Abstract
Nonalcoholic fatty liver disease (NAFLD) is currently the most common liver disease worldwide, the prevalence of which had progressively increased over the past 10 years where other liver diseases remained at the same prevalence rates or are expected to decrease as in the case of hepatitis C virus (HCV). The treatment of NAFLD is of prime concern to health care professionals and patients due to the significant mortality and morbidity it implies; the problem is further escalated by the fact that standard of care medications targeting NAFLD remain experimental and without evidence base. Treatment nowadays is focused on lifestyle modification and managing the comorbid associated diseases, with a possible role for some hepatic protective agents. This review presents all the medications that had been proposed and used for the treatment of NAFLD with or without scientific rationale and includes agents for weight loss, insulin sensitizers, drugs that reduce blood lipids, glucagon-mimetics, drugs that may reduce fibrosis, angiotensin receptor blockers, and medicines believed to reduce endoplasmic reticular stress such as vitamin E, ursodeoxycholic acid, and S-adenosyl methionine. A quick review of the newer agents that proved to be promising such as obeticholic acid and GFT505 and the medicines that are still in the pipeline is also presented.
Keywords: Insulin sensitizers, liver protectors, Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis, Ursodeoxycholic acid, vitamin E
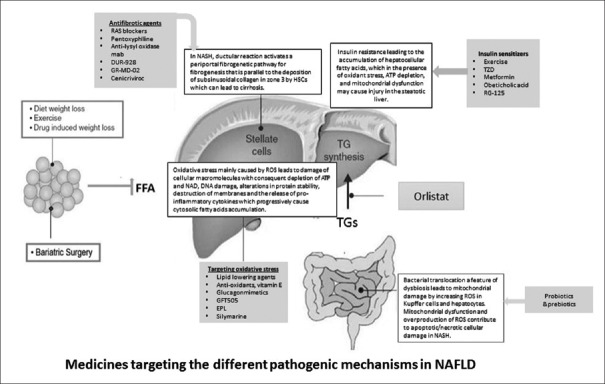
Nonalcoholic fatty liver disease (NAFLD) is probably the most common liver disorder in the world.[1] Globally, NAFLD is pandemic with an estimated prevalence of 20%–30% in Western countries and 5%–18% in Asia, which is expected to increase over time. The prevalence is rising in recent years due to changes in dietary habits being more westernized and owing to increase of sedentary lifestyle thus placing a worry on NAFLD becoming the main cause of liver disease among young adults and children.[2] There is a considerable need to treat NAFLD patients because they have a 1.7-fold increase in standardized age and gender-matched mortality.[3] The most common cause of death in patients with NAFLD and nonalcoholic steatohepatitis (NASH) is cardiovascular events, but they also have an increased liver-related mortality rate particularly when advanced fibrosis, cirrhosis, and hepatocellular carcinoma (HCC) develop.[3,4] Currently, there is no medicine that can be considered as the standard of care for the treatment of NASH.[5] All available medications that are recommended for the treatment of NAFLD patients target the established pathophysiologic mechanisms of NASH in accordance with the presence of an associated disease and remains experimental with inconsistent supporting evidence-based responses. The need for an effective medicinal agent is high, particularly when NAFLD is isolated without other comorbidities. Until then, treatment should focus on lifestyle modifications, therapeutic medicines used for associated conditions, and probably liver-protecting agents. Figure 1 shows the different pathophysiologic mechanisms of NAFLD and the medicines targeting each of these mechanisms.
Figure 1.
Diagram showing the different pathophysiologic mechanisms: Insulin resistance, oxidative stress, lipogenesis, fibrogenesis, and dysbiosis with medicines and therapeutics targeting each of these mechanisms
LIFESTYLE MODIFICATIONS AND WEIGHT LOSS
Life style modifications include attention to patient's physical activity and in addition to that of encouraging weight loss, which could be achieved by dietary control in conjunction, in some instances, of medicines that help losing weight such as orlistat, or of bariatric surgery. Lifestyle modification should be stressed as being central to all therapeutic modalities despite the difficulties in implementing it by the patients.
Weight loss
In a recent meta-analysis of randomized trials, it was observed that weight loss of at least 7% is effective in improving histological disease activity, although it was achieved by less than 50% of patients.[6] A greater weight loss (up to 10%) may be needed to improve necro-inflammation as well.[7] The optimal dietary composition for NAFLD patients is variable as per different authors but physicians should plan a clear target that genuinely leads to loss of weight through carbohydrate and lipid restriction. The Mediterranean dietary pattern has gained interest as it showed that it might have a beneficial effect on both prevention and resolution of the metabolic syndrome.[8] It should be watched, however, that severe caloric restriction should be avoided, as it may be harmful and lead to decompensation of liver function thus aggravating NAFLD.[9]
Physical activity
It is known that physical activity reduces the risk of impaired fasting glucose, type 2 diabetes mellitus (T2DM), insulin resistance (IR), hypertension, dyslipidemia, and the metabolic syndrome, which are main conditions that co-exist with NAFLD and contribute to its pathogenesis. Two analyses carried out in the cohort of the Look AHEAD Study-a multicenter prospective study that compared intensive lifestyle intervention, including caloric restriction and at least 175 min of moderate aerobic physical activity per week, to standard care in diabetic subjects-reported that after 1 year of intervention there was a significant decrease in hepatic fat content and a reduced incidence of NAFLD in patients randomized to the intensive lifestyle group.[10,11] Improving physical activity is the most powerful determinant of lifestyle intervention to correct metabolic indices, and the elevated liver enzymes due to NAFLD. Patients who could perform a 150- to 300-min exercise per week for 16 weeks had a significant improvement in their hepatic steatosis. In this respect both aerobic and endurance exercises were found to have a positive impact on steatosis even if the patient could not lose weight.
Orlistat
The results from studies evaluating treatment with orlistat were inconsistent. An earlier randomized controlled study showed that orlistat improved alanine transaminase (ALT) and steatosis for NAFLD patients.[12] Whereas a subsequent randomized controlled trial (RCT) showed that orlistat in combination with calorie restriction and vitamin E (800 IU/day) did not enhance weight loss, improve liver enzymes, or improve histopathology compared with calorie restriction and vitamin E only.[13] In that study, the authors found that ≥ 9% weight loss resulted in improved histology irrespective of whether patients received orlistat or placebo. Of note, when treatment with orlistat is initiated, it should be discontinued after 12 weeks if patients have been unable to lose at least 5% of the body weight as measured at the start of therapy. Furthermore, treatment duration should not be greater than 1 year as there is potential for fat-soluble vitamin deficiency if continued for longer.[14]
Bariatric surgery (gastric bypass)
NAFLD per se is not an indication for bariatric surgery.[15] However, bariatric surgery-induced weight loss appears to have beneficial effects on lipid profile, hepatic steatosis, steatohepatitis, as well as reducing long-term mortality.[16,17] The type of surgery seems to be relevant to the effect on NAFLD; gastric bypass produces the largest sustained weight loss compared with other bariatric procedures.[15] Because long-term outcome data about bariatric surgery as a specific treatment for NAFLD is lacking, bariatric surgery cannot be considered as a primary treatment for NASH. Surgery should be considered, however, as a firstline option for the treatment of obesity in adults with a body mass index (BMI) greater than 50 kg/m2. It is also important to note that bariatric surgery should be avoided in subjects with advanced cirrhosis and portal hypertension because rapid weight loss in these patients may increase the risk of hepatic decompensation.[18] More RCTs may be needed to validate the fact that bariatric surgery could be contemplated as a firstline treatment for NASH.[16,19]
Therapeutic medicines
The medicines that are being used in the management of NAFLD in every day practice include the standard medications that are usually used to treat the associated conditions. Table 1 summarizes the common medicines used in this respect and their role in treating NAFLD. However, evaluation of investigational trends targeting pathological concepts of the disease itself is being considered and so is the situation with newer medicines in development in the pipeline. Table 2 displays the most important medicines being investigated or studied for future production. Traditionally some conventional medicines and phytomedications have been used in certain cultures, together with hepatoprotective agents in the management of NAFLD; these drugs are briefly discussed in the following sections.
Table 1.
Medicines that are used for treatment of associated comorbid disease and may benefit NAFLD at the same time
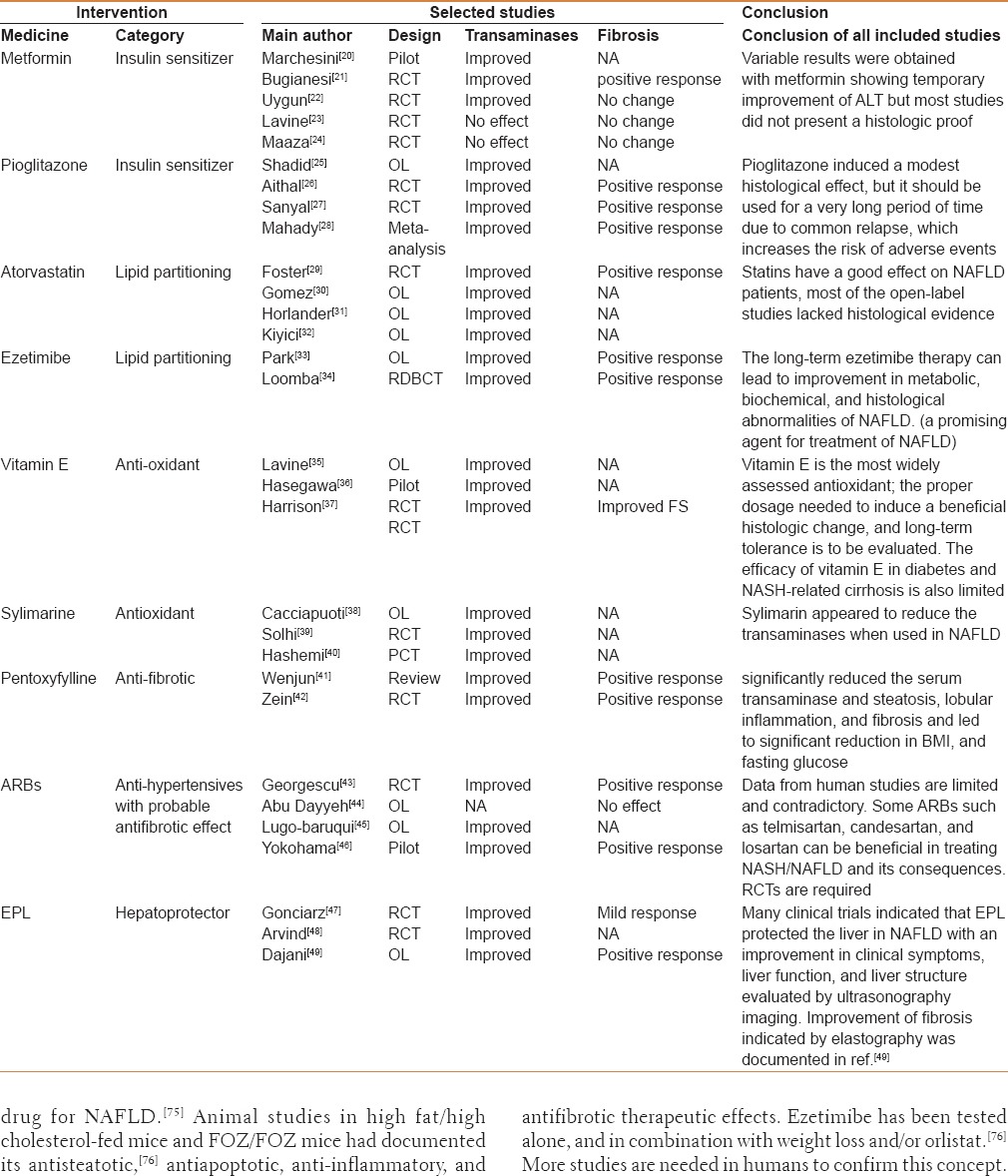
Table 2.
