Abstract
Staphylococcus aureus is a common cause of severe bloodstream infection. We performed a systematic review to assess whether consultation with infectious disease specialists decreased all-cause mortality or rate of complications of S aureus bloodstream infections. The review also assessed parameters associated with the quality of management of the infection. We searched for eligible studies in PubMed, Embase, Scopus, and clinical trials.gov as well as the references of included studies. We identified 22 observational studies and 1 study protocol for a randomized trial. A meta-analysis was not performed because of the high risk of bias in the included studies. The outcomes are reported in a narrative review. Most included studies reported survival benefit, in the adjusted analysis. Recommended management strategies were carried out significantly more often among patients seen by an infectious disease specialist. Trials, such as cluster-randomized controlled trials, can more validly assess the studies at low risk of bias.
Keywords: bloodstream infection, infectious disease specialist consultation, Staphylococcus aureus
BACKGROUND
Staphylococcus aureus (S aureus) is a common cause of severe bloodstream infection [1]. The incidence was found to be 26/100 000 population per year, and the 30-day all-cause mortality is approximately 20% [3]. S aureus possesses a great affinity for foreign bodies and has a propensity to produce biofilm, making patients vulnerable to infections of catheters, prosthetic joints, heart valves, and pacemakers. They are also prone to metastatic infections and abscess formation. S aureus bloodstream infections may result in severe sepsis with organ failure and septic shock [4]. Risk factors for acquiring S aureus bloodstream infection include older age, dialysis treatment, diabetes mellitus, and immunosuppression [1, 5]. Factors associated with a poor prognosis of the infection include older age, comorbid conditions, severity of the infection, certain foci of infection including endocarditis, pneumonia, and undetermined focus, inadequate antibiotic treatment, and nonremoval of a removable infectious focus [3]. Echocardiography is recommended for all patients with S aureus bacteremia [6]. A recent review paper recommends that although the evidence in this field is weak, transthoracic echocardiography may be adequate for patients with a low risk of endocarditis [7]. Removal of the source of infection is important because nonremoval of an intravascular device has been associated with treatment failure [8], and a noneradicated focus has been found to be a predictor of mortality [9]. Timing and choice of antibiotic are important, because both delay in treatment and inappropriate choice of antibiotic are associated with decreased survival [3].
Expected Effect of the Intervention
The intervention consists of implementing infectious disease specialist consultations for patients with S aureus bacteremia. Current management recommendations may vary over time, but the intervention is an attempt to implement the best available practice. Four previous articles have summarized part of this evidence [7, 10–12]. When this article was submitted for publication, no full systematic review of the literature regarding this topic had been published; however, since then, an article has been published on this subject and will be discussed under Agreements and Disagreements With Other Studies or Reviews [13]. Our primary objective was to assess whether consultation with an infectious disease specialist among patients with S aureus bloodstream infection decreased mortality rates or rates of recurrence of the infection compared with those who did not receive the intervention. We also studied whether the intervention increased the quality of patient management.
METHODS
Criteria for Considering Studies for This Review
All controlled trials and prospective or retrospective observational studies on this topic were eligible for inclusion in our study. The studies were grouped according to their design. The consultation could occur in person or by review of patient records. We included (1) studies comparing those receiving the intervention with those who did not and (2) studies comparing time periods with varying degrees of implementation of infectious disease specialist consultation.
Types of Outcome Measures
The primary outcome of interest was all-cause mortality within 7, 30, or 90 days of onset of infection as well as in-hospital mortality. Secondary outcomes included recurrence of bacteremia as well as parameters indicating quality of patient management. The latter included rates of examination by echocardiography, frequency of follow-up blood cultures, frequency of detection of focus of infection including endocarditis and metastatic infection, whether a removable focus was removed or drained, and adequacy of antibiotic treatment.
Search Methods for Identification of Studies
PubMed was searched from 1944 through August 26, 2015 with a combination of medical subject heading (MeSH) and free text terms. The search included terms to identify S aureus, the presence of bloodstream infection, and the presence of infectious disease specialist consultation. Embase and Scopus were searched through August 26, 2015. The detailed search strategy is provided in the Supplementary Material. Clinical trials.gov was searched for completed or ongoing randomized trials. Reference lists of all included studies were searched. Studies in all languages were eligible for inclusion in the review. A librarian experienced with literature search for systematic reviews was consulted. The identified articles were screened for relevance based on title or abstract. For studies that met the inclusion criteria, or cases in which the relevance was not clear, the full text was studied.
