Abstract
Background:
Spinal cord injury (SCI) is not likely to recover by current therapeutic modalities. Stem cell (SC) therapy (SCT) has promising results in regenerative medicine. We present our experience of co-infusion of autologous adipose tissue derived mesenchymal SC differentiated neuronal cells (N-Ad-MSC) and hematopoietic SCs (HSCs) in a set of patients with posttraumatic paraplegia.
Materials and Methods:
Ten patients with posttraumatic paraplegia of mean age 3.42 years were volunteered for SCT. Their mean age was 28 years, and they had variable associated complications. They were subjected to adipose tissue resection for in vitro generation of N-Ad-MSC and bone marrow aspiration for generation of HSC. Generated SCs were infused into the cerebrospinal fluid (CSF) below injury site in all patients.
Results:
Total mean quantum of SC infused was 4.04 ml with a mean nucleated cell count of 4.5 × 104/μL and mean CD34+ of 0.35%, CD45−/90+ and CD45−/73+ of 41.4%, and 10.04%, respectively. All of them expressed transcription factors beta-3 tubulin and glial fibrillary acid protein. No untoward effect of SCT was noted. Variable and sustained improvement in Hauser's index and American Spinal Injury Association score was noted in all patients over a mean follow-up of 2.95 years. Mean injury duration was 3.42 years against the period of approximately 1-year required for natural recovery, suggesting a positive role of SCs.
Conclusion:
Co-infusion of N-Ad-MSC and HSC in CSF is safe and viable therapeutic approach for SCIs.
Keywords: Neuronal stem cells, spinal cord injuries, stem cell infusion, traumatic paraplegia
INTRODUCTION
The incidence of traumatic injuries is estimated as >500,000 new patients annually in the world.[1] Traumatic spinal cord injuries (SCIs) are known to lead to severe and permanent neurologic deficit. Posttraumatic SCI can result in complete sensory and motor function loss, which is challenging for reversal of the damage, but not untreatable. In spite of the advancement of neurological science, there is no definite permanent complete recovery recorded in these patients. Many treatments have been tested in clinical trials, including the use of methylprednisolone, GM1 ganglioside, decompression, and 4-aminopyridine, all of which have only produced marginal benefits with side effects. The present standard of care for SCI is methyl prednisolone and/or decompression. However, neither of these treatments has prevented the pathological cascade triggered by SCI, and the efficacy of methyl prednisolone is questionable. Few therapeutic options such as neurotization by total and hemi-contralateral nerve root transfer are available with limited benefits.[2]
Reinnervation of denervated targets can be achieved by regeneration of injured axons or by collateral branching of undamaged axons in the vicinity. Nevertheless, these mechanisms do not provide for satisfactory functional recovery, especially after severe injuries.[1] At present, there is no cure or effective standard of care for SCI. Postinjury medical interventions are aimed at treatment of complications such as autonomic dysreflexia, pain, and urinary tract infections (UTIs). Regenerative approaches using growth factors and various cell therapies are particularly appealing with early clinical reports of improvement using autologous bone marrow (BM) cells, olfactory ensheathing cells, and Schwann cells.[3] Stem cell (SC) therapy (SCT) is building up hope for all such disorders.[4] We present our experience of co-transplantation of neuronal SCs generated from autologous adipose tissue derived mesenchymal SCs (N-Ad-MSC) and cultured BM (CBM) derived hematopoietic SCs (HSCs) in posttraumatic paraplegics. Primary endpoints of the study were safety and efficacy of SCT, and secondary endpoints were recovery in motor and sensory functions.
