenal hyperplasia, virilizing tumor, Cushing syndrome, and prolactinoma was ruled out.[21] Clinical hyperandrogenism was defined as hirsutism (Ferriman–Gallwey score > 7) and/or acne, and/or androgenic pattern of alopecia.[22,23]
Biochemical hyperandrogenemia was defined by elevated testosterone (>1.2 ng/ml). A luteinizing hormone (LH)-to-follicle stimulating hormone (FSH) ratio above 2 was considered elevated. Transabdominal pelvic USG was performed to detect the presence of cystic ovaries. Other reasons for hyperandrogenism were excluded by adrenocorticotropin-stimulated 17-OH progesterone, dexamethasone suppression test and/or 24-h urine cortisol excretion. Women of the same age group visiting the outpatient clinics with problems unrelated to PCOS or thyroid dysfunction and with normal menstrual cycle served as control population. Subjects suffering from any systemic illness and those on any medications known to interfere with thyroid function, hypothalamic–pituitary–adrenal axis, insulin sensitivity, or glucose tolerance were excluded from the study.
Detailed clinical history, elaborate clinical examination and laboratory investigations such as blood glucose (fasting and 2 h post 75 g glucose), serum LH, FSH, thyroid stimulating hormone (TSH), thyroxine levels (T3 and T4), anti-TPO antibody, anti-thyroglobulin (Tg), serum testosterone were measured by an automated immune-enzyme assay systems, performed in both PCOS and control population. The blood samples were drawn on the 2nd–3rd day of the menstrual cycle.
Normal serum levels of different hormones and peptide were defined as (1) T3 - 0.6–2.02 ng/ml, (2) T4 - 4.4–11.6 ng/dl, (3) TSH - 0.4–6.2 mIU/ml, (4) anti-TPO taken antibody as 7.6–27 IU/ml, (5) anti-Tg < 125 IU/ml, (6) prolactin - 1.9–25 ng/ml, (7) 17-OH progesterone - 15–70 ng/dl, (8) free testosterone - 3–19 pg/ml, (9) LH - 0.8–10.5 IU/ml, and(10) FSH - 5–20 mIU/ml. LH and FSH were measured on the 2nd day of menstruation.
Statistical analysis was performed using IBM SPSS version for Windows. The quantitative variables are described as mean ± standard deviation. To compare quantitative variables between two groups, unpaired t-test was used. The Chi-square/Fischer's exact test was used to compare qualitative variables. ANOVA was used to compare the PCOS and non-PCOS groups. P < 0.05 was considered significant.
RESULTS
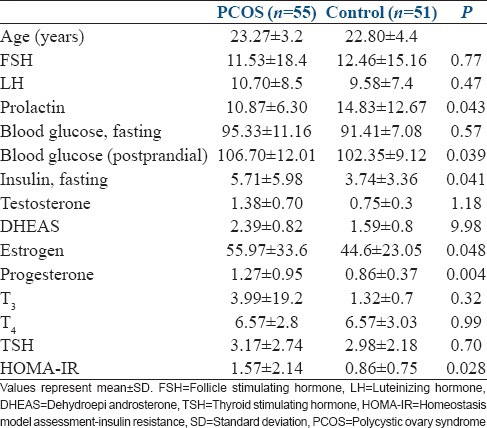
Clinical, biochemical, and hormonal parameters were screened in the patients with PCOS and in the healthy control subjects [Table 1]. We studied 55 patients with PCOS (mean age 23.27 ± 5.83 years, range 18–33 years) and 51 age matched healthy controls (mean age 22.80 ± 4.4 years, range 18–32 years).
Table 1.
Comparative table between subjects with polycystic ovary syndrome and subjects without polycystic ovary syndrome
The study showed that the mean anti-TPO level was higher in PCOS patients in comparison to controls (49.54 ± 136.9 vs. 22.63 ± 38.5) although it was not statistically significant [Figure 1]. Anti-Tg level was significantly higher in PCOS patients (P = 0.004) in comparison to controls [Figure 2]. Serum prolactin was higher in the non-PCOS subjects (P < 0.05). As expected, the metabolic measures of blood glucose and fasting insulin levels were both significantly higher in subjects with PCOS. More number of subjects with PCOS had higher LH: FSH ratio as compared to control subjects. Serum estrogen and progesterone were significantly higher in PCOS subjects (P < 0.05) although the difference was much more pronounced in the case of progesterone levels.
