Abstract
Background:
Niosomes are non-ionic surfactant vesicles used as drug carriers for encapsulating both hydrophobic and hydrophilic drugs. The aim of this study is to evaluate the effect of different surfactants on the physical properties and stability of carvedilol niosomes designed to improve oral bioavailability.
Materials and Methods:
Different niosomal formulations were prepared using a film hydration method, with various mixtures of different non-ionic surfactants including Span 20, 40, and 60, and also Tween 20, 40, and 60, along with cholesterol. The physicochemical characteristics of the formulations were evaluated in vitro.
Results:
The drug encapsulation efficiency was reduced by using lauryl (C12) chain containing surfactants, that is, Span/Tween. Cholesterol content and drug entrapment were the main factors affecting the mean particle size of the niosomes. The drug release profiles from most of the formulations were fitted well with the Baker-Lonsdale model, indicating a diffusion-based drug release mechanism. Niosomes prepared from 50 and 40% of the cholesterol with 25 or 30% of Span/Tween 60 showed the highest stability due to their high transition temperature and solid state feature of these surfactants.
Conclusions:
From the results obtained, it may be concluded that nanoniosomes are promising stable carriers for the oral delivery of carvedilol.
Keywords: Carvedilol, film hydration method, niosome, nonionicsurfactant, stability
INTRODUCTION
The oral route is the preferred route of drug administration to patients.[1] However, oral administration of drugs often leads to degradation, due to the highly acidic environment of the stomach and enzymes of the mucosa or liver, before they enter the systemic circulation.[2] Nanotechnology is a promising approach to oral delivery. Nanoparticles have a potential to improve the delivery of poorly water-soluble drugs, transport drugs to the specific site in the gastrointestinal (GI) tract, enhance transmucosal transport of large macromolecules, protect the encapsulated drug from the harsh environment of the GI, and control release of the encapsulated drug.
Carvedilol [Figure 1] is a nonselective beta/alpha-1 blocker indicated in the treatment of mild-to-severe congestive heart failure (CHF), coronary artery disease (CAD), and in the postmyocardial settings. It also has other activities such as an antioxidant property, inhibition of smooth muscle proliferation, and calcium antagonistic blocking activity.
Figure 1.
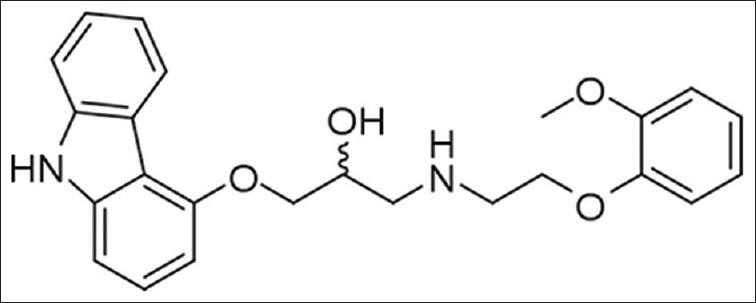
Carvedilol chemical structure
Carvedilol is completely absorbed from the GI tract, but its systemic availability is limited (approximately 25 – 35%) because of its high first-pass metabolism.[3] It also has a short biological half-life. As a result, multiple-dose administration is required for the maintenance of its therapeutic effect throughout the day. Hence, a sustained oral drug delivery will be promising for long-term treatment. Many researchers have attempted to improve the bioavailability of carvedilol by developing new formulations, including buccoadhesive carvedilol tablets,[3,4] a polymer-coated solid lipid nanoparticle of the drug,[5] and solid dispersion.[6] Among the different nanoparticulate systems, vesicular carriers such as liposomes or niosomes are considered as promising drug delivery systems, because these particles can act as drug-containing reservoirs and control drug release by modification of their compositions. Liposomes are phospholipid vesicles with biocompatible, non-toxic, non-immunogenic, non-carcinogenic, non-thrombogenic, and biodegradable properties. In addition, they are recognized as efficient drug carriers to the GI system.[7,8,9] Non-ionic surfactant-based vesicles (niosomes) are similar to liposomes and are able to encapsulate both hydrophilic and lipophilic drugs and serve as drug carriers. The low cost, greater stability, and resultant ease of storage of non-ionic surfactants has led to the development of these carriers as alternatives to liposomes.[10] The niosomal systems are supposed to enhance the bioavailability of poorly water-soluble drugs by enhancing their uptake by the M cells of Peyer's patches at the intestinal lymphatic tissues.[11] This pathway overcomes the first pass metabolism, and therefore, increases the bioavailability. On the basis of this hypothesis, the encapsulation of carvedilol in niosomes can increase its blood circulation time and enhance the bioavailability. The objective of this study is the in vitro development of carvedilol-loaded nonionic surfactant vesicles. The effect of various parameters on the different physicochemical characteristics of the prepared formulations, including, their vesicle size, encapsulation efficiency, release of the encapsulated drug, and their stability, were evaluated.
