Abstract
The human host and the intestinal microbiota co-exist in a mutually beneficial relationship, which contributes to host and microbial metabolism as well as maturation of the host’s immune system, among many other pathways (Tremaroli and Backhed, 2012; Hooper et al., 2012). At mucosal surfaces, and particularly in the intestine, the commensal microbiota provides ‘colonization resistance’ to invading pathogens and maintains homeostasis through microbial regulation of mucosal innate and adaptive immunity (Renz et al., 2012). Recent evidence suggests that natural killer T cells (NKT cells), a subgroup of lipid-reactive T cells, play central roles in bidirectional interactions between the host and the commensal microbiota, which govern intestinal homeostasis and prevent inflammation. Here, we provide a brief overview of recently identified pathways of commensal microbial regulation of NKT cells, discuss feedback mechanisms of NKT cell-dependent control of microbial colonization and composition, and highlight the critical role of host-microbial cross-talk for prevention of NKT cell-dependent mucosal inflammation.
Keywords: CD1d, Natural killer T cells, Microbiota, Inflammatory bowel disease
1. Natural killer T cells
NKT cells are an unconventional subgroup of T cells, initially recognized to co-express T cell and natural killer cell (NK cell) markers (hence, the name NKT cells) and later defined by their ability to recognize self and non-self lipid antigens presented by the atypical MHC class I molecule CD1d, which is also required for NKT cell positive selection in the thymus [4]. Based on their T cell receptor (TCR) repertoire, NKT cells can be distinguished into invariant (i) or type I NKT cells and non-invariant or type II NKT cells [5]. iNKT cells express a semi-invariant TCR consisting of an invariant TCR-α chain (Vα14-Jα18 in mice and Vα24-Jα18 in humans), which pairs with a restricted set of TCR-β chains. iNKT cells recognize α-galactosylceramide (α-GalCer) in the context of CD1d and can be directly detected using α-GalCer-loaded CD1d tetramers [5]. Numerous studies have investigated the phenotype and function of iNKT cells and have demonstrated that these cells exhibit innate- or effector/memory-like functions characterized by immediate and robust cytokine secretion upon antigen recognition [6]. In addition, iNKT cells are potently activated by myeloid-derived cytokines including IL-12 and IL-18, which are secreted by antigen presenting cells in response to recognition of microbe-associated molecular patterns (MAMPS) [7–9]. Further, neurotransmitter-dependent control of iNKT cells has recently been described [10]. Consistent with the wide variety of stimuli associated with the activation of iNKT cells and their potent effector functions, critical roles of iNKT cells have been described in various contexts ranging from antimicrobial immunity to the regulation of autoimmunity and cancer immunosurveillance [5,6,11].
CD1d-restricted T cells which do not express the semi-invariant TCR of iNKT cells are termed non-invariant NKT cells and are less well defined due to the absence of cell-specific markers. Different approaches to identify non-invariant NKT cells, either based on indirect detection as CD1d-dependent T cells in mice deficient for the invariant Jα18 TCR segment or based on direct detection through CD1d-tetramers loaded with sulfatide and other lipids recognized by non-invariant NKT cells, have revealed considerable phenotypic and functional diversity among non-invariant NKT cells. As such, both regulatory and pro-inflammatory roles of non-invariant NKT cells have been described in immunity [12–17].
2. Mechanisms of commensal microbial regulation of NKT cells
The commensal microbiota is critical for postnatal development and maturation of host mucosal immunity [3]. Recent studies have demonstrated that microbial control of the immune system extends to systemic and mucosal iNKT cells and is centrally involved in maintaining homeostasis at mucosal surfaces, while disruption of these interactions is associated with susceptibility to intestinal inflammation and asthma [18].
