Abstract
Intrauterine infection–inflammation is a major cause of early preterm birth and subsequent neonatal mortality and acute or long-term morbidity. Antibiotics can be administered in pregnancy to prevent preterm birth either prophylactically to women at high risk for preterm delivery, or to women with diagnosed intrauterine infection, prelabor rupture of membranes, or in suspected preterm labor. The therapeutic goals of each of these scenarios are different, with different pharmacological considerations, although effective antimicrobial therapy is an essential requirement. An ideal antibiotic for these clinical indications would be (a) one that is easily administered and orally bioactive, (b) has a favorable adverse effect profile (devoid of reproductive toxicity or teratogenicity), (c) is effective against the wide range of microorganisms known to be commonly associated with intra-amniotic infection, (d) provides effective antimicrobial protection within both the fetal and amniotic compartments after maternal delivery, (e) has anti-inflammatory properties, and (f) is effective against antibiotic-resistant microorganisms. Here, we review the evidence from clinical, animal, and ex vivo/in vitro studies that demonstrate that a new macrolide-derived antibiotic – solithromycin – has all of these properties and, hence, may be an ideal antibiotic for the treatment and prevention of intrauterine infection-related pregnancy complications. While this evidence is extremely encouraging, it is still preliminary. A number of key studies need to be completed before solithromycin’s true potential for use in pregnancy can be ascertained.
Keywords: macrolide antibiotics, intrauterine infection, prelabor rupture of membranes, Ureaplasma, Mycoplasma, pregnancy
Introduction
Preterm infants are at high risk of adverse outcomes, including both acute and long-term disability and death (1–4). Evidence from multiple clinical and animal studies suggests that the majority of early preterm deliveries (before 34 weeks’ gestation) arise as the result of intrauterine infection and inflammation (5, 6), although causation is hard to prove in any individual case. Ascending intrauterine infection occurs when bacteria residing in the vagina ascend and breach the cervical barrier, colonize and invade the fetal membranes and amniotic fluid (AF) – and sometimes infect the fetus itself (7, 8). When a vigorous inflammatory response ensues (typically manifested as histologic chorioamnionitis), this may trigger preterm labor and delivery (7, 9, 10). Microbial colonization of the amniotic cavity without a significant inflammatory response rarely manifests as a cause of preterm delivery (11–13).
In order to successfully prevent intra-amniotic infection-associated preterm birth and associated neonatal sequelae, an effective antibiotic therapy needs to be a core component of any pharmaceutical solution. Ideally an antibiotic administered antenatally for preterm birth prevention should (a) be easily administered and orally bioactive; (b) have a favorable adverse effect profile in pregnancy (devoid of reproductive toxicity or teratogenicity); (c) exhibit efficacy against the wide range of microorganisms known to be commonly associated with intra-amniotic infection; (d) be able to provide effective antimicrobial protection within both the fetal and amniotic compartments after maternal delivery; (e) possess anti-inflammatory properties; and (f) be effective against antibiotic-resistant microorganisms.
In this review, we discuss the key properties, benefits, and potential obstetric and perinatal applications of the novel antibiotic solithromycin. We suggest that solithromycin is the first antibiotic that may meet all of the above-mentioned criteria and as such has the potential to represent an exciting and major new advance in obstetric and perinatal medicine. We highlight the key indications where solithromycin may be of most benefit, and identify areas where further research is needed in order to facilitate the introduction of solithromycin into obstetric practice.
Intrauterine Infection, Antibiotics, and Preterm Birth
Intrauterine infection and inflammation play a well-recognized role in the etiology of spontaneous preterm labor and birth, particularly in early preterm deliveries or those complicated by preterm prelabor rupture of membranes (PPROM) (6, 9, 12). A large number of microorganisms have been implicated in the etiology of preterm birth, including many organisms commonly found in normal vaginal microbiota as well as conditions associated with vaginal dysbiosis (Figure 1) (14–16). Some of the bacteria that commonly cause infection-driven preterm birth are common Gram-positive bacteria frequently found in the reproductive tract of pregnant women; some are more closely associated with oral microbes, while others are often found in women with abnormal vaginal microbiota [bacterial vaginosis (BV)] and/or are associated with reproductive tract infections (14, 17–19). In many preterm deliveries, multiple bacteria are present in the amniotic cavity (14, 17, 20). The incidence of confirmed intra-amniotic infection in preterm deliveries varies according to a variety of factors, including race and gestational age. In a recent analysis, Romero et al. reported that AF bacterial colonization rates are around 10–15% in preterm births overall, approaching 30% in extreme preterm births delivered before 30 weeks’ gestation (12). In an earlier review of the topic, DiGiulio described a frequency of AF infection ranging from 15 to 50%, with pregnancies complicated by PPROM having a similar infection rate (14). Intra-amniotic inflammation (with or without infection) is considerably more common, and increases more markedly with decreasing gestational age at delivery (12, 21, 22).
Figure 1.
Relative frequency of colonization by different bacteria of the amniotic cavity in preterm deliveries with intact membranes and intra-amniotic infection. Note that more than one bacterium are frequently detected. Data, compiled from Ref. (16–19), are indicative only and will vary according to clinical and demographic characteristics, plus methodological differences.
Bacteria of the class Mollicutes, in particular the “genital mycoplasmas,” such as Ureaplasma parvum, Ureaplasma urealyticum, and Mycoplasma hominis, are the most common group of microorganisms isolated from the amniotic cavity of preterm deliveries (9, 14). Vaginal colonization rates of these organisms in pregnant women ranges from 35 to 90% for Ureaplasma spp. and 5–75% for Mycoplasma hominis (23). Dual colonization with both microorganisms is approximately fourfold more common in women with preterm vs. term deliveries (23, 24). Most studies with a preterm birth endpoint have reported a significant association with intrauterine Ureaplasma sp. colonization and preterm birth (25); studies of AF and placental tissues obtained from preterm deliveries show a clear link between Ureaplasma colonization, a vigorous inflammatory response, and preterm delivery (24–29).
The clinical evidence is supported by experimental studies consistent with causality (30). Using a pregnant sheep model (31), we reported that intra-amniotic injection with Ureaplasma parvum resulted in chronic chorioamnionitis accompanied by pro-inflammatory cytokines in the AF and enhanced lung maturation. Experiments in Rhesus macaques have shown that intra-amniotic Ureaplasma sp. injection also drives intrauterine cytokine and prostaglandin production, preterm labor, and chorioamnionitis, replicating the disease pathogenesis and ontogeny observed in human pregnancy (32, 33). Together, these and other studies have shown that robust intrauterine inflammation sufficient to cause preterm birth can be induced by Ureaplasma sp. colonization of the amniotic cavity (25). However, it is important to note that around half of all preterm deliveries with intra-amniotic infection contain bacteria other than the genital Mycoplasmataceae, and a large number of bacterial species have been associated with inflammation-driven preterm birth (14, 17, 18, 34).