Spinal cord injuries background
Nerve damage in SCI occurs in the majority of cases as a result of the combined effects of the initial physical injury, and subsequent inflammatory response caused in part by physical damage to the blood-brain barrier, immune cell response to injury, and local ischemia. Typical causes of injury include contusive, compressive, or stretch damage, which is associated with severing of axons at the nodes of Ranvier, leading to axon retraction. Furthermore, axons proximal to the area of injury that do not retract are known to develop abnormalities such as loss of myelination and swelling of the axonal body, resulting in loss of excitability. Demyelination is in part believed to result from the death of oligodendrocytes surrounding the axon, a process, which occurs even at 3 weeks after the initial injury. Importance of demyelination in this process is seen in experiments where remyelination induced by administration of Schwann cells has been demonstrated to elicit benefit in animal models of SCI. Mechanistically, oligodendrocyte death appears to be related to the death receptor Fas based on: (a) Pattern of expression is temporarily correlated with oligodendrocyte apoptosis in SCI models; (b) genetic inactivation of Fas results in reduced oligodendrocyte death; and (c) administration of soluble Fas has a protective effect on SCI associated demyelination. Interestingly, administration of human umbilical cord blood SCs in a rat SCI model results in therapeutic benefit, which seems to be mediated by reduction of Fas expression. Death of neurons themselves subsequent to SCI is associated with the release of glutamate and other excitotoxins such as free adenosine triphosphate. Interestingly, excitotoxicity occurs not only as a result of initial injury but has also been implicated in secondary, more long-term neuronal damage. Associated with demyelination is the exposure of potassium channels which causes accumulation of the ion intraneuronally, thus, further modifying ability to transmit electrical signals. Inhibition of fast acting potassium channel, channels using 4-aminopyridine has demonstrated some therapeutic effects in animal models of SCI, and in clinical trials. Thus, the initial injury process seems to cause: (a) Direct transection of neurons; (b) inflammatory responses that stimulate a self-perpetuating cascade of axon retraction; (c) inflammatory mediated death of oligodendrocytes; and (d) stimulation of mediators such as NOGO that prevent endogenous axonal reattachment.[3]
MATERIALS AND METHODS
Aim
This was a prospective single arm open-labeled clinical trial conducted from December 2008 to October 2012 to test the efficacy and safety of combined N-Ad-MSC and HSC infusion in posttraumatic paraplegics approved by the Institutional Review Board. We obtained informed consent for participation in research activities in accordance with the committee's standards from the individual described herein.
HSC co-infusion with N-Ad-MSC was designed to augment the effect of the later. Cerebrospinal fluid (CSF) infusion was carried out below the site of injury to facilitate faster movement of the cells to injured sites across blood brain barrier without losing them in the pathway.
Inclusion and exclusion criteria
Inclusion criteria were patients of any gender between 8 and 70 years of age, with posttraumatic paraplegia for ≥12 months, and confirmed with magnetic resonance imaging (MRI) of the spinal cord. Patients with positive serology for HIV/hepatitis B surface antigen/hepatitis C virus, malignancy, advanced systemic illness, active infective focus in the body, and pregnant women were excluded.
Study design
Patients
Ten paraplegics (8 males, 2 females) with mean age of 28 (range: 9–42) years and mean disease duration of 3.42 (range: 2.5–5.4) years volunteered for SCT. Site of injury was lower dorsal vertebral column in all; D6-D8 in 6, D12-L1 in 3, and L1-L2 in 1 patient. MRI of all patients was performed before starting the SCT to document the level of SCI with myelomalacic changes and to rule out the complete spinal cord transection. All patients had bowel-bladder incontinence, 7 had neurogenic pain, 6 had pressure sores, 4 had recurrent UTIs, 3 had kyphosis/scoliosis, 1 had bladder calculi, and 5 had depression. All adults had sexual dysfunction. Mean Hauser ambulation index (HAI) was 9 (normal index: 1). The HAI is scored using a 10-point scale (0–9), with 0 representing no impairment (fully active) and 9 representing confinement to a wheelchair with the inability to transfer oneself independently [Table 1]. Nerve conduction studies showed motor axonopathy in 4, upper motor neuron lesion in 2, and radiculoneuropathy in 4 patients.
Table 1.