MATERIALS AND METHODS
Materials
Carvedilol was obtained from the Darupakhsh Company (Iran). The nonionic surfactants used as vesicle-forming materials were sorbitan monolaurate (Span 20), sorbitan monopalmitate (Span 40), sorbitan monostearate (Span 60), polyoxyethylene-20-sorbitan monolaurate (Tween 20), polyoxyethylene-20-sorbitan monopalmitate (Tween 40), polyoxyethylene-20-sorbitan monostearate (Tween 60), and cholesterol, which were purchased from Fluka (Switzerland). All the organic solvents and other chemicals were of analytical grade, and were obtained from the Merck Chemical Company (Germany).
Preparation of drug-loaded niosomes
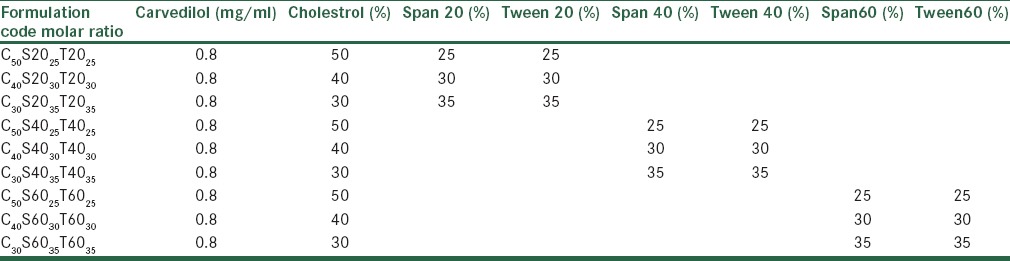
Niosomes containing carvedilol were prepared by using the film hydration method,[12] with various mixtures of nonionic surfactant/cholesterol. The compositions of the prepared vesicles are shown in Table 1.
Table 1.
Composition of the different prepared nanoniosomes of carvedilol
Briefly, 400 µmol of surfactants/cholesterol and 8 mg of carvedilol were dissolved in chloroform, in a round-bottomed flask. The organic solvent was evaporated under vacuum at 55°C. The resultant thin lipid film produced on the inner wall of the flask was then hydrated with 10 mL of phosphate buffer at 55°C, for 30 minutes. The niosomal suspension was then submitted to a sonication procedure of four cycles of two seconds, followed by a pause of two seconds, by using a probe sonicator (Bandeline, Berlin, Germany), with the instrument set at 40% of its maximum power, to reduce the mean size of the vesicles. The final formulations were stored in a refrigerator (4–8°C) for further studies. To evaluate the formation of the niosomes, the niosomal suspensions were observed before sonication by an optical microscope (HFX-DX, Nikon, Japan) and photomicrographs were taken by a camera attached to the microscope in ×450 magnifications.
Vesicle size, polydispersity index, and zeta potential measurements
The mean particle size, polydispersity index, and zeta potential of the nanoparticles was estimated by photon correlation spectroscopy (PCS, Zetasizer 3000, Malvern, UK) at a fixed angle of 90°. Samples were diluted with dust-free water, to give the recommended scattering intensity of 200000 counts/second.