A number of clinical trials of maternal antibiotic administration have been performed to attempt to prevent or treat intrauterine infection with the aim of reducing the rates of preterm birth and associated neonatal morbidities. As discussed in detail in this series by Lamont (35), some recent meta-analyses have concluded that antibiotic treatment of BV does not prevent preterm birth or improve neonatal outcomes (36–41). Metronidazole and clindamycin are the two most studied antibiotics. It should be noted here that conventional treatment of BV results in relatively high recurrence rates (42–44), and that the antibiotics commonly used to treat BV show only weak activity against Mycoplasma hominis (erythromycin, azithromycin, metronidazole) or Ureaplasma spp. (metronidazole, clindamycin) (14). High concentrations of these antibiotics may be required for efficacy that may not be achievable with standard oral doses due to their comparatively low oral bioavailability or adverse effects profile.
However, there are some studies that suggest that prophylactic antibiotic administration can be effective – if given before 20 weeks’ gestation (35). This is presumably because antimicrobial therapy is most effective and beneficial when administered prior to colonization of the amniotic cavity (45, 46). A retrospective study of clindamycin treatment of women with genital mycoplasmas at high risk of preterm birth found a small but significant reduction in preterm birth rates and neonatal complications (47). In addition to clindamycin, azithromycin may also be effective. In non-human primates, Grigsby and colleagues showed that 10 days of high-dose maternal azithromycin treatment delays preterm labor induced by experimental intra-amniotic Ureaplasma spp. infection and prevents fetal inflammatory response (32). We recently showed in our ovine model that a 4-day course of azithromycin-delivered maternally (10 mg/kg i.v.) eradicated intra-amniotic Ureaplasma parvum infection (48). Surprisingly, there are only two clinical studies of macrolide treatment of vaginal Ureaplasma spp. colonization on pregnancy outcome, the results of which are inconclusive (49, 50).
In addition to difficulties surrounding diagnosis of infection and the appropriate selection of antibiotics, a fundamental reason for the lack of success of antibiotic trials for preterm birth prevention may lie in the limitations of the antibiotics employed. While macrolide antibiotics, such as erythromycin and azithromycin, are considered effective in treating important microorganisms, such as Ureaplasma spp., and are generally free of serious maternal and fetal side effects, their potency against genital mycoplasmas is not high, and there is growing prevalence of antibiotic resistance in these organisms (23). Studies have shown that maternal erythromycin administration is largely ineffective in eradicating intrauterine infection (39, 51, 52). This is likely due to poor transplacental passage of macrolides, estimated to be only 2–4% (53, 54). We previously showed in our pregnant sheep model that maternal macrolide administration fails to deliver effective levels of antibiotic to either the fetal circulation or the amniotic cavity (55) and does not eradicate intra-amniotic Ureaplasma parvum infection (52). Human studies confirm that the degree of maternal-to-fetal (M:F) passage of macrolides, such as erythromycin and azithromycin, is low and variable (53, 54), while the extent of maternal-to-amniotic transfer is only marginally greater. Antibiotics with better maternal-amniotic-fetal transfer properties and enhanced potencies against key bacterial pathogens are required to eliminate intra-amniotic infection and prevent significant neonatal morbidity and mortality.
Solithromycin: Pharmacodynamics and Antimicrobial Properties
Solithromycin, a fourth-generation macrolide derived from clarithromycin, is a novel fluoroketolide antibiotic being developed by Cempra Inc. (Chapel Hill, NC, USA) for the treatment of community-acquired pneumonia and a variety of other indications (56, 57). It exhibits broad-spectrum activity against Gram-positive and some Gram-negative organisms, including many that are resistant to other macrolide antibiotics (58–67). It is acid stable (63) and has excellent oral bioavailability (~70%), superior to the approved macrolides (68–70). Solithromycin also demonstrates excellent tissue uptake and accumulation, important when considering its activity in tissues infected with intracellular pathogens, such as Ureaplasma sp. (70, 71).
Like other macrolides, solithromycin contains a 14-atom lactone ring structure and selectively binds to the peptide exit tunnel of the bacterial ribosome, blocking subunit assembly, and mRNA translation and protein synthesis (72). It has three key structural features that distinguish it from first- and second-generation macrolides: a keto group replacing the cladinose moiety (hence, the origin of the class name “ketolide”), a fluoro group at the C2 position of the lactone ring, and an aryl–aryl side chain at C11–C12. Deletion of the cladinose structure renders the molecule insensitive to methylation-dependent resistance. Hydrogen bonding via the amino-phenyl headgroup of the C11,C12 side chain is primarily responsible for solithromycin’s high-affinity bacterial ribosomal binding properties, while the fluoro group enhances binding in some macrolide-resistant strains. Ketolides are generally less susceptible than macrolides to bacterial efflux pumps, enhancing their efficacy in some species. In addition, there is some evidence that solithromycin selectively blocks translation of specific polypeptides, which confers additional antimicrobial efficacy over traditional macrolides (72).
Solithromycin has been shown to exhibit excellent activity against many of the microbial species known to be associated with infection-associated preterm delivery (Table 1), including Ureaplasma spp., Mycoplasma spp., Group B streptococci, staphylococci, and Chlamydia trachomatis (59, 61, 65–67, 73, 74). Indeed, although solithromycin has not yet been tested on all relevant microorganisms, from the established efficacy profile of its parent drug clarithromycin, it is likely that solithromycin will be effective against all bacteria known to be associated with intra-amniotic infection. We recently showed that its potency against Ureaplasma spp. is ~30 times greater than azithromycin in vitro (75). Importantly, no strains of Ureaplasma were resistant to solithromycin, and both Ureaplasma parvum and Ureaplasma urealyticum were susceptible, with overall MIC90 values 125 ng/ml (compared to 2000 ng/ml for azithromycin).
Table 1.