Figure 1.
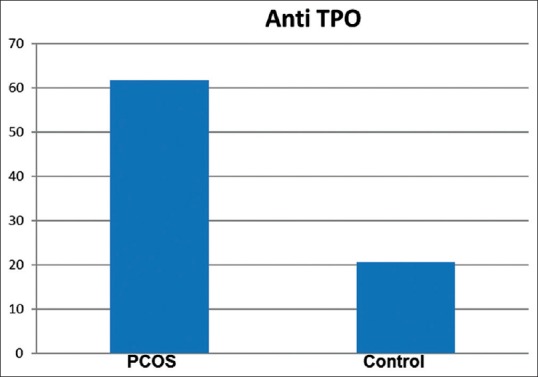
Anti-thyroid peroxidase levels (IU/ml) in polycystic ovary syndrome group and control group
Figure 2.
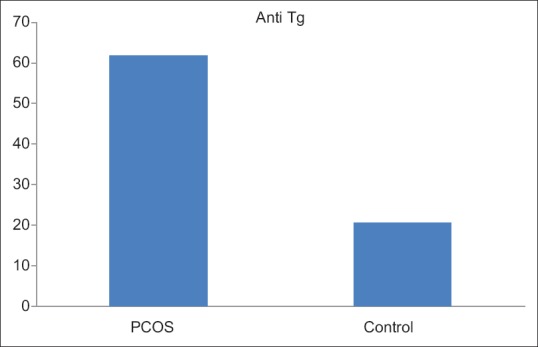
Anti-thyroglobulin levels (IU/ml) in polycystic ovary syndrome group and control group
IR as calculated by homeostasis model assessment (HOMA)-IR was also significantly higher (P = 0.028) in PCOS subjects as compared to non-PCOS subjects.
DISCUSSION
AIT represents the most common endocrine disorders in women at reproductive age. AIT, with a prevalence varying from 5% to 15% is 5–10 times more common in women than men. This might be explained by genetic factors, the effect of estrogen and perhaps chromosome X abnormalities. In addition, what makes AIT often ignored is that it may be present without thyroid dysfunction for many years[24] and result in hypothyroidism much later. Although there seems to be no correlations between the underlying causes of hypothyroidism and PCOS, these two diseases have many characteristics in common, such as chronic anovulation, decreased serum sex hormone binding globulin, increased serum testosterone, increased LH, and cholesterol.[25] Furthermore, a strong interaction between thyroid and ovary is implied by many in vitro researches, both in humans and animals. As human chorionic gonadotropin has a TSH-like effect,[26] thyroid activity affects the functionality of the reproductive axis[27] and TSH has been reported highly elevated in ovarian hyperstimulation syndrome[28] and PCOS patients.
As seen in our study, there was significantly higher prevalence of thyroid autoantibodies in patients with PCOS in comparison to the controls. Our study showed that anti-Tg and anti-TPO antibodies were significantly raised in PCOS patients (P = 0.004). 37.72% of PCOS patients have raised levels of either or both anti-TPO and/or anti-Tg antibody level, while 15.6% controls showed raised levels of either or both, which although were not statistically significant but nevertheless signify an important trend. Our results were consistent with other studies, for example, Janssen et al.[14] found 26.9% and Ozdemir et al.[29] found the prevalence of 30.5% of autoantibodies in PCOS subjects.
These two apparently diverse disorders with familial aggregation and genetic predisposition show high prevalence of other serologic parameters of autoimmunity (anti-histone and anti-double stranded DNA), which further suggest the role of autoimmunity in the pathogenesis of PCOS. Similar findings supporting a potential link between PCOS and autoimmunity have been reported by another study.[30] In our study TSH, T3 and T4 did not differ significantly between patients with PCOS and controls, i.e., most of our patients were euthyroid.