Investigational medicines and specific medicines targeting NAFLD or its pathologic components being produced in the pipeline
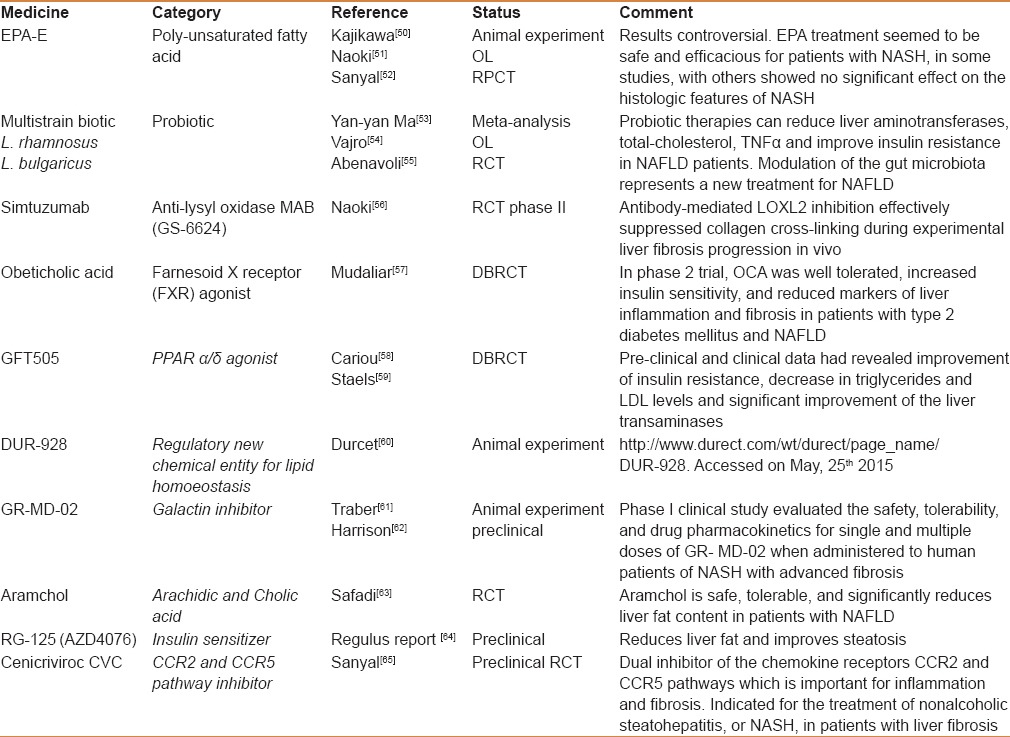
Insulin sensitizers
These include medicines that affect hepatic liver partitioning by decreasing insulin resistance, modifying adipocyte differentiation, inhibiting vascular endothelial growth factor (VEGF)-induced angiogenesis, decreasing leptin and increasing adiponectin levels, and decreasing levels of certain interleukins (eg, IL-6).[66] Metformin, rosiglitazone, and pioglitazone had been used in this respect alone or in different combinations with other medicines.[67]
Clinical experience revealed variable results with metformin with temporary improvement of transaminases but most studies that were done did not have a histologic proof.[20]
Only pioglitazone was believed to induce a convincing effect, however, it should be noted that relapse was common when these medicines were stopped and hence it should be used for a very long period of time, which raises worries with pioglitazone about the risk of cardiovascular events,[66] increase in body weight,[68] increase in fracture risk,[66,68,69] and increase in the incidence of bladder and prostatic cancer.[68] Pioglitazone also appeared to work best and have a better prognosis in the nondiabetic patient where its effects seem to be least relevant to clinical outcome (steatosis, ballooning).[25]
Drugs that reduce blood lipids
Fibrates, peroxisome proliferator-activated receptor (PPAR)-α agonists, were found to have anti-inflammatory and antifibrotic effects on the liver of experimental animals. In particular they were successful in preventing tamoxifen-induced steatosis in rats. In humans, however, this was not confirmed. A pilot study revealed that gemfibrozil had resulted in biochemical improvement of transaminases, but histologic data were lacking.[70]
Statins appeared to have some good effect in NAFLD in many open-label studies [71] particularly atorvastatin,[29] which has been extensively studied in the literature. Given the lack of evidence to show that patients with NAFLD and NASH are at increased risk for serious drug-induced liver injury from statins, statins can be used to treat dyslipidemia in patients with NAFLD and NASH.[71] However, until more RCTs with histological endpoints prove their efficacy, statins should not be used to specifically treat NASH alone.[71]
Rosuvastatin used for NAFLD had also improved glucose intolerance, insulin sensitivity, and liver histology [7,71] in a dose-dependent manner. Animal experiments in obese mouse model indicated that it would change the fat distribution from visceral to subcutaneous fat depot.[72] Consequently, rosuvastatin therapy may be of great help to patients with metabolic syndrome because it has a wide range of beneficial pleiotropic effects.[72,73] In a case study when rosuvastatin was combined with pioglitazone they led to rapid and sustained improvement of biochemical and ultrasonographic markers of resistant, severe NAFLD.[74]
Ezetimibe which blocks Niemann-Pick C1-Like 1 (NPC1L1) pathway of hepatic cholesterol uptake [75,76] to lower hepatic free and total cholesterol stores emerged as a promising drug for NAFLD.[75] Animal studies in high fat/high cholesterol-fed mice and FOZ/FOZ mice had documented its antisteatotic,[76] antiapoptotic, anti-inflammatory, and antifibrotic therapeutic effects. Ezetimibe has been tested alone, and in combination with weight loss and/or orlistat.[76] More studies are needed in humans to confirm this concept.
MEDICINES THAT TARGET ANTIOXIDANT ACTIVITY AND REDUCE ENDOPLASMIC RETICULAR STRESS
Vitamin E was studied extensively alone and in combination with pioglitazone, vitamin C, “essential” phospholipids (EPL), sylimarin, and metformin with variable results and a general consensus that its effects are very limited and dubious.[77] Vitamin E is currently the most widely assessed antioxidant;[77,78] several questions yet need to be answered, including the proper dosage needed to induce a beneficial histologic change, long-term tolerance, and efficacy of vitamin E in particular subsets, such as diabetes and NASH-related cirrhosis. Based on the available evidence, vitamin E (RRR-α-tocopherol) is currently only recommended in NASH adults without diabetes or cirrhosis and with aggressive histology.[77,78] Validation is needed in children before its use can be recommended, although a recent study indicated that Vitamin E has been shown to be effective and safe in improving NASH histology in children.[78,79] A meta-analysis warned that the high doses of vitamin E that are usually effective to treat NASH should be used with extreme caution as they had increased all-cause mortality [5,77,78,79,80] from any disease, prostatic cancer,[5,77,78,79] and hemorrhagic strokes.[5,77,78] Longer follow-up of RCTs are needed to assess long-term vitamin E safety.[77]
Ursodeoxycholic acid
Ursodeoxycholic acid (UDCA) was theoretically believed to improve patients with steatosis,[7] however, results of treatment with UDCA were variable and nonconclusive. Recent multicenter clinical studies of large scale populations with UDCA in NAFLD and NASH revealed inconsistent and disappointing results;[7,80,81] hence UDCA was not recommended by the AASLD, EASL, and ACG for the treatment of NAFLD or NASH as per their treatment guidelines.[5,80] Furthermore, UDCA is known to inhibit DNA repair, co-enzyme A, cyclic AMP, p53, phagocytosis, and induction of nitric oxide synthetase.[82] This renders it with a possible genotoxic effect.[82] UDCA toxicity is related to its interference with drug detoxification, being hydrophilic and antiapoptotic, which leads to a remarkable list of unanticipated toxicity events.[82]
Glucagonmimetics
The Glucagonmimetics, including the dipeptidyl peptidase-4(DPP-4) inhibitors (sitagliptin, vidagliptin, saxigliptin, linagliptin, and alogliptin),[83] and the GLP-1 agonists (Exanitide,[83] Liraglutide [83] and Exenidin-4[84]) appeared to reduce hepatocyte steatosis [83] and improve survival of the hepatocyte by enhancing the unfolded protein response (UPR) and promoting macroautophagy.[84] The serum DPP-4 activity invariably increases in cirrhosis and several liver diseases.[85] This increase is correlated with the prognosis of liver disease.[85] DPP-4 activity was higher in NASH patients when compared with controls and the activity correlated with BMI. These changes in NASH might result either primarily from the underlying liver disease or secondary to insulin resistance.[85]
A study on NASH aimed at developing markers such ase DPP-4 that would enable detecting patients with high risk of progressive fibrosis and to develop effective treatment for the disease suggested that DDP-4 inhibitors could be potential candidate for NASH treatment.[85] Incretin-based therapies, including DPP-4 inhibitors and GLP-1 analogues have appreciable experimental and clinical data available in this respect so far.