Comparison of antimicrobial efficacy (MIC50 and MIC90 values) of solithromycin vs. four other relevant antibiotics against a range of important bacteria.
| Organism (number of strains) | Solithromycin | Macrolidesa | Levofloxacin | Penicillinsb | Doxycyclin | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | # | |
| Streptococcus pneumoniae (1363) | <30 | 120 | <250 | >2,000(Er) | 1,000 | 1,000 | <30 | 2000(P) | (67) | ||
| Streptococcus pyogenes (124) | 60 | 500 | 8,000 | >64,000(Az) | 500 | 1000 | 15 | 15(AC) | (76) | ||
| Streptococcus agalactiaec (GBS)R (62) | 30 | 125 | >8,000 | >8,000(Az) | (59) | ||||||
| Streptococcus agalactiaec (GBS)S (10) | 8 | 15 | <125 | <125(Az) | 32 | 47(P) | (59) | ||||
| Staphylococcus aureus (4729) | 60 | >4000 | >2,000 | >2,000(Er) | <500 | >4,000 | 1,000 | >2,000(O) | (67) | ||
| Coagulase-neg Staph. (CoNS) (862) | 60 | >4,000 | >2,000 | >2,000(Er) | 4,000 | >4,000 | >2,000 | >2,000(O) | (67) | ||
| Haemophilus influenzae (150) | 1,000 | 2,000 | 1,000 | 4,000(Az) | <500 | <500 | <1,000 | 2,000(AC) | (67) | ||
| Neisseria gonorrhoeae (246) | 125 | 250 | 500 | 8,000(Az) | 1,000 | 16,000(A) | (65) | ||||
| Chlamydia trachomatis (10) | 250 | 250 | 125 | 125(Az) | 60 | 60 | (77) | ||||
| Mycoplasma pneumoniae (38) | 0.03 | 0.125 | 0.25 | 0.5(Az) | 500 | 500 | 125 | 250 | (74) | ||
| Mycoplasma hominis (13) | 4 | 8 | 2,000 | 4,000(Az) | 250 | 500 | 125 | 8,000 | (74) | ||
| Mycoplasma genitalium (40) | <1 | 1,000 | 8 | >8,000(Az) | 250 | 1,000 | (61) | ||||
| Ureaplasma urealyticumd (10) | 8 | 31 | 2,000 | 4,000(Az) | 500 | 1,000 | 1,000 | 16,000 | (74) | ||
| Ureaplasma parvumd (10) | 8 | 16 | 2,000 | 4,000(Az) | 500 | 2,000 | 8,000 | 16,000 | (74) | ||
a(Er) erythromycin; (Az) azithromycin.
b(P) penicillin G; (AC) Amoxicillin–clavulanic acid; (O) oxacillin; (A) ampicillin.
cR, macrolide resistant; S, macrolide susceptible.
dMore recent data from the analysis of 100 strains of Ureaplasma spp. (U. parvum and U. Urealyticum combined) suggest that the solithromycin MIC90 is 125 ng/ml and 2000 ng/ml for azithromycin (75).
Solithromycin data are highlighted in the shaded text.
Solithromycin has been shown to be well tolerated and relatively free of adverse effects, with the most frequent complaint being GI disturbance and nausea that has not been dose-limiting (57, 69, 78, 79). It is not extensively metabolized in humans and is eliminated essentially unchanged via biliary excretion; furthermore, its plasma pharmacokinetics are not altered in patients with mild and moderate renal impairment (80). Two large global Phase 3 trials in community-acquired bacterial pneumonia have recently been completed using both oral and intravenous solithromycin administration (57). The standard oral regimen for solithromycin is 800 mg on day 1 followed by 400 mg daily for 4 days (78). However, a recent clinical trial demonstrated that a single 1000 mg dose of solithromycin eradicates Neisseria gonorrhoeae infection at oral, rectal, and genital sites (79).
Transplacental Pharmacokinetics of Solithromycin: Significance and Implications
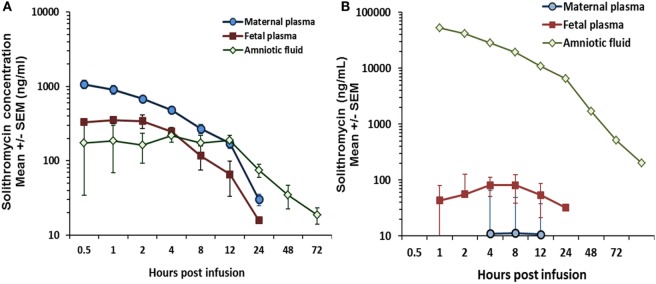
Critically, unlike preexisting macrolides, there is strong evidence that the M:F passage of solithromycin is comparatively efficient. We have shown in an ex vivo perfusion model that solithromycin readily crosses the human placenta and reaches effective concentrations in the fetal compartment (81). The M:F transfer ratio is around 0.4–0.6 for the human placenta, while in the pregnant sheep model the M:F transfer ratio of solithromycin was 0.3–0.5 – more than 10-fold greater than erythromycin and azithromycin at similar doses (5–10 mg/kg) (Figure 2) (82). Although ovine and human placentation differ, the similarity in apparent M:F transfer between the species (53) suggests that, in this case, the sheep is a good model to investigate biodistribution of this and other macrolides. Our data would indicate that a daily oral regimen of around 10 mg/kg would maintain effective antimicrobial protection to the fetus, achieving fetal plasma concentrations of several hundred nanogram/milliliter (Figure 3A) (82). Levels are likely to increase further with multiple doses, such that the standard dosing regimen is likely to achieve effective levels in the fetus and intra-amniotically, but this requires experimental confirmation. The key structural features responsible for solithromycin’s dramatically enhanced placental permeability are not known.
Figure 2.
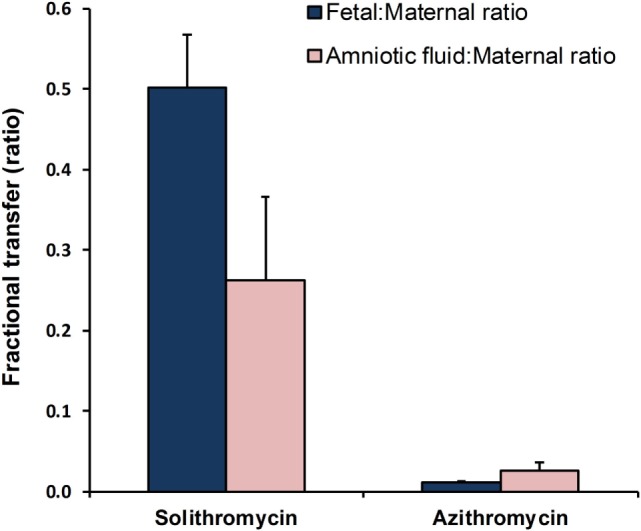
Comparisons of maternal-to-fetal and maternal-to-amniotic transfer efficiency of solithromycin vs. azithromycin in the pregnant sheep model (10 and 5 mg/kg, respectively). Data are mean ± SD; taken from Refs. (55, 82), respectively.
Figure 3.
(A) Biodistribution of solithromycin in pregnant sheep, showing concentrations in the maternal, fetal, and amniotic fluid (AF) compartments after maternal intravenous administration (10 mg/kg); (B) Plasma and AF concentration data in the same model after intra-amniotic injection (1.4 mg/kg fetal weight); azithromycin and solithromycin data taken from Ref (82).