Assessment of Risk of Bias in Included Studies
Risk of bias was assessed using the Newcastle-Ottawa scale for assessing the quality of nonrandomized studies. This scale was subdivided into 3 categories, which were evaluated based on the selection of the exposed and nonexposed groups, the comparability of the groups, and the ascertainment of exposure or outcome. A star was awarded if the exposed cohort in the study was truly or somewhat representative of the cohort with this disease in the community, if the nonexposed cohort was drawn from the same community as the exposed cohort, if the outcome was assessed by record linkage or structured interview, and if there was demonstration that the outcome of interest was not present at the start of the study. For comparability, a star was given for adjustment for the most important confounding factor, and an additional star was given for adjustment of any other factor. For outcome, a star was awarded if the outcome measure was ascertained blindly or by independent record linkage, if the follow up was long enough for the outcome to occur, and if there was little loss to follow up. A study was awarded a maximum of 4 stars for selection, 2 stars for comparability, and 3 stars for outcome ascertainment [14]. All studies were assessed by 2 authors, the first author and one coauthor. Any disagreement regarding the assessment was resolved by discussion within the group. Particular attention was paid to selection bias and confounding and how these were identified and adjusted.
Measures of Treatment Effect
The Revman data analysis tool, developed by the Cochrane Collaboration, was used for summarizing the outcomes. Data were entered in outcome tables, and odds ratios (ORs) with 95% confidence intervals (CIs) were calculated by the Mantel-Haenzsel method. For adjusted analysis, the log OR and standard error were entered, and ORs were calculated by inverse variance methods. All results were reported for those receiving the intervention compared with those who did not. If the included study reported results for the control group compared with the intervention group, the results were inversed for the purpose of this review so that the magnitude of the results from all studies and subgroup analyses could be compared. A pooled analysis was not performed because of the high risk of bias in the included studies, irrespective of the statistical measures of heterogeneity. The estimates of treatment effect and CIs for each study were displayed by forest plots, and a funnel plot was used to assess the existence of publication bias.
RESULTS
Results of the Search
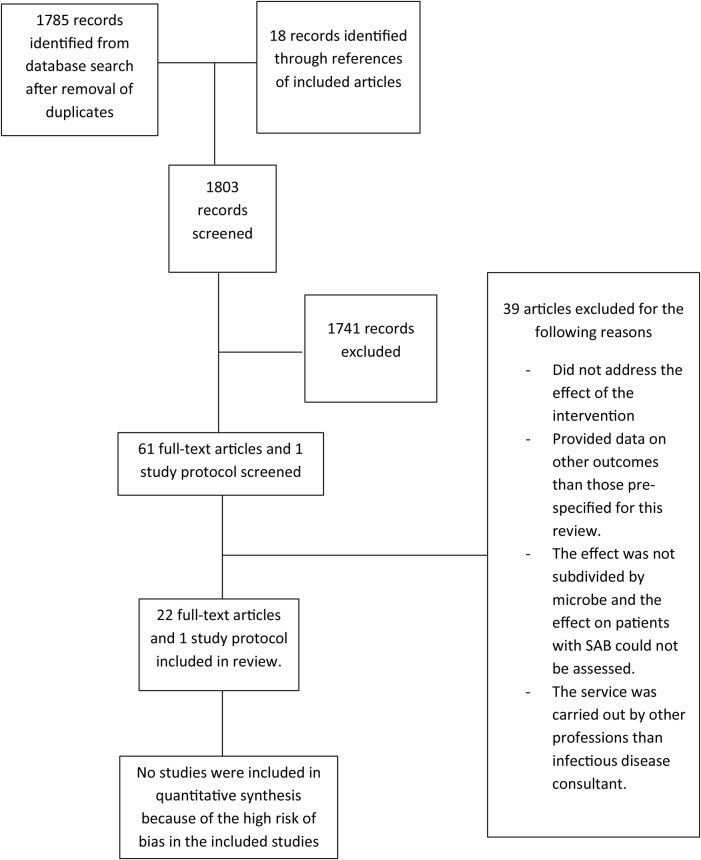
The final database search was conducted on August 26, 2015 and revealed 1785 records identified after removal of duplicates. In addition, 18 studies were identified from the references of included studies. In total, 1803 record abstracts or titles were screened and 1741 were excluded during the screening. Sixty-one full-text articles and 1 study protocol were assessed, and 22 studies and 1 study protocol met the inclusion criteria for this systematic review. Thirty-nine studies were excluded because the intervention did not specify information for S aureus or primary outcomes of interest for this review (Figure 1).
Figure 1.
Literature search flowchart. Abbreviation: SAB, Staphylococcus aureus bloodstream infection.
Among the 22 studies included, 16 assessed the effect of infectious disease consultation by comparing those receiving the consultation with those who did not [10, 15–28] and whether the advice given was heeded or not [8]. Five studies compared time periods in which an intervention with infectious disease consultation was offered or implemented on a mandatory basis to a time period in which this intervention was not systematically offered [29–33], and 1 study compared early and late time periods after implementation of mandatory infectious disease consultation for patients with S aureus bloodstream infection [34] (Table 1).
Table 1.