Conventional TCRαβ T cells exhibit decreased numbers in the intestines of germfree (GF) mice and expand in response to microbial exposure [19–22]. Surprisingly, the opposite has been observed for iNKT cells wherein increased relative and absolute numbers were present in the intestines, but not the spleen or liver, of GF compared to specific-pathogen-free (SPF) mice [23,24] (Fig. 1). In accordance with these findings, intestinal iNKT cell numbers correlated indirectly with bacterial density and proximity to the microbiota. Specifically, mice showed reduced iNKT cell numbers in the large intestine compared to the small intestine and had fewer iNKT cells in the intraepithelial compartment compared to the lamina propria [23]. Similar principles of commensal microbial regulation of iNKT cells were observed at other mucosal surfaces since GF mice also harbored increased numbers of iNKT cells in the lung [24]. Moreover, microbial regulation of iNKT cells extended beyond mucosal compartments to primary and secondary lymphoid organs with effects that differed from those observed for mucosal iNKT cells. GF mice, compared to SPF mice, showed an altered TCR Vβ usage of thymic and splenic iNKT cells, reduced expression of activation markers by thymic, splenic, and hepatic iNKT cells, and impaired cytokine secretion by splenic iNKT cells in response to CD1d-restricted antigen presentation [23].
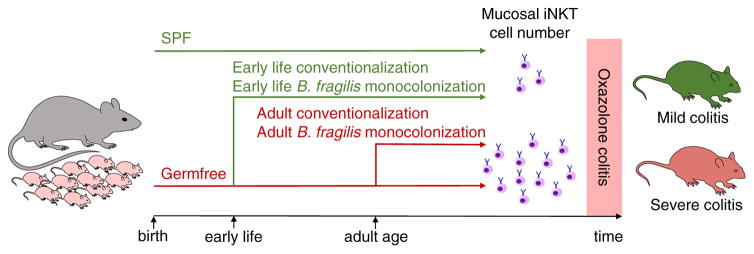
Figure 1.
Commensal microbial regulation of iNKT cell-dependent intestinal inflammation. Germfree mice, compared to specific pathogen-free (SPF) mice, exhibit increased numbers of mucosal iNKT cells due to increased CXCL16-dependent iNKT cell homing into the intestinal mucosa as well as CD1d-dependent activation and proliferation of tissue-resident iNKT cells in the lamina propria [23–25]. Conventionalization of germfree mice or monocolonization with Bacteroides fragilis at a critical window of opportunity during early postnatal life restores mucosal iNKT cell numbers and prevents susceptibility to intestinal inflammation [24,25]. In contrast, conventionalization of germfree mice or monocolonization with B. fragilis fails to regulate iNKT cell levels and susceptibility to intestinal inflammation when performed at adult age [24,25].
Commensal microbial regulation of host immunity is often temporally controlled and restricted to particular periods of postnatal development [3]. Accordingly, impaired host-microbial interactions during such ‘windows of opportunity’ may lead to persistent alterations in host immunity [18]. Consistent with this concept, microbial colonization of GF mice at neonatal but not adult age restored iNKT cell levels at mucosal surfaces of the lung and the intestine [24] (Fig. 1). While the molecular basis of these ‘windows of opportunity’ is incompletely understood, epigenetic processes such as methylation likely contribute to their presence. It was thus demonstrated that a locus 5′ of the gene encoding Cxcl16 is hypermethylated in GF mice leading to increased CXCL16 expression and CXCL16-dependent mucosal recruitment of iNKT cells [24]. Therefore, temporal control of epigenetic modifications in response to microbial exposure seems critical for the regulation of mucosal iNKT cells.
