Abstract
This investigation tested the hypotheses that: (a) N-Acetyl Cysteine (N-AC) attenuates hyper-acute intermittent hypoxia induced sympathoexcitation, (b) without elevating superoxide measured in peripheral venous blood. Twenty-eight healthy human subjects were recruited to the study. One hour prior to experimentation, each subject randomly ingested either 70 mg·kg−1 of N-Acetyl Cysteine (N-AC, n =16) or vehicle placebo (n =12). Three lead electrocardiogram (ECG) and arterial blood pressure (ABP), muscle sympathetic nerve activity (MSNA, n = 17) and whole blood superoxide concentration using electron paramagnetic resonance (EPR, n =12) spectroscopy were measured. Subjects underwent a 20 minute hyper-acute intermittent hypoxia training (hAIHT) protocol consisting of cyclical end-expiratory apneas with 100% Nitrogen. N-AC decreased MSNA after hAIHT compared to placebo (P < 0.02). However, N-AC did not alter superoxide concentrations compared to placebo (P > 0.05) in venous blood. Moreover, hAIHT did not increase superoxide concentrations in the peripheral circulation as measured by EPR (P > 0.05). Based on these findings, we contend that (a) hAIHT and (b) the actions of N-AC in hAIHT are primarily centrally vs. peripherally mediated, although central measurements of ROS are difficult to obtain in human subjects thus making this assertion difficult to verify. This investigation suggests the possibility of developing a pharmaceutical therapy for inhibiting the sympathoexcitation associated with OSA.
Keywords: chemoreflex, sleep apnea, reactive oxygen species, oxidative stress, sympathetic nerve activity, intermittent hypoxia training
Introduction
Obstructive Sleep Apnea (OSA) is associated with a significant increase in cardiovascular morbidity and mortality due to intermittent hypoxia (IH) induced sympathetic activation (Somers et al., 1995; Malpas, 2010). Animal studies have elucidated the causal role of free radicals and reactive oxygen species (ROS) in this phenomenon. The findings of these studies demonstrate that ROS generated at the carotid body, in the nucleus of the solitary tract (NTS) and within the rostral ventral lateral medulla (RVLM) in response to chronic intermittent hypoxia (CIH; i.e. 3–14 days of IH exposure) elicits increases in sympathetic nerve activity (SNA) and hypertension (Peng et al., 2011; Peng et al., 2013; Peng et al., 2014). Further, it has been reported that antioxidant administration substantially reduces the efflux of catecholamines from ex-vivo adrenal medullae in rats conditioned to CIH (Kumar et al., 2006). In addition, CIH has been found to reduce the activities of critical antioxidant enzymes in the carotid body, the NTS and RVLM (Peng et al., 2011; Peng et al., 2013; Peng et al., 2014). Human (Foster et al., 2010; Pialoux et al., 2011) and animal (Marcus et al., 2010) studies have demonstrated that oxidative stress is also elevated after acute intermittent hypoxia (AIH; i.e. 4–24 hours of IH exposure), and plays a crucial role in the blood pressure (BP) response to AIH through activation of Angiotensin II Type Ia (AT1a) receptors. It is also known that hyper-acute IH (i.e. <1 hour of IH exposure) alters chemoreflex control of SNA and arterial pressure in human subjects (Morgan et al., 1995; Cutler et al., 2004; Leuenberger et al., 2005); yet, the extent of: (a) the elevation in systemic ROS; and (b) the causal relationship of ROS to elevated SNA is poorly understood in human subjects exposed to hAIH. Animal investigations have reported that 20 minutes to 1 hour of IH produces sensory long-term facilitation (sLTF) of ventilation and enhances neural transmission to central cardiovascular control centers via a superoxide dependent mechanism (Griffioen et al., 2007; MacFarlane & Mitchell, 2008). Furthermore, Yamamoto et al (2015) demonstrated that activating NTS neurons hyper-acutely and intermittently via optogenetic mechanisms produces similar increases in renal SNA compared to hAIH. These findings indicate that hypoxia, chemoreceptor stimulation or peripherally generated oxidative stress is not necessary to produce the sympathetic dysregulation observed with hAIH. Indeed, at least 3 days of IH are required to induce carotid body sensory plasticity and hAIH stimulation is not sufficient to elicit changes in carotid body sensory activity (Prabhakar et al., 2007) These investigations suggest that hyper-acute intermittent hypoxia mediated increases in sympathetic nerve discharge can be reduced with an antioxidant such as N-Acetyl Cysteine (N-AC) (Holdiness, 1991; Hashimoto et al., 2004; Harvey et al., 2008; Jantzie et al., 2010), without altering systemic ROS. The present investigation tested the hypothesis that N-AC, which interacts within the CNS (e.g. NTS and RVLM) tissues, will reduce the sympathoexcitation associated with hyper-acute intermittent hypoxia, while not altering circulating superoxide concentrations measured in the peripheral venous circulation.
Methods
Ethical Approval
The research described herein was approved by the University of North Texas Health Science Center Institutional Review Board (IRB #2011-079). Written informed consent was obtained from all participants in this study in accordance with the Declaration of Helsinki.