GLP-1 analogues are known to improve insulin resistance and are even more attractive because they have anorexigenic potential leading to weight loss, serum glucose and lipids improvement.[83] Those, as well as DPP-4 inhibitors, might also be involved in direct pathways of liver fat elimination.[83] A growing body of literature suggests that GLP-1 and DPP-4 activity have numerous effects on the cells of various organs. The data regarding GLP-1 analogues and DPP-4 inhibitors action on hepatocytes are convincing, but so far only T2DM patients with NAFLD/NASH have been studied with incretin analogues or DPP-4 inhibitors.[83] Further studies are needed in order to assess the long-term effect of incretin-based therapies on NAFLD.
MEDICINES TARGETING NALFD-INDUCED FIBROSIS
Pentoxifylline, the phosphodiesterase inhibitor (PDE I), had been investigated in the management of NAFLD-induced fibrosis. A few RCTs, several open-label studies, and a meta-analysis have shown that it may have an antioxidant [86] and antifibrotic effect.[86] Pooled results showed that pentoxifylline significantly reduced the serum transaminase activity in NAFLD patients compared with placebo.[41,86,87] It also significantly improved steatosis, lobular inflammation, and fibrosis and led to significant reduction in BMI, and fasting glucose,[86,87] but did not significantly affect the serum tumor necrosis factor-α (TNFα)[41,86,87] and adiponectin levels when compared with placebo.[86] It is therefore a potential agent that may be useful to induce regression or at least halt fibrosis progression.[41,87] It is generally safe and effective.[41,86,87] Patients with T2DM and advanced proliferative retinopathy or tendency to retinal bleeding should use this medicine under strict observation and close follow up.
POSSIBLE ROLE FOR THE RENIN–ANGIOTENSIN SYSTEM
Angiotensin Receptor Blockers (ARBs) are also in the focus of investigation as a possible treatment for NAFLD.[88] It is believed that ARBs by targeting the pancreatic effects of angiotensin should be able to preserve an adequate insulin secretion and acquire a better metabolic balance.[88] Several clinical and research studies documented these effects and had shown that ARBs improve the intracellular insulin signaling pathway, provide better control of adipose tissue proliferation and adipokine production, produce more balanced local and systemic levels of various cytokines, prevent fibrosis, and slow down the vicious cycle that links steatosis to necroinflammation.[88] There are a few RCTs on the effects of ACE-I or ARB in patients with NAFLD and most data are from retrospective studies, pilot prospective studies and post hoc analyses of clinical trials. Accordingly, more and larger RCTs are needed to directly assess the effectiveness of ACE-I and ARBs in NAFLD.
S-adenosyl methionine
S-adenosyl methionine (SAMe) is a medication that has been used for some chronic liver disorders.[89] SAMe is a nutritional supplement that theoretically exerts its effect by donating a (CH3) group to an acceptor substrate in the trans-methylation process, and other metabolic reactions involving substrates, such as nucleic acids, proteins, lipids, and secondary metabolites.[89] It was initially recommended for the treatment of depression, osteoarthritis, noncalculous chronic cholecystitis, cholangitis,[90] intrahepatic cholestasis, and liver damage caused by drugs (antibiotics, antiviral preparation, and tricyclic antidepressants). There are a few uncontrolled pilot studies in the literature about its therapeutic applications in humans with no strong evidence base yet furnished; most of the data we know are based mainly on animal studies.
A clinical study evaluating its potential use in NAFLD is being investigated and is in phase III.[91] It is noteworthy that, this medicine should be used with extreme caution in patients with bipolar disorders where depression could be converted to mania, in patients with Parkinson's disease which could worsen with the use of SAMe and in patients planned for or undergoing any surgery because of its effects on the central nervous system.
Polyunsaturated fats 3n PUF (ethyl-eicosapentaenoic Acid EPA-E)
Animal experiments demonstrated that EPA-E attenuates progression of hepatic fibrosis that has already developed in steatohepatitis.[50] Moreover, the antifibrotic effect of EPA-E may be mediated by direct inhibition of reactive oxygen species (ROS) production, which involves a TGF-β-stimulated signaling pathway and/or fatty acid oxidation. These mechanisms, independent of the effect against triglyceride accumulation, may contribute to the therapeutic efficacy of EPA-E in NASH patients. A few clinical pilot studies indicated that it may exert a positive effect on reducing hepatic triglycerides and improving NAFLD.[50]
In one of these studies using highly purified EPA-E the serum alanine aminotransferase levels were significantly improved, and serum free fatty acids, plasma soluble TNF receptor 1 and 2 levels, and serum ferritin and thioredoxin levels, which may reflect hepatic oxidative stress, were significantly decreased. Body weight, blood glucose, insulin, and adiponectin concentrations remained unchanged. Hepatic steatosis and fibrosis, improved in seven out of the 23 patients endorsing post-treatment biopsy.[51] More research is ongoing to evaluate this concept and to furnish convincing evidence that it really might work; however, in a recent large scale phase II trial, EPA-E reduced subjects' levels of triglyceride as compared with placebo but had revealed no significant effect on the histologic features of NASH.[52]
Probiotics
It is well known that dysbiosis may lead to insulin resistance, and obesity by virtue of bacterial translocation. Both are important components of the metabolic syndrome and NAFLD.[92] Bacterial translocation leads to mitochondrial dysfunction by causing an increased production of ROS in the Kupffer cells and hepatocytes, by upregulating nitric oxide synthetase (NOS) through the activation of both endothelial and inducible NOS.[55] Furthermore LPS compounds that arise from bacterial translocation will also activate the Kupffer cells and induce them to produce pro-inflammatory cytokines besides the ROS.[53,55,92,93,94] The net result will be fat accumulation in the liver.[55] Clinical research had shown that bacterial translocation from the gut to the liver has an important role in the pathogenesis of NAFLD with its entire spectrum.[55] Re-instating the microbiota of the gut therefore appears an interesting target to ameliorate dysbiosis and probably treat NAFLD.[53,93,94] The preliminary results of research testing different probiotic preparations in this respect had yielded encouraging results and indicated that it could be an emerging therapeutic tool of potential great benefits.[95] It remains to decide how and when to use this modality of treatment.
Obeticholic acid
Obeticholic acids (OCAs) are modified bile acid that change the body metabolism of lipids and might be beneficial for NASH.[57] It was found to improve insulin sensitivity,[57,96] and reduce serum triglyceride levels.[57] OCAs were initially recommended as a monotherapy for primary biliary cirrhosis and were being investigated in NAFLD.[57]
NASH-FLINT study is a multicenter, randomized, double-blind, placebo-controlled, phase IIb clinical trial of treatment with either OCA or placebo in patients with NASH.[96] The trial aimed to determine if administration of the farnesoid X receptor (FXR) ligand OCA for 72 weeks to subjects with biopsy evidence of NASH will result in improvement in their liver disease [96] as measured by changes in the NAFLD activity score (NAS). An interim analysis, however, found that OCA showed improved efficacy on NASH-related liver health (P = 0.0024) and supported a decision to not perform end-of-treatment biopsies in 64 of the patients. Forty-five percent of the patients in the OCA group who were meant to have biopsies showed improved liver histology versus 21% of the patients in the placebo group (RR 1.9, 95% CI 1.3-2.8; P = 0.0002).[96] Results also showed increases in total low-density lipoprotein (LDL) cholesterol and decreases in high-density lipoprotein (HDL) cholesterol in the OCA treatment group; it was then decided to discontinue treatment but continue the study. No continued cholesterol increases were seen after discontinuing OCA. Furthermore, improvements in liver enzymes with OCA were not sustained after discontinuing treatment. The authors noted that further research is needed to better understand the long-term effects of OCA on cholesterol and arterial atherosclerosis.[96]
GFT505
GFT505 is a unique PPARα,/δ agonist.[97] PPARα activation, in combination with PPARβ/δ agonism, improves steatosis, inflammation, and fibrosis in preclinical models of NAFLD, identifying a new potential therapeutic area.[98] Preclinical and clinical data had revealed improvement of insulin resistance,[98] decrease in triglycerides [97,98] and LDL levels, and significant improvement of the liver transaminases with this product.[98] GFT505 had no induction of PPAR-driven genes in the skeletal muscles and hence will not produce any myalgia.[98]
Anti-lysyl oxidase (mab)
Anti-lysyl oxidase is a promising monoclonal antibody still in phase II evaluation, which is believed to halt fibrosis and probably reverse some cirrhotic changes associated with NAFLD by inhibiting lysyl oxidase-like 2(LOXL2), which promotes the cross-linking of type I collagen (a key component in the core regulatory pathway of fibrogenesis). This effect is expected to be exerted in a wide range of liver diseases leading to fibrosis and cirrhosis, regardless of the etiology.[56]
Metreleptin
The effect of leptin treatment is different when circulating leptin levels are low, normal, or high.[100] When the levels are low, leptin supplementation acquires adiponectin-like properties which render it appealing as a therapeutic target for NAFLD by decreasing hepatic and systematic IR and attenuating liver inflammation and fibrosis, as indicated from the hepatoprotective effect of the recombinant adiponectin against NASH.[100] Recombinant leptin (metreleptin) administered in patients with NAFLD and lipodystrophy decreased hepatic volume and steatosis, although had no effect on hepatic inflammation and fibrosis.[100] The discovery of adiponectin analogues, such as osmotin (a ligand for the yeast homologue of the adiponectin receptor), might provide a therapeutic alternative.[100]
Roflumilast and pioglitazone
Roflumilast and pioglitazone combination therapy is hypothesized to produce additive anti-inflammatory and hepatoprotective effects without overlapping toxicities. A randomized, double-blind, controlled, multicenter proof of mechanism phase II study is ongoing; it aims to evaluate the effect of roflumilast plus pioglitazone versus roflumilast and pioglitazone monotherapy versus placebo on liver enzymes and liver fat content based on MRI in patients with NASH after 4 months of treatment.[100]
Vitamin D
NAFLD patients have decreased serum 25-hydroxy vitamin D concentrations, suggesting that vitamin D may play a role in the development of NAFLD.[101] NAFLD and Vitamin D deficiency are often found associated, and while this is not unexpected, given their similar associations with obesity and sedentary lifestyle, a growing body of evidence points to a closely linked and potentially causative relationship between Vitamin D deficiency and NAFLD.[102] However it should be mentioned that vitamin D deficiency does exist with many other diseases and even in normal subjects as well hence a cause and effect relationship is extremely difficult to conclude. Vitamin D supplement in the treatment of NAFLD is not studied properly yet and the research seems to be in its earliest stages.