Recent studies have provided insight into the microbial requirements for regulation of mucosal iNKT cells. While iNKT cells are potently activated by cytokines secreted by myeloid cells in response to microbial engagement of pattern recognition receptors, genetic deficiency in IL-12 or the toll-like receptor adaptor MyD88 did not affect mucosal iNKT cell numbers [23,24]. Intriguingly, however, monocolonization of neonatal but not adult GF mice with Bacteroides fragilis, an abundant intestinal commensal enriched in sphingolipids, restored iNKT cell levels to those found in SPF mice [25] (Fig. 1). Such regulation was not observed with bacteria devoid of iNKT cell-activating lipids suggesting that commensal regulation of mucosal iNKT cells is dependent on CD1d-restricted bacterial lipids [25]. Indeed, an isogenic strain of B. fragilis with genetic deficiency in serine palmitoyl-transferase, an enzyme required for sphingolipid biosynthesis, failed to regulate mucosal iNKT cell abundance. Mass spectrometry of the lipid content of B. fragilis revealed the presence of an abundant α-GalCer named Bf717, which bound to CD1d but failed to activate iNKT cells explaining negative iNKT cell regulation by B. fragilis [25]. An et al. further demonstrated that B. fragilis sphingolipids are critical for inhibition of local, mucosal iNKT cell activation and proliferation thus revealing how single commensal-derived CD1d-restricted lipids can elicit early and persistent effects on mucosal iNKT cells [25]. Importantly, local proliferation of mucosal iNKT cells occurred during a short period of early postnatal development and was not observed in adult mice thus explaining selective effects of the commensal microbiota in neonatal but not adult mice. Recent studies have also highlighted considerable functional and spatial heterogeneity among microbial-derived lipids. Wieland Brown et al. reported another α-GalCer derived from B. fragilis, which, in contrast to Bf717, activated iNKT cells and promoted their expansion [26]. In addition, Chang et al. described a CD1d-binding cholesteryl glucoside derived from Helicobacter pylori (PI57), which was shown to be associated with expansion of a subset of iNKT cells with regulatory properties [27]. Further work will therefore be required to delineate the structural and functional diversity of commensal microbial lipids and potential microbial-derived non-lipid mediators involved in the control of mucosal iNKT cells. In addition, the mechanisms underlying distinct effects of the commensal microbiota on mucosal iNKT cells compared to splenic, hepatic, and thymic iNKT cells remain to be identified.
3. CD1d and NKT cells in the control of the intestinal microbiota
Interactions between the host and the microbiota at the intestinal mucosa are tightly regulated in networks of bidirectional interactions [1,2]. While the commensal microbiota influences host mucosal immunity, the host’s immune system in turn shapes the composition of the intestinal microbiota [2]. In accordance with this concept, feedback mechanisms of CD1d- and NKT cell-dependent regulation of the commensal microbiota have been described. Specifically, mice deficient in CD1d, and thus also lacking NKT cells due to absent CD1d-restricted positive selection, showed impaired restriction of commensal microbial growth associated with accelerated microbial colonization and persistence of increased numbers of commensal bacteria in the small intestine of CD1d-deficient mice compared to wild type mice [28] (Fig. 2). Mechanistically, CD1d knockout mice exhibited impaired secretion of antimicrobial peptides (AMPs) by small intestinal Paneth cells, thus contributing to increased and accelerated commensal microbial colonization [28]. Further insight into the underlying mechanisms was provided by the observation that IFN-γ derived from activated iNKT cells potently stimulated AMP release through extrusion from Paneth cells in the epithelial layer [29]. As expected from these observations, mice with genetic ablation of CD1d, similar to mice with altered processing of Paneth cell-derived α-defensins [30], showed alterations in the composition of the intestinal microbiota [28]. These results suggest that early postnatal microbial colonization, which is known to be associated with activation of inflammatory signaling pathways [31], may in turn inhibit microbial growth through CD1d-, NKT cell-, and IFN-γ-dependent AMP release. Given the critical role of AMPs in the pathogenesis of IBD and of Crohn’s disease in particular [32], it remains to be investigated whether alterations in iNKT cell-dependent release of AMPs contribute to the pathogenesis of intestinal inflammation in IBD.
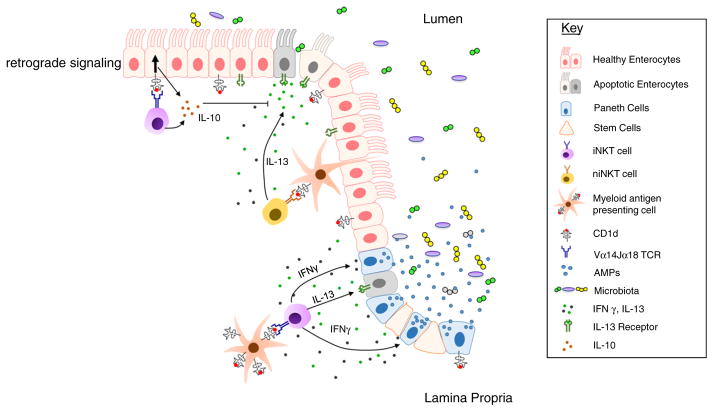
Figure 2.