Hauser ambulation index

Generation and infusion of stem cells
MSC were generated using our standard technique from 10 g adipose tissue resected from patients’ own anterior abdominal wall on day 1, and sent to SC lab for culture in self-designed proliferation medium [Figure 1] comprised of minimum essential medium with alpha modification (Sigma, USA), 20% human albumin (Reliance Life Sciences, India), basic-human fibroblast growth factor, 1% sodium pyruvate, and standard antibiotics which included penicillin-streptomycin solution (10 mg/L) (Hi-media, India), cefotaxime (1 mg/ml), and anti-fungal agent fluconazol (10 mg/ml).[5] After subjecting to in vitro expansion for 10 days in proliferation medium without passaging, the cells were harvested by trypsinization, quantified and checked for viability and sterility. Harvested MSCs were checked for viability using trypan blue, sterility (Bactec, Franklin Lakes, NJ, USA), and cell counts in a modified Neubauer chamber. Hematoxylin and eosin stain used for morphologic analysis of MSCs, showing round to elongate or fibroblastoid, with centrally placed round nucleus with prominent nuclear margins, and surrounding fine granular eosinophilic cytoplasm under a microscope.
Figure 1.
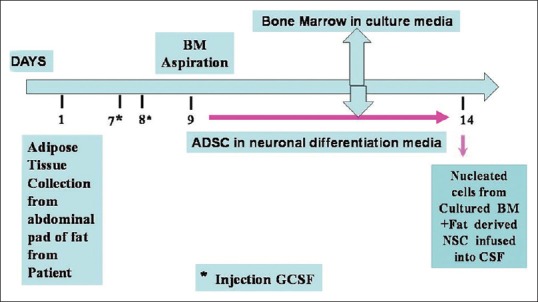
Paradigm for in vitro generation and co-infusion of bone marrow derived hematopoietic stem cells and adipose tissue-derived mesenchymal stem cells differentiated into neuronal cells, for posttraumatic spinal cord injury
MSCs were confirmed with CD45−/CD90+/CD73+ (Beckton Dickinson, Franklin Lakes, NJ, USA) by flow-cytometric analysis [Figure 2], which revealed negligible amount at the very 1st day of culture from h-AD. Increase in the CD45−/90+/73+ population was found after culture of the MSC. Media were replenished on alternate days. Ad-MSC were further subjected to differentiation into N-Ad-MSC using neuronal differentiation medium for 4 days, comprising neurobasal medium (Invitrogen, Germany), Dulbecco's modified Eagle's medium (DMEM) F-12 (Sigma, USA), nonessential amino acids (Sigma, USA), and growth factors such as epidermal growth factor (10 ng/ml) (Sigma, USA), brain-derived neurotrophic factor (BDNF) (10 ng/ml) (Sigma, USA), glial derived neurotrophic factor (10 ng/ml) (Sigma, USA), cyclic adenosine monophosphate (60 µg/ml) (Sigma, USA), L-glutamine (0.5 mM) (Sigma, USA), laminin (5 µg/ml) (Sigma, USA), and N2 and B27 serum supplements (Sigma, USA). Neuronal cells were isolated by concentration gradient separation media and then the inoculum was set ready for mixing with CBM derived HSC. Presence of neuronal markers, ß-3 tubulin, and glial fibrillary acid protein (GFAP) were confirmed by immunofluorescence (IF) studies using biotinylated mouse monoclonal IgG2A and purified sheep IgG, respectively (R and D system, USA).
Figure 2.
