Abstract
Obesity and chronic pain are often comorbid and their rates are rising. It is currently unknown whether increased pain is due to greater weight or poor diet quality, or both. Therefore, we utilized a Total Western Diet (TWD) to investigate the functional and physiological consequences of nutritionally-poor diet in mice. During thirteen weeks on the commercially-available TWD, based on the National Health and Nutrition Examination Survey (NHANES), thresholds of TWD-fed mice significantly increased in both thermal and mechanical tests. Quantitative magnetic resonance (QMR) imaging revealed a significant increase in fat mass with a concomitant decrease in lean mass, in the TWD-fed mice. Additionally, there were significant increases in serum leptin and inflammatory cytokines. Following chronic pain induction using Complete Freund’s Adjuvant (CFA), hypersensitivity was more pronounced and significantly prolonged in the TWD-fed mice. Therefore, prolonged exposure to poor diet quality resulted in altered acute nociceptive sensitivity, systemic inflammation and persistent pain following inflammatory pain induction.
Keywords: Diet, pain, mice, inflammation, hypersensitivity
Introduction
Obesity and chronic pain are increasing morbidities that have significant public health consequences2. In fact, of the many clinical components of metabolic syndrome, obesity is significantly associated with chronic pain 23. Pain is most prevalent in the load-bearing joints 46, but is also common in non-weight bearing joints23, suggesting another underlying factor 21. It is possible that excess body weight results in a persistent pro-inflammatory state that worsens widespread and local pain 2, but we hypothesize that diet may be the root of the inflammatory state that may predispose individuals to chronic pain.
Obesity is generally the result of excess energy intake and an increase in adipose tissue stores. Adipose tissue releases the adipokine leptin, which activates the innate immune system directly 1, 9. In addition, infiltrating macrophages are often recruited by adipose tissue to release pro-inflammatory cytokines 41. Thus obesity is a pro-inflammatory state. In many cases, obesity is the result of diets that are energy-dense and nutrient poor 29, 40. These diets are often high in carbohydrates, saturated fatty acids and omega-6 polyunsaturated fatty acids. Carbohydrates contribute to oxidative stress 12 and saturated fatty acids directly activate toll-like receptor 4 (TLR4) 20 and the inflammasome 35. Omega-6 polyunsaturated fatty acids are precursors for prostaglandins and have a major role in production of cytokines (i.e. tumor necrosis factor α (TNFα) and interleukin-6 (IL-6))17. Thus, both a gain in adipose tissue stores and a poor quality diet may independently contribute to a chronic inflammatory state.
In general, most preclinical explorations of diet quality are focused on metabolic outcomes related to obesity. One study, using a “cafeteria diet”, demonstrated greater diet-induced changes in both body fat and markers of metabolic health (i.e. glucose intolerance and inflammation) compared to a high-fat diet 34. More specifically, the cafeteria diet led to accumulation of pro-inflammatory mediators in the adipose tissue and elevated peripheral inflammatory markers 33, 34. Furthermore, the standard high-fat diet (HFD) in mice was linked with a greater than 2 fold increase in the numbers of circulating monocytes and neutrophils 24. Interestingly, once mice were returned to a low-fat diet they had a significant loss of visceral adipose tissue that was accompanied by decreases of both monocytes and neutrophil levels 24. Thus, a HFD is pro-inflammatory as a result of immune system activation. These findings may have a direct impact on pain as administration of cytokines elicits pain in animals 6, 10, 26 and we have shown that blockade of glial receptors 36, 38 and glial cells themselves 37 , the primary source of cytokines in the central nervous system, reduces pain in mice. It is currently unknown whether a poor quality diet and the resulting systemic inflammation have an effect on pain sensitivity and recovery from inflammatory pain induction in mice. This is a critical gap in knowledge given the current rise of obesity and the possibility that diet can negatively affect chronic pain susceptibility and recovery.
In order to explore the relationship between diet and pain, the present study aimed to investigate the effect of diet quality on behavioral and physiological indices of pain and inflammation in mice. To accomplish this, the Total Western Diet (TWD) was utilized modeled on the National Health and Nutrition Examination Survey (NHANES)14. The TWD contains the median values of a number of micro- and macro-nutrients from the NHANES and we feel more accurately represents the diet quality of a significant proportion of Americans as compared to the standard HFD.