CD1d- and iNKT cell-dependent regulation of epithelial function and commensal microbial colonization and composition. CD1d-restricted antigen presentation to intestinal iNKT cells and subsequent IFN-γ production by iNKT cells supports the release of antimicrobial peptides (AMPs) by Paneth cells, which inhibits commensal microbial colonization and influences the composition of the intestinal microbiota [28,29]. Myeloid APCs are potent activators of NKT cells [33] and may thus contribute to NKT cell-derived barrier-disruptive IFN-γ and IL-13 production. In addition, intestinal epithelial cells express CD1d, which can promote retrograde epithelial CD1d signaling resulting in secretion of barrier protective IL-10 [33].
It was recently demonstrated that interactions between iNKT cells and intestinal epithelial cells (IECs) not only provide the basis for iNKT cell activation but also for retrograde CD1d signaling by IECs (Fig. 2) [33]. As such, engagement of epithelial CD1d by iNKT cells was associated with CD1d-dependent activation of STAT3 and STAT3-mediated transcription of Cd1d1 (encoding CD1d), Hsph1 (encoding heat-shock protein (HSP) 110) and Il10 (encoding IL-10) [33]. HSP110 and IL-10 in turn further supported this pathway through signaling in a STAT3-dependent manner [33]. Consistent with a central role of this pathway in the regulation of intestinal inflammation, mice with IEC-specific deletion of Cd1d1 and Il10 or with deletion of Hsph1 in the radioresistant compartment exhibited severe iNKT cell-dependent morbidity and mortality in the oxazolone-induced colitis model [33]. While it remains to be investigated whether self or microbial lipids provide the basis for CD1d-dependent interactions between IECs and intestinal iNKT cells, it is possible that retrograde epithelial CD1d signaling, in addition to iNKT cell-dependent IFN-γ secretion, affects the function of Paneth cells and regulates intestinal microbial colonization and composition (Fig. 2).
4. Regulation of mucosal homeostasis through crosstalk between the microbiota and iNKT cells
NKT cells play critical roles in antimicrobial immunity, but can also contribute to inflammation and damage at mucosal surfaces [5,34,35]. Oxazolone colitis, a mouse model of the human inflammatory bowel disease (IBD) ulcerative colitis (UC), is characterized by superficial mucosal inflammation and epithelial destruction caused by iNKT cell-dependent secretion of IL-13 as well as IFN-γ [36–38]. In addition, transgenic expression of CD1d together with a non-invariant NKT-TCR in mice is associated with spontaneous intestinal inflammation [39]. Thus, both invariant and non-invariant NKT cells can contribute to mouse models of intestinal inflammation. In human UC, T cells co-expressing NK cell markers secreted barrier-disruptive IL-13 in a manner dependent on CD1d and CD1d-restricted presentation of endogenous lysosphingolipids [15,16]. These cells did not stain with α-GalCer-loaded CD1d tetramers in accordance with the presence of non-invariant NKT cells. Together, these results demonstrate a critical role of NKT cells in the pathogenesis of human IBD and mouse models of intestinal inflammation. Pathogenic roles of NKT cells in inflammatory processes at mucosal surfaces are not limited to IBD, but also found at the respiratory mucosa, where critical roles of NKT cells in the promotion of airway hyperreactivity (AHR), a hallmark of asthma, have been described [40,41].