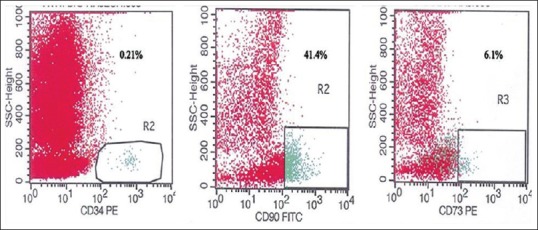
Flow cytometric analysis revealed CD45−/90+/73+ as adipose tissue-derived mesenchymal stem cells and CD34+ as hematopoietic stem cells
HSC were also generated using our standard technique and confirmed with flow cytometry as CD34+ [Figure 2]. After stimulation with granulocyte colony-stimulating factor, 7.5 µg/kg body weight twice daily, subcutaneously for 2 days, 100 ml BM was aspirated from patients’ posterior superior iliac crest under local anesthesia on day 9. The aspirated BM was subjected to in vitro expansion for 5 days to generate HSC under self-designed medium [Figure 1] using DMEM with nonessential amino acids, growth factors, and antibiotics in CO2 incubator at 37°C with 5% CO2 under humid conditions. On day 14th, N-Ad-MSC with HSC was infused into CSF intrathecally via lumbar puncture using 23 gauge spinal needle below the site of SCI, under all aseptic and antiseptic precautions [Figure 1].[5]
Patient monitoring
Patients were monitored closely for 24 h post SC infusion for spinal headache, giddiness, behavioral disturbances, convulsions, and hypertension, and discharged if this phase was uneventful. Broad spectrum antibiotics were given for 5 days with 3 monthly follow-up for further evaluation with reference to American Spinal Injury Association (ASIA) impairment scale and Hauser's Ambulation Index.[6,7] After discharge they were advised to continue with physiotherapy exercises for muscle strengthening. Their neurological status was determined in terms of ASIA scale, divided into five subdivision: Scale A: Complete loss of motor and sensory function; Scale B: Sensory but not motor function preserved below neurological level and includes sacral segments S4-S5; Scale C: Motor function preserved below the neurological level and >half of the key muscles below the neurological level having muscle Grade <3; Scale D: Motor function preserved below the neurological level, and at least half of the key muscles below the neurological level having muscle Grade ≥3; Scale E: Motor and sensory functions normal. HAI was also used to determine the disability.
RESULTS
Mean quantum of CBM-HSC and N-Ad-MSC was 1.96 (range: 1–4) ml and 2.08 (range: 1.2–4) ml, respectively, with total mean quantum infusion of 4.04 (range: 2.8–7) ml. Mean nucleated cell counts were 4.5 × 104/µL (range: 0.53–1.24 × 104/µL) and mean N-Ad-MSC counts were 2.0 × 102/µL (range: 1.5–2 × 102/µL) with mean HSC CD34+, 0.35% (range: 0.17–0.85%), mean Ad-MSC, CD45−/90+ and CD45−/73+ were 41.4% (range: 21.74–65.6%) and 10.04% (range: 0.8–22.2%), respectively. CD marker evaluation for HSC and Ad-MSC were carried out using flow-cytometric analysis, and transcription factors using IF technique were checked for neuronal cells. (Note: No CD marker evaluation carried out for neuronal cells). The neuronal cells expressed transcription factors beta-3 tubulin and GFAP using IF technique [Figure 3]. No untoward effect of SCT was noted.
Figure 3.
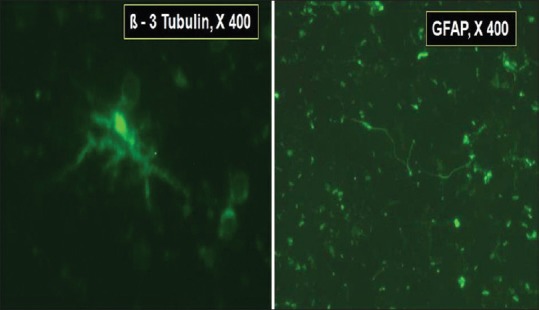
Photomicrograph of neuronal stem cells exhibiting ß-3 tubulin (×400) on left and glial fibrillary acid protein (×400) on the right, by immunofluorescence staining
Temporal course of improvement
Variable and sustained improvement in Hauser's index and ASIA score was noted in all patients over a mean follow-up of 2.95 (range: 1.2–4.0) years. The first sign of recovery was subjective crude pain sensation which is noted in all patients around the 15th day of infusion in lower extremities. Crude touch sensation was achieved after 1-month, followed by fine touch sensation and deep pain sensation after 3 months up to 3–4 dermatomes below the level of injury. This was observed in 6 patients. Deep pressure sensation was regained in 3 patients at 4 dermatomes below the level of injury. This was followed by gradual improvement in motor power-up to Grade 3. By the end of 3 months of infusion, all the patients were able to stand with the support of calipers and walker. Initially, they were able to stand for 1-min and within few weeks the duration of standing improved. By the end of 4 months, 8 patients could stand comfortably for 15–20 min using the