Methods
Animals
Male CD1 (ICR:Crl) mice were housed in groups of 2, under a 12 h light cycle (lights on at 07:00 h) and provided with standard chow (Harlan Teklad) and sterile water (Hydropac) ad libitum. Male mice were used in this study based on pilot data showing that male mice showed greater diet-induced weight gain. All mice were fed standard chow for two weeks before introduction to experimental diet. Upon assignment of diets, mice were assigned to either ad libitum AIN-93M (Harlan-Teklad) chow as a maintenance and comparator diet (Control, n=8) or provided commercially available Total Western Diet (TWD, n=10)14. The TWD has fewer calories from protein and carbohydrates and increased calories from saturated and monounsaturated fats over the control diet 14. The diet exposure lasted for 13 weeks. In contrast to typical HFD (60% kcal from fat, 20% from carbohydrates), the TWD has 34.5% and 54.5% daily kcal from fat and carbohydrates, respectively. Thus, the TWD is a high-carbohydrate diet, as opposed to high-fat. All of the animals used in the present study have been obtained, housed, cared for and used in accordance with the guidelines of the University of Alabama at Birmingham Institutional Animal Care and Use Committee.
von Frey Testing
Mice were placed individually in transparent Plexiglas cubicles placed on a perforated metal floor and habituated for 2 h prior to behavioral testing. Nylon monofilaments (Stoelting Touch Test Sensory Evaluator Kit #2 to #9; 0.015–1.3 g) were firmly applied to the plantar surface of the hind paw. Both paws were tested and data presented represent the average of the two paws. The up-down method of Dixon 5, 8 was used to estimate 50% withdrawal thresholds. Mice were only tested when alert or resting. Testing for mechanical sensitivity was carried out at baseline and once per week during diet exposure.
Radiant Heat Paw Withdrawal Testing
Thermal sensitivity was tested using a modified Hargreaves’ method 13. Mice were placed individually in transparent Plexiglas cubicles placed on an elevated glass table with a portable radiant heat source (IITC Inc.) under the glass table. The heat source was focused on the ventral surface during testing. Both paws were tested and data presented represent the average of the two hind paws. The withdrawal latency was defined as the time to withdraw the hind paw from the heat source with a maximum of 40 sec set as the cut-off point.
Quantitative Magnetic Resonance (QMR) Imaging
At the end of 13 weeks of TWD exposure, mice were sent to the Small Animal Phenotyping Subcore at the University of Alabama at Birmingham. Body composition (fat and lean mass) was measured in vivo using quantitative magnetic resonance (QMR, Echo 3-in-1, Echo Medical System, Houston, TX, USA). Three hours prior to the scans, food was removed from the cage. This fasting period helped avoid any potential effect of gut fill on body composition results.
Inflammatory chronic pain
After testing for mechanical and thermal sensitivity (as described above) on two separate occasions separated by at least 24 h (after 13 weeks of TWD exposure), mice were injected with Complete Freund’s Adjuvant (CFA; 50%, in a 20 μl injection volume) into one hind paw. Mice were retested 24 h later to confirm the presence of mechanical allodynia and thermal hyperalgesia, and then on days 3, 5, 8, 11 and 14 following CFA injection.
Serum Leptin
At baseline, blood was collected by submandibular bleed 11, serum was isolated and frozen until final analysis. Following recovery from inflammatory pain (day 15 post-CFA) mice were sacrificed via rapid decapitation and trunk blood was collected between 0900–1100 h. Whole blood (clotted for 1 h at room temperature) aliquots were centrifuged and the supernatant fractions were collected. Serum was collected and frozen until final analysis. Samples were sent to the Human Physiology Core (Diabetes Research Center) at the University of Alabama at Birmingham. Leptin concentrations were determined in duplicate using a Millipore leptin ELISA kit. The intra-assay coefficient of variation was 6.66% and the detection limit was 0.2 ng/ml.
Inflammatory Cytokines
Samples were analyzed by the Human Physiology Core using 7-plex mouse assay for Interferon gamma (IFN-γ), interleukin-10 (IL-10), interleukin-12 (IL-12p70), interleukin-1β (IL-1β), IL-6, TNFα, and keratinocyte chemoattractant/growth-regulated oncogene (KC/GRO), as per manufacturer instructions (MesoScale Discovery).
Corticosterone
Serum samples were analyzed using a corticosterone ELISA kit from Abcam (ab108821). Directions were followed as per included instructions.
Statistics
Data are shown as means ± SEM. A linear regression was performed for mechanical and thermal sensitivity data. The slopes of the two lines were compared to zero (change from baseline) and to each other. Post-hoc t-tests were performed to compare weekly mechanical and thermal sensitivity measures between groups, corrected for multiple comparisons. A t-test was performed on QMR and corticosterone results and on allodynia/hyperalgesia measures, corrected for multiple comparisons. In addition, analysis of covariance was used to determine the effects of diet (independent variable) and fat mass (covariate) on leptin and cytokine levels in separate analyses. Following CFA, area above the curve was calculated using the trapezoid method and analyzed by one-way ANOVA. Group data were further analyzed by paired sample t-test comparing each day to the week 13 baseline. A t-test at baseline and week 16 was used to analyze the leptin levels. A multivariate ANOVA was used to analyze the cytokine levels.
