Abstract
BACKGROUND:
Serum estradiol (E2) levels are measured in in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI), to assess the ovarian response and to predict ovarian hyperstimulation syndrome. The impact of peak E2 levels on IVF-ICSI outcome was found to be inconsistent in the previous studies.
AIM:
To evaluate the impact of the serum E2 levels on the day of ovulation trigger with the reproductive outcome of ICSI.
SETTINGS AND DESIGN:
Retrospective observational study. ART Center, at a Tertiary Care University Teaching Hospital.
SUBJECTS AND METHODS:
Eighty-nine infertile women, who underwent ICSI with fresh embryo transfer over a period of 3 years, were included in the study. The study subjects were grouped based on the serum E2 level on the day of ovulation trigger:- Group I - <1000 pg/ml, Group II - 1000–2000 pg/ml, Group III – 2000.1-3000 pg/ml, Group IV – 3000.1–4000 pg/ml, and Group V >4000 pg/ml. The baseline characteristics and controlled ovarian hyperstimulation (COH) outcome were compared among the study groups.
STATISTICAL ANALYSIS USED:
Chi-square test, Student's t-test, ANOVA, and logistic regression analysis.
RESULTS:
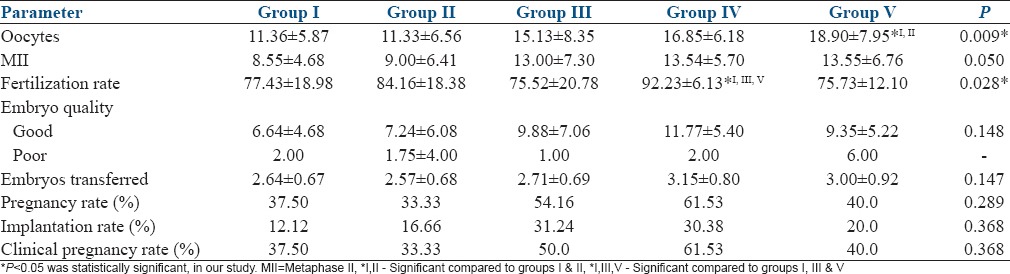
The study groups were comparable with regard to age, body mass index, ovarian reserve. Group V had significantly higher number of oocytes retrieved than I and II (18.90 vs. 11.36 and 11.33; P = 0.009). Group IV showed significantly higher fertilization rate than I, III, and V; (92.23 vs. 77.43, 75.52, 75.73; P = 0.028). There were no significant differences in the implantation rates (P = 0.368) and pregnancy rates (P = 0.368).
CONCLUSION:
Higher E2 levels on the day of ovulation trigger would predict increased oocyte yield after COH. E2 levels in the range of 3000–4000 pg/ml would probably predict increased fertilization and pregnancies in ICSI cycles.
Keywords: Embryo quality, estradiol, fertilization, hCG, implantation, intracytoplasmic sperm injection, oocyte, ovulation trigger, pregnancy
INTRODUCTION
Estradiol (E2) or 17β E2 is the primary female sex steroid secreted mainly from the granulosa cells of the ovarian follicle. It is secreted in the follicular phase of the menstrual cycle, peaks at the time of ovulation before luteinizing hormone surge and declines and plateaus thereafter.[1] Measurement of E2 in in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) cycles would be helpful to assess the ovarian response and prediction of ovarian hyperstimulation syndrome (OHSS). It is interesting to note that numerous studies have been performed to assess the impact of E2 levels on the day of human chorionic gonadotropin (hCG) on IVF-ICSI outcome. The results of those studies were heterogenous. Some studies have reported that higher values adversely affected endometrial receptivity, whereas other studies showed no significant effect.[2,3,4,5] Higher E2 level on the day of hCG was found to have a positive impact on embryo quality, but extremely high levels were found to affect embryo quality, implantation, and pregnancy by modulation of leptin levels in the follicular fluid.[5,6] Interestingly, medium cumulative E2 exposure during controlled ovarian hyperstimulation (COH) was found to have significantly higher number of pregnancies than low and high exposure groups.[7] Significantly higher pregnancies reported after cryo-embryo transfer (CET) compared to fresh ET, have been attributed due to low exposure to steroid hormones, such as E2.[8] The present study is aimed to assess the impact of E2 levels on the day of ovulation trigger by hCG, on ICSI outcome viz., oocyte yield, fertilization, embryo quality, implantation, and pregnancy.