support of calipers and walker and had started walking a few steps. Along with this walking, their adductors were toned up by physiotherapy up to Grade 3. By the end of 8 months they could walk for 1–2 h approximately with support. Six out of these 8 patients also learnt taking 1–2 reverse steps and by the end of 9 months they could comfortably take a reverse walk for up to 5–8 steps. Calipers were initially up to hip joints and then by 11 months they were reduced from “above knee” to “below knee” braces. Bladder sensation was taught by catheter clamping and intermittent catheterization to test the bladder holding capacity. By the end of 6 months, urge for micturition with bladder fullness and partial control to hold urine was noted in 5 patients. Voluntary external anal sphincter tone improved by the end of 10 months in 5 patients and they perceive the sense for defecation with poor control. Over a mean follow-up of 2.95 years, variable improvement in muscle power from Grade 0 to 3 was noted in all, touch sensation regained in 60%, bladder-bowel control improved in 50%, pressure sensation regained in 30%, and Hauser's index improved to 7.4 (P = 0.004) [Figure 4]. ASIA score improved from A to B in 6, A to C in 3, and A to D in 1 patient [Figure 5]. Although clinical recovery was noted in muscle power and sensory system, electromyography and nerve conduction velocity studies did not show any re-innervation.
Figure 4.
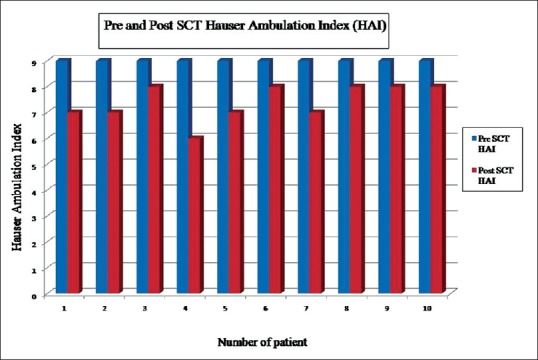
Hauser ambulation index after stem cell therapy were compared with prestem cell therapy Hauser ambulation index
Figure 5.
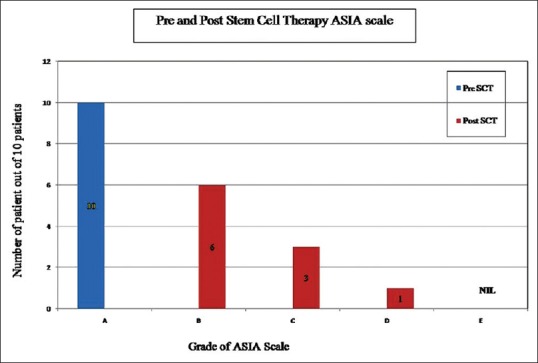
Number of patients improved after stem cell therapy were recorded using American Spinal Injury Association scale as compared to prestem cell therapy
Safety report
No adverse events were noted during and after SCT. We did not notice the procedure related complications such as spinal headache, meningitis, local site hematoma during lumbar puncture, and fever after SCT. We also noticed that involuntary movements were also stopped in 1 patient and reduced in 3 patients, out of 5 patients suffering from the involuntary movements among the total 10 patients. Urinary tract infection episode was well controlled after SCT in all 4 patients who had recurrent UTI.
DISCUSSION
SCI are usually serious since there is no established therapy for neuromuscular recovery of these patients, especially after the lapse of 1-year of injury due to the development of adhesions and fibrosis at and around the site of injury. It is also well known that natural recovery is likely only in the 1st year of injury after surgical stabilization of spinal cord. Less than 1% of these patients with no muscle activity in the lower extremities 1-month after injury learn to walk again and 10% recover enough function to be reclassified as ASIA B or better.[8,9] The literature does not provide a single example of an individual with an ASIA Grade A SCI who recovered by more than one grade, 2 years after injury.[10] Some delayed recovery occurred, but the time frame is typically between 1 and 6 months after injury for significant improvement.[11,12] ASIA scale grading is determined by the clinical neurologic findings and not by pathologic severity. As a result, ASIA scale grading does not completely reflect pathologic severity. It is possible that one ASIA grade could include a wide spectrum of severity. Less severely injured patients with an ASIA Grade A could, therefore, have a greater potential for improvement. In this study, all the patients underwent surgical and medical management immediately after SCI. However, none of them had recovery even in the 1st year of injury allotted for the natural recovery period. Since there was no recovery in any patient, they were desperate for any other therapeutic modality available. Hence, they were offered SCT with our indigenously developed N-Ad-MSC in addition to HSC. ASIA score improved from A to B in 6, A to C in 3, and A to D in 1 patient. It is believed that this partial effect was caused by an additive or alleviating role of N-Ad-MSC and HSC rather than the innate ability of natural recovery.