Results
Body weight
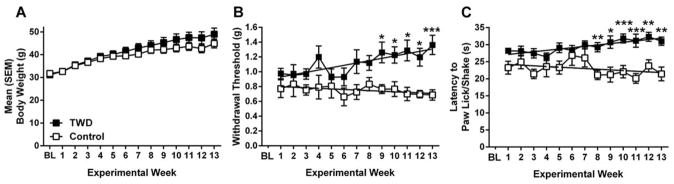
There was a significant main effect of Time for all mice (F(12,204) = 66.451, p <0.001) (Fig 1A). There was a significant Time by Diet interaction (F(12,204) =2.256, p<0.05), but no main effect of Diet (p>0.05).
Figure 1.
Weight and sensitivity change during exposure to the TWD. A. Weight of male mice in TWD (black) or Control (white) conditions over the course of the 13 experimental weeks. B. Mechanical thresholds over the 13-week diet exposure period expressed as 50% withdrawal threshold (in grams). Lines represent linear regression for TWD and Control diets. C. Radiant heat paw-withdrawal thresholds over the 13-week exposure. Linear regression lines are shown. All data are expressed as mean ± SEM. * p<0.05, ** p<0.01, ***p<0.001 when compared to Control (t-test). In spite of no significant weight differences, the TWD-fed mice had progressively higher thresholds over time.
Sensitivity Thresholds
Following linear regression, TWD increased mechanical thresholds over 13 weeks (F(1,11) = 21.33, p<0.001) (Fig 1B). Thresholds for the control diet did not deviate from zero (p>0.05). Importantly, the slopes for TWD and regular diet differed significantly from each other (F(1,22) = 25.6133, p<0.001). The thresholds were significantly different between the groups starting on week 9 until the end of the experiment (p’s<0.05). Thresholds for thermal sensitivity increased over 13 weeks for the TWD (F(1,11) = 33.19, p<0.001) (Fig 1C). Thresholds for control diet-fed mice did not differ from zero (p>0.05). The slopes for the diets were significantly different (F(1,22) = 13.6108, p<0.01). Thresholds were significantly different between the two groups starting on week 8 until the end of the experiment (p’s<0.05). The TWD significantly changed baseline sensitivity to acute pain stimuli.
Inflammatory Chronic Pain
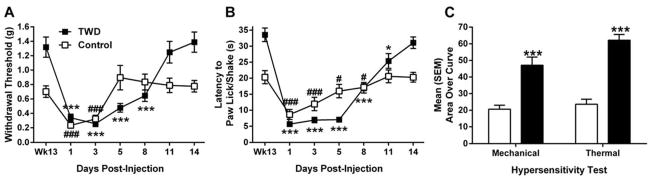
Mice on the TWD displayed significant allodynia on days 1–8 (p<0.001) (Fig 2A) and returned to pre-CFA sensitivity by day 11 (t(9) = 0.489, p>0.05). In contrast, mice on the control diet were allodynic on days 1–3 (p <0.001) and returned to pre-CFA sensitivity by day 5 (p>0.05).
Figure 2.
Hypersensitivity following administration of CFA. A. Allodynia following CFA administration as measured by 50% withdrawal threshold for TWD (black) and control (white) diet-fed mice. B. Hyperalgesia following CFA administration as measured by latency to lick/shake back paw. * p<0.05, ** p<0.01, *** p<0.001, # p<0.05, ### p<0.001 expressed in comparison to baseline thresholds (Wk13) for TWD (*) or Control (#) groups by paired samples t-test. C. Area over the curve for both mechanical and thermal hypersensitivity. TWD significantly increased days to return to baseline sensitivity following CFA. *** p<0.001. All data are expressed as mean ± SEM. Mice fed the TWD had greater hypersensitivity and delayed return to baseline thresholds following CFA administration.
Similarly, mice on the TWD were significantly hyperalgesic from days 1–11 (p<0.05) while mice on control diet were only hyperalgesic for 8 days (p<0.05) (Fig 2B). Mice on the control diet returned to pre-CFA sensitivity on day 11 (t(7) = 0.173, p>0.05) while mice on TWD returned on day 14 (t(9) =0 .867, p >0.05).
Area over the curve analysis revealed that mice on the TWD were significantly more hypersensitive than mice on control diet for mechanical (F(1,16) = 20.273, p<0.001) and thermal (F(1,16) = 67.536, p<0.001) tests (Fig 2C).
