Abstract
Background:
The rising prevalence of obesity in today populations has led obese individuals to seek medical interventions. Aside from special diets, routine exercise and in some cases, medical treatment, most of the obese patients, favoring those with morbid or super obesity can benefit from bariatric surgery to lose weight. Laparoscopic sleeve gastrectomy (LSG) is relatively new method to limit the compliance of stomach. The consequent quick satiety during each meal results in gradual weight loss in patients. We investigated the efficacy and safety of this method among a group of our patients.
Materials and Methods:
This cross-sectional study was conducted in Isfahan, Iran, from January 2012 to January 2013. Thirty-five cases of obesity that had undergone LSG were enrolled and their baseline data of weight, body mass index (BMI), blood sugar, lipid profile, liver function indexes and blood pressure were collected. The patients were followed up for 6 months. The 6-month results were analyzed.
Results:
There was significant reduction in BMI, weight, blood sugar, blood pressure, liver enzymes and lipid profile components (P < 0.05), except for alkaline phosphatase (ALP) (P = 0.3). The average of excess weight loss percentage after 6 months was 69.2 ± 20.9%. No mortality occurred. Two of the patients had micro anastomotic leaks that were treated with nonoperative management. A case of gross leakage was treated with tube jejunostomy.
Conclusion:
Our study confirmed the efficacy and safety of LSG as a single surgical intervention for body weight reduction in morbidly and super obese patients.
Keywords: Bariatric surgery, gastrectomy, laparoscopic, laparoscopic surgery, obesity, sleeve gastrectomy
INTRODUCTION
Obesity is considered as one of the preventable causes of death in the United States. Both genetics and environmental factors are involved in obesity; the rapid growth in the prevalence of obesity in the society points out that components other than genetics play important roles in obesity. In 2004, 32.2% of adults in the Unites States were considered obese.[1,2]
Severe obesity, defined as having body mass index (BMI) over 35, causes several obesity-related problems that affect daily social and occupational activities of obese individual, including abnormal physical appearance in cases of morbid obesity, having difficulty using public transport and lack of variety of choice regarding clothes; these differences lead to development of depression among obese individuals which is more prevalent in them than it is in the general population.[3]
Obesity-related conditions include degenerative joint disease, back pain, hypertension, sleep apnea, gastro-esophageal reflux, cholelithiasis, type II diabetes, elevated lipid profile, asthma, migraine headaches, deep vein thrombosis, cutaneous abscess, infertility and increased rate of specific malignancies.[4]
These factors have made the obese individuals to seek solutions for their problem. Non-surgical methods combine low calorie intake diets with moderate exercise programs and are the easiest and most reliable interventions to reduce weight; however, the success of this method among severely obese individuals is estimated about 3%. Medical treatments for losing excess weight have been suggested, yet only, orlistat has been approved by the food and drug administration (FDA), which is not effective for severe obesity.[5] Consequently a growing tendency has emerged toward bariatric surgery. These methods reduce weight through limiting the compliance of the alimentary tract for food and creating a malabsorption state.
After the assessment of their results from deudenal switch (DS) surgery, Gagner et al. introduced laparoscopic sleeve gastrectomy (LSG) for the first time as a new surgery method. This method was developed to break the main surgical procedure into two shorter operations in order to reduce the mortality rate; this however resulted in recognizing LSG as a separate effective method for treatment of obesity.[6]
Many surgeons have started using this method, and reports of outstanding successful treatment through this procedure have been published.[7,8]
Regarding the increased prevalence of obesity in our population, this procedure is being more frequently used. LSG is a reliable surgery with minimal invasiveness for severe obesity and growing data is being published, confirming its efficacy. In this article, we aimed to evaluate the data on efficacy of LSG in the literature.
MATERIALS AND METHODS
In a prospective study, in a 12-month period, we consecutively enrolled referred patients to the saint Alzahra hospital daily clinic who had underwent LSG surgery and had a BMI of more than 35 before surgery. All patients had a history of failed, non-surgical obesity treatment prior to their surgery. We excluded the patients who did not comply with post surgical modifications of their life style and dietary patterns from the study.
All patients had been tested for fasting blood sugar (FBS), lipid profile including high-density lipoproteins (HDL), low-density lipoproteins (LDL) and triglycerids (TG) and liver function tests before the surgery. The patients had also been assessed for presence of hypertension and sleep apnea through physical examination and medical history.