Testosterone replacement
Androgens may have a role in the pathogenesis of NAFLD particularly in hypogonadal men, those on testosterone replacement as well as the hypogonadism associated with obesity. Testosterone replacement in the hypogonadal men causes a significant reduction in weight, BMI, waist circumference, and circulating TNFα.[103] In obese men with obstructive sleep apnea, testosterone treatment reduced liver fat as measured by computed tomography (CT) without a reduction in BMI or weight.[104] Although altered liver enzymes are poorly predictive of steatohepatitis, testosterone administration has also been shown to decrease alanine transaminase and aspartate transaminase.[104]
Pradigastat
A randomized, multicenter, double-blind, placebo-controlled, parallel-group, 24-week pilot study [105] is ongoing to assess the efficacy, safety, and tolerability of LCQ908 (Pradigastat) in patients with nonalcoholic fatty liver disease (NCT01146522). The purpose of core part of this study is to determine whether LCQ908 effectively lowers liver fat, as assessed by MRI and to assess its safety and tolerability profile in subjects with NAFLD. No study results are available yet.
Medicines in the pipeline
DUR-928 is an endogenous, small-molecule, new chemical entity that modulates the activity of several nuclear receptors, which play an important regulatory role in lipid homeostasis, inflammation, and cell survival and may have broad applicability in metabolic diseases such as NAFLD and NASH. It may also play an important role in protecting against acute kidney injury (AKI) and other types of acute organ injury. A systems biology study involving over 23,000 genes showed that DUR-928 modulates the activity of more than 240 genes, including ACC, FAS, HMGR, Cyp7A1, LXR, PPARγ, NFκB/IκB, TNFα, IL-1α, IL-6, COX-2, PCSK9, and others. The biological activity of DUR-928 has been demonstrated in different animal models including NASH.[60]
GR-MD-02 is a carbohydrate-based galectin inhibitor that had shown in preclinical data from animal models of NASH with fibrosis to reduce hyaluronic acid in 33% of treated animals, as compared with the untreated ones. Phase I clinical study evaluated the safety, tolerability, and drug pharmacokinetics for single and multiple doses of GR-MD-02 when administered to human patients of NASH with advanced fibrosis. The medicine was safe and well tolerated and there were no serious adverse events reported. FibroTest®, was significantly reduced by GR-MD-02 treatment. The treatment effect was due to a statistically significant reduction of alpha-2 macroglobulin, one of the components of the score.[62]
Aramchol is a novel synthetic lipid molecule obtained by conjugating two natural components, cholic acid (a primary bile acid) and arachidic acid (a saturated fatty acid), through a stable amide bond. In a randomized, double-blind, placebo-controlled study of patients with biopsy-confirmed nonalcoholic fatty liver disease (NAFLD) or NASH, aramchol (300 mg daily for 3 months) was associated with lower liver fat content (a mean of 13% less), which is statistically significant compared with an increase in liver fat (a mean of 6% more) in patients who received placebo with improvement in endothelial function, levels of alanine aminotransferase and adiponectin. No serious or drug-related adverse events occurred in the treated patients.[63]
RG-125 (AZD4076) an anti-microRNA targeting miR-103/107 function appeared to be unique insulin sensitizer. Inhibition of miR-103/107 leads to a sustained reduction in fasting glucose and fasting insulin levels and decrease in liver triglycerides and steatosis. Research is evaluating the effect of RG-125 on patients with NAFLD or NASH.[64]
Cenicriviroc CVC is a dual inhibitor of the chemokine receptors CCR2 and CCR5 pathways, which play a key role in the cycle of inflammation and fibrosis. CVC has been shown in clinical trials to bind to both CCR2 and CCR5 targets. Food and Drug Administration has granted Fast Track designation to CVC for the treatment of NASH, in patients with liver fibrosis. A global randomized Phase IIb study CENTAUR (NCT02217475) of CVC versus placebo in patients with NASH and liver fibrosis is being carried out.[65]
Phytomedication
Yo Jyo Hen Shi Ko
Yo jyo hen shi ko (YHK) is a Chinese herb that in a rat model, had prevented NASH, promoted weight loss, lowered serum aminotransferases, and reduced visceral fat.[106] It appeared that it has an antioxidant, hypoglycemic, antifibrotic, and lipid-lowering properties [106,107] and acts by promoting an increase in PPARα and microsomal triglyceride transfer protein with a decrease in PPARγ mRNA contents. In a small clinical study of eight patients, after discontinuing YHK the ALT returned toward baseline at week 12. The mean decrease in ALT compared with that of baseline was significantly greater in the YHK group than in the placebo group at both week 4 and week 8 (P = 0.036, for both).[107]
Folic acid
Folate deficiency is believed to affect NAFLD by causing a disturbance in hepatic methionine metabolism, which was shown to promote hepatic steatosis in the murine model.[99,108] Low levels of vitamin B12 and folate were also observed in NASH and NAFLD patients but these low levels were not associated with either insulin resistance or the severity of liver disease.[109] Neither vitamin B12 nor folate levels were significantly different within any histological category, including steatosis grade, fibrosis stage, lobular inflammation, portal inflammation and ballooning.[109] A 6-month open-label pilot study of folic acid for the treatment of NAFLD did not show any improvement in serum aspartate and alanine aminotransferase levels (P = 0.5 and 0.6, respectively) in 10 patients with NASH.[108]
Resveratrol
Resveratrol could protect the liver from NAFLD by reducing triglyceride accumulation and improving insulin resistance.[110] Typical features of NAFLD, including histological changes, fibrosis, insulin resistance, oxidative stress, and inflammation were ameliorated by resveratrol treatment of knockout mice.[111] This is achieved by activating AMP-activated protein kinase and reducing the activity of nuclear factor-κB (NF-κB) through the restoration of its inhibitor IκBα.[111] Therefore RSV has the therapeutic potential for preventing or treating NAFLD and IR-related metabolic disorders through regulating autophagic and IκBα–NF-κB pathways.[111]
Anthocyanins
Anthocyanin, from different plant foods, have been shown to improve features of experimental NASH,[112] such as oxidative stress,[112,113] dyslipidemia,[112] liver steatosis,[112,113] and inflammation [113] in rodents. ACN-rich foods can be promising for the prevention of NAFLD and its complications.[113] Purified anthocyanins may prevent the progression of liver damage related to NAFLD by three independent mechanisms: Inhibition of lipogenesis by reducing Srebp1c, promotion of lipolysis by induction of PPARα activity, and reduction of oxidative stress by induction of antioxidant enzymes. The effects of anthocyanins on lipid metabolism seem to be dependent on the activation of the AMPK pathway in hepatocytes [113] by targeting protein kinase C, zeta (PKCζ) thus they may contribute to the prevention and treatment of steatotic liver associated with obesity and T2DM.[112] Additional studies are required to clarify the molecular mechanisms and to test the specific effect of single compounds and food extracts in vitro and in vivo.[113] Randomized controlled studies are warranted to test foods on histological damage or noninvasive biomarkers of liver damage progression in patients with NASH.[113]
Quercetin
Treatment with quercetin may help to delay the progression of NAFLD, possibly by adjusting the balance of inflammatory cytokines. NAFLD rats have higher serum levels of IL-18 but lower levels of IL-10 than their healthy counterparts and these differential cytokine expressions may be related to liver inflammation and steatosis.[114] It was found that the intracellular lipid content was significantly reduced following quercetin therapy, which also strongly affected cellular lipid accumulation in a dose-dependent manner.[115] Quercetin with allopurinol contributed to the reduction of liver inflammation and lipid accumulation under hyperglycemic conditions by inhibiting hepatic thioredoxin-interacting protein (TXNIP); this may have therapeutic implications for prevention of type 1 diabetes-associated NAFLD.[116]
Acarbose
Acarbose is an alfa-glucosidase inhibitor, which can lead to an increase in the total adiponectin levels.[117] Together with pioglitazone, they decreased HbA1c values by 0.49% and 0.63%, respectively, in 17 patients with T2DM and 16 for pioglitazone during three months treatment.[117] Treatment with acarbose and pioglitazone increased the serum levels of total adiponectin by 2.1-fold and its high molecular weight isoform by 3.6-fold, which makes the combination a useful agent for the treatment of NAFLD.[117] Both ezetimibe and acarbose improved metabolic and biochemical abnormalities in patients with NASH; however, these effects were more prominent with ezetimibe.[118] The combination therapy with ezetimibe and acarbose for 24 weeks also improved the histopathological findings in a mouse model of NAFLD.[119]
Coffee
Coffee consumption is a popular lifestyle choice worldwide; at least 50% of US adults consume coffee on a daily basis.[120,121] Beneficial effects of coffee have been observed in multiple chronic medical conditions including T2DM, Parkinson disease, prostate cancer, hepatitis C virus (HCV), HCC, and more recently, NAFLD.[120] Moreover, a large population-based study found that increasing coffee intake is associated with a modest decrease in all-cause mortality.[120,121] Coffee has inverse relationships with both T2DM and hepatic fibrosis in patients with NAFLD.[120] Relationships were explored between coffee intake and insulin resistance (IR) with respect to NAFLD histological severity.[120] In one of large studies, among 782 participants (24% of them having diabetes), NASH was present in 79%. Coffee intake was shown to be inversely associated with advanced fibrosis among NAFLD patients with lower HOMA-IR.[120]
HEPATOPROTECTIVE AGENTS
Historically speaking, several compounds have been claimed to have a theoretical protective effect on the liver cells, which in some patients could be clinically relevant and had been applied in practice either on empiric, local experience, or traditional management basis. Some of these products are available as nutritional supplements and may have a convincing rationale, whereas some others such as “essential phospholipids (EPL) may have evidence furnished on research basis. These medicines included N-Acetyl Cysteine, Choline Bitartrate, Artichoke Extract, Dandelion Root, taraxacin and inulin, Turmeric (curcumin), Milk Thistle Extract (Silymarin), Essential phospholipids, UCDA, vitamin E, S-Adenosyl Methionine, Ganoderma spores, traditional Chinese medicine, including Gansu and some herbal compounds.