Importantly, we have also shown in sheep that significant solithromycin levels in the amniotic cavity (the primary site of infection in this context) are achieved after maternal administration (Figure 3A) (82); repeat daily dosing is likely to achieve even higher concentrations due to delayed clearance from AF, and thereby provide enhanced protection against less sensitive organisms (82). The pharmacokinetic profile in the group receiving an intra-amniotic bolus of solithromycin (Figure 3B) showed that high concentrations were achieved and maintained for over 48 h, with a half-life estimate of 16.5 h. However, it should also be pointed out that this route of administration failed to achieve therapeutic levels in either the maternal or fetal circulation (82), highlighting the need for concurrent maternal administration.
Reproductive and Developmental Toxicity Aspects of Solithromycin
Macrolides (clarithromycin being the exception due to evidence of teratogenic effects in animal studies) are considered safe to use in pregnancy and have been administered to pregnant women for decades. Two recent, large studies of pregnancies in Canada and Israel (each including more than 100,000 women studied over a 10-year timeframe) reported no evidence of congenital malformations (including cardiac abnormalities) associated with antenatal exposure to erythromycin, azithromycin, or clarithromycin (first-, second-, and third-generation macrolides) (83, 84). Exposure during the third trimester was not associated with increased risk of perinatal mortality, low birth weight, preterm birth, or low apgar scores (83). The Israeli study also found no evidence of increased risk of pyloric stenosis (83), a complication reported in several studies to be associated with perinatal and postnatal exposure of infants to macrolides during the first few weeks of life (85, 86). It is important to note that exposure to macrolides in pregnancy (excluding the peri-partum period) was not associated with a similar increased risk (83, 85). The topic was recently discussed by de Vries and Ludvigsson et al., who pointed out some potential causes of the disparities in the literature (87); the event is rare even if the association is correct (88).
A potential concern around increased risk of congenital heart disease and maternal erythromycin exposure (odds ratio 1.92, 95% CI: 1.37–2.68) was raised in a study by Kallen (89), again using data from the Swedish Birth Register. However, a later study of over 13,000 pregnancies (280 of which were exposed to macrolides) failed to find evidence to support such an association (90). Subsequently, the risks of antenatal macrolide exposure on pyloric stenosis and congenital cardiac malformations were specifically examined in a study of almost 5000 infants born with major congenital defects over a 20-year period by Lin et al. (91). These investigators found no evidence of an association between incidence of these complications and macrolide exposure, regardless of trimester of exposure, including exposure to erythromycin specifically (91). Collectively, these studies support the conclusion that macrolides are safe to administer in pregnancy and any risks to the mother and fetus are extremely low or non-existent.
Nevertheless, if solithromycin is to be administered in pregnancy, it needs an excellent safety profile, particularly in light of its ability to cross the placenta (unlike other macrolides) that could theoretically increase the potential for adverse fetal effects. Studies to date show that its tolerability is high and its side-effects profile is favorable compared to other antibiotics. A phase 2 comparator study in adults showed that at the standard oral dose the most common adverse event was diarrhea (4.7%) followed by flatulence and nausea (1.6% each); no cardiac or neurological side effects were reported and the adverse event rate was significantly lower than in patients taking levofloxacin (78). In a series of phase 1 pharmacokinetic/safety studies, no clinically significant effects were seen, although transient increases in liver enzyme levels were observed in 40% of subjects receiving 600 mg daily over 5 days (68).
Available data suggest that the risks of developmental toxicity and/or teratogenesis with solithromycin are extremely low. The effects of orally administered solithromycin on male and female fertility and early embryonic development to implantation have been evaluated in rats and rabbits (Cempra Inc.: unpublished findings on file). No changes were noted in estrus cycles or sperm parameters in the Segment I study at the maximum dose tested (220 mg/kg). In Segment II studies, some maternal toxicity was observed at the highest dose (decreases in body weight and food consumption and treatment-related clinical signs). The “no observed adverse effect limit” for developmental toxicity was 110–220 mg/kg. No evidence of a teratogenic effect on the fetuses was evident in any treatment group.
Solithromycin has also been evaluated in three in vitro genetic toxicology assays and was not mutagenic or clastogenic in any of these assays. In our recent sheep studies, solithromycin-treated animals exhibited no clear evidence of hepatotoxicity (48), although the studies were not designed to specifically address toxicity. Detailed, long-term safety studies have not been carried out in this model.
An emerging concern relating to the use of antibiotics during pregnancy relates to potential adverse effects mediated by perturbation of the maternal–neonatal microbiome by, among other things, antibiotics (92–95). There is now strong evidence that maternal antibiotic exposure can cause neonatal dysbiosis and influence perinatal microbiome development, with potentially significant effects on many developmental processes (96, 97). However, it remains to be seen how dose, timing, and duration of exposure, not to mention the nature of the antibiotic itself, impacts upon these effects. It will be important to assess the effects of maternal solithromycin treatment on both the maternal microbiome (gut, vagina, and other sites) and the infant microbiome and correlate these with developmental outcomes, growth, and the incidence of allergic (98) and metabolic disorders (99, 100). At this point in time, there are no data on solithromycin’s effects on the gut microbiome. However, due to its efficient absorption by the upper GI tract, it is expected that solithromycin’s effects on lower GI tract microflora (typically sampled for microbiome studies) will be markedly less than other macrolides. To address this question, and to assess the short-term and longer-term effects of solithromycin administration on maternal microbiota, a series of studies are planned for the near future.
Anti-Inflammatory Properties of Solithromycin
It is now widely accepted that fetal and intra-amniotic inflammation, which occurs as a consequence of intrauterine infection or exposure to non-infection inflammatory agents, must be prevented in order to protect the fetus and maximize the benefits of antenatal/perinatal antimicrobial treatment (4, 101, 102). A number of pharmacological strategies have been evaluated by different researchers in order to achieve this effectively and safely. We have focused on the use of cytokine suppressive anti-inflammatory drugs (CSAIDs), in particular agents that block inflammatory signaling via NF-κB and p38MAPK pathways; an overview of these studies is presented in a companion article in this series (101). A key issue of such approaches, however, is the mode of delivery and the prevention of side effects. When given maternally, the dose administered must be large enough to ensure that the drug achieves effective anti-inflammatory concentrations in the amniotic cavity, but not sufficiently high to cause maternal toxicity or off-target side effects. To address this problem, and also overcome the lack of permeability of some agents across the human placenta, we have investigated intra-amniotic delivery of agents. We have been able to demonstrate benefits of this approach with several agents in animal and ex vivo models (103–105). However, while this mode of delivery has some clear benefits, including the ability to achieve quite high drug concentrations in the amniotic cavity without risk of significant maternal or fetal exposure (where fetal drug uptake is low), it also has drawbacks in that any maternal inflammation remains untreated. This limitation may represent a lost opportunity to improve pregnancy outcomes in a sub-group of women.