Cell-based therapy can offer promising results by generation of new neurons from transplanted cells producing functional recovery, believed to be due to (a) neurons relaying ascending signals disrupted by the injury, (b) demyelinated axons are re-myelinated, and (c) paracrine activity releasing trophic factors inducing endogenous mechanisms of tissue repair and plasticity.[13,14,15,16]
Majority of HSC and MSC transplantation studies in animal models of SCI occur in the acute injury phase. However, there are a number of studies using chronic models of SCI in animals that have reported increased functional recovery following MSC transplantation 6–12 weeks after injuries were induced, which is considered chronic in these model systems.[17,18] Our study shows acceptable results if compared with these studies.
Ying et al. reported that MSCs derived from the inguinal fat of rat model, were characterized by flow-cytometry and immunophenotyped of CD45−/CD90+/CD44+ suggested that ADSCs were positive for mesenchymal stem cell markers CD90 and CD44, but negative for hematopoietic stem cell markers. Adipose tissue derived SCs (ADSCs) maintained self-renewing capacity and could differentiate into adipocytes and neurocytes under special culture condition. ADSCs have been shown to differentiate into neural-like cells in vitro, and have been detected by IF with neuronal markers, GFAP, and beta-3 tubulin.[19] Co-expression of beta 3-tubulin with GFAP was detected in cells at all time points but in specially distinct patterns, and expressed astrocytes isolated from the cerebral hemispheres of two human fetuses at 18 to 20 weeks of gestation. The number of GFAP+ cells gradually decreased from day 1 to day 21 in vitro, whereas beta 3-tubulin immunoreactivity was present in 100% of cells at all time points.[20]
We have been generating MSC from human adipose tissue in vitro, which qualify the definition standardized by the mesenchymal and tissue SC Committee of the International Society for Cellular Therapy.[5,21] HSC and/or MSC without neuronal differentiation have been infused in various routes, including subarachnoid space in the past for neurological injuries, however, none of the studies has shown sustained and progressive improvement in terms of recovery of motor and sensory system.[22,23] MSCs appear to work as neurotrophic generators to promote functional recovery via angiogenesis, neurogenesis, synaptogenesis, and axonal remodeling. Multiple trophic/growth factors secreted by MSCs may contribute enhancing neuroregeneration, including BDNFs, vascular endothelial growth factors, and fibroblast growth factors.[24] Hence, we decided to embark upon this trial, which has shown satisfactory improvement although further research is required in this area for better recovery. NSC have been generated in vitro, from adipose tissue however, to our knowledge this is the first clinical study showing safe and effective generation of N-Ad-MSC and HSC from BM without using xenogeneic material and infusing them in CSF leading to significant recovery. For the added benefit of HSCs, both HSC and N-Ad-MSC were used simultaneously. HSC helps in regeneration, revascularization, and engraftment of neuronal cells derived from the Ad-MSC. HSC co-infusion with N-Ad-MSC was designed to augment the effect of the later.
The other advantage of this therapeutic approach is avoiding the risk of anesthesia and surgery and yet giving added benefits of recovery. We believe that combined N-Ad-MSC and HSC infusion in CSF will open the gateway to therapy of all neuronal disorders, which have otherwise dismal prognosis.