Phosphatidylcholine (PC), a main component of EPL, is one of the most important support nutrients for the liver and is considered as a universal building block for cell membranes, which regulate the vast majority of the activities that make up life. PC helps recovery and maintenance of the consistency of the hepatocytes; it activates the phospholipid-depending enzymes [122] and improves lipid metabolism by accelerating synthesis of lipoproteins in the liver. Studies have demonstrated that EPL will activate synthesis of RNA and as a result lead to the normalization of protein metabolism. It exerts a good effect on glycogenesis and improves the detoxification function of the liver. All of these effects may lead to a reduction of the fatty infiltration of the hepatocytes.[123] Many clinical trials indicated that EPL protected the liver against damage in patients diagnosed with NAFLD with an improvement in clinical symptoms, liver function, and liver structure evaluated by ultrasonography imaging.[47,124,125,126,127,128,129,130,131,132]
In an open-label observational study,[133] we looked into the efficacy of EPL as an adjunctive agent in treating 324 patients of primary NAFLD or NAFLD associated with comorbid clinical conditions such as T2DM and hyperlipidemia. It was concluded from the study that there was a significant improvement for all symptoms, general and gastrointestinal, and a significant reduction of the elevated transaminases (P< 0.001 baseline vs endpoint) associated with NAFLD disease. The study had also demonstrated a mild but noteworthy improvement in fatty infiltration (P = 0.7) as concluded from changes of liver ultrasonography as well as a positive impact on liver stiffness measurement associating fibrosis of the liver as indicated from liver elastography. Extended treatment seemed to further benefit patients leading to stabilization of liver function and maintaining recovery.
Silymarin and silibinin, the active products of milk thistle have shown in several pharmacological studies hepatoprotective, antioxidant, anti-infiammatory, and antifibrotic properties; in addition, they stimulate protein biosynthesis and liver regeneration and have immunomodulatory activity. They also improve insulin resistance, reduce lipidperoxidation, and restore GSH levels, which makes it another suitable herb for use against liver steatosis.[134,135]
CONCLUSION
A decade of clinical trials involving a diversity of pharmaceutical agents targeting the pathological concepts of NAFLD did not reveal a single intervention that has convincingly improved all important outcomes for all NASH patients. Therefore, in 2015, there was no single medication that can be recommended as a target-specific agent for routine clinical use for all patients of NAFLD. It remains that the best available treatment should be instructing patients to follow a reasonable dietary plan and a tailored exercise program. Together with this, patients should be treated for any associated comorbid disease in accordance with a specific medicine from the list of medications discussed in this review. Liver protection should be an important part of the management plan complementing lifestyle modification and other medications that the patient may be taking. A liver-protective agent such as EPL or sylimarin may be added to the management plan. EPL had emerged as an important additive nutritional support that has a favorable effect on most NAFLD patients. There is no enough convincing evidence to support the use of medicines that were long used on empiric basis such as vitamin E, UDCA, and SAMe for treatment of NAFLD, hence they are not recommended in most guidelines as standard treatment of NAFLD. The impact of the treatment adopted for fibrosis caused by NASH is not convincing so far, although pentoxyfylline may have some effect. The anti-lysyl oxidase monoclonal antibodies, proposed to reduce fibrosis and reverse cirrhosis may be the choice in this respect. The new medications in the pipeline being currently evaluated such as OCA, GFT505, metreleptin, and pradigastat and the probiotics should provide a hope for the future as target-specific agents when they furnish good clinical evidence after phase III results. Testosterone replacement may be indicated for hypogonadal men. For the very obese patients, weight reduction surgery may improve NASH but data of long-term outcome and enough RCT are lacking.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
REFERENCES
- 1.Chitturi S, Wong VW, Farrell G. Nonalcoholic fatty liver in Asia: Firmly entrenched and rapidly gaining ground. J Gastroenterol Hepatol. 2011;26(Suppl 1):163–72. doi: 10.1111/j.1440-1746.2010.06548.x. [DOI] [PubMed] [Google Scholar]
- 2.Masarone M, Federico A, Abenavoli L, Loguercio C, Persico M. Non alcoholic fatty liver: Epidemiology and natural history. Rev Recent Clin Trials. 2014;9:126–33. doi: 10.2174/1574887109666141216111143. [DOI] [PubMed] [Google Scholar]
- 3.Söderberg C, Stål P, Askling J, Glaumann H, Lindberg G, Marmur J, et al. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51:595–602. doi: 10.1002/hep.23314. [DOI] [PubMed] [Google Scholar]
- 4.Yatsuji S, Hashimoto E, Tobari M, Taniai M, Tokushige K, Shiratori K. Clinical features and outcomes of cirrhosis due to non-alcoholic steatohepatitis compared with cirrhosis caused by chronic hepatitis C. J Gastroenterol Hepatol. 2009;24:248–54. doi: 10.1111/j.1440-1746.2008.05640.x. [DOI] [PubMed] [Google Scholar]
- 5.Nascimbeni F, Pais R, Bellentani S, Day CP, Ratziu V, Loria P, et al. From NAFLD in clinical practice to answers from guidelines. J Hepatol. 2013;59:859–71. doi: 10.1016/j.jhep.2013.05.044. [DOI] [PubMed] [Google Scholar]
- 6.Musso G, Cassader M, Rosina F, Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): A systematic review and meta-analysis of randomised trials. Diabetologia. 2012;55:885–904. doi: 10.1007/s00125-011-2446-4. [DOI] [PubMed] [Google Scholar]
- 7.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. American Association for the Study of Liver Diseases; American College of Gastroenterology; American Gastroenterological Association. The diagnosis and management of non-alcoholic fatty liver disease: Practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Am J Gastroenterol. 2012;107:811–26. doi: 10.1038/ajg.2012.128. [DOI] [PubMed] [Google Scholar]
- 8.Babio N, Toledo E, Estruch R, Ros E, Martínez-González MA, Castañer O, et al. Mediterranean diets and metabolic syndrome status in the PREDIMED randomized trial. CMAJ. 2014;186:E649–57. doi: 10.1503/cmaj.140764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gasteyger C, Larsen TM, Vercruysse F, Astrup A. Effect of a dietary-induced weight loss on liver enzymes in obese subjects. Am J Clin Nutr. 2008;87:1141–7. doi: 10.1093/ajcn/87.5.1141. [DOI] [PubMed] [Google Scholar]
- 10.Lazo M, Solga SF, Horska A, Bonekamp S, Diehl AM, Brancati FL, et al. Fatty Liver Subgroup of the Look AHEAD Research Group. Effect of a 12-month intensive lifestyle intervention on hepatic steatosis in adults with type 2 diabetes. Diabetes Care. 2010;33:2156–63. doi: 10.2337/dc10-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albu JB, Heilbronn LK, Kelley DE, Smith SR, Azuma K, Berk ES, et al. Look AHEAD Adipose Research Group. Metabolic changes following a 1-year diet and exercise intervention in patients with type 2 diabetes. Diabetes. 2010;59:627–33. doi: 10.2337/db09-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zelber-Sagi S, Kessler A, Brazowsky E, Webb M, Lurie Y, Santo M, et al. A double-blind randomized placebo-controlled trial of orlistat for the treatment of nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2006;4:639–44. doi: 10.1016/j.cgh.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Harrison SA, Fecht W, Brunt EM, Neuschwander-Tetri BA. Orlistat for overweight subjects with nonalcoholic steatohepatitis: A randomized, prospective trial. Hepatology. 2009;49:80–6. doi: 10.1002/hep.22575. [DOI] [PubMed] [Google Scholar]
- 14.European Medicine Agency. Xenical 120 mg Summary of Product Characteristics. [Last accessed on 2015 Apr 19]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000154/WC500058428.pdf .