Description of Included Studies
| Study | Setting | Study Design | Intervention/Control | Number of Patients (Intervention/Control) |
|---|---|---|---|---|
| Lundberg et al [15] | 900-bed teaching hospital | Matched retrospective cohort study | IDC/No IDC | 18 (9/9) |
| Fowler et al [8] | University medical center | Prospective cohort study | Advice from IDC heeded/not heeded | 244 (112/132) |
| Mylotte and Tayara [16] | 300-bed public university affiliated hospital | Retrospective cohort study | IDC/No IDC | 281 (100/181) |
| Kaech et al [17] | 800-bed university hospital | Retrospective cohort study | IDC/No IDC | 308 |
| Jenkins et al [29] | 400-bed teaching hospital | Retrospective cohort study | Preintervention period/Intervention period | 234 (100/134) |
| Rieg et al [18] | 1600-bed tertiary care center | Cohort study. First period retrospective, second period prospective. | IDC/No IDC | 521 (350/171) |
| Lahey et al [19] | Tertiary care hospital | Prospective cohort study | IDC/No IDC | 240 (122/118) |
| Nagao et al [34] | 1240-bed tertiary hospital | Retrospective cohort study | Early and late period after mandatory IDC was implemented | 346 (194/152) |
| Honda et al [20] | Large tertiary care hospital | Prospective cohort study | IDC/No IDC | 341 (111/230) |
| Choi et al [21] | Hospital with <400 beds | Retrospective cohort study | IDC/No IDC | 100 (42/58) |
| Robinson et al [22] | 955-bed tertiary referral center | Retrospective cohort study | IDC/No IDC | 599 (162/437) |
| Isobe et al [23] | University hospital | Retrospective cohort study | IDC/No IDC | 115 (28/87) |
| Pragman et al [10] | 279-bed medical center | Retrospective cohort study | IDC/No IDC | 233 (179/54) |
| Pastagia et al [24] | 1171-bed tertiary care center | Retrospective cohort study | IDC/No IDC | 699 (461/238) |
| Forsblom et al [25] | University hospital | Retrospective cohort study | IDC/telephone consultation only/No IDC | 342 (245/62/35) |
| Lopez-Cortes et al [30] | 12 tertiary hospitals | Quazi-experimental study | Preintervention period/Intervention period. | 508 (221/287) |
| Fries et al [26] | Tertiary care hospital | Retrospective cohort study | IDC/No IDC | 177 (142/35) |
| Tissot et al [27] | 1000-bed tertiary care center | Retrospective cohort study | IDC/No IDC | 148 (118/30) |
| Borde et al [31] | 200-bed community hospital | Cohort study. First period retrospective, second period prospective. | Preintervention period/Intervention period | 59 (20/39) |
| Saunderson et al [32] | Large acute university hospital | Cohort study. First period retrospective, second period prospective. | Preintervention period/Intervention period | 63 (35/28) |
| Bai et al [28] | 6 academic and community hospitals | Retrospective cohort study | IDC/No IDC | 847 (506/341) |
| Saunderson et al [33] | Teaching hospital | Cohort study. First period retrospective, second period prospective. | Preintervention period/Intervention period. | 477 (183/294) |
Abbreviation: IDC, infectious disease consultation.
The studies were published between 1998 and 2015 and included between 18 and 847 subjects. In total, there were data on 6927 patients. Eight studies were carried out in Europe [17, 18, 25, 27, 30–33], 3 studies were carried out in Asia [21, 23, 34], 1 study was carried out in Australia [22], and 10 studies were carried out in North America [8, 10, 15, 16, 19, 20, 24, 26, 28, 29].
Risk of Bias in Included Studies
All of the included studies are observational and as such are at an increased risk of bias, mainly selection bias. Some studies reported incomplete follow-up data [18, 27, 34], which make their outcome assessment somewhat less robust. However, most studies included all patients meeting defined criteria consecutively over a given period, so that the overall outcome assessments were deemed to be reliable. Most studies excluded those who died before the blood culture results were available, or where care was withdrawn, because they would not have been able to benefit from the intervention. In all the studies where baseline variables were displayed, there were differences in factors that could be associated with the risk of mortality between the intervention groups (Supplementary Table 1). Sixteen studies provided effect estimates adjusted for potential confounding factors [10, 15–21, 24, 25, 27–30, 33, 34], and 6 studies provided unadjusted effect estimates only [8, 22, 23, 26, 31, 32]. The degree of adjustment of all important confounders differed between the studies (Supplementary Table 1). The details of the Newcastle-Ottawa score are presented in Table 2. A funnel plot of studies assessing unadjusted outcomes did not show any sign of publication bias (Figure 2).
Table 2.