There were some limitations of this study. Complete double-blind case–control studies were not possible. Patients came to our center with their MRI images and reports for SCT. This helped us to document the level of SCI with myelomalacia changes and to rule out complete spinal cord transection. Follow-up MRI was not feasible in this study due to practical problems, including nonavailability of MRI facilities in the institute where the study was carried out and the unwillingness of patients to repeat MRI after cell infusion. Hence, we assessed all the patients for improvement by manual muscle testing, HAI, and ASIA scale in the follow-up. Neurologic improvements were described as advancing ASIA scale. Therefore, small improvement without changes in ASIA scale could not be qualified. Further large group studies are required to compare the effects of this SCT. This study was performed in a limited number of patients. It is recommended that the therapeutic effects should be established in a more comprehensive multicenter study.
CONCLUSION
Co-infusion of N-Ad-MSC and HSC in CSF is safe and viable therapeutic approach for SCI.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors are thankful to C N Patel, J V Patel, B N Patel, J M Chudasma, and P N Bhavsar for carrying out all the laboratory tests, including flow-cytometry analysis of the cases mentioned in the paper. We are also thankful to Ms. Shobhana Sengunthar, our statistician for analysis, and our librarian J. Suthar for literature search of manuscript.
REFERENCES
- 1.Hsu YC, Chen SL, Wang DY, Chiu IM. Stem cell-based therapy in neural repair. Biomed J. 2013;36:98–105. doi: 10.4103/2319-4170.113226. [DOI] [PubMed] [Google Scholar]
- 2.Tu YK, Tsai YJ, Chang CH, Su FC, Hsiao CK, Tan JS. Surgical treatment for total root avulsion type brachial plexus injuries by neurotization: A prospective comparison study between total and hemicontralateral C7 nerve root transfer. Microsurgery. 2014;34:91–101. doi: 10.1002/micr.22148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ichim TE, Solano F, Lara F, Paris E, Ugalde F, Rodriguez JP, et al. Feasibility of combination allogeneic stem cell therapy for spinal cord injury: A case report. Int Arch Med. 2010;3:30. doi: 10.1186/1755-7682-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindvall O, Kokaia Z. Stem cells for the treatment of neurological disorders. Nature. 2006;441:1094–6. doi: 10.1038/nature04960. [DOI] [PubMed] [Google Scholar]
- 5.Trivedi HL, Vanikar AV, Thakker U, Firoze A, Dave SD, Patel CN, et al. Human adipose tissue-derived mesenchymal stem cells combined with hematopoietic stem cell transplantation synthesize insulin. Transplant Proc. 2008;40:1135–9. doi: 10.1016/j.transproceed.2008.03.113. [DOI] [PubMed] [Google Scholar]
- 6.Marino RJ, Barros T, Biering-Sorensen F, Burns SP, Donovan WH, Graves DE, et al. International standards for neurological classification of spinal cord injury. J Spinal Cord Med. 2003;26(Suppl 1):S50–6. doi: 10.1080/10790268.2003.11754575. [DOI] [PubMed] [Google Scholar]
- 7.Hauser SL, Dawson DM, Lehrich JR, Beal MF, Kevy SV, Propper RD, et al. Intensive immunosuppression in progressive multiple sclerosis. A randomized, three-arm study of high-dose intravenous cyclophosphamide, plasma exchange, and ACTH. N Engl J Med. 1983;308:173–80. doi: 10.1056/NEJM198301273080401. [DOI] [PubMed] [Google Scholar]
- 8.Kirshblum SC, O’Connor KC. Predicting neurologic recovery in traumatic cervical spinal cord injury. Arch Phys Med Rehabil. 1998;79:1456–66. doi: 10.1016/s0003-9993(98)90244-1. [DOI] [PubMed] [Google Scholar]
- 9.Waters RL, Adkins R, Yakura J, Sie I. Donal Munro Lecture: Functional and neurologic recovery following acute SCI. J Spinal Cord Med. 1998;21:195–9. doi: 10.1080/10790268.1998.11719526. [DOI] [PubMed] [Google Scholar]