Encapsulation efficiency determination of carvedilol nano-niosomes
Non-entrapped carvedilol was separated by the centrifugation method (Microcentrifuge Sigma 30k, UK), at 14000 rpm, for 40 minutes, at 25°C. The amount of carvedilol in the nano-niosomes was analyzed by an ultraviolet (UV)/visible spectrophotometer (RF-5301 PC, Shimadzu, Kyoto, Japan) after disrupting it by ethanol, 96%, at 285 nm.
The percent of carvedilol encapsulation efficiency (EE%) was determined from equation 1.
EE% = (Cp/CT) × 100 (Eq. 1)
Where Cp is the carvedilol concentration in the nano-niosomes and CT is the initial drug concentration added to the formulation. Empty nanoniosomes were used as blanks.
Carvedilol release from various formulations
Carvedilol release from the various formulations was evaluated using the dialysis method. One milliliter of nanoparticle dispersion was placed into a dialysis bag (cutoff 12 kDa) and suspended into a beaker containing 70 mL of a phosphate buffer solution (pH 7.4) on a magnetic stirrer, with a speed of 100 rpm at 37°C ± 0.5°C. At pre-determined time intervals, 1 mL samples were withdrawn from the incubation medium and analyzed for the drug content by a UV spectrophotometer (UV-mini-1240 CE-Shimadzu, Japan) at λmax = 255 nm. The drug release tests were performed in triplicate.
Stability studies
Aggregation or fusion of the vesicles was determined by the changes in the vesicle diameter using the laser light scattering method. The formulations were stored at 4°C for two months and assessed for changes in particle size one and two months after preparation. Encapsulation efficiency was also determined two months after preparation of the formulations, as described previously.
RESULTS AND DISCUSSION
Physicochemical characteristics of carvedilol-loaded nano-niosomes
Nanoniosomes were prepared by the thin film hydration method using a mixture of amphiphilic surfactants with different lipophilic side chain lengths and cholesterol, at different molar ratios.
The presence of vesicles in niosomal dispersion was confirmed by viewing the unsonicated system using an optical microscope [Figure 2]. The vesicles were spherical and majority of them were multi-lamellar. Very few large unilamellar vesicles were also seen. In this situation the particles were not in the nanometric size, and therefore, were observable through the optical microscope, but after sonication the multilayered niosomes were shed to nanoniosomes with particle sizes ranging between 167 ± 2.5 and 763 ± 7.8 nm [Table 2]. The niosomes were composed of cholesterol, and Span/Tween 20 also showed many cholesterol crystals.
Figure 2.
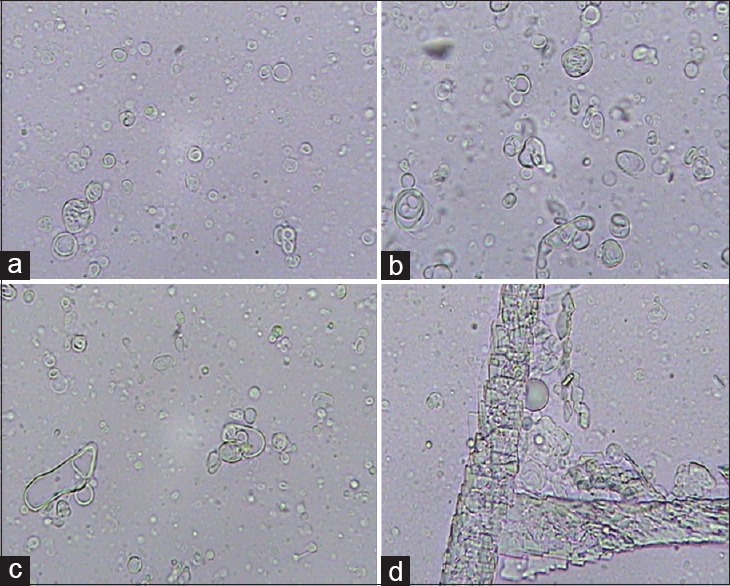
Photomicrographs (450× magnification) of unsonicated carvedilol-containing niosomes prepared by the film hydration method. The niosomes were composed of (a) Span, Tween 40 / CHOL(7:3), (b) Span, Tween 60 / CHOL(5:5), (c) Span, Tween 60 / CHOL(7:3), and (d) Span, Tween 20 / CHOL(6:4)
Table 2.