Subjects
Twenty-eight human subjects (11 females, 17 males) participated in the protocol. Female subjects were tested during the early follicular phase (days 1–4 post menses) of their menstrual cycle. All subjects completed a medical history questionnaire and physical examination prior to experimentation and were free of cardiovascular, metabolic or respiratory diseases including hypertension. Two subjects were excluded due to mild hypertension and a high pre-test probability of OSA present at the time of experimentation. Both subjects were advised to consult with their primary care physician. Subjects were randomly assigned into placebo (n =11) or N-Acetyl Cysteine (NAC, n =15) groups prior to experimentation. Out of these 26 subjects, a reliable MSNA recording was obtained on 17 (N-AC n = 10, Placebo n =7), and blood samples were successfully obtained on 13 (N-AC n = 6, Placebo n = 7). Hemodynamic data were recorded and analysed for all 26 subjects
Cardiovascular and Hemodynamic Measurements
We measured muscle sympathetic nerve activity (MSNA) with standard microneurographic techniques (n = 17) as described elsewhere (Smith et al., 1996a; Smith et al., 1996b). Briefly, a sterile tungsten microelectrode was inserted into a fascicle of the peroneal nerve near the fibular head. The nerve signals were amplified, filtered (700 to 2,000Hz), rectified and discriminated. The nerve signals were then integrated (time constant = 0.1s) to produce a mean voltage display for quantitative analysis (Jouett et al., 2015; White et al., 2015). Muscle sympathetic neural bursts were readily recognized by their tight temporal relationship to the cardiac cycle, their increasing frequency during Valsalva maneuvers and their failure to respond to arousal stimuli or stroking of the skin (Jouett et al., 2015; White et al., 2015).
A three-lead electrocardiogram (ECG) was obtained (Hewlett-Packard, Inc.) along with beat-to-beat photoplethsymographic measurements of arterial pressure (Finometer, Finapres, The Netherlands). Pulse-oximetry (Nellcor, Inc.) was performed to measure the nadir of O2 desaturation while ventilation was measured with a Ventilation Measurement Module (VMM, Laguna Hills, CA). All data were digitally recorded in data acquisition software (WinDaq, Dataq, Akron, OH) and stored offline for later analysis.
Measurements of Superoxide in Peripheral Venous Blood
Superoxide concentrations were measured experimentally (n = 13) via electron paramagnetic resonance (EPR) spectroscopy. An intravenous catheter was inserted into an antecubital fossa vein to withdraw 5 mL samples of venous blood. 200 μL of whole blood samples for each time point (pre-ingestion, post-ingestion and hAIHT, see ‘Experimental Protocol’ below) were incubated in a buffer solution: 3.5 mM deferoxamine methanesulfonate salt (DF), 9.08 mM of diethyldithiocarbamic acid sodium (DETC) and Krebs-HEPES buffer (Noxygen Science Transfer & Diagnostics GmbH, Elzach, Germany) containing the superoxide-sensitive methoxycarbonyl-2,2,5,5-tetramethyl-pyrrolidine (CMH) spin probe at 37°C for 15 min (Deo et al., 2012; Vianna et al., 2015). 50 μl samples of whole blood for each time point in duplicate were then loaded into a 1-cc syringe and flash frozen using liquid nitrogen between buffer solutions to form a continuous frozen plug. Samples were then stored at −80°C and shipped to the University of Nebraska Medical Center’s EPR Spectroscopy Core for analysis (Deo et al., 2012; Vianna et al., 2015). EPR amplitude was measured using a Bruker e-Scan EPR Spectrometer and was averaged for all duplicate data to generate an individual subject’s average for each time point. One subject (placebo) was excluded due to unequal variation among duplicate samples between time points (Levene’s test of equal variance P = 0.004, final EPR n=12).
Experimental Protocol
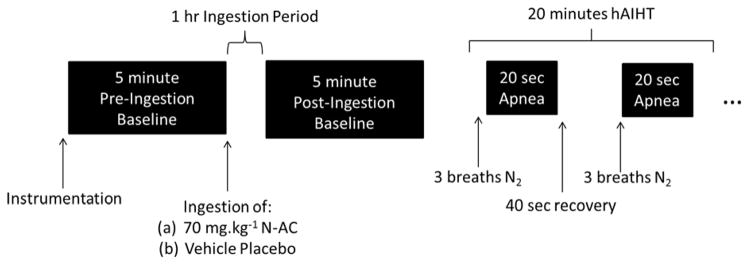
After instrumentation, baseline MSNA and arterial blood pressure data were obtained for a 5 minute period (pre-ingestion, Figure 1). After the baseline period, a 5 mL blood sample was withdrawn. Subjects then ingested either 70 mg.kg−1 of N-AC (PharmaNAC, BioAdvantex Pharma, Mississauga, Ontario, Canada) dissolved in a fruit-tasting drink as a vehicle or vehicle placebo (same volume of fruit-tasting drink). After a 1 hour ingestion period to reach peak N-AC plasma concentrations (De Caro et al., 1989), another 5 minute baseline period (post-ingestion) commenced where experimental data were recorded and another blood sample draw was performed. Subjects then underwent a 20 minute hAIHT protocol (Figure 1 illustrates 1 minute of the 20 minute protocol). Subjects breathed 2–3 breaths at normal tidal volume of 100% Nitrogen prepared in a Douglas bag, and then initiated a 20-second end-expiratory apnea. Subjects were allowed to recover on room-air for 40 seconds, at which point the N2 breathing and apnea cycle was repeated and continued for 20 minutes. Desaturations below 90% were targeted for each apnea. The apneic frequency of this protocol approximates severe OSA (AHI ~ 60) for 20 minutes, which is sufficient to elicit significant alterations of sympathetic regulation in human subjects (Cutler et al., 2004). A final 5 mL venous blood sample was obtained immediately after hAIHT to capture the ROS, particularly superoxide, in circulating venous blood post-intermittent hypoxia.
Figure 1.
Representation of the experimental protocol over time. Black boxes represent measurement of hemodynamic, microneurographic and EPR data. Experimental data were averaged over the entire 5 minute period for the pre- and post-ingestion periods. MSNA data were averaged over the entire 20 minute hAIHT period, while hemodynamic data were averaged only during the last 5 minutes of the hAIHT period (see ‘Data Analysis’ for rationale).