- 15.Hafeez S, Ahmed MH. Bariatric surgery as potential treatment for nonalcoholic fatty liver disease: A future treatment by choice or by chance? J Obes. 2013;2013:839275. doi: 10.1155/2013/839275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chavez-Tapia NC, Tellez-Avila FI, Barrientos-Gutierrez T, Mendez-Sanchez N, Lizardi-Cervera J, Uribe M. Bariatric surgery for non-alcoholic steatohepatitis in obese patients. Cochrane Database Syst Rev. 2010:CD007340. doi: 10.1002/14651858.CD007340.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mummadi RR, Kasturi KS, Chennareddygari S, Sood GK. Effect of bariatric surgery on nonalcoholic fatty liver disease: Systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2008;6:1396–402. doi: 10.1016/j.cgh.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 18.D'Albuquerque LA, Gonzalez AM, Wahle RC, de Oliveira Souza E, Mancero JM, de Oliveira e Silva A. Liver transplantation for subacute hepatocellular failure due to massive steatohepatitis after bariatric surgery. Liver Transpl. 2008;14:881–5. doi: 10.1002/lt.21472. [DOI] [PubMed] [Google Scholar]
- 19.Sasaki A, Nitta H, Otsuka K, Umemura A, Baba S, Obuchi T, et al. Bariatric surgery and non-alcoholic fatty liver disease: Current and potential future treatments. Front Endocrinol (Lausanne) 2014;5:164. doi: 10.3389/fendo.2014.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Zoli M, Melchionda N. Metformin in non-alcoholic steatohepatitis. Lancet. 2001;358:893–4. doi: 10.1016/s0140-6736(01)06042-1. [DOI] [PubMed] [Google Scholar]
- 21.Uygun A, Kadayifci A, Isik AT, Ozgurtas T, Deveci S, Tuzun A, et al. Metformin in the treatment of patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2004;19:537–44. doi: 10.1111/j.1365-2036.2004.01888.x. [DOI] [PubMed] [Google Scholar]
- 22.Bugianesi E, Gentilcore E, Manini R, Natale S, Vanni E, Villanova N, et al. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005;100:1082–90. doi: 10.1111/j.1572-0241.2005.41583.x. [DOI] [PubMed] [Google Scholar]
- 23.Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: The TONIC randomized controlled trial. JAMA. 2011;305:1659–68. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazza A, Fruci B, Garinis GA, Giuliano S, Malaguarnera R, Belfiore A. The role of metformin in the management of NAFLD. Exp Diabetes Res. 2012;2012:716404. doi: 10.1155/2012/716404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shadid S, Jensen MD. Effect of pioglitazone on biochemical indices of non-alcoholic fatty liver disease in upper body obesity. Clin Gastroenterol Hepatol. 2003;1:384–7. doi: 10.1053/s1542-3565(03)00198-8. [DOI] [PubMed] [Google Scholar]
- 26.Aithal GP, Thomas JA, Kaye PV, Lawson A, Ryder SD, Spendlove I, et al. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1176–84. doi: 10.1053/j.gastro.2008.06.047. [DOI] [PubMed] [Google Scholar]
- 27.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. NASH CRN. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–85. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahady SE, Webster AC, Walker S, Sanyal A, George J. The role of thiazolidinediones in non-alcoholic steatohepatitis - a systematic review andmeta analysis. J Hepatol. 2011;55:1383–90. doi: 10.1016/j.jhep.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 29.Foster T, Budoff MJ, Saab S, Ahmadi N, Gordon C, Guerci AD. Atorvastatin and antioxidants for the treatment of nonalcoholic fatty liver disease: The St Francis Heart Study randomized clinical trial. Am J Gastroenterol. 2011;106:71–7. doi: 10.1038/ajg.2010.299. [DOI] [PubMed] [Google Scholar]
- 30.Gómez-Domínguez E, Gisbert JP, Moreno-Monteagudo JA, García-Buey L, Moreno-Otero R. A pilot study of atorvastatin treatment in dyslipemid, non-alcoholic fatty liver patients. Aliment Pharmacol Ther. 2006;23:1643–7. doi: 10.1111/j.1365-2036.2006.02926.x. [DOI] [PubMed] [Google Scholar]
- 31.Horlander J, Kwo P. Atorvastatin for the treatment of NASH. Hepatology. 1997;26:544A. [Google Scholar]
- 32.Kiyici M, Gulten M, Gurel S, Nak SG, Dolar E, Savci G, et al. Ursodeoxycholic acid and atorvastatin in the treatment of nonalcoholic steatohepatitis. Can J Gastroenterol. 2003;17:713–8. doi: 10.1155/2003/857869. [DOI] [PubMed] [Google Scholar]
- 33.Park H, Shima T, Yamaguchi K, Mitsuyoshi H, Minami M, Yasui K, et al. Efficacy of long-term ezetimibe therapy in patients with nonalcoholic fatty liver disease. J Gastroenterol. 2011;46:101–7. doi: 10.1007/s00535-010-0291-8. [DOI] [PubMed] [Google Scholar]
- 34.Loomba R, Sirlin CB, Ang B, Bettencourt R, Jain R, Salotti J, et al. San Diego Integrated NAFLD Research Consortium (SINC) Ezetimibe for the treatment of nonalcoholic steatohepatitis: Assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial) Hepatology. 2015;61:239–50. doi: 10.1002/hep.27647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lavine JE. Vitamin E treatment of nonalcoholic steatohepatitis in children: A pilot study. J Pediatr. 2000;136:734–8. [PubMed] [Google Scholar]
- 36.Hasegawa T, Yoneda M, Nakamura K, Makino I, Terano A. Plasma transforming growth factor-beta1 level and efficacy of alpha-tocopherol in patients with non-alcoholic steatohepatitis: A pilot study. Aliment Pharmacol Ther. 2001;15:1667–72. doi: 10.1046/j.1365-2036.2001.01083.x. [DOI] [PubMed] [Google Scholar]
- 37.Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003;98:2348–50. doi: 10.1111/j.1572-0241.2003.08699.x. [DOI] [PubMed] [Google Scholar]
- 38.Cacciapuoti F, Scognamiglio A, Palumbo R, Forte R, Cacciapuoti F. Silymarin in non alcoholic fatty liver disease. World J Hepatol. 2013;5:109–13. doi: 10.4254/wjh.v5.i3.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Solhi H, Ghahremani R, Kazemifar AM, Hoseini Yazdi Z. Silymarin in treatment of non-alcoholic steatohepatitis: A randomized clinical trial. Caspian J Intern Med. 2014;5:9–12. [PMC free article] [PubMed] [Google Scholar]
- 40.Hashemi SJ, Hajiani E, Sardabi EH. A placebo-controlled trial of silymarin in patients with nonalcoholic fatty liver disease. Hepat Mon. 2009;9:265–70. [Google Scholar]
- 41.Li W, Zheng L, Sheng C, Cheng X, Qing L, Qu S. Systematic review on the treatment of pentoxifylline in patients with non-alcoholic fatty liver disease. Lipids Health Dis. 2011;10:49. doi: 10.1186/1476-511X-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zein CO, Yerian LM, Gogate P, Lopez R, Kirwan JP, Feldstein AE, et al. Pentoxifylline improves nonalcoholic steatohepatitis: A randomized placebo-controlled trial. Hepatology. 2011;54:1610–9. doi: 10.1002/hep.24544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Georgescu EF. Angiotensin receptor blockers in the treatment of NASH/NAFLD: Could they be a first-class option? Adv Ther. 2008;25:1141–74. doi: 10.1007/s12325-008-0110-2. [DOI] [PubMed] [Google Scholar]
- 44.Abu Dayyeh BK, Yang M, Dienstag JL, Chung RT. The effects of angiotensin blocking agents on the progression of liver fibrosis in the HALT-C trial cohort. Dig Dis Sci. 2011;56:564–8. doi: 10.1007/s10620-010-1507-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lugo-Baruqui A, Muñoz-Valle JF, Arévalo-Gallegos S, Armendáriz-Borunda J. Role of angiotensin II in liver fibrosis-induced portal hypertension and therapeutic implications. Hepatol Res. 2009;40:95–104. doi: 10.1111/j.1872-034X.2009.00581.x. [DOI] [PubMed] [Google Scholar]
- 46.Yokohama S, Yoneda M, Haneda M, Okamoto S, Okada M, Aso K, et al. Therapeutic efficacy of an angiotensin II receptor antagonist in patients with nonalcoholic steatohepatitis. Hepatology. 2004;40:1222–5. doi: 10.1002/hep.20420. [DOI] [PubMed] [Google Scholar]
- 47.Gonciarz Z, Besser P, Lelek E, Gundermann KJ, Johannes KJ. Randomized placebo-controlled double-blind trial on “essential” phospholipids in the treatment of fatty liver associated with diabetes. Med Chir Dig. 1988;17:61–5. [Google Scholar]
- 48.Arvind N, Savaikar P, Rajkumar JS. Therapy for NAFLD: A comparative study of essential phospholipids vs. ursodeoxycholic acid. Ind J Clin Pract. 2006;16:21–4. [Google Scholar]
- 49.Dajani AI, Abu Hammour AM, Zakaria MA, Al Jaberi MR, Nounou MA, Semrin AI. Essential phospholipids as a supportive adjunct in the management of patients with NAFLD. Arab J Gastroenterol. 2015 doi: 10.1016/j.ajg.2015.09.001. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 50.Kajikawa S, Imada K, Takeuchi T, Shimizu Y, Kawashima A, Harada T, et al. Eicosapentaenoic acid attenuates progression of hepatic fibrosis with inhibition of reactive oxygen species production in rats fed methionine- and choline-deficient diet. Dig Dis Sci. 2011;56:1065–74. doi: 10.1007/s10620-010-1400-5. [DOI] [PubMed] [Google Scholar]
- 51.Tanaka N, Sano K, Horiuchi A, Tanaka E, Kiyosawa K, Aoyama T. Highly purified eicosapentaenoic acid treatment improves nonalcoholic steatohepatitis. J Clin Gastroenterol. 2008;42:413–8. doi: 10.1097/MCG.0b013e31815591aa. [DOI] [PubMed] [Google Scholar]
- 52.Sanyal AJ, Abdelmalek MF, Suzuki A, Cummings OW, Chojkier M. EPE-A Study Group. No significant effects of ethyl-eicosapentanoic acid on histologic features of nonalcoholic steatohepatitis in a phase 2 trial. Gastroenterology. 2014;147:377–84.e1. doi: 10.1053/j.gastro.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 53.Ma YY, Li L, Yu CH, Shen Z, Chen LH, Li YM. Effects of probiotics on nonalcoholic fatty liver disease: A meta-analysis. World J Gastroenterol. 2013;19:6911–8. doi: 10.3748/wjg.v19.i40.6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vajro P, Mandato C, Licenziati MR, Franzese A, Vitale DF, Lenta S, et al. Effects of Lactobacillus rhamnosus strain GG in pediatric obesity-related liver disease. J Pediatr Gastroenterol Nutr. 2011;52:740–3. doi: 10.1097/MPG.0b013e31821f9b85. [DOI] [PubMed] [Google Scholar]
- 55.Abenavoli L, Scarpellini E, Rouabhia S, Balsano C, Luzza F. Probiotics in non-alcoholic fatty liver disease: Which and when. Ann Hepatol. 2013;12:357–63. [PubMed] [Google Scholar]
- 56.Ikenaga N, Yoshida S, Liu SB, Chung J, Sverdlov D, Marshall D, et al. Targeting Lysyl Oxidase like 2 (LOXL2) Inhibits Collagen Cross-Linking and Accelerates Reversal of Pre-Established Liver Fibrosis. Hepatology. 2013;58:219A–20A. [Google Scholar]
- 57.Mudaliar S, Henry RR, Sanyal AJ, Morrow L, Marschall HU, Kipnes M, et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145:574–82.e1. doi: 10.1053/j.gastro.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 58.Cariou B, Staels B. GFT505 for the treatment of nonalcoholic steatohepatitis and type 2 diabetes. Expert Opin Investig Drugs. 2014;23:1441–8. doi: 10.1517/13543784.2014.954034. [DOI] [PubMed] [Google Scholar]
- 59.Staels B, Rubenstrunk A, Noel B, Rigou G, Delataille P, Millatt LJ, et al. Hepatoprotective effects of the dual peroxisome proliferator-activated receptor alpha/delta agonist, GFT505, in rodent models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology. 2013;58:1941–52. doi: 10.1002/hep.26461. [DOI] [PubMed] [Google Scholar]
- 60.DUR-928. [Last accessed on 2015 May 25]. Available from: http://www.durect.com/wt/durect/page_name/DUR-928 .
- 61.Traber PG, Chou H, Zomer E, Hong F, Klyosov A, Fiel MI, et al. Regression of fibrosis and reversal of cirrhosis in rats by galectin inhibitors in thioacetamide-induced liver disease. PLoS One. 2013;8:e75361. doi: 10.1371/journal.pone.0075361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harrison S. Galectin Therapeutics': Phase 1 Data Advances GR-MD-02 in to Phase 2 Clinical Development. Presented at AASLD Annual Meeting. 2014. [Last accessed on 2015 May 25]. Available from: www.galactintherapeutics.com/releasedetail.cfm?ReleaseID=897897 .