Particle size, polydispersity index, and zeta potential of carvedilol niosomal formulations (mean±SD, n=3)
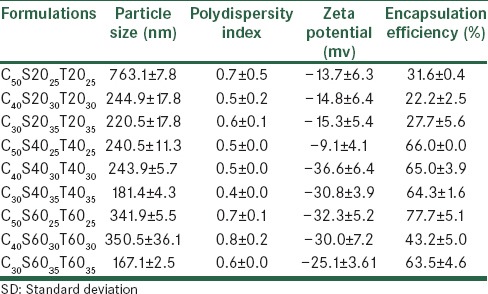
Carvedilol seems to have an impact on the lipid membrane structure and stability, especially in short lauryl (C12) chains of Span/Tween 20, which is in a liquid form at room temperature. Carvedilol is a lipophilic molecule and it is located in bilayers of the hydrophobic core. This result can be explained by the presence of a possible competition between carvedilol and cholesterol, incorporated into the nano-niosomes. This has also been previously observed in a niosomal model enriched with cholesterol and carotenoids.[13] The mean volume diameters (dv), poly dispersity index, and zeta potential of the prepared carvedilol vesicles and blank vesicles are presented in Tables 2 and 3. The mean diameter size of the different carvedilol formulations ranged between 167 ± 2.5 and 763 ± 7.8 nm.
Table 3.
Particle size, polydispersity index, and zeta potential of blank niosomal formulations (mean±SD, n=3)
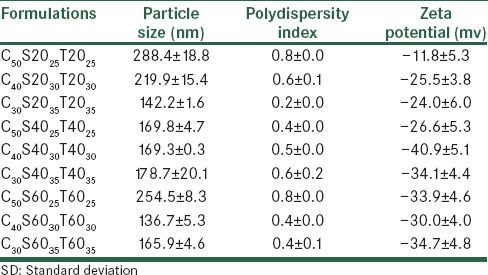
Decreasing the amount of cholesterol content from five to three percent molar ratio, reduced the mean volume diameter of the particles significantly (P < 0.05). This result was in agreement with the previous data, showing that an increment in the amount of cholesterol caused the size of the vesicles to increase.[14,15] As shown in Table 2, the maximum particle size was observed for the C50S2025T2025 formulation, due to the production of cholesterol crystals. The incorporation of the drug had a significant effect on the particle size of the vesicles (P < 0.05). It was revealed that the incorporation of carvedilol in all formulations led to an increment in particle size compared to blank niosomes, as previously reported by Vangala et al.[16] The size distribution could be observed from the poly dispersity index (PDI). The PDI ranged from zero to one. Values close to zero indicated a homogenous dispersion. The PDI results are shown in Table 2, which indicate that all the formulations are multi-dispersed nanoniosomes. By incorporation of carvedilol in bilayers, the zeta potential decreased compared to the blank formulation. This could be because of the NH group in the drug structure and its basic properties [Figure 1].
Encapsulation efficiency
Carvedilol encapsulation efficiencies (EE) of all the studied formulations are shown in Table 2. The percentage of drug entrapped in all formulations changed between 22 and 77%. Carvedilol encapsulation efficiency depended on the hydrophilic–lipophilic balance (HLB) of the different surfactants. The least encapsulation efficiency was observed for C40S2030T2030 and C30S2035T2035 and C50S2025T2025 as the higher HLB of the mixture of Tween/Span 20 with respect to Tween/Span 40 and Tween/Span 60 reduced its potential in solubilizing the lipophilic molecule of carvedilol. Palozza et al.[13] reported the lowest encapsulation efficiency of carotene in niosomes of Tween 20. The encapsulation efficiency improved when the cholesterol content was increased to 50% molar ratio due to reduction of drug permeability. A similar result was reported by Mokhtar et al.,[17] who studied the effect of some formulation parameters such as the cholesterol content of niosomes on flurbiprofen encapsulation and release rates of niosomes prepared from proniosomes. The vesicle size is another parameter that affects the encapsulation efficiency. Changes in vesicles size have had no significant effect (P > 0.05) on carvedilol encapsulation efficiency in the studied formulations [Table 2].