SUBJECTS AND METHODS
Data collection
Data of the infertile couple who underwent ICSI with fresh embryo transfer (ET), from May 2011 to June 2014, in our ART clinic, at a tertiary care hospital, were included in the study. Data were collected from the medical records department. Of the 222 patients, 183 cases were excluded due to lack of proper data, E2 assay not performed on the day of ovulation trigger, donor-recipient cycles, oocyte sharing cycles, patients in whom fresh ET was withheld due to increased serum progesterone on the day of hCG, OHSS or increased risk of OHSS, agonist-triggered cycles, and thin endometrium.
Controlled ovarian hyperstimulation
COH was performed using long agonist protocol or antagonist protocol, individualized depending on the patient characteristics. In long agonist protocol, leuprolide acetate 3.75 mg depot was given on D21 of periods or after 15 days of combined oral contraceptive pills (OCPs) containing 0.03 mg Ethinyl estradiol + 0.15 mg Levonorgestrel. Gonadotropins were started after 3 weeks of GnRH depot administration. In antagonist protocol, OCPs were administered from 2nd or 3rd day of the previous cycle for 21 days. The gonadotropins used were mainly recombinant follicle-stimulating hormone (FSH). Human menopausal gonadotropin (HMG) was added from day one or later in case of expected poor response, poor initial response, or tardy follicular growth. The doses of gonadotropins were decided according to the individual patient characteristics, in both the protocols. The follicular study was done by transvaginal ultrasound with 6 MHz probe, starting from D6 of COH and every 2 days or daily, thereafter. In antagonist protocol, cetrorelix 0.25 mg was administered by flexible regimen, when lead follicle reached 1.4 cm and/or E2 levels >300 pg/ml on D6 of COH. Ovulation was triggered by 250 mcg of recombinant hCG when at least two lead follicles have reached ≥2.0 cm. Serum E2 was estimated by chemiluminescence assay, on the morning of the day of ovulation trigger. Oocyte retrieval was performed after 35–36 h of ovulation trigger.
Intracytoplasmic sperm injection
Oocytes retrieved were collected in the Quinns advantage medium with HEPES, and examined under the stereomicroscope, for quality and maturity. Denudation was performed by 0.5 ml (80 IU) hyase. ICSI was performed in all the cycles, within 2–4 h of egg collection. The culture medium used was Quinns advantage cleavage medium. Embryos were examined on D2 of culture for cleavage and embryo quality. Embryo grading was performed by ASEBIR embryo assessment criteria.[9] All the patients underwent fresh ET on D2 (majority) or D3 of embryo culture.
Analysis
The study subjects were divided into five groups based on the serum E2 level on the day of ovulation trigger; Group I - <1000 pg/ml, Group II - 1000–2000 pg/ml, Group III – 2000.1–3000 pg/ml, Group IV – 3000.1–4000 pg/ml, and Group V - >4000 pg/ml. The demographic parameters such as age, body mass index (BMI), duration of infertility, indication for ART, were compared among these five study groups. The results of ovarian reserve tests, COH protocol, duration of stimulation, and gonadotropin requirement, were compared among these five study groups. The outcome parameters of ICSI viz., oocytes retrieved, M-II oocytes, fertilization rate, embryo quality, implantation rates, pregnancies, and clinical pregnancies, were compared among these five groups.
Pregnancy was defined as serum β-hCG ≥10 mIU/ml, on the 14th day after ET. Clinical pregnancy was defined as the presence of atleast a single intrauterine gestational sac visualized by transvaginal ultrasound, at 7 weeks gestation. Implantation was defined as the number of intrauterine gestational sacs seen by transvaginal ultrasound at 7 weeks gestation. Statistical analysis is performed by SPSS Version 13 software (IBM Corporation, Armonk, New York, U.S.). Chi-square tests, Student's t-test, ANOVA, and logistic regression analyses were used to analyze our data.
Ethical clearance was not required for retrospective studies, in our institute.