Summary of the Newcastle-Ottawa Score of Included Studiesa
| Article | Selection | Comparability | Outcome |
|---|---|---|---|
| Lundberg et al [15] | **** | ** | *** |
| Fowler et al [8]b | **** | *** | |
| Mylotte and Tayara [16] | **** | ** | *** |
| Kaech et al [17] | **** | ** | *** |
| Jenkins et al [29]c | *** | ** | *** |
| Rieg et al [18]d | **** | ** | ** |
| Lahey et al [19] | **** | ** | *** |
| Honda et al [20] | **** | ** | *** |
| Choi et al [21] | **** | ** | *** |
| Robinson et al [22] | **** | *** | |
| Isobe et al [23] | **** | *** | |
| Pragman et al [10] | **** | ** | ** |
| Pastagia et al [24] | **** | ** | ** |
| Forsblom et al [25] | **** | ** | *** |
| Lopez-Cortes et al [30]c | *** | ** | *** |
| Fries et al [26] | **** | ** | |
| Tissot et al [27] | **** | ** | *** |
| Borde et al [31] | *** | *** | |
| Saunderson et al [32]c | *** | *** | |
| Bai et al [28] | **** | ** | *** |
| Saunderson et al [33]c | *** | ** | *** |
a Content of the Newcastle-Ottawa scale: selection is graded based on representativeness of the exposed cohort, selection of the nonexposed cohort, ascertainment of exposure, and demonstration that the outcome of interest was not present at the start of the study. Comparability refers to control of cofactors with 1 star for adjustment of the most important cofactor and an additional star for adjustment for additional cofactors. Outcome refers to how the outcome was assessed, if the follow-up time was long enough, and whether it was complete. A study can be awarded a maximum of 4 stars for selection, 2 stars for comparability, and 3 stars for outcome ascertainment.
b Adjusted analysis was reported to have been performed, but results are not included in the article.
c Compares different time periods: the exposed cohort is from the same community but not the same time, and as such the study has not been awarded a star in question 2 under selection (not fully comparable communities).
d Ninety patients lost to follow up for 90-day mortality.
Figure 2.
Funnel plot of unadjusted all-cause mortality and recurrence of studies comparing infectious disease consultation (IDC) to no IDC. Abbreviations: OR, odds ratio; SE, standard error.
Effects of Interventions
All-Cause Mortality and Recurrence Rates
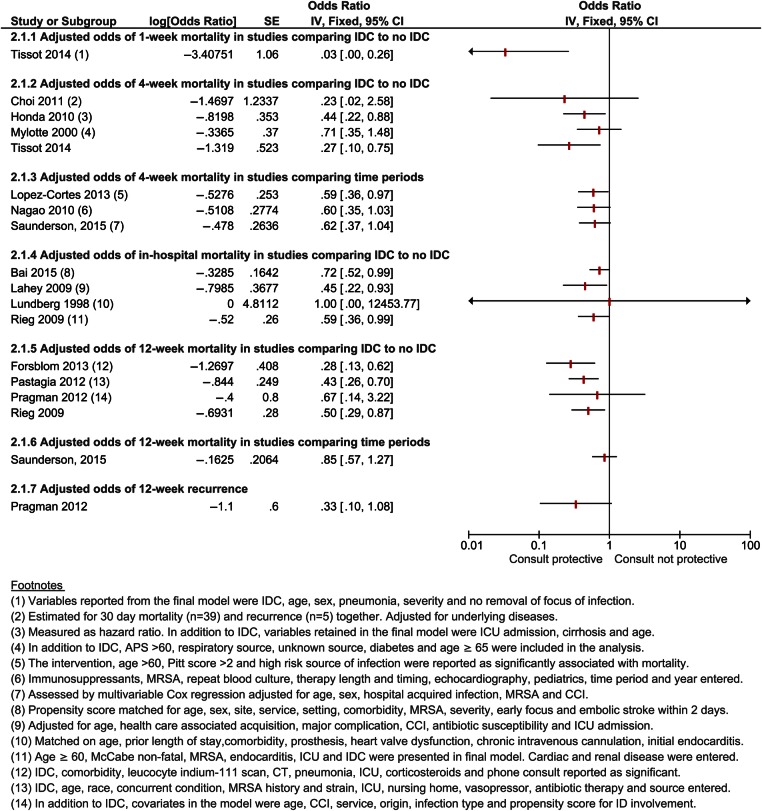
Most studies comparing those who received the intervention with those who did not reported a clear benefit of infectious disease consultation in unadjusted analysis for all-cause mortality after 1 week, 4 weeks, and in-hospital. The effect was less clear for 12-week mortality, with 2 studies showing a benefit [18, 25] and 2 studies showing no significant benefit [8, 10] (Figure 3). Eleven studies provided adjusted estimates of 1 or multiple of these outcomes: 1 study showed benefit after 1 week (OR = 0.03; 95% CI, .00–.26) [27]; 2 studies showed benefit after 4 weeks (OR = 0.44 [95% CI, .22–.88] [20] and OR = 0.27 [95% CI, .10–.75]) [27]; and 2 studies suggested a benefit, although the statistical evidence for this effect was insufficient (OR = 0.23 [95% CI, 0.02–2.56] [21] and OR = 0.71 [95% CI, .35–1.48]) [16]. Three studies showed benefit in adjusted analysis after 12 weeks [18, 24, 25], with OR from 0.28 (95% CI, .13–.62) [25] to 0.50 (95% CI, .29–.87) [18], and 1 study suggested a benefit, although this was not statistically significant (OR = 0.67; 95% CI, .14–3.22) [10]. Most studies that assessed in-hospital mortality showed benefit in adjusted analysis with OR estimates ranging from 0.45 (95% CI, .22–.93) [19] to 0.72 (95% CI, .52–.99) [28], except for Kaech [17]. Bai [28] assessed in-hospital mortality after 12 weeks both by a propensity score-matched analysis and by a thoroughly adjusted logistic regression model and showed a benefit of the intervention (hazard ratio = 0.72; 95% CI, .52–.99) (Figure 4).