The central role of NKT cells in mucosal inflammation raised the question of whether alterations in host-microbial interactions during early postnatal development may promote susceptibility to atopic and immune-mediated diseases such as asthma and IBD. Support for these concepts originates from the observation that reduced exposure to infectious agents and microbial elements in general, as a consequence of increased hygiene as well as antibiotic use, are associated with an increasing incidence of atopic and immune-mediated diseases [18]. Several recent studies provided evidence for a role of NKT cells in this process. Increased numbers of mucosal iNKT cells in the lung and intestine of GF mice were associated with increased disease severity in mouse models of AHR and intestinal inflammation [24] (Fig. 1). Disease susceptibility was prevented upon neonatal but not adult exposure of GF mice to the commensal microbiota or to B. fragilis [24,25] (Fig. 1). Similarly, exposure of suckling mice to a CD1d-binding cholesteryl glucoside derived from Helicobacter pylori (PI57) led to an expansion of a regulatory subset of iNKT cells in the lung, which contributed to prevention of AHR in mice [27]. These results demonstrate that host-microbial interactions during critical ‘windows of opportunity’ for microbiota-dependent licensing of the immune system are centrally involved in the control of NKT cell homeostasis and the prevention of NKT cell-mediated inflammation at mucosal surfaces.
While iNKT cells elicit pathogenic effects in predominantly Th2-driven models of intestinal inflammation such as oxazolone colitis, protective iNKT cell-dependent effects have been described in other mouse models of colitis, namely in dextran sulfate sodium (DSS) colitis [42,43]. It therefore remains to be investigated whether commensal microbial regulation of iNKT cells also affects protective roles of NKT cells in the intestine.
5. Conclusion and future perspective
Recent studies have highlighted numerous pathways of bidirectional interactions between host immunity and the commensal microbiota, which are central to homeostasis at mucosal surfaces. NKT cells are potent immune cells, whose tight control at the interface of the host and the microbiota is critical to balance protective effects of NKT cells as a first-line defense at the mucosa, while preventing NKT cell-mediated overt inflammation. Recent work has revealed a complex network of temporal and spatial control of mucosal iNKT cells through the commensal microbiota. In addition, a bidirectional nature of these interactions was demonstrated through the discovery that CD1d and NKT cells regulate intestinal microbial composition through control of Paneth cell function and AMP release. Intriguingly, these studies have also revealed that missed ‘windows of opportunities’ for early postnatal host-microbial interactions provide the basis for susceptibility to NKT cell-mediated inflammation later in life.
Further studies are required to delineate the structural and functional diversity of microbial lipids and other microbial mediators involved in the regulation of mucosal and systemic NKT cells. In addition, it will be critical to define whether commensal microbial regulation of NKT cells elicits distal effects, such as the regulation of host metabolism, and whether such pathways are involved in the control of malignant processes at mucosal surfaces. Finally, it remains to be investigated whether interactions between the commensal microbiota and NKT cells modulate immunity at other host-microbial interfaces such as the skin.
Acknowledgments
This work was supported by: the European Research Council (ERC Starting Grant agreement no. 336528), the Deutsche Forschungsgemeinschaft (DFG) (ZE 814/4-1, ZE 814/5-1, ZE 814/6-1; DFG Priority Program SPP 1656 “Intestinal Microbiota”), the Crohn’s and Colitis Foundation of America (Postdoctoral Fellowship Award), the European Commission (Marie Curie International Reintegration Grant No. 256363) and the DFG Excellence Cluster “Inflammation at Interfaces” (S.Z.), the National Institutes of Health (NIH) (grants DK044319, DK051362, DK053056, DK088199) and the Harvard Digestive Diseases Center (DK0034854) (R.S.B.).
Footnotes
Conflict of interest statement
The author(s) declare that there are no conflicts of interest.
Contributor Information
Richard S. Blumberg, Email: rblumberg@partners.org.
Sebastian Zeissig, Email: sebastian.zeissig@uniklinikum-dresden.de.