QMR
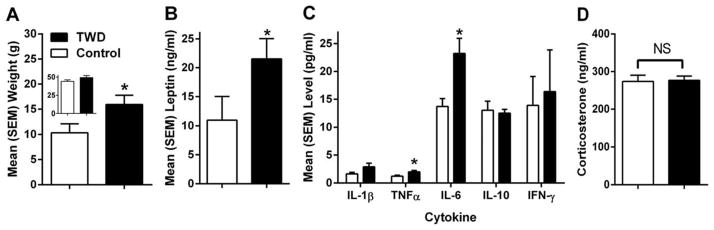
Mice on TWD had more fat mass than mice on control diet (F(1,16) = 4.576, p<0.05) (Fig 3A), despite there being no significant difference in weight at the time of imaging (F(1,16) = 2.093, p>0.05) (Fig 3A inset). Consequently, TWD-fed mice had a greater percentage of their body weight as fat (not shown, F(1,16) = 5.857, p<0.05).
Figure 3.
Physiological effects of the TWD in mice. A. Weight (in grams) of all fat mass in mice as measured by QMR – weight (in grams) of the mice at the time of QMR imaging shown in inset. B. Leptin levels in serum following 16 weeks of diet exposure. C. Levels of cytokines in serum samples of mice following 16 weeks of diet exposure. D. Corticosterone in serum following 13 weeks of diet exposure. The TWD increased fat mass, leptin and pro-inflammatory cytokines, but not corticosterone. *p<0.05. All data are expressed as mean ± SEM.
Serum Leptin
There was no difference in leptin levels between the diet groups at baseline, prior to diet exposure (data not shown, p>0.05).There was an increase over time for both diet groups with increased adipose tissue deposits accompanying normal aging. Following diet exposure, mice who consumed the TWD had higher serum leptin levels compared to the mice who consumed the control diet (t = 1.979, p<0.05) (Fig 3B). When final leptin levels were adjusted for fat mass (covariate), there was no effect of Diet (p>0.05), but there was an effect of Fat Mass (F(1,15) = 46.546, p<0.001), suggesting that fat mass itself was contributing to the increased leptin levels. This supports the known relationship between adipose tissue and leptin release.
Inflammatory Cytokines
Mice on the TWD had significantly elevated TNF-α (F(1,16) = 6.04, p<0.05) and IL-6 (F(1,16) = 8.276, p<0.05) compared to control diet (Fig 3C). There was a similar trend in IL-1β, though not significant (p = 0.059). When adjusted for fat mass (covariate), there were consistent effects of Diet alone (p<0.05), but not Fat Mass (p>0.05), indicating that the diet quality was influencing the pro-inflammatory state and not obesity per se. That is, consuming the TWD resulted in more pro-inflammatory cytokine activity irrespective of adipose tissue mass or obesity.
Corticosterone
As stress is known to increase IL-6 3, we examined corticosterone levels in the same serum samples. There were no effects of Diet on corticosterone levels (Fig 3D) in our mice (p>0.05), suggesting similar levels of stress as a result of the sacrificing and that cytokine levels elevations were the result of the TWD and not a byproduct of stress.
Discussion
Obesity rates in the United States are climbing rapidly and obesity is a significant risk factor for many disorders (e.g., cardiovascular disease, diabetes, metabolic syndrome), including chronic pain 16. In a population-based survey in the US, 26% of participants reported chronic pain conditions. Of those reporting chronic pain, 58% were overweight or obese 42, indicating that more chronic pain sufferers are above “normal” weight than not. Obesity is often the result of excess caloric intake and/ or poor diet quality, thus we undertook to examine the effect of diet quality on measures of nociception in mice. We utilized a Total Western Diet (TWD) to investigate the functional and physiological consequences in mice in order to model an American-like diet with translatable levels of micro- and macro-nutrients.
In our study, thresholds of the TWD-fed mice were significantly elevated in both thermal and mechanical tests during diet exposure, suggesting changes in baseline sensitivity. QMR analysis revealed a significant increase in fat mass in the TWD mice in spite of no significant difference in weights at the time of imaging. Interestingly, this suggests that the distribution of weight was shifted from lean to fat mass as a result of TWD exposure. Perhaps as a direct consequence of increased adipose tissue, serum leptin levels were elevated. Leptin, an adipokine satiety signal released from adipose tissue, directly activates the innate immune system and can stimulate the release of pro-inflammatory cytokines 1, 9. As expected, pro-inflammatory cytokine (IL-6 and TNF-α) levels were also elevated in TWD mice. However, this increased systemic inflammation was the result of the diet and not correlated with the increased fat mass in these mice. We believe that this increase was due to diet for two possible reasons. First, both groups received CFA 15 days prior to blood collection. If the rise in inflammatory cytokines was the result of CFA, we would expect a similar rise in both groups. The differential effect suggests that the TWD may have enhanced the responsiveness to CFA administration. Second, following CFA administration, inflammatory cytokines peak in the local tissue in a matter of hours to days 48. The local levels decline shortly after that, though hypersensitivity persists for a number of days. In our hands, the circulating levels of inflammatory cytokines are elevated beyond the presence of hypersensitivity. Together, we believe it highly unlikely that the circulating cytokines are the result of CFA itself, but that either the diet elevated these levels alone or caused an increase in immune system responsiveness to CFA that persisted beyond the appearance of hypersensitivity. We believe that the most parsimonious conclusion is that the diet itself elevated inflammatory cytokines and that this chronic activation resulted in prolonged hypersensitivity. This is a critical finding in that those consuming a poor quality diet high in carbohydrates and saturated fats may be experiencing chronic inflammation even in the absence of obesity.