RESULTS
There was no significance in the demographic characteristics such as age, BMI, duration of infertility, and the indications of ART among the five study groups. There was a significant variation in the BMI of the study subjects, with the highest mean BMI of the subjects in Group I [Table 1]. There was no significant difference in the ovarian reserve assessed by FSH, E2, and Antral follicle count (AFC), among the subjects of the five study groups [Table 2]. There was no significant difference in the distribution of agonist and antagonist protocols followed for COH, among the study groups. The mean duration of stimulation and gonadotropin requirement did not significantly differ among the five study groups [Table 3]. The number of oocytes retrieved was highest in Group V, significantly higher than Groups I and II. The number of mature oocytes was highest in Group V compared to other groups, but did not reach statistical significance. Fertilization rate was highest in Group IV, significantly higher than Groups I, III, and V. There was no significant difference between the good quality and poor quality embryos obtained, among the five groups. The pregnancy rate of the whole study population was 43.82%. There was no significant difference between implantation rate and the number of pregnancies obtained among the five groups [Table 4]. However, the pregnancy rate was highest in Group IV. There were four OHSS cases in our study, two in Group II, and two in Group IV. The OHSS rate in our study was 4.4%.
Table 1.
Comparison of the demographic characteristics of the study subjects
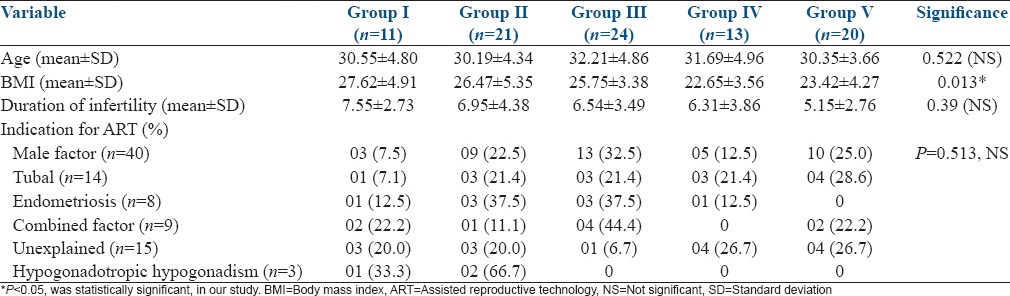
Table 2.
Comparison of ovarian reserve tests among the study groups
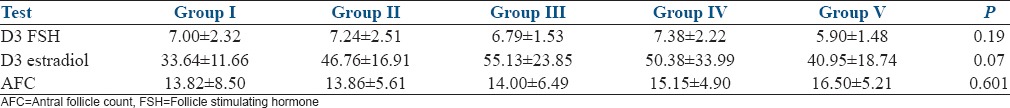
Table 3.
Comparison of controlled ovarian hyperstimulation characteristics among the study groups
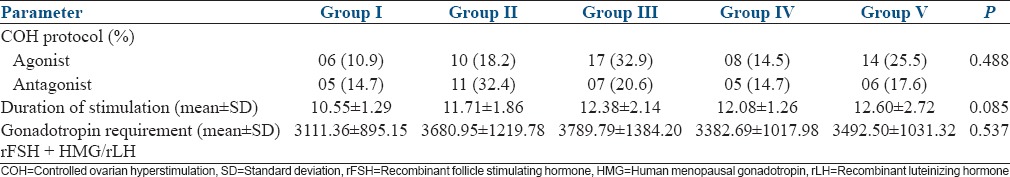
Table 4.
Comparison of controlled ovarian hyperstimulation outcome among the study groups
DISCUSSION
In our study, there was significantly higher BMI in Group I, with E2 <1000 pg/ml. Rehman et al. did a prospective study on the effect of E2 levels on pregnancy outcome in ICSI. That study concluded that overweight women with BMI ≥26 Kg/m2 had significantly decreased E2 levels on the day of hCG. That finding was similar to our study.[10] There were no significant differences in other demographic parameters, such as age, duration of infertility, indications of ART, among the study groups.