Elevated fasting insulin and HOMA-IR, indicative of significantly higher IR in our PCOS group compared to controls, may indicate some pathogenic link between autoimmunity and IR.[15,16] The association of IR and hyperinsulinemia of PCOS with elevation of various cytokines such as IL-4, IL-6, TNF-α, and their alteration after treatment with insulin sensitizers is well established.[12,13]
It may be speculated that the imbalance of normal to high estrogens and low progesterone levels, the so called “unopposed estrogens” thought to be responsible for the apparent increase in prevalence of AIT during the menopause,[31] also accounts for the higher prevalence of AIT in PCOS. Estrogens are known to increase IL-4 expression in TH2 cells, IL-1 in monocytes, IL-6 in T-cells, and interferon gamma in TH1 cells. During normal menstrual cycles in young women, IL-6 is elevated in the follicular phase and decreased in the luteal phase, and inversely correlated to progesterone levels.[31] Hence, the immune stimulatory activity of estrogens seems to be counteracted by progesterone. In our patients also, estrogen levels were significantly high in patients in comparison to controls. Hence, measures aimed to re-establish ovulatory cycles would be likely to prevent the development of AIT in PCOS patients.
The presence of thyroid antibodies in euthyroid women has been reported to be associated with an adverse outcome in an in vitro fertilization – embryo transfer program.[32] Furthermore, hypothyroidism worsens PCOS by further decreasing sex hormone binding globulin levels, increasing the conversion of androstenedione to testosterone and aromatization to estradiol and reducing the metabolic clearance rates of androstenedione and estrone.[33] Correction of hypothyroidism, when present, would certainly form an important aspect in the management of PCOS.
CONCLUSION
This study demonstrates higher prevalence of AIT in PCOS. Our data suggest that all patients with PCOS should be screened for thyroid function and thyroid-specific autoantibodies even without evidence of overt thyroid dysfunction, as it is known that patients with TPO and Tg autoantibodies are likely to develop thyroid dysfunction later in life. We do not yet know whether AIT predisposes subjects to develop PCOS or if PCOS is a forerunner of AIT and requires more evidence base in form of follow-up longitudinal studies.
Limitations
Our study had several limitations. First, the sample size was low and the power of the study was affected due to this. Second, longitudinal follow-up was not done so the reliability of the laboratory results over time was not established. Third, blinding was not done between subjects and controls, which may have resulted in researcher bias.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
Acknowledgment
The authors acknowledge the assistance and material provided by Mr. MS Rana, Senior Technician, Department of Pathology, Safdarjung Hospital and Vardhaman Mahavir Medical College. The authors also express their gratitude to Dr. Manushree Gupta, Assistant Professor, Department of Psychiatry, Govind Ballabh Pant Post Graduate Institute of Medical Education and Research, New Delhi for statistical analysis and in preparation of this manuscript.
REFERENCES
- 1.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–9. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 2.Wild RA, Carmina E, Diamanti-Kandarakis E, Dokras A, Escobar-Morreale HF, Futterweit W, et al. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: A consensus statement by the androgen excess and polycystic ovary syndrome (AE-PCOS) society. J Clin Endocrinol Metab. 2010;95:2038–49. doi: 10.1210/jc.2009-2724. [DOI] [PubMed] [Google Scholar]