Data Analysis
Data were sampled at 500Hz and recorded directly to a computer with data acquisition software (WINDAQ, Dataq Instruments, Akron, OH). Data were then analyzed using a commercially available biomedical analysis software program (WinCPRS, Absolute Aliens, Turku, Finland). R-waves generated from the ECG were detected and marked at their occurrence in time while diastolic and systolic pressures were marked from the Finometer for arterial blood pressure (Moralez et al., 2012). MSNA bursts were identified by a single investigator and average MSNA burst frequency (bursts/min) and burst incidence (bursts/100 heart beats) were calculated during the entire hAIHT period and the entire baseline periods according to published guidelines (White et al., 2015). Briefly, MSNA bursts were selected by the investigator: if (a) they occurred within 1–1.4 s of an R-wave on ECG; and (b) they exhibited a 3:1 signal-to-noise ratio (White et al., 2015). Hemodynamic and MSNA variables were averaged over the entire 5 minute period to generate averages for the pre-ingestion and post-ingestion periods. Hemodynamic data during hAIHT were averaged over the last five minutes of the protocol, since it has been identified that hemodynamic variables require at least 15 minutes to increase (Cutler et al., 2004; Leuenberger et al., 2005). In contrast to the increase in MAP the, MSNA increases almost immediately during hAIHT and remains elevated to a similar extent throughout the hAIHT protocol (Cutler et al., 2004). Hence, MSNA was averaged over the entire 20 minute period to generate an hAIHT average for MSNA burst frequency and incidence.
Statistical Analysis
All data were analyzed with commercially available statistical software (SigmaPlot, Systat Software Inc., California, USA). Microneurographic and EPR data were compared for each time point using a three (time point: pre-ingestion, post-ingestion or hAIHT)-by two (treatment: N-AC or placebo) ANOVA, which was followed by Student-Newman-Keuls post-hoc tests. Hemodynamic data were first compared pre-ingestion and post-ingestion by Student’s t-test, where no statistical differences were observed (P > 0.05). Subsequently, a two (time point: post-ingestion or hAIHT)-by two (treatment: N-AC or placebo) ANOVA was performed with subsequent Student-Newman-Keuls post-hoc tests. Statistical normality was verified using a Kolmogorov-Smirnov test. Significance was set at α = 0.05. Results are reported as means ± SEM unless otherwise stated.
Results
Subject Demographics and Representative Subject
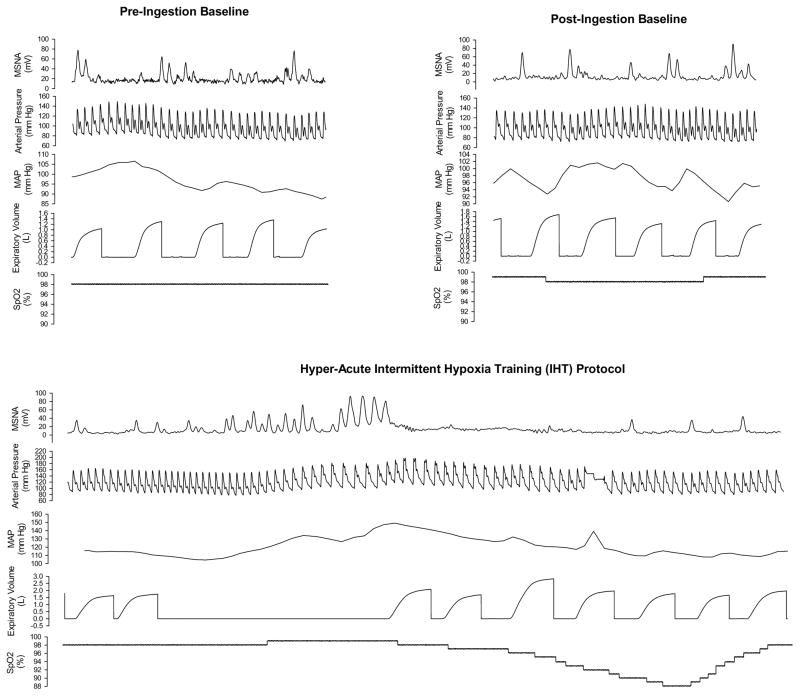
Subject demographics are provided in Table 1. Placebo control and N-AC subjects did not differ in age, height, weight or body mass index (BMI) (all P > 0.05). Furthermore, there were no statistical differences in baseline measures of arterial blood pressure (systolic, diastolic or mean) or HR between placebo and N-AC subjects (P > 0.05, Table 1). One minute of experimental data from a representative subject ingesting N-AC for each of the time points are provided in Figure 2. The hAIHT protocol with apnea elicited increases in MSNA as described previously (Smith et al., 1996b) and caused substantial (11%–30%) oxyhemoglobin desaturation.
Table 1.
Anthropometric and Hemodynamic Variables of Study Sample
| Group | Placebo | N-AC | P-Value |
|---|---|---|---|
| Age (years) | 32 ± 3 | 31 ± 3 | 0.54 |
| Sex (M/F) | 6/5 | 9/6 | --- |
| Height (cm) | 174.2 ± 3.5 | 174.5 ± 2.4 | 0.95 |
| Weight (kg) | 83.1 ± 5.9 | 76.5 ± 3.2 | 0.30 |
| BMI (kg/m2) | 27.2 ± 1.5 | 25.1 ± 0.80 | 0.23 |
| SBP (mm Hg) | 129.2 ± 5.2 | 127.8 ± 3.1 | 0.81 |
| DBP (mm Hg) | 74.4 ± 3.0 | 72.5 ± 1.6 | 0.54 |
| MAP (mm Hg) | 96.6 ± 3.7 | 93.2 ± 1.9 | 0.39 |
| HR (beats per minute) | 65.6 ± 2.6 | 65.3 ± 1.4 | 0.86 |
Figure 2.