All included patients had undergone LSG surgery by the same surgical team, under the same surgical settings. Briefly, the surgeries were performed under general anesthesia; patients were placed in a supine trendelenburg position. Five trocars were used in the procedure, a supra umbilical incision for passing the 11 mm 30 degree lens trocar, a 5 mm epigastric incision, a 12 mm incision at the right side above the umbilicus and two 5 mm incisions at both left and right sides of the umbilicus. An endoGIA™ stapler was used in this procedure. Right before stapling, a size-36 tube was passed through patient's mouth into the stomach to mark the stapling line; using this guide the surgeon removed the stomach from 4 cm proximal to the pyloric sphincter all the way to the angle of Hiss at the side of stomach's greater curvature, longitudinally. Duration of the procedure was registered and patients’ vital signs were monitored post surgically. Upper gastrointestinal (GI) imaging was performed the next day via X-ray, to search for anastomotic leak using Gastrografin. Patients were discharged from the hospital after they were able to start oral diet. Patients’ days of stay at the hospital after the surgery were registered. The patients were revisited for a follow-up at 2 weeks, 1 month, 3 months and 6 months. The patients’ BMI and weight were registered at each visit; FBS, lipid profile, blood pressure, liver enzymes were evaluated through a routine biochemistry test and presence of sleep apnea was assessed via medical history at 6 months again.
We calculated patients’ excess weight before surgery by using BMI of 23 as a reference. At the 6-month visit, excess weight loss percentage (EWL%) was defined as the amount of weight patients had lost as a percentage of excess weight (weight loss/excess weight × 100).[1]
We used independent t test to compare means between two groups and paired sample-t test for analysis of dependant data, using the SPSS, version 19 (Chicago, IL) software. A two-tailed P value of less than 0.05 was considered statistically significant.
RESULTS
During the study, 35 patients were enrolled. The mean ± standard deviation (SD) for age was 31.45 ± 8.84. The demographic data is presented in Table 1.
Table 1.
Demographic data
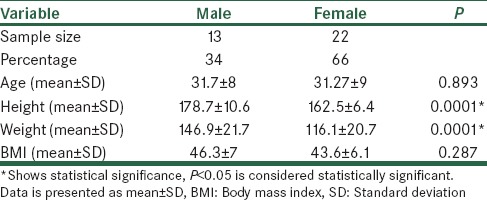
There was a significant difference between male and female patients regarding their height and weight, with males being taller and heavier (P < 0.05); however, there was not any significant BMI difference between males and females (P = 0.287). The age between men and women was not significantly different (P = 0.893).
The values of weight, BMI, FBS, lipid profile, liver function tests and systolic (SBP) and diastolic blood pressure (DBP) in patients were registered at baseline and 6 months from the surgery. The data is shown in Table 2.
Table 2.
Comparison of biochemical variables between baseline and 6 months after the surgery
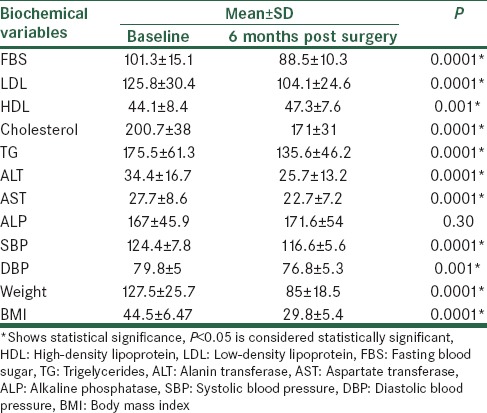
There was statistically significant reduction of the mean value of FBS between the baseline data and the data from 6 months after the surgery (P < 0.05). The lipid profile values between the baseline time and after 6 months were significantly lower (P < 0.05) except for HDL level that had a significant increase during the 6 months of the study (P < 0.05). Evaluations of blood pressure revealed that there was a significant decrease in systolic and diastolic blood pressure before the surgery and after 6 months (P < 0.05). Comparison of the results of liver function tests indicated that although the differences in ALT and AST before the surgery and after 6 months were significant (P < 0.05); the difference in ALP between these times was not statistically significant (P = 0.3). Patients had significantly lower weight and BMI after 6 months than at the beginning of the study (P < 0.05).
For each patient, BMI and weight were also measured at 2 weeks, 1 month and 3 months from the surgery. Data is presented in Table 3.
Table 3.
Comparison of BMI and weight between visits
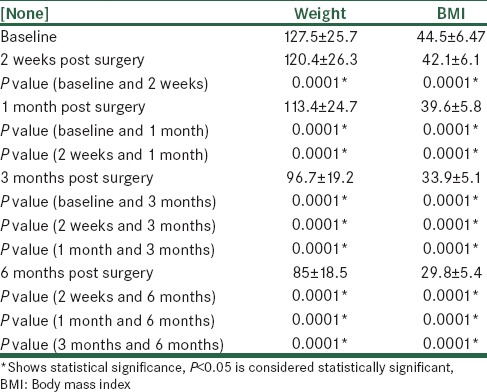
The data indicated that between the baseline values and visits at 2 weeks, 1 month, 3 months and 6 months after surgery, and also between the visits themselves, there were significant differences showing a general tendency to weight loss (P < 0.05). The mean ± SD of EWL% at 6 months was 69.2 ± 20.9% among the patients.