Estradiol levels and the number of oocytes retrieved
Pena et al. did a retrospective study on the association of supraphysiological E2 levels and embryo quality in 332 consequent fresh oocyte donation cycles.[11] They concluded that E2 levels >3000 pg/ml had significantly higher number of oocytes retrieved than other groups with lower E2 levels, of 1500–3000 pg/ml and <1500 pg/ml: 26.2 ± 0.8 versus 16.2 ± 0.5 and 10 ± 0.5, respectively. Wu et al. did a retrospective study on serum E2 levels on the day of hCG and IVF outcome, in 274 IVF cycles. In that study, there were significantly higher oocytes retrieved in the group with E2 levels >5000 pg/ml (27.3 ± 10), than other groups.[12] Kara et al. did a retrospective study on the E2 levels on the day of hCG and ICSI outcome, in 203 cycles. In that study, it was observed that women who had E2 levels >4000 pg/ml on the day of hCG, had significantly higher number of number of oocytes retrieved (17.2 ± 4.4) compared to other groups.[13] Joo et al. did a retrospective study of E2 levels on the day of hCG and IVF outcome, in 455 fresh IVF-ET cycles. In that study, it was observed that significantly higher number of oocytes were retrieved in the group with serum E2 levels >4000 pg/ml (15.3 ± 6.6), compared to other groups.[14] Similar results were obtained in our study.
Estradiol levels and fertilization
Pena et al. showed that there was no significant difference in the fertilization rates between the group with E2 levels >3000 pg/ml compared to other groups (E2 levels 1500–3000 pg/ml and <1500 pg/ml): 59% versus 59 and 60%, respectively.[11] Mittal et al. did a retrospective study on prediction of IVF success by serum E2 levels on the day of hCG, in 342 IVF cycles. In this study, the subjects were grouped by serum E2 levels per mature follicle and compared. There was no significant difference in the fertilization rates between the study groups (72.41 ± 24.8%).[15] Kara et al. observed a mean fertilization rate of 55.7 ± 24.8%, and did not find a significant difference in the fertilization rates between the study groups.[13] Wu et al. observed no significant difference in the fertilization rates among the five study groups.[12] Valbuena et al. reviewed data on ovarian stimulation and endometrial receptivity. In that review, it was reported that there was no effect of serum E2 levels on the day of hCG and fertilization rate.[16] In our study, fertilization rate was highest in Group IV, significantly higher than Groups I, III, and V. This finding was not reported in any of the studies, prior. However, further large studies with less confounding variables, are required to confirm this finding.
Estradiol levels and embryo quality
Kyrou et al. did a prospective study on the impact of E2 levels on the day of hCG on pregnancy rates in IVF-ICSI, in 207 subjects. In his study, there was significantly higher total embryo score (12 ± 12.6) of the embryos in 75th centile E2 group (>2446 pg/ml) than 25th and 50th centile groups.[5] Mittal et al. found no effect of E2/mature follicle on the day of hCG and the number of Grade I embryos obtained.[15] Pena et al. observed significantly higher average embryo score in groups with E2 levels on the day of hCG of 1500–3000 pg/ml (30.2 ± 0.8) and > 3000 pg/ml (31.2 ± 0.8), compared to that of < 1500 pg/ml (27.1 ± 1.2).[11] In our study, we had not obtained any significant difference between the number of good and poor quality embryos between the study groups.