Figure 3.
Unadjusted outcome analysis for mortality and recurrence. Abbreviations: CI, confidence interval; IDC, infectious disease consultation.
Figure 4.
Adjusted outcome analysis for mortality and recurrence. Abbreviations: APS, acute physiology score; CCI, Charlson comorbidity index; CI, confidence interval; ICU, intensive care unit; IDC, infectious disease consultation; MRSA, methicillin-resistant Staphylococcus aureus; SE, standard error.
Among studies comparing 2 time periods, 6 provided unadjusted measures and 3 provided adjusted measures of all-cause mortality. In unadjusted analysis, most studies showed reduced or borderline reduced all-cause 4-week mortality [30, 33, 34], except for the study on pediatric patients by Saunderson [32]. In adjusted analysis, Lopez-Cortes [30] showed benefit for 4-week mortality (OR = 0.59; 95% CI, .36–.97), and 2 studies showed borderline statistical evidence for benefit with OR = 0.60 (95% CI, .35–1.03) [34] and OR = 0.62 (95% CI, .37–1.04) [33], respectively. For 12-week mortality, 3 studies did not show a clear benefit in the unadjusted analysis [29, 30, 32] (Figure 3), whereas 1 study showed a benefit in the intervention period in unadjusted analysis with OR = 0.62 (95% CI, .40–.96) but not in adjusted analysis (OR = 0.85; 95% CI, .57–1.27) [33] (Figure 4).
Eight studies examined the recurrence of bloodstream infection within 12 weeks. In the study by Fowler et al [8], there was less recurrence among those who heeded the advice of the infectious disease consultant compared with those who did not (OR = 0.28; 95% CI, .10–.77); other studies were inconclusive (OR = 0.22 [95% CI, .04–1.23] [25] and OR = 1.29 [95% CI, .42–4.02]) [18] (Figure 3). One study examined the adjusted risk of 12-week relapse and suggested a protective effect of the intervention (OR = 0.33; 95% CI, .10–1.08) [10] (Figure 4).
Patient Management Strategies
The intervention led to increased rates of examination with echocardiography and an increased rate of acquisition of repeat blood cultures in most studies. There was also an increase in detection of metastatic complications and focus of infection. Studies that assessed adequacy of choice and timing of antibiotic treatment reported a positive effect of the intervention. The effect was less clear when it came to removal of a removable infectious focus, with some studies reporting increased rates and some not showing a clear effect (Figure 5).
Figure 5.
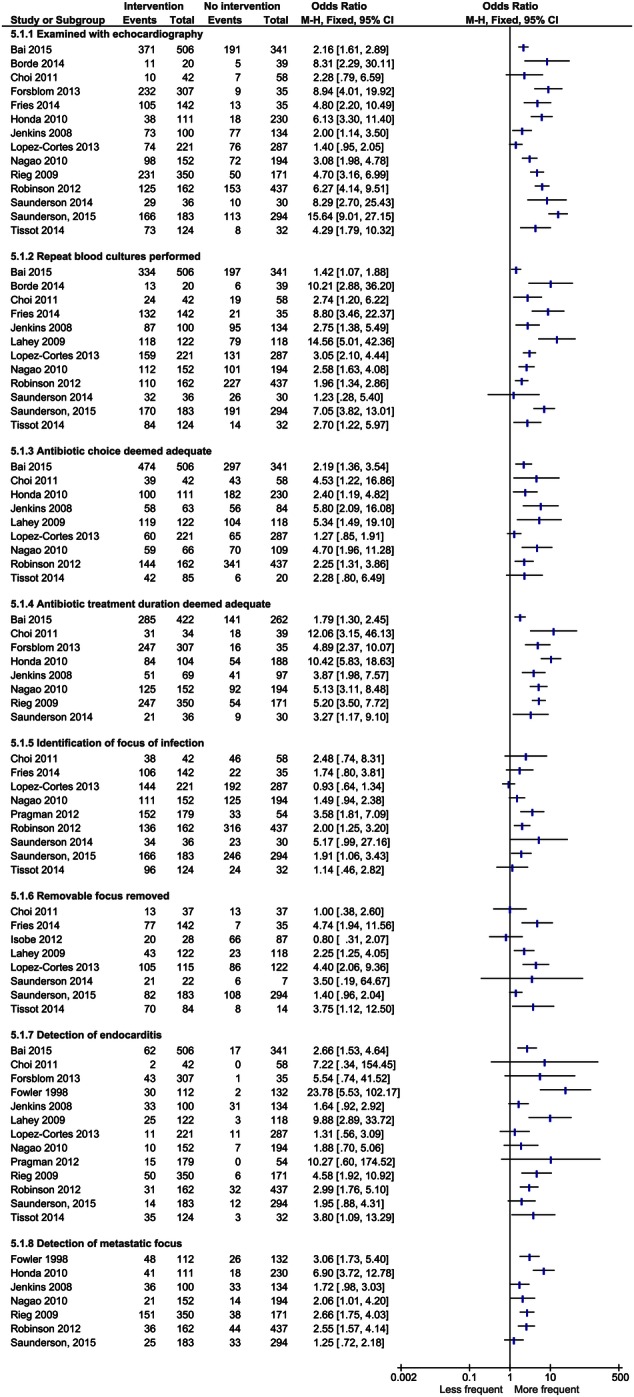
Effect of the intervention on patient management. Abbreviation: CI, confidence interval.