The mean ± SD for duration of surgery was 117 ± 26.5 min. Patients stayed at the hospital for an average of 3.5 ± 1.3 days after the surgery.
Patients were evaluated for the presence of sleep apnea before they had the surgery; 22.8% had no history related to sleep apnea, 42.8% had mild to moderate sleep apnea and 20% had severe sleep apnea. A remaining 14.2% of the patients had only snoring during the night time. At the end of the study, no patient had a complaint of symptoms related to sleep apnea.
We evaluated the outcome of the surgery by performing upper GI imaging to look for probable anastomotic leaks. One of the enrolled patients had gross leak from the site of surgery, two patients had micro leaks, one micro leak developed into a sub-diaphragmatic abscess. There were no reports of other post operative complications including pulmonary embolism, wound infection or mortalities among our patients.
DISCUSSION
Several methods are being used as surgical interventions for obesity; none of them are without complications.[9] LSG is relatively new method to limit the compliance of stomach. The consequent quick satiety during each meal results in gradual weight loss in patients.[10] We investigated the efficacy and safety of this method among a group of our patients in a short-term follow-up.
We compared several biochemical indexes to evaluate the efficacy of this procedure in our patients. FBS levels decreased significantly within 6 months, which points to the effectiveness of this method to control diabetes related co-morbidities in obese patients. Nosso et al., claimed in their study that LSG provides a sustained controlled serum level of glucose among obese type 2 diabetic patients.[11]
Imbalanced lipid profile and metabolic syndrome accompanies obesity. Pe’qiugnot et al. showed that sleeve gastrectomy is capable of reducing the incidence of metabolic syndrome.[12] While our data supports this idea, the reduction in LDL, Cholesterol and triglycerides along with rising levels of HDL shows a potential for management of cardiovascular risk factors. The reduction in patients’ systemic blood pressure can be the first sign of a less stressed circulatory system. Weight loss and resolution of major cardiovascular risk factor in obese patients may be able to considerably lengthen their life expectancy. This idea is further enlightened if we consider significant reductions of hepatic transaminase levels in our patients. The treatment of obesity could have indirect benefits to hepatic cells and prevent damage from conditions such as non-alcoholic fatty liver.
The main goal of bariatric surgery is to help obese patients achieve a desired body weight. Studies have shown that LSG benefits the obese adults by both weight loss and decreasing their obesity-related co-morbidities in a mid-term follow-up period and that efficacy of LSG is similar to that of a Roux-en-Y gastric bypass.[13,14] It has also been noted that despite favorable 5-year outcomes of LSG, a major lifestyle modification is necessary for this method to be most effective.[15] This type of surgery has also been approved as an alternative procedure in patients whose attempts for weight loss with other methods have failed;[16] however, the benefit of a second LSG on patients is controversial and carries a risk of increased post surgical complication.[17] The efficacy and safety of this surgery has been reported in the literature regarding short-term start of weight loss and acceptable EWL% with a 6-year EWL% of over 50%,[18,19] sustained weight control, reduction of co morbidities and very few complications.[10,20,21] The procedure has even been considered safe to be used in patients over the age of 60.[22] The reported complications include development of gastro-esophageal reflux disease (GERD),[21] and post surgical anastomotic leaks. The first signs that indicate a post surgical leakage or bleeding include tachycardia, pain, fever and hypotension.[23] As the main complication for LSG, Kehagias et al. stated that a non-surgical approach can help with the leaks.[24] A combination of percutaneous drainage, stents, use of antibiotics and parenteral support of the patient is suggested as treatment and early usage of stents is associated with a faster time of recovery.[25,26] In order to decrease the risk of post surgical leaks, use of stapling line reinforcement methods such as over sewing, application of bovine pericardium or synthetic polyester and thrombin matrix have been proposed.[27]
In our study, we compared weight and BMI of the patients between baseline values and subsequent follow up visits for 6 months. A significant reduction in weight and BMI was observed indicating a gradual weight loss trend. Our patients had an average EWL% of 69.2 ± 20.9% in the first 6 months. These results suggest the efficacy of LSG among our patients.