In this context, solithromycin may provide additional pharmacological benefits as it is also an effective anti-inflammatory agent. As it would be given maternally, and crosses the placenta relatively efficiently, solithromycin therapy may be able to achieve the benefits of inhibiting both maternal and intrauterine inflammation in addition to its antimicrobial actions – depending upon the dose administered. In a key publication, Kobayashi et al. reported that solithromycin exhibits significant NF-κB-mediated anti-inflammatory effects (reduced cytokine and matrix-metalloproteinase (MMP)-9 expression) in human monocytes and peripheral blood mononuclear cells at concentrations ~10–40 μM (106, 107). Importantly, the anti-inflammatory effects were ≥10-fold more potent than erythromycin, clarithromycin, or azithromycin. The structural features responsible for the anti-inflammatory properties of macrolides have not been identified, although the macrocyclic ring is likely to be crucial (106); the specific structural characteristics responsible for solithromycin’s enhanced anti-inflammatory properties are unknown. In vivo suppression of neutrophilia and MMP-9 activity in a mouse model was also achieved with solithromycin treatment (100 mg/kg) after exposure to a non-infectious inflammatory stimulus (107). The mechanism of action appears to be, in part, a combination of effects on HDAC2 activity (enhanced) and Akt phosphorylation (inhibited), via increased protein phosphatase PP2A activity (106). Solithromycin also has significant effects on NF-κB activity, probably mediated through enhanced dissociation of IκBα from p65/RelA, as has been demonstrated for other macrolides (107–110). These findings have stimulated interest in the use of solithromycin administration for treatment of chronic obstructive lung disease, asthma, and non-alcoholic steatohepatitis, with a series of investigational studies now underway.
In an in vitro study of human placental tissues, we confirmed the anti-inflammatory properties of solithromycin in human placentas, reporting inhibition of pro-inflammatory cytokine production by the antibiotic; however, this effect was only observed at relatively high concentrations (≥33 μg/ml, or ~40 μM) at which a decline in cell viability was observed in this model (81). Effects of a similar magnitude and potency were also observed in (maternal) decidual cells. Furthermore, data from the pregnant sheep model also support an anti-inflammatory effect of solithromycin in pregnancy. We previously reported that solithromycin, delivered maternally (10 mg/kg i.v.), decreases the levels of mRNA expression of IL-1beta, IL-6, IL-8, and MCP2 in fetal skin of Ureaplasma parvum-exposed animals (48). No significant effects on the inflammation scoring of lung or chorioamnion were observed in this study, although some non-significant trends were observed toward reductions in lung cytokine expression, inflammatory histology, and cord blood white blood cell count (48).
These findings raise questions as to whether a sufficiently high dose of the antibiotic can be given to achieve anti-inflammatory benefits without toxicity. Our studies, assuming that they can be extrapolated to the pregnant woman, would suggest that large amounts of solithromycin would need to be given maternally to suppress placental and intra-amniotic inflammation, running the risk of placental toxicity and possibly other adverse effects. Administration via the intra-amniotic route by ultrasound-guided injection would be able to achieve the levels necessary to exert significant anti-inflammatory effects within the amniotic cavity without maternal or placental exposure. This may be an advantageous strategy in some clinical situations, in which the fetus is at risk and particularly rapid antimicrobial and anti-inflammatory therapy is required. Appropriate randomized clinical trials in pregnancy would be required to ensure that the benefits outweigh the potential risks of the intervention.
Conclusions, Applications, and Future Research Directions
We believe solithromycin has exciting potential for the treatment of intrauterine infections, prevention of preterm birth, and also treatment of perinatal and postnatal infections. Its pharmacodynamic and pharmacokinetic properties are ideally suited to these applications, and our data on transplacental passage and AF accumulation suggest that this antibiotic may represent a major advance in antimicrobial therapy in pregnancy.
There are three main obstetric scenarios where solithromycin therapy may be particularly beneficial. The first is in the prophylactic treatment of asymptomatic women at high risk of preterm birth in the first half of pregnancy. The strategy requires the ability to identify women who are at risk of intrauterine infection and, thus, target them for solithromycin treatment (35). Prognostic indications, in addition to standard clinical risk factors, could be abnormal vaginal microbiota or presence of particular microbial profiles or species (15, 16, 111–113), or a short cervix with evidence of inflammatory changes (114–116). The second situation is in women with PPROM. In these pregnancies, macrolides have been shown to have significant benefits in terms of neonatal outcomes (117); with its far superior efficacy profile and ability to treat the fetus in utero, it is likely that solithromycin would be much more beneficial than erythromycin for this indication. Finally, solithromycin may be effective in improving neonatal outcomes in women presenting with preterm labor and intact membranes, providing both antimicrobial and anti-inflammatory benefits to the fetus prior to delivery. In all of these scenarios, co-administration with a more potent anti-inflammatory agent may further improve outcomes. Clinical trials to explore all of these applications are warranted, once pharmacokinetic studies have been conducted to establish safe and effective dosing regimens in early, mid, and late pregnancy. Assessment of the short- and long-term effects of antenatal solithromycin therapy on the vaginal, gastrointestinal, and neonatal microbiomes would also be warranted prior to trials of its therapeutic effectiveness in pregnancy.
Author Contributions
JK conceptualized the article and prepared the first draft, including the figures and tables. MP, MK, DI, and JN contributed to various sections of the text and provided input, comment, and changes to the entire manuscript.
Conflict of Interest Statement
Dr. Prabha Fernandes of Cempra Inc. (Chapel Hill, NC, USA) supplied the solithromycin used in the pertinent studies included in this review, and provided comments on the manuscript, but had no involvement in the design of the studies or the analysis of the data. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are indebted to Dr Prabha Fernandes of Cempra Inc. (Chapel Hill, NC, USA) for her support, advice, and comments on this manuscript. Cempra supplied the solithromycin used in the pertinent studies included in this review, but had no involvement in the design of the studies or the analysis or interpretation of the data. The financial support of the Women and Infants Research Foundation, National Health, and Medical Research Council (NHMRC) of Australia (APP1049148, 1077931 and APP1010315) and Channel 7 Telethon Trust is gratefully acknowledged.