Perhaps as a result of this chronic inflammatory state, the mice fed the TWD showed prolonged hypersensitivity following CFA administration. There is evidence that high levels of inflammatory cytokines such as TNFα can contribute to the demyelination of A-δ fibers which are responsible for the rapid “first pain” transmission 39, which could explain the decreased sensitivity to acute pain as assessed during the weekly diet exposure tests. Chronic inflammation, and increased TNF specifically, can contribute to sensitization of C fibers 31. Thus, when injury occurs, the nociceptive system is hypersensitive for a prolonged period of time. While we have no direct evidence that demyelination of A-δ fibers or C-fiber sensitization has occurred as a result of the TWD exposure, we are suggesting this as a possible explanation for our results. We measured elevated levels of pro-inflammatory cytokines (TNFα and IL-6) that are unlikely the result of any acute event that preferentially affected the TWD-fed mice. These levels were associated with the diet exposure and likely were elevated chronically.
The hyper-responsive state following TWD exposure may have been due to increased immune system activation and/or obesity itself. Although not examined in our experiment, the HFD has been shown to increase the extent of damage following ischemic injury in mice 22 and to increase immune cell entry into the central nervous system 4. We hypothesize that the TWD led to a hyper-responsive immune system that enhanced the hypersensitivity and prolonged the recovery from inflammatory pain induction. In addition, obesity is considered a pro-inflammatory state with increased adipose tissue recruitment of macrophages that promote pro-inflammatory cytokine production and release. In rats, obesity is associated with greater sickness response to LPS challenge27, suggesting the presence of a hyper-responsive immune system. In humans, obesity is associated with longer recovery from injury compared to normal weight controls in youths 47 and obesity reduced the functional recovery of trauma patients upon discharge7. Therefore, both obesity and diet are likely to have activated the immune system and led to protracted hypersensitivity.
A number of studies reveal that obesity treatment (e.g. surgical or lifestyle intervention) reduces chronic pain as a secondary result to weight loss but few studies examine pain as a primary outcome 16. However, certain diets have been investigated and shown to affect pain sensitivity and particular foods have direct immune-cell activating/suppressing effects 43. For example, saturated fats are known to activate innate immune cell receptors 20 while omega-6 fatty acids are precursors for prostaglandins. Both TLR4 activation and prostaglandin synthesis can be inhibited by administration/consumption of omega-3 fatty acids 20. In terms of pain sensitivity, mice fed concentrated fish oil showed significant reduction in thermal nociception compared to mice fed safflower oil 45, likely the result of higher omega-3 fatty acid content. A ketogenic (high-fat, low-carbohydrate) diet has also been shown to increase withdrawal latency 32 and reverse diabetic neuropathy pain 28 in rodents. In contrast, rats fed a HFD had a significant increase in tail flick latency compared to rats on a control diet that was correlated with body weight 30. This is in line with our results showing a gradual decline in sensitivity (increase in thresholds) with the TWD. Similar to our findings in mice, obese individuals are also less sensitive to acute pain 19, suggesting a similar underlying mechanism.
In summary, results from the present experiments indicate that the TWD resulted in a pro-inflammatory state that may have prolonged hypersensitivity following CFA administration. Protracted exposure to an energy-dense, nutrient-poor diet resulted in behavioral and physiological changes without significant effects on body weight. Our data support that diet itself was the cause of the effects observed herein. Although a time course of cytokine levels was not investigated, levels of CRP and pro-inflammatory cytokine concentration (IL-6 and TNFα) have been linked to measures of obesity 49. Elevated levels of CRP are thought to be a blood-borne marker of inflammation in chronic pain conditions 25 and there is evidence that CRP levels are high in obese patients, indicative of chronic inflammation in the absence of pain 15 and may be reflective of adipose tissue macrophage activity 18. Therefore, it can be assumed that the mice with high levels of cytokines at the end of the experiment were in a chronic inflammatory state. Elevated microglia activation has been seen in the hypothalamus after a single day on the HFD and persists for weeks 44, suggesting that the effects of poor diet quality are immediate and long-lasting. Consequently, patients who consume a high-fat diet are likely living in a state of low-grade inflammation that can have significant effects on recovery from injury or surgery. Therefore, obesity itself is a chronic inflammatory condition that may result in chronic pain, but diet quality itself may have a greater impact. Energy-dense and nutrient-poor diets cause a pro-inflammatory response that contributes to poor recovery from injury in addition to the well-characterized metabolic and obesogenic effects. Thus, it is important to consider that diet quality affects pain and inflammation independent of weight.