DISCUSSION
Summary of Main Results
Most studies comparing those who received infectious disease consultation with those who did not showed benefit on all-cause mortality in unadjusted analysis and many also in adjusted analysis, with varying degree of adjustment for confounding variables. At least 1 study with very thorough adjustment for covariates showed benefit of the intervention [28].
Among studies comparing time periods with and without mandatory infectious disease consultation, most studies showed benefit after 4 weeks, but a less clear effect after 12 weeks. Due to the design of these studies, other events having an effect on mortality may have occurred at the same time as the implementation of mandatory infectious disease consultation so that the effect of the program itself is less discernable. On the other hand, these studies analyze the entire population with S aureus bloodstream infection, and they may be more likely to reflect the overall effect of the intervention. The reason why there is a difference in effect on 4-week and 12-week mortality in studies comparing time periods could be because the outcome at 4 weeks is more likely to reflect mortality secondary to the infection, whereas 12-week mortality may also reflect the severity of underlying conditions. None of the included studies reported data which indicated that the intervention was harmful for patients.
The included studies detected an increase in quality of the management of patients with S aureus bloodstream infections. Data from observational studies show that these management strategies have been shown to increase the success rates of treatment [3, 9, 35]. The increase in detection of complications such as endocarditis and metastatic infection could be due to a higher proportion of high-risk patients being referred to a specialist. However, increased rates of examination with echocardiography have also led to increased rates of detection of endocarditis in patients who exhibited no specific clinical signs or symptoms [36]. Moreover, this increase was also noted in studies comparing time periods before and after implementation of routine consultation, which supports that the increase reported is associated with an increase in detection [29].
Overall Completeness and Applicability of Evidence
Most included studies were carried out at larger tertiary referral centers or university hospitals, but some were from smaller hospitals. The studies were conducted in North America, Europe, Asia, and Australia, and as such they provided a fairly good representation of the situation in many industrialized countries. The studies themselves were heterogeneous in their recruitment, size of the intervention groups, types and timing of outcomes assessed, and how potential confounders were adjusted in the analysis. This is a cause for concern regarding the overall robustness of the results and the true effect of the intervention. It is unclear how much of a pooled estimate of such studies is due to the actual effect of the intervention and how much is due to the baseline difference in mortality risk between those who received the intervention and those who did not. On one hand, in some studies, patients with an ultimately or rapidly fatal disease or those residing in a nursing home were underrepresented among those being seen by the infectious disease specialist. These factors may affect the outcome estimate in favor of the intervention, even if the intervention is not truly beneficial. On the other hand, in some studies, those receiving the intervention had indicators of more severe disease, as indicated by admissions to the intensive care unit. This could affect the outcome estimate in favor of the nonexposed group, although the intervention was actually beneficial. For this reason, we chose not to meta-analyze the data and opted instead to summarize the findings of these studies and describe their strengths and weaknesses.
One randomized controlled trial for this intervention is registered (clinical trials.gov identifier NCT00622882), and if the study is completed it may give a more robust estimate of its overall effect. In general, it can be problematic to perform randomized trials with no specialist consultation because the risk of this suboptimal management can cause harm to patients. The study in question was registered in 2008, before many of the studies referenced in this review were conducted, so the uncertainty about the benefit of the intervention was more pronounced. However, one approach for currently studying this clinical intervention could be to randomize patients to mandatory specialist consultation rather than referral for consultation with a specialist, at the physician's discretion. Cluster-randomized trials at the institution level would be one way of performing such a trial, avoiding cross-contamination of the intervention within an institution.
There are limitations to our study. The literature search was carried out with help from a qualified searcher, but the primary screening of abstracts and papers was carried out by 1 author, and, as such, there is a risk of overlooking papers and introducing selection bias. However, we searched 3 databases in addition to the references of all included studies, and when there was doubt about the inclusion criteria, it was discussed among the coauthors. In addition, the included papers were reviewed by experts in epidemiology and infectious diseases. We chose to use the Newcastle-Ottawa scale to assess the quality of the studies, because this reveals more details than the Grade system, where all of the included studies would be classified as low or very low quality because of the study design. However, the Newcastle-Ottawa scale may not be optimal for assessing clinical observational studies, and it may not be sufficiently sensitive to detect subtle but important differences in quality between the included studies [37, 38]. In general, there is a need for improved instruments for assessing the quality of observational studies included in systematic reviews [39].