References
- 1.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet (2008) 371:261–9. 10.1016/S0140-6736(08)60136-1 [DOI] [PubMed] [Google Scholar]
- 2.Platt MJ. Outcomes in preterm infants. Public Health (2014) 128:399–403. 10.1016/j.puhe.2014.03.010 [DOI] [PubMed] [Google Scholar]
- 3.Strunk T, Inder T, Wang X, Burgner D, Mallard C, Levy O. Infection-induced inflammation and cerebral injury in preterm infants. Lancet Infect Dis (2014) 14:751–62. 10.1016/S1473-3099(14)70710-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bastek JA, Weber AL, McShea MA, Ryan ME, Elovitz MA. Prenatal inflammation is associated with adverse neonatal outcomes. Am J Obstet Gynecol (2014) 210:450.e1–10. 10.1016/j.ajog.2013 [DOI] [PubMed] [Google Scholar]
- 5.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med (2000) 342:1500–7. 10.1056/NEJM200005183422007 [DOI] [PubMed] [Google Scholar]
- 6.Iams JD, Papatsonis DN, van Geijn HP, Bleker OP, Ader HJ. The epidemiology of preterm birth. Clin Perinatol (2003) 30:651–64. 10.1016/S0095-5108(03)00101-5 [DOI] [PubMed] [Google Scholar]
- 7.Romero R, Espinoza J, Gonçalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med (2007) 25:21–39. 10.1055/s-2006-956773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim MJ, Romero R, Gervasi MT, Kim JS, Yoo W, Lee DC, et al. Widespread microbial invasion of the chorioamniotic membranes is a consequence and not a cause of intra-amniotic infection. Lab Invest (2009) 89:924–36. 10.1038/labinvest.2009.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, et al. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol (2014) 71(4):330–58. 10.1111/aji.12189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romero R, Chaemsaithong P, Korzeniewski SJ, Tarca AL, Bhatti G, Xu Z, et al. Clinical chorioamnionitis at term II: the intra-amniotic inflammatory response. J Perinat Med (2015) 44(1):5–22. 10.1515/jpm-2015-0045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Combs CA, Gravett M, Garite TJ, Hickok DE, Lapidus J, Porreco R, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol (2014) 210:125.e1–15. 10.1016/j.ajog.2013.11.032 [DOI] [PubMed] [Google Scholar]
- 12.Romero R, Miranda J, Chaiworapongsa T, Korzeniewski SJ, Chaemsaithong P, Gotsch F, et al. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol (2014) 72:458–74. 10.1111/aji.12296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romero R, Miranda J, Kusanovic JP, Chaiworapongsa T, Chaemsaithong P, Martinez A, et al. Clinical chorioamnionitis at term I: microbiology of the amniotic cavity using cultivation and molecular techniques. J Perinat Med (2015) 43:19–36. 10.1515/jpm-2014-0249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiGiulio DB. Diversity of microbes in amniotic fluid. Semin Fetal Neonatal Med (2012) 17:2–11. 10.1016/j.siny.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 15.Mysorekar IU, Cao B. Microbiome in parturition and preterm birth. Semin Reprod Med (2014) 32:50–5. 10.1055/s-0033-1361830 [DOI] [PubMed] [Google Scholar]
- 16.Romero R, Hassan SS, Gajer P, Tarca AL, Fadrosh DW, Bieda J, et al. The vaginal microbiota of pregnant women who subsequently have spontaneous preterm labor and delivery and those with a normal delivery at term. Microbiome (2014) 2:18. 10.1186/2049-2618-2-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DiGiulio DB, Romero R, Kusanovic JP, Gómez R, Kim CJ, Seok KS, et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol (2010) 64:38–57. 10.1111/j.1600-0897.2010.00830.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendz GL, Kaakoush NO, Quinlivan JA. Bacterial aetiological agents of intra-amniotic infections and preterm birth in pregnant women. Front Cell Infect Microbiol (2013) 3:58. 10.3389/fcimb.2013.00058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pararas MV, Skevaki CL, Kafetzis DA. Preterm birth due to maternal infection: causative pathogens and modes of prevention. Eur J Clin Microbiol Infect Dis (2006) 25:562–9. 10.1007/s10096-006-0190-3 [DOI] [PubMed] [Google Scholar]
- 20.Payne MS, Bayatibojakhi S. Exploring preterm birth as a polymicrobial disease: an overview of the uterine microbiome. Front Immunol (2014) 5:595. 10.3389/fimmu.2014.00595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science (2014) 345:760–5. 10.1126/science.1251816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romero R, Miranda J, Chaemsaithong P, Chaiworapongsa T, Kusanovic JP, Dong Z, et al. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med (2014) 28(12):1394–409. 10.3109/14767058.2014.958463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Capoccia R, Greub G, Baud D. Ureaplasma urealyticum, Mycoplasma hominis and adverse pregnancy outcomes. Curr Opin Infect Dis (2013) 26:231–40. 10.1097/QCO.0b013e328360db58 [DOI] [PubMed] [Google Scholar]
- 24.Kwak DW, Hwang HS, Kwon JY, Park YW, Kim YH. Co-infection with vaginal Ureaplasma urealyticum and Mycoplasma hominis increases adverse pregnancy outcomes in patients with preterm labor or preterm premature rupture of membranes. J Matern Fetal Neonatal Med (2014) 27:333–7. 10.3109/14767058.2013.818124 [DOI] [PubMed] [Google Scholar]
- 25.Murtha AP, Edwards JM. The role of Mycoplasma and Ureaplasma in adverse pregnancy outcomes. Obstet Gynecol Clin North Am (2014) 41:615–27. 10.1016/j.ogc.2014.08.010 [DOI] [PubMed] [Google Scholar]
- 26.Kasper DC, Mechtler TP, Reischer GH, Witt A, Langgartner M, Pollak A, et al. The bacterial load of Ureaplasma parvum in amniotic fluid is correlated with an increased intrauterine inflammatory response. Diagn Microbiol Infect Dis (2010) 67:117–21. 10.1016/j.diagmicrobio.2009.12.023 [DOI] [PubMed] [Google Scholar]
- 27.Kundsin RB, Leviton A, Allred EN, Poulin SA. Ureaplasma urealyticum infection of the placenta in pregnancies that ended prematurely. Obstet Gynecol (1996) 87:122–7. 10.1016/0029-7844(95)00376-2 [DOI] [PubMed] [Google Scholar]
- 28.Kirchner L, Helmer H, Heinze G, Wald M, Brunbauer M, Weninger M, et al. Amnionitis with Ureaplasma urealyticum or other microbes leads to increased morbidity and prolonged hospitalization in very low birth weight infants. Eur J Obstet Gynecol Reprod Biol (2007) 134:44–50. 10.1016/j.ejogrb.2006.09.013 [DOI] [PubMed] [Google Scholar]