Perspective.
These results highlight the negative effects of poor diet quality with respect to recovery from hypersensitivity and susceptibility to chronic pain. A complete understanding of the impact of diet can aid in treatment and recovery dynamics in human clinical patients.
Acknowledgments
Funding Source: This work was supported by a University of Alabama at Birmingham College of Arts and Science Interdisciplinary Team Innovation Award received by BAW and RES
Footnotes
Disclosures
The authors have no conflict of interest involving this work.
References
- 1.Agrawal S, Gollapudi S, Su H, Gupta S. Leptin activates human B cells to secrete TNF-alpha, IL-6, and IL-10 via JAK2/STAT3 and p38MAPK/ERK1/2 signaling pathway. Journal of clinical immunology. 2011;31:472–478. doi: 10.1007/s10875-010-9507-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arranz LI, Rafecas M, Alegre C. Effects of obesity on function and quality of life in chronic pain conditions. Current rheumatology reports. 2014;16:390. doi: 10.1007/s11926-013-0390-7. [DOI] [PubMed] [Google Scholar]
- 3.Bernberg E, Ulleryd MA, Johansson ME, Bergstrom GM. Social disruption stress increases IL-6 levels and accelerates atherosclerosis in ApoE−/− mice. Atherosclerosis. 2012;221:359–365. doi: 10.1016/j.atherosclerosis.2011.11.041. [DOI] [PubMed] [Google Scholar]
- 4.Buckman LB, Hasty AH, Flaherty DK, Buckman CT, Thompson MM, Matlock BK, Weller K, Ellacott KL. Obesity induced by a high-fat diet is associated with increased immune cell entry into the central nervous system. Brain, behavior, and immunity. 2014;35:33–42. doi: 10.1016/j.bbi.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. Journal of neuroscience methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 6.Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. British journal of pharmacology. 1992;107:660–664. doi: 10.1111/j.1476-5381.1992.tb14503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhungel V, Liao J, Raut H, Lilienthal MA, Garcia LJ, Born J, Choi KC. Obesity delays functional recovery in trauma patients. The Journal of surgical research. 2015;193:415–420. doi: 10.1016/j.jss.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 8.Dixon WJ. Staircase bioassay: the up-and-down method. Neuroscience and biobehavioral reviews. 1991;15:47–50. doi: 10.1016/s0149-7634(05)80090-9. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez-Riejos P, Najib S, Santos-Alvarez J, Martin-Romero C, Perez-Perez A, Gonzalez-Yanes C, Sanchez-Margalet V. Role of leptin in the activation of immune cells. Mediators of inflammation. 2010;2010:568343. doi: 10.1155/2010/568343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferreira SH, Lorenzetti BB, Bristow AF, Poole S. Interleukin-1 beta as a potent hyperalgesic agent antagonized by a tripeptide analogue. Nature. 1988;334:698–700. doi: 10.1038/334698a0. [DOI] [PubMed] [Google Scholar]
- 11.Golde WT, Gollobin P, Rodriguez LL. A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab animal. 2005;34:39–43. doi: 10.1038/laban1005-39. [DOI] [PubMed] [Google Scholar]
- 12.Gregersen S, Samocha-Bonet D, Heilbronn LK, Campbell LV. Inflammatory and oxidative stress responses to high-carbohydrate and high-fat meals in healthy humans. Journal of nutrition and metabolism. 2012;2012:238056. doi: 10.1155/2012/238056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 14.Hintze KJ, Benninghoff AD, Ward RE. Formulation of the Total Western Diet (TWD) as a basal diet for rodent cancer studies. Journal of agricultural and food chemistry. 2012;60:6736–6742. doi: 10.1021/jf204509a. [DOI] [PubMed] [Google Scholar]
- 15.Ishii S, Karlamangla AS, Bote M, Irwin MR, Jacobs DR, Jr, Cho HJ, Seeman TE. Gender, obesity and repeated elevation of C-reactive protein: data from the CARDIA cohort. PloS one. 2012;7:e36062. doi: 10.1371/journal.pone.0036062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janke EA, Collins A, Kozak AT. Overview of the relationship between pain and obesity: What do we know? Where do we go next? Journal of rehabilitation research and development. 2007;44:245–262. doi: 10.1682/jrrd.2006.06.0060. [DOI] [PubMed] [Google Scholar]