Agreements and Disagreements With Other Studies or Reviews
Another systematic review on the same topic was published by Vogel et al [13] while this article was under review. The selection of papers is quite similar, and the conclusion regarding the beneficial effect of the intervention is also similar. Our approaches differ in that we have chosen not to conduct a meta-analysis of these studies because of the methodological differences and uncertainty about the true causal effect, to avoid producing a biased pooled estimate. Some studies have discussed a subset of these papers as part of the discussion of their own research or as part of narrative reviews [7, 10–12]. All of these reviews emphasized the improved management of these patients by the intervention, and some recommended that the intervention should be offered to all patients with S aureus bloodstream infection [7, 10].
CONCLUSIONS
Infectious disease specialist consultation for patients with S aureus bloodstream infection is associated with improved patient management and, plausibly, improved survival. Because of the inherent difficulties of assessing the true effect of interventions in observational studies, more robust methods, such as cluster-randomized controlled trials at the institution level, should be developed.
Supplementary Data
Supplementary material is available online at Open Forum Infectious Diseases online (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Acknowledgments
We thank Jan Glover for kindly assisting with the literature search.
Financial support. This work was supported by the Liaison Committee between the Central Norway Regional Health Authority (RHA) and the Norwegian University of Science and Technology (NTNU). J. P. received a travel grant from the Fulbright Foundation.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Laupland KB, Church DL, Mucenski M et al. . Population-based study of the epidemiology of and the risk factors for invasive Staphylococcus aureus infections. J Infect Dis 2003; 187:1452–9. [DOI] [PubMed] [Google Scholar]
- 2.Laupland KB, Lyytikainen O, Sogaard M et al. . The changing epidemiology of Staphylococcus aureus bloodstream infection: a multinational population-based surveillance study. Clin Microbiol Infect 2013; 19:465–71. [DOI] [PubMed] [Google Scholar]
- 3.van Hal SJ, Jensen SO, Vaska VL et al. . Predictors of mortality in Staphylococcus aureus bacteremia. Clin Microbiol Rev 2012; 25:362–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lowy FD. Staphylococcus aureus infections. N Engl J Med 1998; 339:520–32. [DOI] [PubMed] [Google Scholar]
- 5.Jacobsson G, Dashti S, Wahlberg T, Andersson R. The epidemiology of and risk factors for invasive Staphylococcus aureus infections in western Sweden. Scand J Infect Dis 2007; 39:6–13. [DOI] [PubMed] [Google Scholar]
- 6.Liu C, Bayer A, Cosgrove SE et al. . Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52:e18–55. [DOI] [PubMed] [Google Scholar]
- 7.Holland TL, Arnold C, Fowler VG Jr. Clinical management of Staphylococcus aureus bacteremia: a review. JAMA 2014; 312:1330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fowler VG Jr, Sanders LL, Sexton DJ et al. . Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin Infect Dis 1998; 27:478–86. [DOI] [PubMed] [Google Scholar]
- 9.Jensen AG, Wachmann CH, Espersen F et al. . Treatment and outcome of Staphylococcus aureus bacteremia: a prospective study of 278 cases. Arch Intern Med 2002; 162:25–32. [DOI] [PubMed] [Google Scholar]
- 10.Pragman AA, Kuskowski MA, Abraham JM, Filice GA. Infectious disease consultation for Staphylococcus aureus bacteremia improves patient management and outcomes. Infect Dis Clin Pract (Baltim MD) 2012; 20:261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Granwehr BP, Kontoyiannis DP. The impact of infectious diseases consultation on oncology practice. Curr Opin Oncol 2013; 25:353–9. [DOI] [PubMed] [Google Scholar]
- 12.Kern WV. Management of Staphylococcus aureus bacteremia and endocarditis: progresses and challenges. Curr Opin Infect Dis 2010; 23:346–58. [DOI] [PubMed] [Google Scholar]
- 13.Vogel M, Schmitz RP, Hagel S et al. . Infectious disease consultation for Staphylococcus aureus bacteremia - A systematic review and meta-analysis. J Infect 2016; 72:19–28. [DOI] [PubMed] [Google Scholar]
- 14.Wells G, Shea B, Peterson J et al. . The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 29 January 2015.