- 29.Oh KJ, Lee SE, Jung H, Kim G, Romero R, Yoon BH. Detection of Ureaplasmas by the polymerase chain reaction in the amniotic fluid of patients with cervical insufficiency. J Perinat Med (2010) 38:261–8. 10.1515/JPM.2010.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kemp MW. Preterm birth, intrauterine infection, and fetal inflammation. Front Immunol (2014) 5:574. 10.3389/fimmu.2014.00574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kemp MW, Saito M, Kallapur SG, Jobe AH, Keelan JA, Li S, et al. Inflammation of the fetal ovine skin following in utero exposure to Ureaplasma parvum. Reprod Sci (2011) 18:1128–37. 10.1177/1933719111408114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grigsby PL, Novy MJ, Sadowsky DW, Morgan TK, Long M, Acosta E, et al. Maternal azithromycin therapy for Ureaplasma intraamniotic infection delays preterm delivery and reduces fetal lung injury in a primate model. Am J Obstet Gynecol (2012) 207:475.e1–14. 10.1016/j.ajog.2012.10.871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Novy MJ, Duffy L, Axthelm MK, Sadowsky DW, Witkin SS, Gravett MG, et al. Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery, and fetal pneumonia in rhesus macaques. Reprod Sci (2009) 16:56–70. 10.1177/1933719108325508 [DOI] [PubMed] [Google Scholar]
- 34.Han YW, Shen T, Chung P, Buhimschi IA, Buhimschi CS. Uncultivated bacteria as etiologic agents of intra-amniotic inflammation leading to preterm birth. J Clin Microbiol (2009) 47:38–47. 10.1128/JCM.01206-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamont RF. Advances in the prevention of infection-related preterm birth. Front Inflamm (2015) 16:566. 10.3389/fimmu.2015.00566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bujold E, Morency AM. Antibiotics for the prevention of preterm birth. Aust N Z J Obstet Gynaecol (2008) 48:124–5. 10.1111/j.1479-828X.2007.00816.x [DOI] [PubMed] [Google Scholar]
- 37.Simcox R, Sin WT, Seed PT, Briley A, Shennan AH. Prophylactic antibiotics for the prevention of preterm birth in women at risk: a meta-analysis. Aust N Z J Obstet Gynaecol (2007) 47:368–77. 10.1111/j.1479-828X.2007.00759.x [DOI] [PubMed] [Google Scholar]
- 38.Morency AM, Bujold E. The effect of second-trimester antibiotic therapy on the rate of preterm birth. J Obstet Gynaecol Can (2007) 29:35–44. 10.1016/S1701-2163(16)32350-7 [DOI] [PubMed] [Google Scholar]
- 39.Brocklehurst P, Gordon A, Heatley E, Milan SJ. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database Syst Rev (2013) 1:CD000262. 10.1002/14651858.CD000262.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mercer B. Antibiotics in the management of PROM and preterm labor. Obstet Gynecol Clin North Am (2012) 39:65–76. 10.1016/j.ogc.2011.12.007 [DOI] [PubMed] [Google Scholar]
- 41.Thinkhamrop J, Hofmeyr GJ, Adetoro O, Lumbiganon P, Ota E. Antibiotic prophylaxis during the second and third trimester to reduce adverse pregnancy outcomes and morbidity. Cochrane Database Syst Rev (2015) 6:CD002250. 10.1002/14651858.CD002250.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lambert JA, John S, Sobel JD, Akins RA. Longitudinal analysis of vaginal microbiome dynamics in women with recurrent bacterial vaginosis: recognition of the conversion process. PLoS One (2013) 8:e82599. 10.1371/journal.pone.0082599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ugwumadu A. Role of antibiotic therapy for bacterial vaginosis and intermediate flora in pregnancy. Best Pract Res Clin Obstet Gynaecol (2007) 21:391–402. 10.1016/j.bpobgyn.2007.01.001 [DOI] [PubMed] [Google Scholar]
- 44.McDonald HM, Brocklehurst P, Gordon A. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database Syst Rev (2007) (1):CD000262. 10.1002/14651858.CD000262.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lamont RF, Nhan-Chang CL, Sobel JD, Workowski K, Conde-Agudelo A, Romero R. Treatment of abnormal vaginal flora in early pregnancy with clindamycin for the prevention of spontaneous preterm birth: a systematic review and metaanalysis. Am J Obstet Gynecol (2011) 205:177–90. 10.1016/j.ajog.2011.03.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Payne MS, Feng Z, Li S, Doherty DA, Xu B, Li J, et al. Second trimester amniotic fluid cytokine concentrations, Ureaplasma sp. colonisation status and sexual activity as predictors of preterm birth in Chinese and Australian women. BMC Pregnancy Childbirth (2014) 14:340. 10.1186/1471-2393-14-340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vouga M, Greub G, Prod’hom G, Durussel C, Roth-Kleiner M, Vasilevsky S, et al. Treatment of genital Mycoplasma in colonized pregnant women in late pregnancy is associated with a lower rate of premature labour and neonatal complications. Clin Microbiol Infect (2014) 20:1074–9. 10.1111/1469-0691.12686 [DOI] [PubMed] [Google Scholar]
- 48.Miura Y, Payne MS, Keelan JA, Noe A, Carter S, Watts R, et al. Maternal intravenous treatment with either azithromycin or solithromycin clears Ureaplasma parvum from the amniotic fluid in an ovine model of intrauterine infection. Antimicrob Agents Chemother (2014) 58(9):5413–20. 10.1128/AAC.03187-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCormack WM, Rosner B, Lee YH, Munoz A, Charles D, Kass EH. Effect on birth weight of erythromycin treatment of pregnant women. Obstet Gynecol (1987) 69:202–7. [PubMed] [Google Scholar]
- 50.Ogasawara KK, Goodwin TM. Efficacy of azithromycin in reducing lower genital Ureaplasma urealyticum colonization in women at risk for preterm delivery. J Matern Fetal Med (1999) 8:12–6. [DOI] [PubMed] [Google Scholar]
- 51.Gomez R, Romero R, Nien JK, Medina L, Carstens M, Kim YM, et al. Antibiotic administration to patients with preterm premature rupture of membranes does not eradicate intra-amniotic infection. J Matern Fetal Neonatal Med (2007) 20:167–73. 10.1080/14767050601135485 [DOI] [PubMed] [Google Scholar]
- 52.Kemp MW, Miura Y, Payne MS, Watts R, Megharaj S, Jobe AH, et al. Repeated maternal intramuscular or intraamniotic erythromycin incompletely resolves intrauterine Ureaplasma parvum infection in a sheep model of pregnancy. Am J Obstet Gynecol (2014) 211:134.e131–9. 10.1016/j.ajog.2014.02.025 [DOI] [PubMed] [Google Scholar]