- 17.Joffe YT, Collins M, Goedecke JH. The relationship between dietary fatty acids and inflammatory genes on the obese phenotype and serum lipids. Nutrients. 2013;5:1672–1705. doi: 10.3390/nu5051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kahn SE, Zinman B, Haffner SM, O'Neill MC, Kravitz BG, Yu D, Freed MI, Herman WH, Holman RR, Jones NP, Lachin JM, Viberti GC, Group AS. Obesity is a major determinant of the association of C- reactive protein levels and the metabolic syndrome in type 2 diabetes. Diabetes. 2006;55:2357–2364. doi: 10.2337/db06-0116. [DOI] [PubMed] [Google Scholar]
- 19.Khimich S. Level of sensitivity of pain in patients with obesity. Acta chirurgica Hungarica. 1997;36:166–167. [PubMed] [Google Scholar]
- 20.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. The Journal of biological chemistry. 2001;276:16683–16689. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- 21.Manek NJ, Hart D, Spector TD, MacGregor AJ. The association of body mass index and osteoarthritis of the knee joint: an examination of genetic and environmental influences. Arthritis and rheumatism. 2003;48:1024–1029. doi: 10.1002/art.10884. [DOI] [PubMed] [Google Scholar]
- 22.Maysami S, Haley MJ, Gorenkova N, Krishnan S, McColl BW, Lawrence CB. Prolonged diet-induced obesity in mice modifies the inflammatory response and leads to worse outcome after stroke. Journal of neuroinflammation. 2015;12:140. doi: 10.1186/s12974-015-0359-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCarthy LH, Bigal ME, Katz M, Derby C, Lipton RB. Chronic pain and obesity in elderly people: results from the Einstein aging study. Journal of the American Geriatrics Society. 2009;57:115–119. doi: 10.1111/j.1532-5415.2008.02089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagareddy PR, Kraakman M, Masters SL, Stirzaker RA, Gorman DJ, Grant RW, Dragoljevic D, Hong ES, Abdel-Latif A, Smyth SS, Choi SH, Korner J, Bornfeldt KE, Fisher EA, Dixit VD, Tall AR, Goldberg IJ, Murphy AJ. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell metabolism. 2014;19:821–835. doi: 10.1016/j.cmet.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Navarro SL, Brasky TM, Schwarz Y, Song X, Wang CY, Kristal AR, Kratz M, White E, Lampe JW. Reliability of serum biomarkers of inflammation from repeated measures in healthy individuals. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2012;21:1167–1170. doi: 10.1158/1055-9965.EPI-12-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perkins MN, Kelly D. Interleukin-1 beta induced-desArg9bradykinin-mediated thermal hyperalgesia in the rat. Neuropharmacology. 1994;33:657–660. doi: 10.1016/0028-3908(94)90171-6. [DOI] [PubMed] [Google Scholar]
- 27.Pohl J, Sheppard M, Luheshi GN, Woodside B. Diet-induced weight gain produces a graded increase in behavioral responses to an acute immune challenge. Brain, behavior, and immunity. 2014;35:43–50. doi: 10.1016/j.bbi.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Poplawski MM, Mastaitis JW, Isoda F, Grosjean F, Zheng F, Mobbs CV. Reversal of diabetic nephropathy by a ketogenic diet. PloS one. 2011;6:e18604. doi: 10.1371/journal.pone.0018604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poppitt SD, Prentice AM. Energy density and its role in the control of food intake: evidence from metabolic and community studies. Appetite. 1996;26:153–174. doi: 10.1006/appe.1996.0013. [DOI] [PubMed] [Google Scholar]
- 30.Ramzan I, Wong BK, Corcoran GB. Pain sensitivity in dietary-induced obese rats. Physiology & behavior. 1993;54:433–435. doi: 10.1016/0031-9384(93)90231-4. [DOI] [PubMed] [Google Scholar]
- 31.Richter F, Natura G, Loser S, Schmidt K, Viisanen H, Schaible HG. Tumor necrosis factor causes persistent sensitization of joint nociceptors to mechanical stimuli in rats. Arthritis and rheumatism. 2010;62:3806–3814. doi: 10.1002/art.27715. [DOI] [PubMed] [Google Scholar]