Data from a representative subject ingesting N-AC. Thirty seconds of continuously recorded data from pre-ingestion and post-ingestion baseline periods are provided, while a full cycle (1 minute) of apnea/recovery data are provided in this figure. N-AC did not alter baseline MSNA, however, hAIHT with apnea produced substantial sympathoexcitation. (note: the sudden increase in MAP during the 4th breath after the apnea represents the re-calibration period of the beat-to-beat blood pressure monitor)
Hemodynamic Changes During hAIHT and the effect of N-AC on Hemodynamics during hAIHT
Hemodynamic data during the pre-ingestion, post-ingestion and hAIHT periods are provided in Table 2. Hemodynamic variables were not statistically different between pre-ingestion and post-ingestion, hence the arterial blood pressure and heart rate averages were compared from post-ingestion to hAIHT (see ‘Data Analysis’ and ‘Statistical Analysis’ sections). Systolic blood pressure (SBP) increased significantly during hAIHT compared to post-ingestion (ANOVA main effect, P = 0.04), while mean arterial blood pressure (MAP) trended towards an increase during hAIHT (ANOVA main effect, P = 0.07). Diastolic blood pressure (DBP) was unaffected by hAIHT (ANOVA main effect, P = 0.17). N-AC significantly reduced SBP and MAP (ANOVA main effect, P < 0.03) and subject ingesting N-AC had decreased SBP and MAP during hAIHT compared to placebo control subjects during hAIHT (P < 0.05). N-AC did not affect DBP (ANOVA main effect, P = 0.30) and no interaction of treatment (e.g. N-AC vs. vehicle placebo control) and time point were observed for any form of arterial pressure or heart rate (ANOVA interaction effect, P > 0.05). Subjects ingesting N-AC increased HR significantly during hAIHT compared to post-ingestion (P = 0.05), while subjects ingesting the vehicle placebo did not change their HR significantly (P > 0.05).
Table 2.
Hemodynamic Data Throughout the Study
| Variable | Placebo | N-AC | ||||
|---|---|---|---|---|---|---|
| Pre Ingestion | Post Ingestion | HAIHT | Pre Ingestion | Post Ingestion | HAIHT | |
| SBP (mm Hg) | 133.9 ± 3.2 | 132.0 ± 2.9 | 138.9 ± 2.9 | 129.3 ± 2.8 | 127.5 ± 2.1 | 131.5 ± 2.1 † |
| DBP (mm Hg) | 75.5 ± 2.2 | 73.9 ± 2.2 | 77.8 ± 2.2 | 72.5 ± 2.2 | 73.1 ± 2.1 | 74.6 ± 1.6 |
| MAP (mm Hg) | 99.0 ± 2.8 | 96.7 ± 2.5 | 102.3 ± 2.5 | 93.1 ± 2.4 | 93.1 ± 1.8 | 95.8 ± 1.8 † |
| HR (bpm) | 64.1 ± 2.6 | 68.2 ± 3.3 | 72.3 ± 3.3 | 65.4 ± 2.3 * | 66.0 ± 2.3 * | 72.7 ± 2.3 |
different from HAIHT, P≤0.05
different from placebo condition, P≤0.05
Systolic arterial pressure: time point, P=0.04; treatment, P=0.03; time point x treatment, P=0.58
Diastolic arterial pressure: time point, P=0.17; treatment, P=0.30; time point x treatment, P=0.53
Mean arterial pressure: time point, P=0.07; treatment, P=0.03; time point x treatment, P=0.58
Heart rate: time point, P=0.07; treatment, P=0.76; time point x treatment, P=0.65
Alterations in MSNA during hAIHT and the effect of N-AC on MSNA during hAIHT
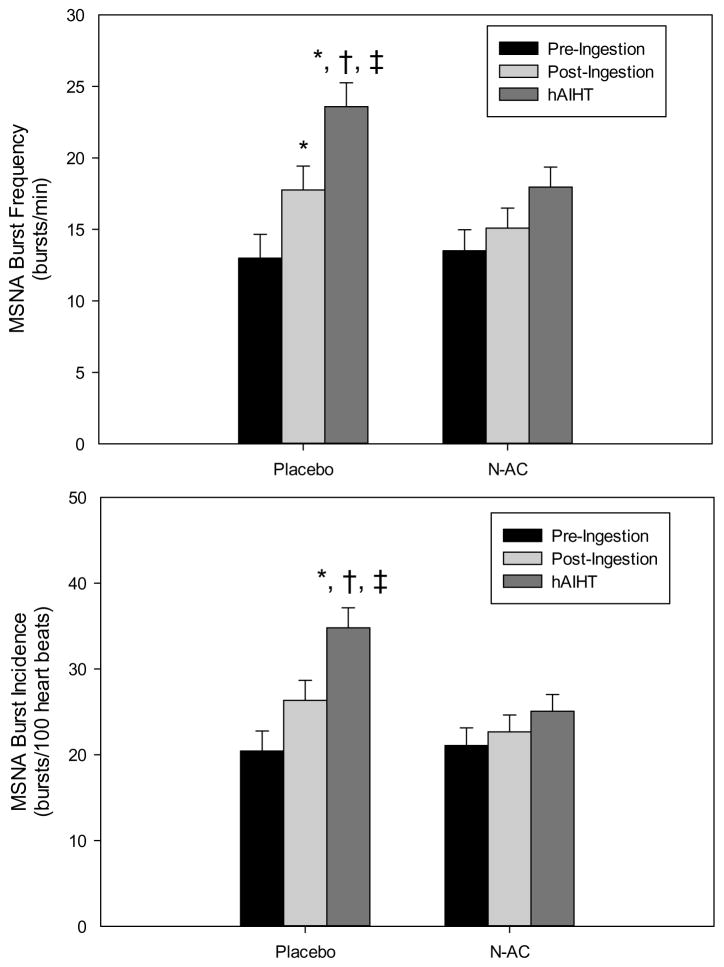
HAIHT increased MSNA burst frequency and burst incidence significantly (ANOVA main effect, P < 0.001), while N-AC reduced the MSNA response to hAIHT (ANOVA main effect, P < 0.047). MSNA burst frequency did not display significant interaction between treatment (i.e. N-AC vs. vehicle placebo) and time point (ANOVA interaction effect, P > 0.05). Subjects ingesting the vehicle placebo significantly increased MSNA burst frequency with hAIHT (P<0.01). In contrast, subjects ingesting N-AC did not significantly increase MSNA during hAIHT (P > 0.05). Furthermore, MSNA (both burst frequency and incidence) was significantly less after hAIHT in subjects who ingested N-AC compared to subjects who ingested placebo (P < 0.03, Figure 3).