Regarding the post surgical complications one of our patients had a gross leak from the site of stapling. We applied a tube jejunostomy which resolved the problem. Two of our patients had micro leaks, one which turned into a sub-diaphragmatic abscess. We used conservative treatment for them, including prophylactic antibiotics, cessation of oral nutrition and hydration. The episode resolved in both cases without the need for a surgical intervention.
Keshet et al. have proposed in their study that a complementary medical support along with the routine supportive care in hospitals can improve pain and anxiety in patients undergoing LSG.[28] This is also suggested by Chang et al., that a proper perioperative care could further improve the safety of LSG.[29]
CONCLUSION
Our study suggested the efficacy and safety of LSG as a single surgical intervention for body weight reduction in morbidly and super obese patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Lee SY, Lim CH, Pasupathy S, Poopalalingam R, Tham KW, Ganguly S, et al. Laparoscopic sleeve gastrectomy: A novel procedure for weight loss. Singapore Med J. 2011;52:794–800. [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 3.Hady HR, Golaszewski P, Zbucki RL, Dadan J. The influence of laparoscopic adjustable gastric banding and laparoscopic sleeve gastrectomy on weight loss, plasma ghrelin, insulin, glucose and lipids. Folia Histochem Cytobiol. 2012;50:292–303. doi: 10.5603/fhc.2012.0039. [DOI] [PubMed] [Google Scholar]
- 4.Garfinkel L. Overweight and cancer. Ann Intern Med. 1985;103:1034–6. doi: 10.7326/0003-4819-103-6-1034. [DOI] [PubMed] [Google Scholar]
- 5.NIH conference. Gastrointestinal surgery for severe obesity. Consensus Development Conference Panel. Ann Intern Med. 1991;115:956–61. [PubMed] [Google Scholar]
- 6.Almogy G, Crookes PF, Anthone GJ. Longitudinal gastrectomy as a treatment for the high-risk super-obese patient. Obes Surg. 2004;14:492–7. doi: 10.1381/096089204323013479. [DOI] [PubMed] [Google Scholar]
- 7.Baltasar A, Serra C, Pérez N, Bou R, Bengochea M, Ferri L. Laparoscopic sleeve gastrectomy: A multi-purpose bariatric operation. Obes Surg. 2005;15:1124–8. doi: 10.1381/0960892055002248. [DOI] [PubMed] [Google Scholar]
- 8.Cottam D, Qureshi FG, Mattar SG, Sharma S, Holover S, Bonanomi G, et al. Laparoscopic sleeve gastrectomy as an initial weight-loss procedure for high-risk patients with morbid obesity. Surg Endosc. 2006;20:859–63. doi: 10.1007/s00464-005-0134-5. [DOI] [PubMed] [Google Scholar]
- 9.Barnett SJ. Bariatric surgical management of adolescents with morbid obesity. Curr Opin Pediatr. 2013;25:515–20. doi: 10.1097/MOP.0b013e328362cd96. [DOI] [PubMed] [Google Scholar]
- 10.Eisenberg D, Bellatorre A, Bellatorre N. Sleeve gastrectomy as a stand-alone bariatric operation for severe, morbid, and super obesity. JSLS. 2013;17:63–7. doi: 10.4293/108680812X13517013317077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nosso G, Angrisani L, Saldalamacchia G, Cutolo PP, Cotugno M, Lupoli R, et al. Impact of sleeve gastrectomy on weight loss, glucose homeostasis, and comorbidities in severely obese type 2 diabetic subjects. J Obes. 2011;2011:340867. doi: 10.1155/2011/340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Péquignot A, Dhahri A, Verhaeghe P, Desailloud R, Lalau JD, Regimbeau JM. Efficiency of laparoscopic sleeve gastrectomy on metabolic syndrome disorders: Two years results. J Visc Surg. 2012;149:e350–5. doi: 10.1016/j.jviscsurg.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 13.Raziel A, Sakran N, Szold A, Teshuva O, Krakovsky M, Rabau O, et al. Mid-term follow-up after laparoscopic sleeve gastrectomy in obese adolescents. Isr Med Assoc J. 2014;16:37–41. [PubMed] [Google Scholar]
- 14.Helmiö M, Victorzon M, Ovaska J, Leivonen M, Juuti A, Peromaa-Haavisto P, et al. Comparison of short-term outcome of laparoscopic sleeve gastrectomy and gastric bypass in the treatment of morbid obesity: A prospective randomized controlled multicenter SLEEVEPASS study with 6-month follow-up. Scand J Surg. 2014;103:175–81. doi: 10.1177/1457496913509984. [DOI] [PubMed] [Google Scholar]