Estradiol levels and pregnancy
Joo et al. reported higher clinical pregnancy rates in the group of peak E2 levels 3000–4000 pg/ml (50%), compared to <1000 pg/ml (22.20%) and 1000–2000 pg/ml (32%).[14] The implantation rate was 12.2%; there was no significant difference in the implantation rate between the study groups. Kara et al. reported implantation and pregnancy rates of 9.0 ± 19.2% and 21 ± 41%, respectively. There were no significant differences between implantation and pregnancy rates between the study groups sratified according to peak E2 levels.[13] Mittal et al. reported significantly increased number of clinical pregnancies in the study group with E2/mature follicle of 200–299.99 pg/ml (clinical pregnancy rate of 32.95%), compared to other groups. The mean serum E2 in that group was 4762 ± 2722 pg/ml.[15] Kyrou et al. reported no significant difference in the implantation and ongoing pregnancy rates between the percentile groups of E2 on the day of hCG.[5] Tao et al. did a study on the effects of E2 level and E2/follicle ratio on IVF pregnancy outcome. In a retrospective analysis of 79 cycles, the authors concluded that higher E2 levels on the day of hCG trigger were associated with significantly higher implantation and clinical pregnancy rates.[17] Pena et al. reported significantly higher implantation rate in the recipients whose donors had an E2 level >3000 pg/ml on the day of hCG, compared to those who had E2 level of <1500 pg/ml (24.5% vs. 17.4%).[11] However, there was no difference in the chemical pregnancy rates and clinical pregnancy rates among the study groups. Wu et al. reported no significant difference in the pregnancy and implantation rates between the study groups. There was no association of E2 on the day of ovulation trigger and pregnancy and implantation, in that study.[12] Soni et al. reported significantly higher chemical and clinical pregnancies (70% and 57.5% respectively), in the group with E2 level of 1000-2500 pg/ml on the day of hCG compared to other groups.[18] Valbuena et al. reviewed data showed a significant decrease in pregnancies and implantation, in the groups with E2 level on the day of hCG of ≥2500 pg/ml, compared to 1500–2000 pg/ml group.[16] Kosmas et al. reviewed the data on the E2 level on the day of hCG and pregnancy achievement in IVF.[19] Their review concluded that there was no positive correlation between the E2 levels on the day of hCG and pregnancy in IVF. In our study, there was no difference in implantation and pregnancy rates among the study groups. Logistic regression analysis was performed to find the confounding factors affecting the association of E2 levels on the day of hCG and pregnancy and implantation. We had found that there was no significant confounding of any of the factors such as age, BMI, ovarian reserve, COH protocol, duration of stimulation and number of embryos transferred, on those associations.
CONCLUSION
Higher serum E2 level on the day of ovulation trigger is associated with increased oocyte yield. Peak E2 levels between 3000 and 4000 pg/ml seem to be associated with increased fertilization and higher pregnancies. Further large studies are required to confirm these findings.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
Acknowledgment
Mrs. Manjula D, Chief Embryologist, Department of Reproductive Medicine, SRMC
Mr. Sudhakar C, Assistant Embryologist, Department of Reproductive Medicine, SRMC
Dr. Pallavi C, Senior Resident, Department of Reproductive Medicine, SRMC
Dr. Latha G, Postgraduate in DM Reproductive Medicine, SRMC
Dr. Anitha N, Postgraduate in DM Reproductive Medicine, SRMC
Dr. Arun M, Postgraduate in DM Reproductive Medicine, SRMC
Dr. Erika Desai, Postgraduate in DM Reproductive Medicine, SRMC.
REFERENCES
- 1.Speroff L. Regulation of menstrual cycle. In: Fritz MA, Speroff L, editors. Clinical Gynecologic Endocrinology and Infertility. 8th ed. Philadelphia: Lippincott Williams and Wilkins; 2011. pp. 199–242. [Google Scholar]
- 2.Kolibianakis EM, Albano C, Kahn J, Camus M, Tournaye H, Van Steirteghem AC, et al. Exposure to high levels of luteinizing hormone and estradiol in the early follicular phase of gonadotropin-releasing hormone antagonist cycles is associated with a reduced chance of pregnancy. Fertil Steril. 2003;79:873–80. doi: 10.1016/s0015-0282(02)04920-8. [DOI] [PubMed] [Google Scholar]