- 63.Safadi R, Konikoff FM, Mahamid M, Zelber-Sagi S, Halpern M, Gilat T, et al. The fatty acid-bile acid conjugate aramchol reduces liver fat content in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2014;12:2085–91.e1. doi: 10.1016/j.cgh.2014.04.038. [DOI] [PubMed] [Google Scholar]
- 64.Regulus Reports First Quarter 2015 Financial Results and Recent Highlights. [Last accessed on 2015 May 25]. Available from: http://www.researchviews.com/healthcare/pharma/infectiousdisease/NewsReport.aspx?Type=1andsector=Infectious DiseaseandArticleID=4571857 .
- 65.Sanyal AJ, Friedman SL, McCullough AJ, Dimick-Santos L. American Association for the Study of Liver Diseases; United States Food and Drug Administration. Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: Findings and recommendations from an American Association for the Study of Liver Diseases-U.S. Food and Drug Administration Joint Workshop. Hepatology. 2015;61:1392–405. doi: 10.1002/hep.27678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stein LL, Dong MH, Loomba R. Insulin sensitizers in nonalcoholic fatty liver disease and steatohepatitis: Current status. Adv Ther. 2009;26:893–907. doi: 10.1007/s12325-009-0072-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nair S, Diehl AM, Wiseman M, Farr GH, Jr, Perrillo RP. Metformin in the treatment of non-alcoholic steatohepatitis: A pilot open label trial. Aliment Pharmacol Ther. 2004;20:23–8. doi: 10.1111/j.1365-2036.2004.02025.x. [DOI] [PubMed] [Google Scholar]
- 68.Mahady S. Therapies for nonalcoholic steatohepatitis. Gastroenterol Hepatol (N Y) 2013;9:40–2. [PMC free article] [PubMed] [Google Scholar]
- 69.Xiao WH, Wang YR, Hou WF, Xie C, Wang HN, Hong TP, et al. The effects of pioglitazone on biochemical markers of bone turnover in the patients with type 2 diabetes. Int J Endocrinol. 2013;2013:290734. doi: 10.1155/2013/290734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fernández-Miranda C, Pérez-Carreras M, Colina F, López-Alonso G, Vargas C, Solís-Herruzo JA. A pilot trial of fenofibrate for the treatment of non-alcoholic fatty liver disease. Dig Liver Dis. 2008;40:200–5. doi: 10.1016/j.dld.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 71.Nseir W, Mahamid M. Statins in nonalcoholic fatty liver disease and steatohepatitis: Updated review. Curr Atheroscler Rep. 2013;15:305. doi: 10.1007/s11883-012-0305-5. [DOI] [PubMed] [Google Scholar]
- 72.Neto-Ferreira R, Rocha VN, Souza-Mello V, Mandarim-de-Lacerda CA, de Carvalho JJ. Pleiotropic effects of rosuvastatin on the glucose metabolism and the subcutaneous and visceral adipose tissue behavior in C57Bl/6 mice. Diabetol Metab Syndr. 2013;5:32. doi: 10.1186/1758-5996-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Argo CK, Loria P, Caldwell SH, Lonardo A. Statins in liver disease: A molehill, an iceberg, or neither? Hepatology. 2008;48:662–9. doi: 10.1002/hep.22402. [DOI] [PubMed] [Google Scholar]
- 74.Riche DM, Fleming JW, Malinowski SS, Black CA, Miller KH, Wofford MR. Resistant nonalcoholic fatty liver disease amelioration with rosuvastatin and pioglitazone combination therapy in a patient with metabolic syndrome. Ann Pharmacother. 2014;48:137–41. doi: 10.1177/1060028013507239. [DOI] [PubMed] [Google Scholar]
- 75.Nakou ES, Filippatos TD, Agouridis AP, Kostara C, Bairaktari ET, Elisaf MS. The effects of ezetimibe and/or orlistat on triglyceride-rich lipoprotein metabolism in obese hypercholesterolemic patients. Lipids. 2010;45:445–50. doi: 10.1007/s11745-010-3409-0. [DOI] [PubMed] [Google Scholar]
- 76.Filippatos TD, Elisaf MS. Role of ezetimibe in non-alcoholic fatty liver disease. World J Hepatol. 2011;30:265–7. doi: 10.4254/wjh.v3.i10.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shu X, Zhang L, Ji G. Vitamin E therapy in non-alcoholic fatty liver disease. Int J Clin Med. 2014;5:87–92. [Google Scholar]
- 78.Pacana T, Sanyal AJ. Vitamin E and nonalcoholic fatty liver disease. Curr Opin Clin Nutr Metab Care. 2012;15:641–8. doi: 10.1097/MCO.0b013e328357f747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mitchel EB, Lavine JE. Review article: The management of paediatric nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2014;40:1155–70. doi: 10.1111/apt.12972. [DOI] [PubMed] [Google Scholar]
- 80.Adams LA, Angulo P. Treatment of non-alcoholic fatty liver disease. Postgrad Med J. 2006;82:315–22. doi: 10.1136/pgmj.2005.042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mouzaki M, Allard J. Non-alcoholic steatohepatitis: The therapeutic challenge of a global epidemic. Ann Gastroenterol. 2012;25:207–17. [PMC free article] [PubMed] [Google Scholar]
- 82.Kotb MA. Molecular mechanisms of ursodeoxycholic Acid toxicity and side effects: Ursodeoxycholic Acid freezes regeneration & induces hibernation mode. Int J Mol Sci. 2012;13:8882–914. doi: 10.3390/ijms13078882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Blaslov K, Bulum T, Zibar K, Duvnjak L. Incretin based therapies: A novel treatment approach for non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20:7356–65. doi: 10.3748/wjg.v20.i23.7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu J, Wang G, Jia Y, Xu Y. GLP-1 receptor agonists: Effects on the progression of non-alcoholic fatty liver disease. Diabetes Metab Res Rev. 2015;31:329–35. doi: 10.1002/dmrr.2580. [DOI] [PubMed] [Google Scholar]
- 85.Balaban YH, Korkusuz P, Simsek H, Gokcan H, Gedikoglu G, Pinar A, et al. Dipeptidyl peptidase IV (DDP IV) in NASH patients. Ann Hepatol. 2007;6:242–50. [PubMed] [Google Scholar]
- 86.Zeng T, Zhang CL, Zhao XL, Xie KQ. Pentoxifylline for the treatment of nonalcoholic fatty liver disease: A meta-analysis of randomized double-blind, placebo-controlled studies. Eur J Gastroenterol Hepatol. 2014;26:646–53. doi: 10.1097/MEG.0000000000000068. [DOI] [PubMed] [Google Scholar]
- 87.Du J, Ma YY, Yu CH, Li YM. Effects of pentoxifylline on nonalcoholic fatty liver disease: A meta-analysis. World J Gastroenterol. 2014;20:569–77. doi: 10.3748/wjg.v20.i2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Paschos P, Tziomalos K. Nonalcoholic fatty liver disease and the renin-angiotensin system: Implications for treatment. World J Hepatol. 2012;4:327–31. doi: 10.4254/wjh.v4.i12.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Anstee QM, Day CP. S-adenosylmethionine (SAMe) therapy in liver disease: A review of current evidence and clinical utility. J Hepatol. 2012;57:1097–109. doi: 10.1016/j.jhep.2012.04.041. [DOI] [PubMed] [Google Scholar]
- 90.Lam B, Younossi ZM. Treatment options for nonalcoholic fatty liver disease. Therap Adv Gastroenterol. 2010;3:121–37. doi: 10.1177/1756283X09359964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Study to Investigate the Effects of Different Doses of S-adenosyl-L-methionine (SAMe) in Subjects With Nonalcoholic Fatty Liver Disease and Non-treated Matched Healthy Volunteers as Control Group (EXPO) [Last accessed on 2015 May 25]. Available from: https://clinicaltrials.gov/ct2/show/NCT01754714?term=S-Adenosyl+Methionine+and+NAFLDandrank=1 .
- 92.Wong VW, Won GL, Chim AM, Chu WC, Yeung DK, Li KC, et al. Treatment of nonalcoholic steatohepatitis with probiotics. A proof-of-concept study. Ann Hepatol. 2013;12:256–62. [PubMed] [Google Scholar]
- 93.Mencarelli A, Cipriani S, Renga B, Bruno A, D'Amore C, Distrutti E, et al. VSL#3 resets insulin signaling and protects against NASH and atherosclerosis in a model of genetic dyslipidemia and intestinal inflammation. PLoS One. 2012;7:e45425. doi: 10.1371/journal.pone.0045425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kelishadi R, Farajian S, Mirlohi M. Probiotics as a novel treatment for non-alcoholic fatty liver disease; a systematic review on the current evidences. Hepat Mon. 2013;13:e7233. doi: 10.5812/hepatmon.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Paolella G, Mandato C, Pierri L, Poeta M, Di Stasi M, Vajro P. Gut-liver axis and probiotics: Their role in non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20:15518–31. doi: 10.3748/wjg.v20.i42.15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ali AH, Carey EJ, Lindor KD. Recent advances in the development of farnesoid X receptor agonists. Ann Transl Med. 2015;3:5. doi: 10.3978/j.issn.2305-5839.2014.12.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cariou B, Zair Y, Staels B, Bruckert E. Effects of the new dual PPAR α/δ agonist GFT505 on lipid and glucose homeostasis in abdominally obese patients with combined dyslipidemia or impaired glucose metabolism. Diabetes Care. 2011;34:2008–14. doi: 10.2337/dc11-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cariou B, Hanf R, Lambert-Porcheron S, Zaïr Y, Sauvinet V, Noël B, et al. Dual peroxisome proliferator-activated receptor α/δ agonist GFT505 improves hepatic and peripheral Insulin sensitivity in abdominally obese subjects. Diabetes Care. 2013;36:2923–30. doi: 10.2337/dc12-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Polyzos SA, Kountouras J, Patsiaoura K, Katsiki E, Zafeiriadou E, Zavos C, et al. Serum vitamin B12 and folate levels in patients with non-alcoholic fatty liver disease. Int J Food Sci Nutr. 2012;63:659–66. doi: 10.3109/09637486.2011.649249. [DOI] [PubMed] [Google Scholar]
- 100.Pearlman M, Loomba R. State of the art: Treatment of nonalcoholic steatohepatitis. Curr Opin Gastroenterol. 2014;30:223–37. doi: 10.1097/MOG.0000000000000060. [DOI] [PubMed] [Google Scholar]
- 101.Eliades M, Spyrou E, Agrawal N, Lazo M, Brancati FL, Potter JJ, et al. Meta-analysis: Vitamin D and non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2013;38:246–54. doi: 10.1111/apt.12377. [DOI] [PubMed] [Google Scholar]
- 102.Kwok RM, Torres DM, Harrison SA. Vitamin D and nonalcoholic fatty liver disease (NAFLD): Is it more than just an association? Hepatology. 2013;58:1166–74. doi: 10.1002/hep.26390. [DOI] [PubMed] [Google Scholar]
- 103.Kalinchenko SY, Tishova YA, Mskhalaya GJ, Gooren LJ, Giltay EJ, Saad F. Effects of testosterone supplementation on markers of the metabolic syndrome and inflammation in hypogonadal men with the metabolic syndrome: The double-blinded placebo-controlled Moscow study. Clin Endocrinol (Oxf) 2010;73:602–12. doi: 10.1111/j.1365-2265.2010.03845.x. [DOI] [PubMed] [Google Scholar]
- 104.Hoyos CM, Yee BJ, Phillips CL, Machan EA, Grunstein RR, Liu PY. Body compositional and cardiometabolic effects of testosterone therapy in obese men with severe obstructive sleep apnoea: A randomised placebo-controlled trial. Eur J Endocrinol. 2012;167:531–41. doi: 10.1530/EJE-12-0525. [DOI] [PubMed] [Google Scholar]
- 105.ClinicalTrials.gov, Safety, Tolerability, Pharmacokinetics (PK) and Pharmacodynamics (PD) Assessment of LCQ908 in Patients with Severe Hypertriglyceridemia. [Last accessed on 2013 Jun 1]. Available from: http://www.clinicaltrials.gov/ct2/show/study/NCT01146522 .