Figure 3.
MSNA burst frequency (bursts/minute, top) and incidence (bursts/100 heart beats, bottom) absolute data during the pre-ingestion, post-ingestion and hAIHT time points. *, different from pre-ingestion; †, different from post-ingestion; ‡; different from N-AC corresponding timepoint. N-AC substantially reduced hAIHT sympathoexcitation. MSNA bursts/min: time point, P<0.001; treatment, P=0.047; time point x treatment, P=0.15. MSNA bursts/100 heart beats: time point, P <0.001; treatment, P=0.02; time point x treatment, P=0.07.
Changes in Superoxide Concentrations during hAIHT and N-AC effects
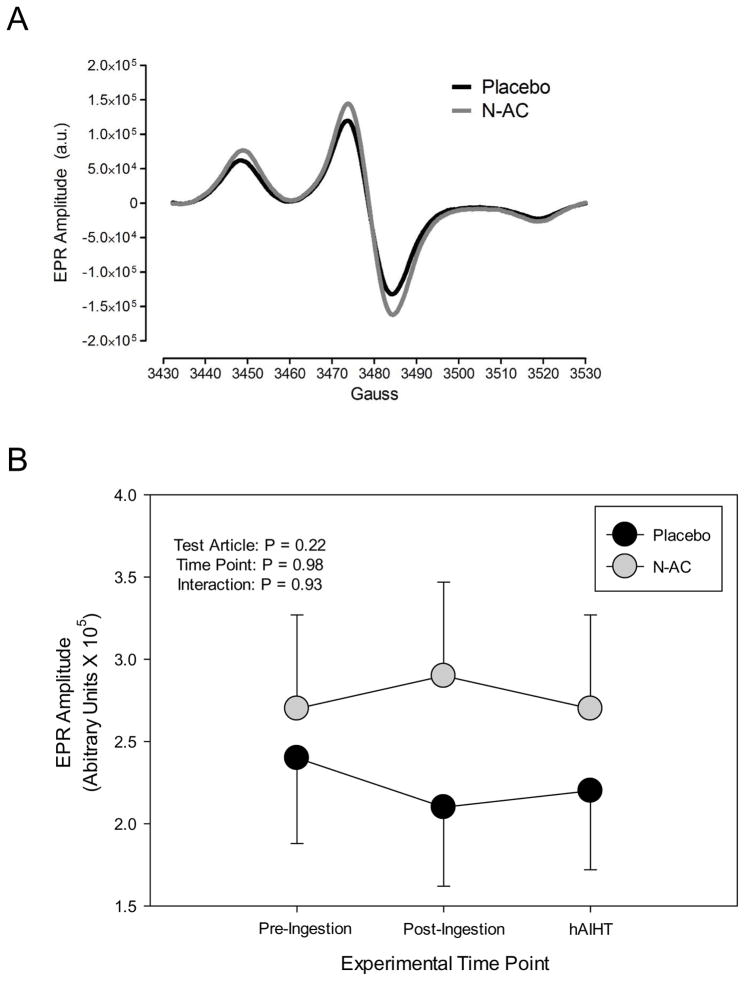
Two-way ANOVA demonstrated no main effects of hAIHT or N-AC and no interaction effects on superoxide concentrations in whole blood (all ANOVA main and interactions effects P > 0.05, Figure 4). Furthermore, neither N-AC nor placebo subjects nor all subjects combined had significant changes in blood concentrations of superoxide in response to hAIHT (one-sample t-test, all P > 0.05), indicating that hAIHT did not significantly elevate superoxide concentrations in the peripheral venous circulation.
Figure 4.
(A) Representative EPR spectra from blood collected from subjects that received either placebo or N-AC. The EPR amplitude (arbitrary units) is directly proportional to the amount of free radicals in the sample. (B) Summary data of EPR amplitude from subjects that received placebo or N-AC at the pre-ingestion, post-ingestion, and hAIHT time-points. ANOVA main effects P values are provided in the top left. Neither hAIHT nor N-AC altered the concentrations of circulating free radicals in peripheral venous blood in the study subjects.
Discussion
This study demonstrated that N-AC attenuated the MSNA response to hAIHT but did so without altering peripherally circulating superoxide concentrations as measured by EPR spectroscopy. Furthermore, peripherally circulating superoxide concentrations did not increase after hAIHT. Therefore, we contend that hAIHT and the actions of N-AC in hAIHT are likely centrally versus peripherally mediated, although we were limited in identifying across-the-brain effects of the antioxidant, as discussed below.
Hemodynamic Responses to hAIHT
Our hAIHT protocol elevated arterial blood pressure in a manner consistent with that of Cutler et al. (2004) and Leuenberger et al (2005), who utilized a similar hypoxic apnea protocol to the present study. However, arterial blood pressure during hAIHT has been shown to normalize to baseline shortly after the IH exposure while MSNA remains elevated (Cutler et al., 2004). Animal studies utilizing IH exposures indicate that at least 1 day of IH is required to elicit sustained increases in MAP that persist beyond the IH exposure (Marcus et al., 2009; Mifflin et al., 2015), while human studies suggest that as little as 4–6 hours is required (Foster et al., 2010). Hence, we postulate that sustained arterial blood pressure elevations between hyperacute versus longer IH protocols are likely due to slow elevations in circulating neurohormones, such as endothelin and catecholamines (Kanagy et al., 2001). The increases in HR that we observed only with N-AC during IH are probably baroreflex mediated, as subjects ingesting N-AC had significantly decreased MAP during IH compared to subjects ingesting vehicle placebo (Table 2).