- 3.Yu Ng EH, Yeung WS, Yee Lan Lau E, So WW, Ho PC. High serum oestradiol concentrations in fresh IVF cycles do not impair implantation and pregnancy rates in subsequent frozen-thawed embryo transfer cycles. Hum Reprod. 2000;15:250–5. doi: 10.1093/humrep/15.2.250. [DOI] [PubMed] [Google Scholar]
- 4.Arslan M, Bocca S, Arslan EO, Duran HE, Stadtmauer L, Oehninger S. Cumulative exposure to high estradiol levels during the follicular phase of IVF cycles negatively affects implantation. J Assist Reprod Genet. 2007;24:111–7. doi: 10.1007/s10815-006-9101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kyrou D, Popovic-Todorovic B, Fatemi HM, Bourgain C, Haentjens P, Van Landuyt L, et al. Does the estradiol level on the day of human chorionic gonadotrophin administration have an impact on pregnancy rates in patients treated with rec-FSH/GnRH antagonist? Hum Reprod. 2009;24:2902–9. doi: 10.1093/humrep/dep290. [DOI] [PubMed] [Google Scholar]
- 6.Anifandis G, Koutselini E, Louridas K, Liakopoulos V, Leivaditis K, Mantzavinos T, et al. Estradiol and leptin as conditional prognostic IVF markers. Reproduction. 2005;129:531–4. doi: 10.1530/rep.1.00567. [DOI] [PubMed] [Google Scholar]
- 7.Mitwally MF, Bhakoo HS, Crickard K, Sullivan MW, Batt RE, Yeh J. Estradiol production during controlled ovarian hyperstimulation correlates with treatment outcome in women undergoing in vitro fertilization-embryo transfer. Fertil Steril. 2006;86:588–96. doi: 10.1016/j.fertnstert.2006.02.086. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: A prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil Steril. 2011;96:344–8. doi: 10.1016/j.fertnstert.2011.05.050. [DOI] [PubMed] [Google Scholar]
- 9.Balaban B, Brison D, Calderón G, Catt J, Conaghan J, Cowan L, et al. The Istanbul consensus workshop on embryo assessment: Proceedings of an expert meeting. Hum Reprod. 2011;26:1270–83. doi: 10.1093/humrep/der037. [DOI] [PubMed] [Google Scholar]
- 10.Rehman R, Hussain Z, Faraz N. Effect of estradiol levels on pregnancy outcome in obese women. J Ayub Med Coll Abbottabad. 2012;24:3–5. [PubMed] [Google Scholar]
- 11.Pena JE, Chang PL, Chan LK, Zeitoun K, Thornton MH, 2nd, Sauer MV. Supraphysiological estradiol levels do not affect oocyte and embryo quality in oocyte donation cycles. Hum Reprod. 2002;17:83–7. doi: 10.1093/humrep/17.1.83. [DOI] [PubMed] [Google Scholar]
- 12.Wu CH, Kuo TC, Wu HH, Yeh GP, Tsai HD. High serum estradiol levels are not detrimental to in vitro fertilization outcome. Taiwan J Obstet Gynecol. 2007;46:54–9. doi: 10.1016/S1028-4559(08)60108-4. [DOI] [PubMed] [Google Scholar]
- 13.Kara M, Kutlu T, Sofuoglu K, Devranoglu B, Cetinkaya T. Association between serum estradiol level on the hCG administration day and IVF-ICSI outcome. Iran J Reprod Med. 2012;10:53–8. [PMC free article] [PubMed] [Google Scholar]
- 14.Joo BS, Park SH, An BM, Kim KS, Moon SE, Moon HS. Serum estradiol levels during controlled ovarian hyperstimulation influence the pregnancy outcome of in vitro fertilization in a concentration-dependent manner. Fertil Steril. 2010;93:442–6. doi: 10.1016/j.fertnstert.2009.02.066. [DOI] [PubMed] [Google Scholar]
- 15.Mittal S, Gupta P, Malhotra N, Singh N. Serum estradiol as a predictor of success of in vitro fertilization. J Obstet Gynaecol India. 2014;64:124–9. doi: 10.1007/s13224-013-0470-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valbuena D, Jasper M, Remohí J, Pellicer A, Simón C. Ovarian stimulation and endometrial receptivity. Hum Reprod. 1999;14(Suppl 2):107–11. doi: 10.1093/humrep/14.suppl_2.107. [DOI] [PubMed] [Google Scholar]
- 17.Tao T, Robichaud A, Heudes R, Ouellette R. Effects of estradiol levels and estradiol/follicle ratio on trigger day on the IVF pregnancy outcome. Fertil Steril. 2013;100:S262–3. [Google Scholar]
- 18.Soni R, Fiza B, Mathur R, Sinha M. Association of 17 β estradiol levels on the day of hCG administration with pregnancy rate in IVF (in vitro fertilization) patients. Int J Basic Appl Med Sci. 2015;5:108–13. [Google Scholar]
- 19.Kosmas IP, Kolibianakis EM, Devroey P. Association of estradiol levels on the day of hCG administration and pregnancy achievement in IVF: A systematic review. Hum Reprod. 2004;19:2446–53. doi: 10.1093/humrep/deh473. [DOI] [PubMed] [Google Scholar]