References
- 1.Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–249. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 2.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Renz H, Brandtzaeg P, Hornef M. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nat Rev Immunol. 2012;12:9–23. doi: 10.1038/nri3112. [DOI] [PubMed] [Google Scholar]
- 4.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what’s in a name? Nat Rev Immunol. 2004;4:231–237. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 5.Dowds CM, Kornell SC, Blumberg RS, Zeissig S. Lipid antigens in immunity. Biol Chem. 2014;395:61–81. doi: 10.1515/hsz-2013-0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nat Rev Immunol. 2013;13:101–117. doi: 10.1038/nri3369. [DOI] [PubMed] [Google Scholar]
- 7.Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;4:1230–1237. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 8.Brigl M, Tatituri RV, Watts GF, Bhowruth V, Leadbetter EA, Barton N, Cohen NR, Hsu FF, Besra GS, Brenner MB. Innate and cytokine-driven signals, rather than microbial antigens, dominate in natural killer T cell activation during microbial infection. J Exp Med. 2011;208:1163–1177. doi: 10.1084/jem.20102555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagarajan NA, Kronenberg M. Invariant NKT cells amplify the innate immune response to lipopolysaccharide. J Immunol. 2007;178:2706–2713. doi: 10.4049/jimmunol.178.5.2706. [DOI] [PubMed] [Google Scholar]
- 10.Wong CH, Jenne CN, Lee WY, Leger C, Kubes P. Functional innervation of hepatic iNKT cells is immunosuppressive following stroke. Science. 2011;334:101–105. doi: 10.1126/science.1210301. [DOI] [PubMed] [Google Scholar]
- 11.Berzofsky JA, Terabe M. The contrasting roles of NKT cells in tumor immunity. Curr Mol Med. 2009;9:667–672. doi: 10.2174/156652409788970706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halder RC, Aguilera C, Maricic I, Kumar V. Type II NKT cell-mediated anergy induction in type I NKT cells prevents inflammatory liver disease. J Clin Invest. 2007;117:2302–2312. doi: 10.1172/JCI31602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jahng A, Maricic I, Aguilera C, Cardell S, Halder RC, Kumar V. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J Exp Med. 2004;199:947–957. doi: 10.1084/jem.20031389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeissig S, Murata K, Sweet L, Publicover J, Hu Z, Kaser A, Bosse E, Iqbal J, Hussain MM, Balschun K, Rocken C, Arlt A, Gunther R, Hampe J, Schreiber S, Baron JL, Moody DB, Liang TJ, Blumberg RS. Hepatitis B virus-induced lipid alterations contribute to natural killer T cell-dependent protective immunity. Nat Med. 2012;18:1060–1068. doi: 10.1038/nm.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuss IJ, Heller F, Boirivant M, Leon F, Yoshida M, Fichtner-Feigl S, Yang Z, Exley M, Kitani A, Blumberg RS, Mannon P, Strober W. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J Clin Invest. 2004;113:1490–1497. doi: 10.1172/JCI19836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuss IJ, Joshi B, Yang Z, Degheidy H, Fichtner-Feigl S, de Souza H, Rieder F, Scaldaferri F, Schirbel A, Scarpa M, West G, Yi C, Xu L, Leland P, Yao M, Mannon P, Puri RK, Fiocchi C, Strober W. IL-13Ralpha2-bearing, type II NKT cells reactive to sulfatide self-antigen populate the mucosa of ulcerative colitis. Gut. 2014;63(11):1728–1736. doi: 10.1136/gutjnl-2013-305671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeissig S, Blumberg RS. Commensal microbiota and NKT cells in the control of inflammatory diseases at mucosal surfaces. Curr Opin Immunol. 2013;25(6):690–696. doi: 10.1016/j.coi.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeissig S, Blumberg RS. Life at the beginning: perturbation of the microbiota by antibiotics in early life and its role in health and disease. Nat Immunol. 2014;15:307–310. doi: 10.1038/ni.2847. [DOI] [PubMed] [Google Scholar]