- 15.Lundberg J, Nettleman MD, Costigan M et al. . Staphylococcus aureus bacteremia: the cost-effectiveness of long-term therapy associated with infectious diseases consultation. Clin Perform Qual Health Care 1998; 6:9–11. [PubMed] [Google Scholar]
- 16.Mylotte JM, Tayara A. Staphylococcus aureus bacteremia: predictors of 30-day mortality in a large cohort. Clin Infect Dis 2000; 31:1170–4. [DOI] [PubMed] [Google Scholar]
- 17.Kaech C, Elzi L, Sendi P et al. . Course and outcome of Staphylococcus aureus bacteraemia: a retrospective analysis of 308 episodes in a Swiss tertiary-care centre. Clin Microbiol Infect 2006; 12:345–52. [DOI] [PubMed] [Google Scholar]
- 18.Rieg S, Peyerl-Hoffmann G, de With K et al. . Mortality of S. aureus bacteremia and infectious diseases specialist consultation--a study of 521 patients in Germany. J Infect 2009; 59:232–9. [DOI] [PubMed] [Google Scholar]
- 19.Lahey T, Shah R, Gittzus J et al. . Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine 2009; 88:263–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Honda H, Krauss MJ, Jones JC et al. . The value of infectious diseases consultation in Staphylococcus aureus bacteremia. Am J Med 2010; 123:631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi SH, Cho SY, Park JH, Chung JW. Impact of infectious-disease specialist consultations on outcomes of Staphylococcus aureus bacteremia in a hospital with a low volume of patients with S. aureus bacteremia. J Infect 2011; 62:181–5. [DOI] [PubMed] [Google Scholar]
- 22.Robinson JO, Pozzi-Langhi S, Phillips M et al. . Formal infectious diseases consultation is associated with decreased mortality in Staphylococcus aureus bacteraemia. Eur J Clin Microbiol Infect Dis 2012; 31:2421–8. [DOI] [PubMed] [Google Scholar]
- 23.Isobe M, Uejima E, Seki M et al. . Methicillin-resistant Staphylococcus aureus bacteremia at a university hospital in Japan. J Infect Chemother 2012; 18:841–7. [DOI] [PubMed] [Google Scholar]
- 24.Pastagia M, Kleinman LC, Lacerda de la Cruz EG, Jenkins SG. Predicting risk for death from MRSA bacteremia. Emerg Infect Dis 2012; 18:1072–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forsblom E, Ruotsalainen E, Ollgren J, Jarvinen A. Telephone consultation cannot replace bedside infectious disease consultation in the management of Staphylococcus aureus Bacteremia. Clin Infect Dis 2013; 56:527–35. [DOI] [PubMed] [Google Scholar]
- 26.Fries BL, Licitra C, Crespo A et al. . Infectious diseases consultation and the management of Staphylococcus aureus bacteremia. Clin Infect Dis 2014; 58:598–9. [DOI] [PubMed] [Google Scholar]
- 27.Tissot F, Calandra T, Prod'hom G et al. . Mandatory infectious diseases consultation for MRSA bacteremia is associated with reduced mortality. J Infect 2014; 69:226–34. [DOI] [PubMed] [Google Scholar]
- 28.Bai AD, Showler A, Burry L et al. . Impact of infectious disease consultation on quality of care, mortality, and length of stay in Staphylococcus aureus bacteremia: results from a large multicenter cohort study. Clin Infect Dis 2015; 60:1451–61. [DOI] [PubMed] [Google Scholar]
- 29.Jenkins TC, Price CS, Sabel AL et al. . Impact of routine infectious diseases service consultation on the evaluation, management, and outcomes of Staphylococcus aureus bacteremia. Clin Infect Dis 2008; 46:1000–8. [DOI] [PubMed] [Google Scholar]
- 30.Lopez-Cortes LE, Del Toro MD, Galvez-Acebal J et al. . Impact of an evidence-based bundle intervention in the quality-of-care management and outcome of Staphylococcus aureus bacteremia. Clin Infect Dis 2013; 57:1225–33. [DOI] [PubMed] [Google Scholar]
- 31.Borde JP, Batin N, Rieg S et al. . Adherence to an antibiotic stewardship bundle targeting Staphylococcus aureus blood stream infections at a 200-bed community hospital. Infection 2014; 42:713–9. [DOI] [PubMed] [Google Scholar]
- 32.Saunderson RB, Gouliouris T, Cartwright EJ et al. . Impact of infectious diseases consultation on the management of Staphylococcus aureus bacteraemia in children. BMJ Open 2014; 4:e004659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saunderson RB, Gouliouris T, Nickerson EK et al. . Impact of routine bedside infectious disease consultation on clinical management and outcome of Staphylococcus aureus bacteraemia in adults. Clin Microbiol Infect 2015; 21:779–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagao M, Iinuma Y, Saito T et al. . Close cooperation between infectious disease physicians and attending physicians can result in better management and outcome for patients with Staphylococcus aureus bacteraemia. Clin Microbiol Infect 2010; 16:1783–8. [DOI] [PubMed] [Google Scholar]
- 35.Fowler VG Jr, Olsen MK, Corey GR et al. . Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med 2003; 163:2066–72. [DOI] [PubMed] [Google Scholar]
- 36.Fowler VG Jr, Li J, Corey GR et al. . Role of echocardiography in evaluation of patients with Staphylococcus aureus bacteremia: experience in 103 patients. J Am Coll Cardiol 1997; 30:1072–8. [DOI] [PubMed] [Google Scholar]
- 37.Oremus M, Oremus C, Hall GB, McKinnon MC; ECT, Team CSR. Inter-rater and test–retest reliability of quality assessments by novice student raters using the Jadad and Newcastle–Ottawa Scales. BMJ Open 2012; 2:e001368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hartling L, Hamm M, Milne A et al. . Rockville (MD): Agency for Healthcare Research and Quality (US); 2012 Mar. Report No.: 12-EHC039-EF. Available at: http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0077153/. Accessed 19 January 2016. [Google Scholar]
- 39.Sanderson S, Tatt ID, Higgins JP. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol 2007; 36:666–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.