- 3.Fux Otta C, Fiol de Cuneo M, Szafryk de Mereshian P. Polycystic ovary syndrome: Physiopathology review. Rev Fac Cien Med Univ Nac Cordoba. 2013;70:27–30. [PubMed] [Google Scholar]
- 4.Buccola JM, Reynolds EE. Polycystic ovary syndrome: A review for primary providers. Prim Care. 2003;30:697–710. doi: 10.1016/s0095-4543(03)00089-7. [DOI] [PubMed] [Google Scholar]
- 5.Hefler-Frischmuth K, Walch K, Huebl W, Baumuehlner K, Tempfer C, Hefler L. Serologic markers of autoimmunity in women with polycystic ovary syndrome. Fertil Steril. 2010;93:2291–4. doi: 10.1016/j.fertnstert.2009.01.056. [DOI] [PubMed] [Google Scholar]
- 6.Gleicher N, Barad D, Weghofer A. Functional autoantibodies, a new paradigm in autoimmunity? Autoimmun Rev. 2007;7:42–5. doi: 10.1016/j.autrev.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Artini PG, Uccelli A, Papini F, Simi G, Di Berardino OM, Ruggiero M, et al. Infertility and pregnancy loss in euthyroid women with thyroid autoimmunity. Gynecol Endocrinol. 2013;29:36–41. doi: 10.3109/09513590.2012.705391. [DOI] [PubMed] [Google Scholar]
- 8.Cooper DS. Clinical practice. Subclinical hypothyroidism. N Engl J Med. 2001;345:260–5. doi: 10.1056/NEJM200107263450406. [DOI] [PubMed] [Google Scholar]
- 9.Dayan CM, Daniels GH. Chronic autoimmune thyroiditis. N Engl J Med. 1996;335:99–107. doi: 10.1056/NEJM199607113350206. [DOI] [PubMed] [Google Scholar]
- 10.Nieuwenhuis-Ruifrok AE, Kuchenbecker WK, Hoek A, Middleton P, Norman RJ. Insulin sensitizing drugs for weight loss in women of reproductive age who are overweight or obese: Systematic review and meta-analysis. Hum Reprod Update. 2009;15:57–68. doi: 10.1093/humupd/dmn043. [DOI] [PubMed] [Google Scholar]
- 11.Ganie MA, Khurana ML, Eunice M, Gupta N, Gulati M, Dwivedi SN, et al. Comparison of efficacy of spironolactone with metformin in the management of polycystic ovary syndrome: An open-labeled study. J Clin Endocrinol Metab. 2004;89:2756–62. doi: 10.1210/jc.2003-031780. [DOI] [PubMed] [Google Scholar]
- 12.Tarkun I, Cetinarslan B, Turemen E, Canturk Z, Biyikli M. Association between circulating tumor necrosis factor-alpha, interleukin-6, and insulin resistance in low grade inflammatory serum markers in patients with polycystic ovary syndrome (PCOS) and their relationship to PPAR gamma gene variants. Exp Clin Endocrinol Diabetes. 2008;116:481–6. [Google Scholar]
- 13.Jakubowska J, Bohdanowicz-Pawlak A, Milewicz A, Szymczak J, Bednarek-Tupikowska G, Demissie M. Plasma cytokines in obese women with polycystic ovary syndrome, before and after metformin treatment. Gynecol Endocrinol. 2008;24:378–84. doi: 10.1080/09513590802128968. [DOI] [PubMed] [Google Scholar]
- 14.Janssen OE, Mehlmauer N, Hahn S, Offner AH, Gärtner R. High prevalence of autoimmune thyroiditis in patients with polycystic ovary syndrome. Eur J Endocrinol. 2004;150:363–9. doi: 10.1530/eje.0.1500363. [DOI] [PubMed] [Google Scholar]
- 15.Idris I, O'Malley BP. Thyrotoxicosis in Down's and Turner's syndromes: The likelihood of Hashimoto's thyroiditis as the underlying aetiology. Int J Clin Pract. 2000;54:272–3. [PubMed] [Google Scholar]
- 16.Marwaha RK, Sen S, Tandon N, Sahoo M, Walia RP, Singh S, et al. Familial aggregation of autoimmune thyroiditis in first-degree relatives of patients with juvenile autoimmune thyroid disease. Thyroid. 2003;13:297–300. doi: 10.1089/105072503321582114. [DOI] [PubMed] [Google Scholar]