- 10.Piepmeier JM, Jenkins NR. Late neurological changes following traumatic spinal cord injury. J Neurosurg. 1988;69:399–402. doi: 10.3171/jns.1988.69.3.0399. [DOI] [PubMed] [Google Scholar]
- 11.Waters RL, Adkins RH, Yakura JS. Definition of complete spinal cord injury. Paraplegia. 1991;29:573–81. doi: 10.1038/sc.1991.85. [DOI] [PubMed] [Google Scholar]
- 12.Waters RL, Adkins RH, Yakura JS, Sie I. Motor and sensory recovery following complete tetraplegia. Arch Phys Med Rehabil. 1993;74:242–7. [PubMed] [Google Scholar]
- 13.Chopp M, Li Y, Zhang ZG. Mechanisms underlying improved recovery of neurological function after stroke in the rodent after treatment with neurorestorative cell-based therapies. Stroke. 2009;40(3 Suppl):S143–5. doi: 10.1161/STROKEAHA.108.533141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Locatelli F, Bersano A, Ballabio E, Lanfranconi S, Papadimitriou D, Strazzer S, et al. Stem cell therapy in stroke. Cell Mol Life Sci. 2009;66:757–72. doi: 10.1007/s00018-008-8346-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bakondi B, Shimada IS, Perry A, Munoz JR, Ylostalo J, Howard AB, et al. CD133 identifies a human bone marrow stem/progenitor cell sub-population with a repertoire of secreted factors that protect against stroke. Mol Ther. 2009;17:1938–47. doi: 10.1038/mt.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stroemer P, Patel S, Hope A, Oliveira C, Pollock K, Sinden J. The neural stem cell line CTX0E03 promotes behavioral recovery and endogenous neurogenesis after experimental stroke in a dose-dependent fashion. Neurorehabil Neural Repair. 2009;23:895–909. doi: 10.1177/1545968309335978. [DOI] [PubMed] [Google Scholar]
- 17.Vaquero J, Zurita M, Oya S, Santos M. Cell therapy using bone marrow stromal cells in chronic paraplegic rats: Systemic or local administration? Neurosci Lett. 2006;398:129–34. doi: 10.1016/j.neulet.2005.12.072. [DOI] [PubMed] [Google Scholar]
- 18.Zurita M, Vaquero J. Functional recovery in chronic paraplegia after bone marrow stromal cells transplantation. Neuroreport. 2004;15:1105–8. doi: 10.1097/00001756-200405190-00004. [DOI] [PubMed] [Google Scholar]
- 19.Ying C, Hu W, Cheng B, Zheng X, Li S. Neural differentiation of rat adipose-derived stem cells in vitro . Cell Mol Neurobiol. 2012;32:1255–63. doi: 10.1007/s10571-012-9850-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dráberová E, Del Valle L, Gordon J, Marková V, Smejkalová B, Bertrand L, et al. Class III beta-tubulin is constitutively coexpressed with glial fibrillary acidic protein and nestin in midgestational human fetal astrocytes: Implications for phenotypic identity. J Neuropathol Exp Neurol. 2008;67:341–54. doi: 10.1097/NEN.0b013e31816a686d. [DOI] [PubMed] [Google Scholar]
- 21.Yañez R, Lamana ML, García-Castro J, Colmenero I, Ramírez M, Bueren JA. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells. 2006;24:2582–91. doi: 10.1634/stemcells.2006-0228. [DOI] [PubMed] [Google Scholar]
- 22.Mehta T, Feroz A, Thakkar U, Vanikar A, Shah V, Trivedi H. Subarachnoid placement of stem cells in neurological disorders. Transplant Proc. 2008;40:1145–7. doi: 10.1016/j.transproceed.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 23.Kulbatski I, Mothe AJ, Nomura H, Tator CH. Endogenous and exogenous CNS derived stem/progenitor cell approaches for neurotrauma. Curr Drug Targets. 2005;6:111–26. doi: 10.2174/1389450053345037. [DOI] [PubMed] [Google Scholar]
- 24.Xiong Y, Mahmood A, Chopp M. Angiogenesis, neurogenesis and brain recovery of function following injury. Curr Opin Investig Drugs. 2010;11:298–308. [PMC free article] [PubMed] [Google Scholar]