Carvedilol release
In vitro release studies of carvedilol from nano-niosomes were performed using a dialysis bag containing the appropriate volume of carvedilol-loaded niosomal dispersion. The dialysis bag was placed in a flask containing 70 mL of phosphate buffer with 0.5% Tween 80 (pH 7.4). In the present study, the formulations containing Span/Tween 20 were withdrawn from further studies due to their low encapsulation efficiency. As shown in Figures 3 and 4, drug release from niosomes in all formulations were followed by a biphasic process consisting of an initial relatively fast release and a lower release phase. The rapid initial phase may be related to the penetration of free carvedilol and desorption of the drug from the surface of the niosomes and the slower phase could be related primarily to the diffusion of the drug through the bilayers.[18] All the formulations released almost 100% of the loaded drug with no significant difference in their release data [Figures 3 and 4].
Figure 3.
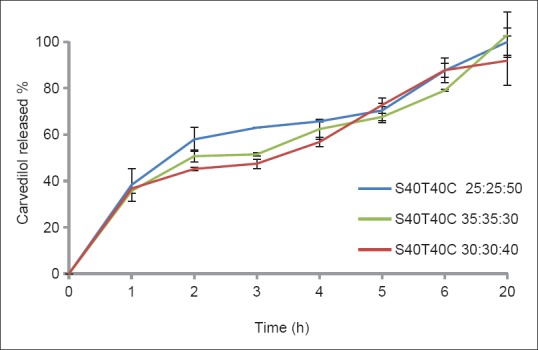
Release profiles of carvedilol from nano-niosomes composed of Span-Tween 40 / cholesterol in a phosphate buffer at 37oC (mean ± SD, n = 3)
Figure 4.
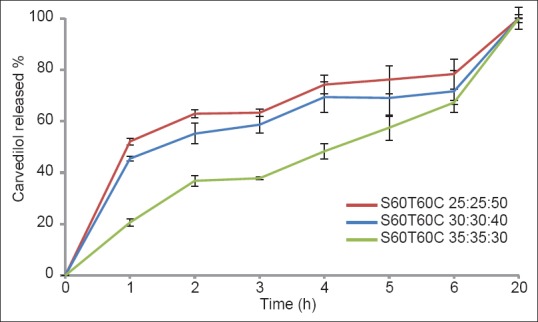
Release profiles of carvedilol from nano-niosomes composed of Span-Tween 60 / cholesterol in phosphate buffer at 37oC (mean ± SD, n = 3)
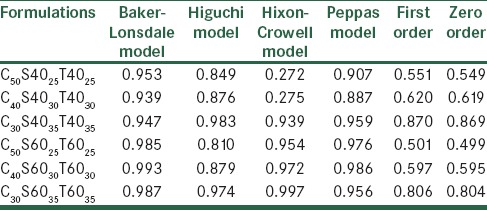
As shown in Table 4, the release profile of most of the formulations were fitted by the Baker and Lonsdale equation, which indicated that carvedilol release from the vesicles might be attributed to the diffusion mechanism.
Table 4.