- 32.Ruskin DN, Kawamura M, Masino SA. Reduced pain and inflammation in juvenile and adult rats fed a ketogenic diet. PloS one. 2009;4:e8349. doi: 10.1371/journal.pone.0008349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sampey BP, Freemerman AJ, Zhang J, Kuan PF, Galanko JA, O'Connell TM, Ilkayeva OR, Muehlbauer MJ, Stevens RD, Newgard CB, Brauer HA, Troester MA, Makowski L. Metabolomic profiling reveals mitochondrial-derived lipid biomarkers that drive obesity-associated inflammation. PloS one. 2012;7:e38812. doi: 10.1371/journal.pone.0038812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sampey BP, Vanhoose AM, Winfield HM, Freemerman AJ, Muehlbauer MJ, Fueger PT, Newgard CB, Makowski L. Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: comparison to high-fat diet. Obesity. 2011;19:1109–1117. doi: 10.1038/oby.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Snodgrass RG, Huang S, Choi IW, Rutledge JC, Hwang DH. Inflammasome-mediated secretion of IL-1beta in human monocytes through TLR2 activation; modulation by dietary fatty acids. Journal of immunology. 2013;191:4337–4347. doi: 10.4049/jimmunol.1300298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sorge RE, LaCroix-Fralish ML, Tuttle AH, Sotocinal SG, Austin JS, Ritchie J, Chanda ML, Graham AC, Topham L, Beggs S, Salter MW, Mogil JS. Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:15450–15454. doi: 10.1523/JNEUROSCI.3859-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, Martin LJ, Austin JS, Sotocinal SG, Chen D, Yang M, Shi XQ, Huang H, Pillon NJ, Bilan PJ, Tu Y, Klip A, Ji RR, Zhang J, Salter MW, Mogil JS. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nature neuroscience. 2015 doi: 10.1038/nn.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sorge RE, Trang T, Dorfman R, Smith SB, Beggs S, Ritchie J, Austin JS, Zaykin DV, Vander Meulen H, Costigan M, Herbert TA, Yarkoni-Abitbul M, Tichauer D, Livneh J, Gershon E, Zheng M, Tan K, John SL, Slade GD, Jordan J, Woolf CJ, Peltz G, Maixner W, Diatchenko L, Seltzer Z, Salter MW, Mogil JS. Genetically determined P2X7 receptor pore formation regulates variability in chronic pain sensitivity. Nature medicine. 2012;18:595–599. doi: 10.1038/nm.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stoll G, Jung S, Jander S, van der Meide P, Hartung HP. Tumor necrosis factor-alpha in immune-mediated demyelination and Wallerian degeneration of the rat peripheral nervous system. Journal of neuroimmunology. 1993;45:175–182. doi: 10.1016/0165-5728(93)90178-2. [DOI] [PubMed] [Google Scholar]
- 40.Stubbs RJ, Whybrow S. Energy density, diet composition and palatability: influences on overall food energy intake in humans. Physiology & behavior. 2004;81:755–764. doi: 10.1016/j.physbeh.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 41.Surmi BK, Hasty AH. Macrophage infiltration into adipose tissue: initiation, propagation and remodeling. Future lipidology. 2008;3:545–556. doi: 10.2217/17460875.3.5.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toblin RL, Mack KA, Perveen G, Paulozzi LJ. A population-based survey of chronic pain and its treatment with prescription drugs. Pain. 2011;152:1249–1255. doi: 10.1016/j.pain.2010.12.036. [DOI] [PubMed] [Google Scholar]
- 43.Totsch SK, Waite ME, Sorge RE. Dietary Influence on Pain via the Immune System. Progress in molecular biology and translational science. 2015;131:435–469. doi: 10.1016/bs.pmbts.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 44.Valdearcos M, Robblee MM, Benjamin DI, Nomura DK, Xu AW, Koliwad SK. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell reports. 2014;9:2124–2138. doi: 10.1016/j.celrep.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Veigas JM, Williams PJ, Halade G, Rahman MM, Yoneda T, Fernandes G. Fish oil concentrate delays sensitivity to thermal nociception in mice. Pharmacological research : the official journal of the Italian Pharmacological Society. 2011;63:377–382. doi: 10.1016/j.phrs.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vincent HK, Heywood K, Connelly J, Hurley RW. Obesity and weight loss in the treatment and prevention of osteoarthritis. PM & R : the journal of injury, function, and rehabilitation. 2012;4:S59–67. doi: 10.1016/j.pmrj.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Warsh J, Janssen I, Pickett W. Do overweight and obese youth take longer to recover from injury? International journal of injury control and safety promotion. 2011;18:143–149. doi: 10.1080/17457300.2010.540329. [DOI] [PubMed] [Google Scholar]
- 48.Woolf CJ, Allchorne A, Safieh-Garabedian B, Poole S. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor alpha. British journal of pharmacology. 1997;121:417–424. doi: 10.1038/sj.bjp.0701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arteriosclerosis, thrombosis, and vascular biology. 1999;19:972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]