- 17.Kanga U, Tandon N, Marwaha RK, Goswami D, Gupta N, Ray D, et al. Immunogenetic association and thyroid autoantibodies in juvenile autoimmune thyroiditis in North India. Clin Endocrinol. 2006;64:573–9. doi: 10.1111/j.1365-2265.2006.02511.x. [DOI] [PubMed] [Google Scholar]
- 18.Crosignani PG, Nicolosi AE. Polycystic ovarian disease: Heritability and heterogeneity. Hum Reprod Update. 2001;7:3–7. doi: 10.1093/humupd/7.1.3. [DOI] [PubMed] [Google Scholar]
- 19.Franks S, Gharani N, McCarthy M. Candidate genes in polycystic ovary syndrome. Hum Reprod Update. 2001;7:405–10. doi: 10.1093/humupd/7.4.405. [DOI] [PubMed] [Google Scholar]
- 20.Urbanek M, Legro RS, Driscoll DA, Azziz R, Ehrmann DA, Norman RJ, et al. Thirty-seven candidate genes for polycystic ovary syndrome: Strongest evidence for linkage is with follistatin. Proc Natl Acad Sci U S A. 1999;96:8573–8. doi: 10.1073/pnas.96.15.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dewailly D, Hieronimus S, Mirakian P, Hugues JN. Polycystic ovary syndrome (PCOS) Ann Endocrinol (Paris) 2010;71:8–13. doi: 10.1016/j.ando.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961;21:1440–7. doi: 10.1210/jcem-21-11-1440. [DOI] [PubMed] [Google Scholar]
- 23.Carmina E, Lobo RA. A comparison of the relative efficacy of antiandrogens for the treatment of acne in hyperandrogenic women. Clin Endocrinol (Oxf) 2002;57:231–4. doi: 10.1046/j.1365-2265.2002.01594.x. [DOI] [PubMed] [Google Scholar]
- 24.Poppe K, Velkeniers B, Glinoer D. Thyroid disease and female reproduction. Clin Endocrinol (Oxf) 2007;66:309–21. doi: 10.1111/j.1365-2265.2007.02752.x. [DOI] [PubMed] [Google Scholar]
- 25.Muderris II, Boztosun A, Oner G, Bayram F. Effect of thyroid hormone replacement therapy on ovarian volume and androgen hormones in patients with untreated primary hypothyroidism. Ann Saudi Med. 2011;31:145–51. doi: 10.4103/0256-4947.77500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mintziori G, Anagnostis P, Toulis KA, Goulis DG. Thyroid diseases and female reproduction. Minerva Med. 2012;103:47–62. [PubMed] [Google Scholar]
- 27.Mutinati M, Rizzo A, Sciorsci RL. Cystic ovarian follicles and thyroid activity in the dairy cow. Anim Reprod Sci. 2013;138:150–4. doi: 10.1016/j.anireprosci.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 28.Katulande P, Kariyawasam SS, Senanayake HM, Weerakkodi M. Multicystic ovaries and pituitary pseudo-adenoma associated with primary hypothyroidism. J Obstet Gynaecol. 2013;33:17–9. doi: 10.3109/01443615.2011.565388. [DOI] [PubMed] [Google Scholar]
- 29.Ozdemir D, Cuhaci N, Balkan F, Usluogullari A, Ersoy R, Cakir B. Prevalence of thyroid pathologies in patients with polycystic ovary syndrome. Eur Congr Endocrinol. 2011;26:92. [Google Scholar]
- 30.Reimand K, Talja I, Metsküla K, Kadastik U, Matt K, Uibo R. Autoantibody studies of female patients with reproductive failure. J Reprod Immunol. 2001;51:167–76. doi: 10.1016/s0165-0378(01)00075-4. [DOI] [PubMed] [Google Scholar]
- 31.Paavonen T. Hormonal regulation of immune responses. Ann Med. 1994;26:255–8. doi: 10.3109/07853899409147900. [DOI] [PubMed] [Google Scholar]
- 32.Angstwurm MW, Gärtner R, Ziegler-Heitbrock HW. Cyclic plasma IL-6 levels during normal menstrual cycle. Cytokine. 1997;9:370–4. doi: 10.1006/cyto.1996.0178. [DOI] [PubMed] [Google Scholar]
- 33.Bussen S, Steck T, Dietl J. Increased prevalence of thyroid antibodies in euthyroid women with a history of recurrent in-vitro fertilization failure. Hum Reprod. 2000;15:545–8. doi: 10.1093/humrep/15.3.545. [DOI] [PubMed] [Google Scholar]