Regression coefficient (r2) of carvedilol release data from studied nano-niosomes according to the different kinetic models
Stability studies
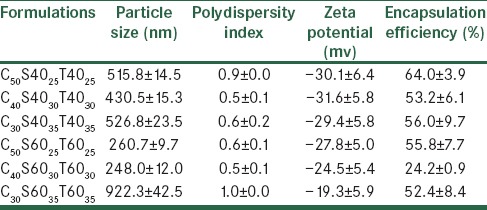
A stable niosome dispersion must exhibit a constant particle size and a constant level of entrapped drug, with no precipitation of the membrane components, which are to a large extent insoluble in an aqueous media.[19] In the present study, changes in particle size of the Span/Tween 40 and Span/Tween 60 formulations during storage at 4°C, one and two months after preparation, were investigated [Tables 5 and 6]. During storage, drug leakage was observed in all formulations during the two months [Table 6]. Size distribution experiments often revealed an increase in the mean diameter of the vesicles due to their fusion or aggregation.[15] Increment of particle size and poly dispersity index in nano-niosomes was observed during storage for two months. Uchegbu et al.[19] reported the effect of the original size of liposomes on the stability of the system. Smaller niosomes, according to thermodynamic theory have more surface energy and tend to aggregate to lower surface energy.[15] Therefore, the smaller particles have a more inherent instability than the larger ones. In addition incorporation of cholesterol into bilayers of niosomes could enhance stability and decrease their leakiness.[19] The mean particle size was found to increase on storage, especially after two months [Table 6]. The increase in particle size was greater for C30S6035T6035, due to their smaller original size and lower content of cholesterol. The greatest stability in the vesicle size was observed in C50S6025T6025 and C40S6030T6030 formulations due to the higher transition temperature of Span 60, Tween 60, and good molecular packaging of the surfactant and cholesterol in bilayers using a higher ratio of cholesterol. This result was in agreement with the previous data reported by Moazeni et al.[15]
Table 5.
Evaluation of physical stability carvedilol formulations at 4°C after one month
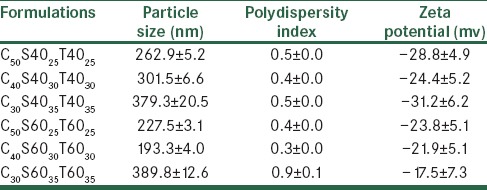
Table 6.
Evaluation of the physical stability of carvedilol formulations at 4°C after two months
CONCLUSIONS
Carvedilol was entrapped in nano niosomal formulations successfully, except in niosomes composed of cholesterol and a mixture of Span/Tween 20, due to the liquid nature of these surfactants, which caused more permeability of the bilayer. Cholesterol content and drug incorporation were the most effective variables on the particle size of nano-niosomes. Nano-niosomes composed of C50S6025T6025 and C40S6030T6030 showed the highest stability during storage, for a two-month period. From the results obtained, it can be concluded that nano-niosomes could be considered as stable carriers for the oral delivery of carvedilol, however, further pharmacokinetic studies are necessary to demonstrate their potential in increasing the drug bioavailability compared to the free drug.
ACKNOWLEDGMENT
The authors acknowledge the financial support from the Isfahan University of Medical Sciences.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Gaucher G, Satturwar P, Jones MC, Furtos A, Leroux JC. Polymeric micelles for oraldrug delivery. Eur J Pharm Biopharm. 2010;76:147–58. doi: 10.1016/j.ejpb.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 2.Jadon PS, Gajbhiye V, Jadon RS, Gajbhiye KR, Ganesh N. Enhanced oral bioavailability of griseofulvin via niosomes. AAPS Pharm Sci Tech. 2009;10:1186–92. doi: 10.1208/s12249-009-9325-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vishnu YV, Chandrasekhar K, Ramesh G, Rao YM. Development of mucoadhesive patches for buccal administration of carvedilol. Curr Drug Deliv. 2007;4:27–39. doi: 10.2174/156720107779314785. [DOI] [PubMed] [Google Scholar]
