Abstract
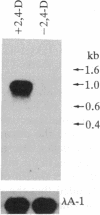
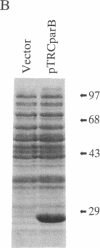
We have isolated an auxin-regulated cDNA, parB, from the early stage of cultured tobacco mesophyll protoplasts. The expression of parB was observed during transition from G0 to the S phase of tobacco mesophyll protoplasts cultured in vitro. The predicted amino acid sequence of parB cDNA has 213 amino acid residues with a relative molecular weight of 23,965. Nucleotide sequence analysis revealed that parB cDNA has homology to glutathione S-transferase (GST; RX:glutathione R-transferase, EC 2.5.1.18) from several sources including plant and animal cells. When we introduced expression vector pKK233-2, which retains parB cDNA, into Escherichia coli, we could detect GST activity in the parB gene product. Accordingly a significant increase of GST activity was detected in the tobacco mesophyll protoplasts cultured in the presence of 2,4-dichlorophenoxyacetic acid. This is an example in which the function of auxin-regulated gene product is shown to be ascribed to a specific enzymatic activity. As GST, and its substrate glutathione, are shown to be related to cell proliferation as well as detoxification of xenobiotics in plant and animal cells, the role of parB is discussed in relation to the induction of proliferative activity in differentiated and nondividing mesophyll protoplasts of tobacco.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amann E., Brosius J. "ATG vectors' for regulated high-level expression of cloned genes in Escherichia coli. Gene. 1985;40(2-3):183–190. doi: 10.1016/0378-1119(85)90041-1. [DOI] [PubMed] [Google Scholar]
- Board P. G., Webb G. C. Isolation of a cDNA clone and localization of human glutathione S-transferase 2 genes to chromosome band 6p12. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2377–2381. doi: 10.1073/pnas.84.8.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasseaud L. F. The role of glutathione and glutathione S-transferases in the metabolism of chemical carcinogens and other electrophilic agents. Adv Cancer Res. 1979;29:175–274. doi: 10.1016/s0065-230x(08)60848-9. [DOI] [PubMed] [Google Scholar]
- Grove G., Zarlengo R. P., Timmerman K. P., Li N. Q., Tam M. F., Tu C. P. Characterization and heterospecific expression of cDNA clones of genes in the maize GSH S-transferase multigene family. Nucleic Acids Res. 1988 Jan 25;16(2):425–438. doi: 10.1093/nar/16.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key J. L., Kroner P., Walker J., Hong J. C., Ulrich T. H., Ainley W. M., Gantt J. S., Nagao R. T. Auxin-regulated gene expression. Philos Trans R Soc Lond B Biol Sci. 1986 Nov 17;314(1166):427–440. doi: 10.1098/rstb.1986.0063. [DOI] [PubMed] [Google Scholar]
- Li Y. C., Seyama T., Godwin A. K., Winokur T. S., Lebovitz R. M., Lieberman M. W. MTrasT24, a metallothionein-ras fusion gene, modulates expression in cultured rat liver cells of two genes associated with in vivo liver cancer. Proc Natl Acad Sci U S A. 1988 Jan;85(2):344–348. doi: 10.1073/pnas.85.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik B., Guthenberg C. Glutathione transferase (human placenta). Methods Enzymol. 1981;77:231–235. doi: 10.1016/s0076-6879(81)77030-7. [DOI] [PubMed] [Google Scholar]
- Meyer Y., Aspart L., Chartier Y. Auxin-induced regulation of protein synthesis in tobacco mesophyll protoplasts cultivated in vitro: I. Characteristics of auxin-sensitive proteins. Plant Physiol. 1984 Aug;75(4):1027–1033. doi: 10.1104/pp.75.4.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushmore T. H., King R. G., Paulson K. E., Pickett C. B. Regulation of glutathione S-transferase Ya subunit gene expression: identification of a unique xenobiotic-responsive element controlling inducible expression by planar aromatic compounds. Proc Natl Acad Sci U S A. 1990 May;87(10):3826–3830. doi: 10.1073/pnas.87.10.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushmore T. H., King R. G., Paulson K. E., Pickett C. B. Regulation of glutathione S-transferase Ya subunit gene expression: identification of a unique xenobiotic-responsive element controlling inducible expression by planar aromatic compounds. Proc Natl Acad Sci U S A. 1990 May;87(10):3826–3830. doi: 10.1073/pnas.87.10.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai M., Okuda A., Muramatsu M. Multiple regulatory elements and phorbol 12-O-tetradecanoate 13-acetate responsiveness of the rat placental glutathione transferase gene. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9456–9460. doi: 10.1073/pnas.85.24.9456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh K., Kitahara A., Soma Y., Inaba Y., Hatayama I., Sato K. Purification, induction, and distribution of placental glutathione transferase: a new marker enzyme for preneoplastic cells in the rat chemical hepatocarcinogenesis. Proc Natl Acad Sci U S A. 1985 Jun;82(12):3964–3968. doi: 10.1073/pnas.82.12.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suguoka Y., Kano T., Okuda A., Sakai M., Kitagawa T., Muramatsu M. Cloning and the nucleotide sequence of rat glutathione S-transferase P cDNA. Nucleic Acids Res. 1985 Sep 11;13(17):6049–6057. doi: 10.1093/nar/13.17.6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthanthiran M., Anderson M. E., Sharma V. K., Meister A. Glutathione regulates activation-dependent DNA synthesis in highly purified normal human T lymphocytes stimulated via the CD2 and CD3 antigens. Proc Natl Acad Sci U S A. 1990 May;87(9):3343–3347. doi: 10.1073/pnas.87.9.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y., Kuroda H., Tanaka T., Machida Y., Takebe I., Nagata T. Isolation of an auxin-regulated gene cDNA expressed during the transition from G0 to S phase in tobacco mesophyll protoplasts. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9279–9283. doi: 10.1073/pnas.86.23.9279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y., Niwa Y., Machida Y., Nagata T. Location of the cis-acting auxin-responsive region in the promoter of the par gene from tobacco mesophyll protoplasts. Proc Natl Acad Sci U S A. 1990 Oct;87(20):8013–8016. doi: 10.1073/pnas.87.20.8013. [DOI] [PMC free article] [PubMed] [Google Scholar]