- 15.Keren D, Matter I, Lavy A. Lifestyle modification parallels to sleeve success. Obes Surg. 2014;24:735–40. doi: 10.1007/s11695-013-1145-2. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs M, Gomez E, Romero R, Jorge I, Fogel R, Celaya C. Failed restrictive surgery: Is sleeve gastrectomy a good revisional procedure? Obes Surg. 2011;21:157–60. doi: 10.1007/s11695-010-0315-8. [DOI] [PubMed] [Google Scholar]
- 17.Rebibo L, Fuks D, Verhaeghe P, Deguines JB, Dhahri A, Regimbeau JM. Repeat sleeve gastrectomy compared with primary sleeve gastrectomy: A single-center, matched case study. Obes Surg. 2012;22:1909–15. doi: 10.1007/s11695-012-0779-9. [DOI] [PubMed] [Google Scholar]
- 18.Paluszkiewicz R, Kalinowski P, Wróblewski T, Bartoszewicz Z, Białobrzeska-Paluszkiewicz J, Ziarkiewicz-Wróblewska B, et al. Prospective randomized clinical trial of laparoscopic sleeve gastrectomy versus open Roux-en-Y gastric bypass for the management of patients with morbid obesity. Wideochir Inne Tech Malo Inwazyjne. 2012;7:225–32. doi: 10.5114/wiitm.2012.32384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Himpens J, Dobbeleir J, Peeters G. Long-term results of laparoscopic sleeve gastrectomy for obesity. Ann Surg. 2010;252:319–24. doi: 10.1097/SLA.0b013e3181e90b31. [DOI] [PubMed] [Google Scholar]
- 20.Abd Ellatif ME, Abdallah E, Askar W, Thabet W, Aboushady M, Abbas AE, et al. Long term predictors of success after laparoscopic sleeve gastrectomy. Int J Surg. 2014;12:504–8. doi: 10.1016/j.ijsu.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Catheline JM, Fysekidis M, Bachner I, Bihan H, Kassem A, Dbouk R, et al. Five-year results of sleeve gastrectomy. J Visc Surg. 2013;150:307–12. doi: 10.1016/j.jviscsurg.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Mizrahi I, Alkurd A, Ghanem M, Zugayar D, Mazeh H, Eid A, et al. Outcomes of laparoscopic sleeve gastrectomy in patients older than 60 telears. Obes Surg. 2014;24:855–60. doi: 10.1007/s11695-014-1177-2. [DOI] [PubMed] [Google Scholar]
- 23.Mittermair R, Sucher R, Perathoner A. Results and complications after laparoscopic sleeve gastrectomy. Surg Today. 2013;44:1307–12. doi: 10.1007/s00595-013-0688-0. [DOI] [PubMed] [Google Scholar]
- 24.Kehagias I, Spyropoulos C, Karamanakos S, Kalfarentzos F. Efficacy of sleeve gastrectomy as sole procedure in patients with clinically severe obesity (BMI≤50 kg/m (2)) Surg Obes Relat Dis. 2013;9:363–9. doi: 10.1016/j.soard.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 25.Simon F, Siciliano I, Gillet A, Castel B, Coffin B, Msika S. Gastric leak after laparoscopic sleeve gastrectomy: Early covered self-expandable stent reduces healing time. Obes Surg. 2013;23:687–92. doi: 10.1007/s11695-012-0861-3. [DOI] [PubMed] [Google Scholar]
- 26.Vix M, Diana M, Marx L, Callari C, Wu HS, Perretta S, et al. Management of staple line leaks after sleeve gastrectomy in a consecutive series of 378 patients. Surg Laparosc Endosc Percutan Tech. 2014 doi: 10.1097/SLE.0000000000000026. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 27.D’Ugo S, Gentileschi P, Benavoli D, Cerci M, Gaspari A, Berta RD, et al. Comparative use of different techniques for leak and bleeding prevention during laparoscopic sleeve gastrectomy: A multicenter study. Surg Obes Relat Dis. 2013;10:450–4. doi: 10.1016/j.soard.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 28.Keshet Y, Attias S, Ben-Arye E, Shaham M, Grimberg O, Schiff E. Integrative complementary medicine for treatment of bariatric perioperative symptoms: Patients’ experiences and staff evaluations. Bariatr Surg Pract Patient Care. 2013;8:108–12. doi: 10.1089/bari.2013.9980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang XS, Yin K, Wang X, Zhuo GZ, Ding D, Guo X, et al. Perioperative managment of laparoscopic sleeve gastrectomy. Zhonghua Wei Chang Wai Ke Za Zhi. 2013;16:993–6. [PubMed] [Google Scholar]