- 19.Bandeira A, Mota-Santos T, Itohara S, Degermann S, Heusser C, Tonegawa S, Coutinho A. Localization of gamma/delta T cells to the intestinal epithelium is independent of normal microbial colonization. J Exp Med. 1990;172:239–244. doi: 10.1084/jem.172.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Camerini V, Sydora BC, Aranda R, Nguyen C, MacLean C, McBride WH, Kronenberg M. Generation of intestinal mucosal lymphocytes in SCID mice reconstituted with mature, thymus-derived T cells. J Immunol. 1998;160:2608–2618. [PubMed] [Google Scholar]
- 21.Kawaguchi M, Nanno M, Umesaki Y, Matsumoto S, Okada Y, Cai Z, Shimamura T, Matsuoka Y, Ohwaki M, Ishikawa H. Cytolytic activity of intestinal intraepithelial lymphocytes in germ-free mice is strain dependent and determined by T cells expressing gamma delta T-cell antigen receptors. Proc Natl Acad Sci U S A. 1993;90:8591–8594. doi: 10.1073/pnas.90.18.8591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Umesaki Y, Setoyama H, Matsumoto S, Imaoka A, Itoh K. Differential roles of segmented filamentous bacteria and clostridia in development of the intestinal immune system. Infect Immun. 1999;67:3504–3511. doi: 10.1128/iai.67.7.3504-3511.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wingender G, Stepniak D, Krebs P, Lin L, McBride S, Wei B, Braun J, Mazmanian SK, Kronenberg M. Intestinal microbes affect phenotypes and functions of invariant natural killer T cells in mice. Gastroenterology. 2012;143:418–428. doi: 10.1053/j.gastro.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, Glickman JN, Siebert R, Baron RM, Kasper DL, Blumberg RS. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336:489–493. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.An D, Oh SF, Olszak T, Neves JF, Avci FY, Erturk-Hasdemir D, Lu X, Zeissig S, Blumberg RS, Kasper DL. Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell. 2014;156:123–133. doi: 10.1016/j.cell.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wieland Brown LC, Penaranda C, Kashyap PC, Williams BB, Clardy J, Kronenberg M, Sonnenburg JL, Comstock LE, Bluestone JA, Fischbach MA. Production of alpha-galactosylceramide by a prominent member of the human gut microbiota. PLoS Biol. 2013;11:e1001610. doi: 10.1371/journal.pbio.1001610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang YJ, Kim HY, Albacker LA, Lee HH, Baumgarth N, Akira S, Savage PB, Endo S, Yamamura T, Maaskant J, Kitano N, Singh A, Bhatt A, Besra GS, van den Elzen P, Appelmelk B, Franck RW, Chen G, DeKruyff RH, Shimamura M, Illarionov P, Umetsu DT. Influenza infection in suckling mice expands an NKT cell subset that protects against airway hyperreactivity. J Clin Invest. 2011;121:57–69. doi: 10.1172/JCI44845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nieuwenhuis EE, Matsumoto T, Lindenbergh D, Willemsen R, Kaser A, Simons-Oosterhuis Y, Brugman S, Yamaguchi K, Ishikawa H, Aiba Y, Koga Y, Samsom JN, Oshima K, Kikuchi M, Escher JC, Hattori M, Onderdonk AB, Blumberg RS. Cd1d-dependent regulation of bacterial colonization in the intestine of mice. J Clin Invest. 2009;119:1241–1250. doi: 10.1172/JCI36509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farin HF, Karthaus WR, Kujala P, Rakhshandehroo M, Schwank G, Vries RG, Kalkhoven E, Nieuwenhuis EE, Clevers H. Paneth cell extrusion and release of antimicrobial products is directly controlled by immune cell-derived IFN-gamma. J Exp Med. 2014;211:1393–1405. doi: 10.1084/jem.20130753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salzman NH, Hung K, Haribhai D, Chu H, Karlsson-Sjoberg J, Amir E, Teggatz P, Barman M, Hayward M, Eastwood D, Stoel M, Zhou Y, Sodergren E, Weinstock GM, Bevins CL, Williams CB, Bos NA. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol. 2010;11:76–83. doi: 10.1038/ni.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karrasch T, Kim JS, Muhlbauer M, Magness ST, Jobin C. Gnotobiotic IL-10−/−;NF-kappa B(EGFP) mice reveal the critical role of TLR/NF-kappa B signaling in commensal bacteria-induced colitis. J Immunol. 2007;178:6522–6532. doi: 10.4049/jimmunol.178.10.6522. [DOI] [PubMed] [Google Scholar]