- 4.Yamsani VV, Gannu R, Kolli C, Rao ME, Yamsani MR. Development and in vitro evaluation of buccoadhesive carvedilol tablets. Acta Pharm. 2007;57:185–97. doi: 10.2478/v10007-007-0015-7. [DOI] [PubMed] [Google Scholar]
- 5.Venishetty VK, Chede R, Komuravelli R, Adepu L, Sistla R, Diwan PV. Design and evaluation of polymer coated carvedilol loaded solid lipid nanoparticles to improve the oral bioavailability: A novel strategy to avoid intraduodenal administration. Colloids Surf B Biointerfaces. 2012;95:1–9. doi: 10.1016/j.colsurfb.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Sharma A, Jain CP. Preparation and characterization of solid dispersions of carvedilol with PVP K30. Res Pharm Sci. 2010;5:49–56. [PMC free article] [PubMed] [Google Scholar]
- 7.Han HK, Shin HJ, Ha DH. Improved oral bioavailability of alendronate via the mucoadhesive liposomal delivery system. Eur J Pharm Sci. 2012;46:500–7. doi: 10.1016/j.ejps.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Jain S, Patil SR, Swarnakar NK, Agrawal AK. Oral delivery of doxorubicin using novel polyelectrolyte-stabilized liposomes (layersomes) Mol Pharm. 2012;9:2626–35. doi: 10.1021/mp300202c. [DOI] [PubMed] [Google Scholar]
- 9.Kowapradit J, Apirakaramwong A, Ngawhirunpat T, Rojanarata T, Sajomsang W, Opanasopit P. Methylated N-(4-N, N-dimethylaminobenzyl) chitosan coated liposomes for oral protein drug delivery. Eur J Pharm Sci. 2012;47:359–66. doi: 10.1016/j.ejps.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 10.Rajera R, Nagpal K, Singh SK, Mishra DN. Niosomes: A controlled and novel drugdelivery system. Biol Pharm Bull. 2011;34:945–53. doi: 10.1248/bpb.34.945. [DOI] [PubMed] [Google Scholar]
- 11.Rentel CO, Bouwstra JA, Naisbett B, Junginger HE. Niosomes as a novel peroral vaccine delivery system. Int J Pharm. 1999;186:161–7. doi: 10.1016/s0378-5173(99)00167-2. [DOI] [PubMed] [Google Scholar]
- 12.Baillie AJ, Florence AT, Hume LR, Muirhead GT, Rogerson A. The preparation and properties of niosomes–non-ionic surfactant vesicles. J Pharm Pharmacol. 1985;37:863–8. doi: 10.1111/j.2042-7158.1985.tb04990.x. [DOI] [PubMed] [Google Scholar]
- 13.Palozza P, Muzzalupo R, Trombino S, Valdannini A, Picci N. Solubilization and stabilization of beta-carotene in niosomes: Delivery to cultured cells. Chem Phys Lipids. 2006;139:32–42. doi: 10.1016/j.chemphyslip.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Varshosaz J, Pardakhty A, Hajhashemi VI, Najafabadi AR. Development and physical characterization of sorbitan monoester niosomes for insulin oral delivery. Drug Deliv. 2003;10:251–62. doi: 10.1080/drd_10_4_251. [DOI] [PubMed] [Google Scholar]
- 15.Moazeni E, Gilani K, Sotoudegan F, Pardakhty A, Najafabadi AR, Ghalandari R, et al. Formulation and in vitro evaluation of ciprofloxacin containing niosomes for pulmonary delivery. J Microencapsul. 2010;27:618–27. doi: 10.3109/02652048.2010.506579. [DOI] [PubMed] [Google Scholar]
- 16.Vangala A, Kirby D, Rosenkrands I, Agger EM, Andersen P, Perrie Y. A comparative study of cationic liposome and niosome-based adjuvant systems for protein subunit vaccines: Characterisation, environmental scanning electron microscopy and immunisation studies in mice. J Pharm Pharmacol. 2006;58:787–99. doi: 10.1211/jpp.58.6.0009. [DOI] [PubMed] [Google Scholar]
- 17.Mokhtar M, Sammour OA, Hammad MA, Megrab NA. Effect of some formulation parameters on flurbiprofen encapsulation and release rates of niosomes prepared from proniosomes. Int J Pharm. 2008;361:104–11. doi: 10.1016/j.ijpharm.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 18.Khazaeli P, Pardakhty A, Shoorabi H. Caffeine-loaded niosomes: Characterization and in vitro release studies. Drug Deliv. 2007;14:447–52. doi: 10.1080/10717540701603597. [DOI] [PubMed] [Google Scholar]
- 19.Uchegbu IF, Vyas SP. Non-ionic surfactant based vesicles (niosomes) in drug delivery. Int J Pharm. 1998;172:33–70. [Google Scholar]