Sympathetic Responses during hAIHT and the Effect of N-AC
Which structures in the cardiovascular neural and humoral arc mediate the hAIHT-dependent increases in MSNA? Although human studies do not permit us to achieve such a distinction, an intact carotid body (Kumar & Prabhakar, 2007; Semenza & Prabhakar, 2015) and neural chemoreflex arc (Peng et al., 2013; Peng et al., 2014) are required to mediate the hypertension observed in CIH conditioned rodents. However, it has been reported that hyper-acute intermittent optogenetic stimulation of NTS neurons increase renal SNA in a fashion similar to hAIHT (Yamamoto et al., 2015), indicating that neither hypoxia, chemoreceptor stimulation nor peripherally generated oxidative stress are required to produce the sympathetic dysregulation observed with hAIHT. Especially considering that at least 3 days of IH exposure are required to alter carotid body responses to hypoxia (Peng et al., 2003; Prabhakar et al., 2007), it is likely that only central (e.g. NTS) components of the chemoreflex mediate sympathetic responses to hAIHT (Mifflin et al., 2015; Prabhakar et al., 2015).
Furthermore, studies in CIH conditioned rodents have identified that both free radicals and ROS generated in the carotid body and the chemoreflex neural arc mediate the carotid body responses to CIH, thereby, activating SNA (Peng et al., 2011; Peng et al., 2013; Prabhakar et al., 2015; Semenza & Prabhakar, 2015). Other animal studies have demonstrated that antioxidants reduce the efflux of catecholamines from the adrenal medulla (Kumar et al., 2006) and reduce the carotid body response to CIH (Peng et al., 2011; Peng et al., 2013; Peng et al., 2014). Furthermore, Pialoux and colleagues (2011) demonstrate that acute intermittent hypoxia (AIH; i.e. 4–24 hours of IH exposure) in human subjects increase markers of oxidative stress, identifying an AT1Ra dependent mechanism, suggesting that shorter exposures of IH can also increase oxidative stress and arterial blood pressure (Foster et al., 2010). However, we have demonstrated that neither hyper-acute exposure to IH nor N-AC altered peripheral superoxide; yet, N-AC significantly lowered the MSNA response to hAIH. We contend that this finding may be explained by N-AC crossing the blood-brain-barrier (BBB) (Holdiness, 1991; Hashimoto et al., 2004; Harvey et al., 2008) and decreasing efferent SNA without altering peripheral redox balance (Figure 4, since hAIHT did not significantly alter peripheral ROS to begin with). Putatively, N-AC could reduce efferent SNA by rescuing nitric oxide (NO), which is rapidly degraded by superoxide and therefore preserves its important sympathoinhibitory function (Paton et al., 2001; Waki et al., 2003; Zimmerman & Davisson, 2004; Zimmerman & Zucker, 2009).
Indeed, our finding that: (a) hAIHT did not increase circulating superoxide concentrations as measured by EPR spectroscopy; and (b) that N-AC had no effect on superoxide concentrations versus placebo in the peripheral circulation suggest that the sympathetic dysregulation and alterations in redox balance during hAIH are primarily centrally-mediated (Griffioen et al., 2007; MacFarlane & Mitchell, 2008). These findings are supported by the following: 1) changes in carotid body activity require at least 3 days of IH exposure (Peng et al., 2003); and 2) stimulation of NTS neurons alone produce similar changes in MSNA and following hAIH (Yamamoto et al., 2015). However, one of the limitations of the present study is that superoxide is a highly reactive molecule and likely experiences substantial chemical decay during sample collection, hence, we were limited by time in not exploring other measures of oxidative stress suggested by Pialoux et al. (2011).
Conclusions
We conclude from these results that N-AC significantly reduces the MSNA response to hAIHT without altering circulating concentrations of free radicals, particularly superoxide. Moreover, peripherally circulating venous superoxide concentrations do not appear to be increased with hAIHT. Since N-AC crosses the BBB these data may be explained by centrally mediated effects of N-AC consistent with previously reported animal studies. Mechanistically, we suggest that N-AC rescues central NO, thereby, decreasing efferent MSNA (Waki et al., 2003) Thus, these findings indicate that centrally active antioxidants, perhaps in combination with angiotensin receptor blockers (ARBs), can provide sympathoinhibitory benefit in clinical conditions of intermittent hypoxia, such as Obstructive Sleep Apnea (OSA) (Somers et al., 1988; Somers et al., 1995)
New Findings.
What is the central question of this study?
This study evaluated the following central question: Does N-Acetyl Cysteine (N-AC), an antioxidant that readily penetrates the blood-brain-barrier, have the capability to reduce the increase in sympathetic nerve activity (SNA) observed during hyper-acute intermittent hypoxia?
What is the main finding and its importance?
We demonstrate that N-AC decreases muscle SNA in response to hyper-acute intermittent hypoxia vs. placebo control. This finding suggests antioxidants such as N-AC have therapeutic potential in Obstructive Sleep Apnea (OSA).
Acknowledgments
Funding
EPR Spectroscopy was performed in the University of Nebraska Medical Center’s EPR Spectroscopy Core, which is supported, in part, by a grant from the National Institute of General Medical Sciences of the National Institutes of Health (P30GM103335) awarded to the University of Nebraska’s Redox Biology Center. All other research described in the manuscript was funded by the National Heart Lung and Blood Institute, Grant #HL106431.
We would like to acknowledge our human subjects for their time and effort
Footnotes
Competing Interests
No authors declare conflicts of interest.