- 32.Ostaff MJ, Stange EF, Wehkamp J. Antimicrobial peptides and gut microbiota in homeostasis and pathology. EMBO Mol Med. 2013;5:1465–1483. doi: 10.1002/emmm.201201773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olszak T, Neves JF, Dowds CM, Baker K, Glickman J, Davidson NO, Lin CS, Jobin C, Brand S, Sotlar K, Wada K, Katayama K, Nakajima A, Mizuguchi H, Kawasaki K, Nagata K, Muller W, Snapper SB, Schreiber S, Kaser A, Zeissig S, Blumberg RS. Protective mucosal immunity mediated by epithelial CD1d and IL-10. Nature. 2014;509:497–502. doi: 10.1038/nature13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeissig S, Kaser A, Dougan SK, Nieuwenhuis EE, Blumberg RS. Role of NKT cells in the digestive system. III. Role of NKT cells in intestinal immunity. Am J Physiol. 2007;293:G1101–G1105. doi: 10.1152/ajpgi.00342.2007. [DOI] [PubMed] [Google Scholar]
- 35.Liao CM, Zimmer MI, Wang CR. The functions of type I and type II natural killer T cells in inflammatory bowel diseases. Inflamm Bowel Dis. 2013;19:1330–1338. doi: 10.1097/MIB.0b013e318280b1e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Burgel N, Fromm M, Zeitz M, Fuss I, Strober W, Schulzke JD. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–564. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Heller F, Fuss IJ, Nieuwenhuis EE, Blumberg RS, Strober W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity. 2002;17:629–638. doi: 10.1016/s1074-7613(02)00453-3. [DOI] [PubMed] [Google Scholar]
- 38.Iijima H, Neurath MF, Nagaishi T, Glickman JN, Nieuwenhuis EE, Nakajima A, Chen D, Fuss IJ, Utku N, Lewicki DN, Becker C, Gallagher TM, Holmes KV, Blumberg RS. Specific regulation of T helper cell 1-mediated murine colitis by CEACAM1. J Exp Med. 2004;199:471–482. doi: 10.1084/jem.20030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liao CM, Zimmer MI, Shanmuganad S, Yu HT, Cardell SL, Wang CR. Dysregulation of CD1d-restricted type ii natural killer T cells leads to spontaneous development of colitis in mice. Gastroenterology. 2012;142:326–334. e321–322. doi: 10.1053/j.gastro.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akbari O, Faul JL, Hoyte EG, Berry GJ, Wahlstrom J, Kronenberg M, DeKruyff RH, Umetsu DT. CD4+ invariant T-cell-receptor+ natural killer T cells in bronchial asthma. N Engl J Med. 2006;354:1117–1129. doi: 10.1056/NEJMoa053614. [DOI] [PubMed] [Google Scholar]
- 41.Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, Taniguchi M, Grusby MJ, DeKruyff RH, Umetsu DT. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003;9:582–588. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 42.Saubermann LJ, Beck P, De Jong YP, Pitman RS, Ryan MS, Kim HS, Exley M, Snapper S, Balk SP, Hagen SJ, Kanauchi O, Motoki K, Sakai T, Terhorst C, Koezuka Y, Podolsky DK, Blumberg RS. Activation of natural killer T cells by alpha-galactosylceramide in the presence of CD1d provides protection against colitis in mice. Gastroenterology. 2000;119:119–128. doi: 10.1053/gast.2000.9114. [DOI] [PubMed] [Google Scholar]
- 43.Ueno Y, Tanaka S, Sumii M, Miyake S, Tazuma S, Taniguchi M, Yamamura T, Chayama K. Single dose of OCH improves mucosal T helper type 1/T helper type 2 cytokine balance and prevents experimental colitis in the presence of valpha14 natural killer T cells in mice. Inflamm Bowel Dis. 2005;11:35–41. doi: 10.1097/00054725-200501000-00005. [DOI] [PubMed] [Google Scholar]