- 106.Stefano JT, de Oliveira CP, Corrêa-Giannella ML, de Lima VM, de Sá SV, de Oliveira EP, et al. Nonalcoholic steatohepatitis (NASH) in ob/ob mice treated with yo jyo hen shi ko (YHK): Effects on peroxisome proliferator-activated receptors (PPARs) and microsomal triglyceride transfer protein (MTP) Dig Dis Sci. 2007;52:3448–54. doi: 10.1007/s10620-007-9810-8. [DOI] [PubMed] [Google Scholar]
- 107.Chande N, Laidlaw M, Adams P, Marotta P. Yo Jyo Hen Shi Ko (YHK) improves transaminases in nonalcoholic steatohepatitis (NASH): A randomized pilot study. Dig Dis Sci. 2006;51:1183–9. doi: 10.1007/s10620-006-8030-y. [DOI] [PubMed] [Google Scholar]
- 108.Charatcharoenwitthaya P, Levy C, Angulo P, Keach J, Jorgensen R, Lindor KD. Open-label pilot study of folic acid in patients with nonalcoholic steatohepatitis. Liver Int. 2007;27:220–6. doi: 10.1111/j.1478-3231.2006.01404.x. [DOI] [PubMed] [Google Scholar]
- 109.Setola E, Monti LD, Galluccio E, Palloshi A, Fragasso G, Paroni R, et al. Insulin resistance and endothelial function are improved after folate and vitamin B12 therapy in patients with metabolic syndrome: Relationship between homocysteine levels and hyperinsulinemia. Eur J Endocrinol. 2004;151:483–9. doi: 10.1530/eje.0.1510483. [DOI] [PubMed] [Google Scholar]
- 110.Shang J, Chen LL, Xiao FX, Sun H, Ding HC, Xiao H. Resveratrol improves non-alcoholic fatty liver disease by activating AMP-activated protein kinase. Acta Pharmacol Sin. 2008;29:698–706. doi: 10.1111/j.1745-7254.2008.00807.x. [DOI] [PubMed] [Google Scholar]
- 111.Li L, Hai J, Li Z, Zhang Y, Peng H, Li K, et al. Resveratrol modulates autophagy and NF-κB activity in a murine model for treating non-alcoholic fatty liver disease. Food Chem Toxicol. 2014;63:166–73. doi: 10.1016/j.fct.2013.08.036. [DOI] [PubMed] [Google Scholar]
- 112.Guo H, Li D, Ling W, Feng X, Xia M. Anthocyanin inhibits high glucose-induced hepatic mtGPAT1 activation and prevents fatty acid synthesis through PKCζ. J Lipid Res. 2011;52:908–22. doi: 10.1194/jlr.M013375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Valenti L, Riso P, Mazzocchi A, Porrini M, Fargion S, Agostoni C. Dietary anthocyanins as nutritional therapy for nonalcoholic fatty liver disease. Oxid Med Cell Longev. 2013;2013:145421. doi: 10.1155/2013/145421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Marcolin E, San-Miguel B, Vallejo D, Tieppo J, Marroni N, González-Gallego J, et al. Quercetin treatment ameliorates inflammation and fibrosis in mice with nonalcoholic steatohepatitis. J Nutr. 2012;142:1821–8. doi: 10.3945/jn.112.165274. [DOI] [PubMed] [Google Scholar]
- 115.Li X, Wang R, Zhou N, Wang X, Liu Q, Bai Y, et al. Quercetin improves insulin resistance and hepatic lipid accumulationin vitro in a NAFLD cell model. Biomed Rep. 2013;1:71–6. doi: 10.3892/br.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang W, Wang C, Ding XQ, Pan Y, Gu TT, Wang MX, et al. Quercetin and allopurinol reduce liver thioredoxin-interacting protein to alleviate inflammation and lipid accumulation in diabetic rats. Br J Pharmacol. 2013;169:1352–71. doi: 10.1111/bph.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ochiai H, Ooka H, Shida C, Ishikawa T, Inoue D, Okazaki R. Acarbose treatment increases serum total adiponectin levels in patients with type 2 diabetes. Endocr J. 2008;55:549–56. doi: 10.1507/endocrj.k07e-107. [DOI] [PubMed] [Google Scholar]
- 118.Hajiaghamohammadi AA, Miroliaee A, Samimi R, Alborzi F, Ziaee A. A comparison of ezetimibe and acarbose in decreasing liver transaminase in nonalcoholic fatty liver disease: A randomized clinical trial. Govaresh. 2013;18:186–90. [Google Scholar]
- 119.Nozaki Y, Fujita K, Yoneda M, Wada K, Shinohara Y, Takahashi H, et al. Long-term combination therapy of ezetimibe and acarbose for non-alcoholic fatty liver disease. J Hepatol. 2009;51:548–56. doi: 10.1016/j.jhep.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 120.Bambha K, Wilson LA, Unalp A, Loomba R, Neuschwander-Tetri BA, Brunt EM, et al. Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN). Coffee consumption in NAFLD patients with lower insulin resistance is associated with lower risk of severe fibrosis. Liver Int. 2014;34:1250–8. doi: 10.1111/liv.12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Saab S, Mallam D, Cox GA, 2nd, Tong MJ. Impact of coffee on liver diseases: A systematic review. Liver Int. 2014;34:495–504. doi: 10.1111/liv.12304. [DOI] [PubMed] [Google Scholar]
- 122.Buang Y, Wang YM, Cha JY, Nagao K, Yanagita T. Dietary phosphatidylcholine alleviates fatty liver induced by orotic acid. Nutrition. 2005;21:867–73. doi: 10.1016/j.nut.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 123.Gundermann KJ, Kuenker A, Kuntz E, Droździk M. Activity of essential phospholipids (EPL) from soybean in liver diseases. Pharmacol Rep. 2011;63:643–59. doi: 10.1016/s1734-1140(11)70576-x. [DOI] [PubMed] [Google Scholar]
- 124.Yin D, Kong L. Observation for curative effect of essentiale in treatment of fatty liver caused by diabetes mellitus. Med J Q Ilu. 2000;15:277–8. [Google Scholar]
- 125.Du Q. Treatment of 52 cases with hepatic dysfunctional fatty liver with essentiale. Chin J Gastro Heap. 2004;13:21–4. [Google Scholar]
- 126.Padma L, Mukaddam Q, Trailokya A. An Observational Study of Essentiale-L in the treatment of Patients with Fatty Liver Disease. Indian J Clin Pract. 2013;23:735–9. [Google Scholar]
- 127.Sun C, Zheng X, Tan Z, Cui F, Zhang R, Zhang H. Clinical observation on polyene phosphatidyl choline and metformin in the treatment of type-2 diabetes and non-alcoholic fatty liver disease. Clinical Focus. 2008;23:1272–3. [Google Scholar]
- 128.Wu Y. Effective analysis of type 2 diabetic united adiposis hepatica with polyene phosphatidylcholine. J Tradit Chin Med. 2009;29:41–2. [Google Scholar]
- 129.Loguercio C, Andreone P, Brisc C, et al. Silybin combined with phosphatidylcholine and vitamin E in patients with nonalcoholic fatty liver disease: A randomized controlled trial. Free Radic Biol Med. 2012;52:1658–65. doi: 10.1016/j.freeradbiomed.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 130.Sas E, Grinevich V, Efimov O, Shcherbina N. Beneficial influence of polyunsaturated Phosphatidylcholine enhances functional liver condition and liver structure in patients with Nonalcoholic steatohepatitis. Results of prolonged randomized blinded prospective clinical study. J Hepatol. 2013;58(Suppl 1):S549. [Google Scholar]
- 131.Arvind N, Savaikar P, Rajkumar J. Therapy for NAFLD. A comparative study of essential phospholipids vs ursodeoxycholic acid. Indian J Clin Pract. 2006;16:21–4. [Google Scholar]
- 132.Zeng JT, Chen J. Clinical observation on the results in treatment of NAFLD with polyene phosphatidylcholine combined with Xuezhikang capsules. China Trop Med. 2008;8:778–9. [Google Scholar]
- 133.Dajani AI, Abu Hammour AM, Zakaria MA, Al Jaberi M, Nounou MA. Essential phospholipids as a supportive adjunct to the management of patients with primary NAFLD and NAFLD associated with type 2 diabetes mellitus or hyperlipidaemia. Hepatol Int. 2013;7(Suppl 1):S1–754. [Google Scholar]
- 134.Abenavoli L, Aviello G, Capasso R, Milic N, Capasso F. Milk thistle for treatment of nonalcoholic fatty liver disease. Hepat Mon. 2015;11:173–7. [Google Scholar]
- 135.Abenavoli L, Capasso R, Milic N, Capasso F. Milk thistle in liver diseases: Past, present, future. Phytother Res. 2010;24:1423–32. doi: 10.1002/ptr.3207. [DOI] [PubMed] [Google Scholar]