Author Contributions
PBR, MLS, DWW, WLE and MCZ were involved in experimental design while NPJ, DWW, WLE and GM were involved in data collection. MCZ and JT analyzed and reported EPR data and were blinded to subject IDs, treatment and time point. NPJ analyzed experimental hemodynamic and MSNA data and drafted the manuscript, while all authors reviewed it critically.
References
- Cutler MJ, Swift NM, Keller DM, Wasmund WL, Smith ML. Hypoxia-mediated prolonged elevation of sympathetic nerve activity after periods of intermittent hypoxic apnea. J Appl Physiol (1985) 2004;96:754–761. doi: 10.1152/japplphysiol.00506.2003. [DOI] [PubMed] [Google Scholar]
- De Caro L, Ghizzi A, Costa R, Longo A, Ventresca GP, Lodola E. Pharmacokinetics and bioavailability of oral acetylcysteine in healthy volunteers. Arzneimittelforschung. 1989;39:382–386. [PubMed] [Google Scholar]
- Deo SH, Fisher JP, Vianna LC, Kim A, Chockalingam A, Zimmerman MC, Zucker IH, Fadel PJ. Statin therapy lowers muscle sympathetic nerve activity and oxidative stress in patients with heart failure. Am J Physiol Heart Circ Physiol. 2012;303:H377–385. doi: 10.1152/ajpheart.00289.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster GE, Hanly PJ, Ahmed SB, Beaudin AE, Pialoux V, Poulin MJ. Intermittent hypoxia increases arterial blood pressure in humans through a Renin-Angiotensin system-dependent mechanism. Hypertension. 2010;56:369–377. doi: 10.1161/HYPERTENSIONAHA.110.152108. [DOI] [PubMed] [Google Scholar]
- Griffioen KJ, Kamendi HW, Gorini CJ, Bouairi E, Mendelowitz D. Reactive oxygen species mediate central cardiorespiratory network responses to acute intermittent hypoxia. J Neurophysiol. 2007;97:2059–2066. doi: 10.1152/jn.00975.2006. [DOI] [PubMed] [Google Scholar]
- Harvey BH, Joubert C, du Preez JL, Berk M. Effect of chronic N-acetyl cysteine administration on oxidative status in the presence and absence of induced oxidative stress in rat striatum. Neurochem Res. 2008;33:508–517. doi: 10.1007/s11064-007-9466-y. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Tsukada H, Nishiyama S, Fukumoto D, Kakiuchi T, Shimizu E, Iyo M. Effects of N-acetyl-L-cysteine on the reduction of brain dopamine transporters in monkey treated with methamphetamine. Ann N Y Acad Sci. 2004;1025:231–235. doi: 10.1196/annals.1316.028. [DOI] [PubMed] [Google Scholar]
- Holdiness MR. Clinical pharmacokinetics of N-acetylcysteine. Clin Pharmacokinet. 1991;20:123–134. doi: 10.2165/00003088-199120020-00004. [DOI] [PubMed] [Google Scholar]
- Jantzie LL, Cheung PY, Johnson ST, Bigam DL, Todd KG. Cerebral amino acid profiles after hypoxia-reoxygenation and N-acetylcysteine treatment in the newborn piglet. Neonatology. 2010;97:195–203. doi: 10.1159/000252972. [DOI] [PubMed] [Google Scholar]
- Jouett NP, Watenpaugh DE, Dunlap ME, Smith ML. Interactive effects of hypoxia, hypercapnia and lung volume on sympathetic nerve activity in humans. Exp Physiol. 2015 doi: 10.1113/EP085092. [DOI] [PubMed] [Google Scholar]
- Kanagy NL, Walker BR, Nelin LD. Role of endothelin in intermittent hypoxia-induced hypertension. Hypertension. 2001;37:511–515. doi: 10.1161/01.hyp.37.2.511. [DOI] [PubMed] [Google Scholar]
- Kumar GK, Rai V, Sharma SD, Ramakrishnan DP, Peng YJ, Souvannakitti D, Prabhakar NR. Chronic intermittent hypoxia induces hypoxia-evoked catecholamine efflux in adult rat adrenal medulla via oxidative stress. J Physiol. 2006;575:229–239. doi: 10.1113/jphysiol.2006.112524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Prabhakar N. Sensing hypoxia: carotid body mechanisms and reflexes in health and disease. Respir Physiol Neurobiol. 2007;157:1–3. doi: 10.1016/j.resp.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Leuenberger UA, Brubaker D, Quraishi SA, Hogeman CS, Imadojemu VA, Gray KS. Effects of intermittent hypoxia on sympathetic activity and blood pressure in humans. Auton Neurosci. 2005;121:87–93. doi: 10.1016/j.autneu.2005.06.003. [DOI] [PubMed] [Google Scholar]
- MacFarlane PM, Mitchell GS. Respiratory long-term facilitation following intermittent hypoxia requires reactive oxygen species formation. Neuroscience. 2008;152:189–197. doi: 10.1016/j.neuroscience.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev. 2010;90:513–557. doi: 10.1152/physrev.00007.2009. [DOI] [PubMed] [Google Scholar]
- Marcus NJ, Li YL, Bird CE, Schultz HD, Morgan BJ. Chronic intermittent hypoxia augments chemoreflex control of sympathetic activity: role of the angiotensin II type 1 receptor. Respir Physiol Neurobiol. 2010;171:36–45. doi: 10.1016/j.resp.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus NJ, Olson EB, Jr, Bird CE, Philippi NR, Morgan BJ. Time-dependent adaptation in the hemodynamic response to hypoxia. Respir Physiol Neurobiol. 2009;165:90–96. doi: 10.1016/j.resp.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mifflin SW, Cunningham JT, Toney GM. Neurogenic mechanisms underlying the rapid onset of sympathetic responses to intermittent hypoxia. J Appl Physiol (1985) 2015 doi: 10.1152/japplphysiol.00198.2015. jap 00198 02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moralez G, Romero SA, Rickards CA, Ryan KL, Convertino VA, Cooke WH. Effects of dehydration on cerebrovascular control during standing after heavy resistance exercise. J Appl Physiol (1985) 2012;112:1875–1883. doi: 10.1152/japplphysiol.01217.2011. [DOI] [PubMed] [Google Scholar]
- Morgan BJ, Crabtree DC, Palta M, Skatrud JB. Combined hypoxia and hypercapnia evokes long-lasting sympathetic activation in humans. J Appl Physiol (1985) 1995;79:205–213. doi: 10.1152/jappl.1995.79.1.205. [DOI] [PubMed] [Google Scholar]
- Paton JF, Deuchars J, Ahmad Z, Wong LF, Murphy D, Kasparov S. Adenoviral vector demonstrates that angiotensin II-induced depression of the cardiac baroreflex is mediated by endothelial nitric oxide synthase in the nucleus tractus solitarii of the rat. J Physiol. 2001;531:445–458. doi: 10.1111/j.1469-7793.2001.0445i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Nanduri J, Raghuraman G, Wang N, Kumar GK, Prabhakar NR. Role of oxidative stress-induced endothelin-converting enzyme activity in the alteration of carotid body function by chronic intermittent hypoxia. Exp Physiol. 2013;98:1620–1630. doi: 10.1113/expphysiol.2013.073700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci U S A. 2003;100:10073–10078. doi: 10.1073/pnas.1734109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Raghuraman G, Khan SA, Kumar GK, Prabhakar NR. Angiotensin II evokes sensory long-term facilitation of the carotid body via NADPH oxidase. J Appl Physiol (1985) 2011;111:964–970. doi: 10.1152/japplphysiol.00022.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YJ, Yuan G, Khan S, Nanduri J, Makarenko VV, Reddy VD, Vasavda C, Kumar GK, Semenza GL, Prabhakar NR. Regulation of hypoxia-inducible factor-alpha isoforms and redox state by carotid body neural activity in rats. J Physiol. 2014;592:3841–3858. doi: 10.1113/jphysiol.2014.273789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pialoux V, Foster GE, Ahmed SB, Beaudin AE, Hanly PJ, Poulin MJ. Losartan abolishes oxidative stress induced by intermittent hypoxia in humans. J Physiol. 2011;589:5529–5537. doi: 10.1113/jphysiol.2011.218156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar NR, Dick TE, Nanduri J, Kumar GK. Systemic, cellular and molecular analysis of chemoreflex-mediated sympathoexcitation by chronic intermittent hypoxia. Exp Physiol. 2007;92:39–44. doi: 10.1113/expphysiol.2006.036434. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR, Peng YJ, Kumar GK, Nanduri J. Peripheral chemoreception and arterial pressure responses to intermittent hypoxia. Compr Physiol. 2015;5:561–577. doi: 10.1002/cphy.c140039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL, Prabhakar NR. Neural Regulation of Hypoxia-Inducible Factors and Redox State Drives the Pathogenesis of Hypertension in a Rodent Model of Sleep Apnea. J Appl Physiol (1985) 2015 doi: 10.1152/japplphysiol.00162.2015. jap 00162 02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, Beightol LA, Fritsch-Yelle JM, Ellenbogen KA, Porter TR, Eckberg DL. Valsalva’s maneuver revisited: a quantitative method yielding insights into human autonomic control. Am J Physiol. 1996a;271:H1240–1249. doi: 10.1152/ajpheart.1996.271.3.H1240. [DOI] [PubMed] [Google Scholar]
- Smith ML, Niedermaier ON, Hardy SM, Decker MJ, Strohl KP. Role of hypoxemia in sleep apnea-induced sympathoexcitation. J Auton Nerv Syst. 1996b;56:184–190. doi: 10.1016/0165-1838(95)00062-3. [DOI] [PubMed] [Google Scholar]
- Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers VK, Mark AL, Abboud FM. Sympathetic activation by hypoxia and hypercapnia--implications for sleep apnea. Clin Exp Hypertens A. 1988;10(Suppl 1):413–422. doi: 10.3109/10641968809075998. [DOI] [PubMed] [Google Scholar]
- Vianna LC, Deo SH, Jensen AK, Holwerda SW, Zimmerman MC, Fadel PJ. Impaired dynamic cerebral autoregulation at rest and during isometric exercise in type 2 diabetes patients. Am J Physiol Heart Circ Physiol. 2015;308:H681–687. doi: 10.1152/ajpheart.00343.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waki H, Kasparov S, Wong LF, Murphy D, Shimizu T, Paton JF. Chronic inhibition of endothelial nitric oxide synthase activity in nucleus tractus solitarii enhances baroreceptor reflex in conscious rats. J Physiol. 2003;546:233–242. doi: 10.1113/jphysiol.2002.030270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DW, Shoemaker JK, Raven PB. Methods and considerations for the analysis and standardization of assessing muscle sympathetic nerve activity in humans. Auton Neurosci. 2015 doi: 10.1016/j.autneu.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Lalley P, Mifflin S. Acute intermittent optogenetic stimulation of nucleus tractus solitarius neurons induces sympathetic long-term facilitation. Am J Physiol Regul Integr Comp Physiol. 2015;308:R266–275. doi: 10.1152/ajpregu.00381.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman MC, Davisson RL. Redox signaling in central neural regulation of cardiovascular function. Prog Biophys Mol Biol. 2004;84:125–149. doi: 10.1016/j.pbiomolbio.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Zimmerman MC, Zucker IH. Mitochondrial dysfunction and mitochondrial-produced reactive oxygen species: new targets for neurogenic hypertension? Hypertension. 2009;53:112–114. doi: 10.1161/HYPERTENSIONAHA.108.125567. [DOI] [PMC free article] [PubMed] [Google Scholar]