- 53.Heikkinen T, Laine K, Neuvonen PJ, Ekblad U. The transplacental transfer of the macrolide antibiotics erythromycin, roxithromycin and azithromycin. Br J Obstet Gynaecol (2000) 107:770–5. 10.1111/j.1471-0528.2000.tb13339.x [DOI] [PubMed] [Google Scholar]
- 54.Philipson A, Sabath LD, Charles D. Transplacental passage of erythromycin and clindamycin. N Engl J Med (1973) 288:1219–21. 10.1056/NEJM197306072882307 [DOI] [PubMed] [Google Scholar]
- 55.Keelan JA, Nitsos I, Saito M, Musk GC, Kemp MW, Timmins M, et al. Maternal-amniotic-fetal distribution of macrolide antibiotics following intravenous, intramuscular, and intraamniotic administration in late pregnant sheep. Am J Obstet Gynecol (2011) 204(546):e510–47. 10.1016/j.ajog.2011.02.035 [DOI] [PubMed] [Google Scholar]
- 56.Mushtaq A. Solithromycin to treat community-acquired pneumonia? Lancet Respir Med (2015) 3:429. 10.1016/S2213-2600(15)00188-5 [DOI] [PubMed] [Google Scholar]
- 57.Van Bambeke F, Tulkens PM. The role of solithromycin in the management of bacterial community-acquired pneumonia. Expert Rev Anti Infect Ther (2016) 14:311–24. 10.1586/14787210.2016.1138857 [DOI] [PubMed] [Google Scholar]
- 58.Farrell DJ, Mendes RE, Jones RN. Antimicrobial activity of solithromycin against serotyped macrolide-resistant Streptococcus pneumoniae isolates collected from U.S. medical centers in 2012. Antimicrob Agents Chemother (2015) 59:2432–4. 10.1128/AAC.04568-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Piccinelli G, Fernandes P, Bonfanti C, Caccuri F, Caruso A, De Francesco MA. In vitro activity of solithromycin against erythromycin-resistant Streptococcus agalactiae. Antimicrob Agents Chemother (2014) 58:1693–8. 10.1128/AAC.02210-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mallegol J, Fernandes P, Melano RG, Guyard C. Antimicrobial activity of solithromycin against clinical isolates of Legionella pneumophila serogroup 1. Antimicrob Agents Chemother (2014) 58:909–15. 10.1128/AAC.01639-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jensen JS, Fernandes P, Unemo M. In vitro activity of the new fluoroketolide solithromycin (CEM-101) against macrolide-resistant and -susceptible Mycoplasma genitalium strains. Antimicrob Agents Chemother (2014) 58:3151–6. 10.1128/AAC.02411-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rodgers W, Frazier AD, Champney WS. Solithromycin inhibition of protein synthesis and ribosome biogenesis in Staphylococcus aureus, Streptococcus pneumoniae, and Haemophilus influenzae. Antimicrob Agents Chemother (2013) 57:1632–7. 10.1128/AAC.02316-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mallegol J, Fernandes P, Seah C, Guyard C, Melano RG. Determination of in vitro activity of solithromycin at different pHs and its intracellular activity tested against clinical isolates of Neisseria gonorrhoeae from a laboratory collection. Antimicrob Agents Chemother (2013). 10.1128/AAC.00564-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wittlin S, Ekland E, Craft JC, Lotharius J, Bathurst I, Fidock DA, et al. In vitro and in vivo activity of solithromycin (CEM-101) against Plasmodium species. Antimicrob Agents Chemother (2012) 56:703–7. 10.1128/AAC.05039-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Golparian D, Fernandes P, Ohnishi M, Jensen JS, Unemo M. In vitro activity of the new fluoroketolide solithromycin (CEM-101) against a large collection of clinical Neisseria gonorrhoeae isolates and international reference strains, including those with high-level antimicrobial resistance: potential treatment option for gonorrhea? Antimicrob Agents Chemother (2012) 56:2739–42. 10.1128/AAC.00036-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Putnam SD, Sader HS, Farrell DJ, Biedenbach DJ, Castanheira M. Antimicrobial characterisation of solithromycin (CEM-101), a novel fluoroketolide: activity against staphylococci and enterococci. Int J Antimicrob Agents (2011) 37:39–45. 10.1016/j.ijantimicag.2010.08.021 [DOI] [PubMed] [Google Scholar]
- 67.Farrell DJ, Castanheira M, Sader HS, Jones RN. The in vitro evaluation of solithromycin (CEM-101) against pathogens isolated in the United States and Europe (2009). J Infect (2010) 61:476–83. 10.1016/j.jinf.2010.08.010 [DOI] [PubMed] [Google Scholar]
- 68.Still JG, Schranz J, Degenhardt TP, Scott D, Fernandes P, Gutierrez MJ, et al. Pharmacokinetics of solithromycin (CEM-101) after single or multiple oral doses and effects of food on single-dose bioavailability in healthy adult subjects. Antimicrob Agents Chemother (2011) 55:1997–2003. 10.1128/AAC.01429-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schranz J, Clark K, Marion A, Ghibellini G, Fernandes P. A phase 1 trial to evaluate the safety and pharmacokinetics of single doses of intravenous (IV) solithromycin (CEM-101) in healthy adult subjects. 21st European Congress of Clinical Microbiology of Infectious Diseases. Milan, Italy: Clinical Microbiology and Infection; (2011). O95 p. [Google Scholar]
- 70.Rodvold KA, Gotfried MH, Still JG, Clark K, Fernandes P. Comparison of plasma, epithelial lining fluid, and alveolar macrophage concentrations of solithromycin (CEM-101) in healthy adult subjects. Antimicrob Agents Chemother (2012) 56:5076–81. 10.1128/AAC.00766-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lemaire S, Van Bambeke F, Tulkens PM. Cellular accumulation and pharmacodynamic evaluation of the intracellular activity of CEM-101, a novel fluoroketolide, against Staphylococcus aureus, Listeria monocytogenes, and Legionella pneumophila in human THP-1 macrophages. Antimicrob Agents Chemother (2009) 53:3734–43. 10.1128/AAC.00203-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Llano-Sotelo B, Dunkle J, Klepacki D, Zhang W, Fernandes P, Cate JH, et al. Binding and action of CEM-101, a new fluoroketolide antibiotic that inhibits protein synthesis. Antimicrob Agents Chemother (2010) 54:4961–70. 10.1128/AAC.00860-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fernandez-Brando R, Yamaguchi N, Tahoun A, McAteer SP, Gillespie T, Wang D, et al. Type 3 secretion-dependent sensitivity of Escherichia coli O157 to specific ketolides. Antimicrob Agents Chemother (2015) 60:459–70. 10.1128/AAC.02085-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Waites KB, Crabb DM, Duffy LB. Comparative in vitro susceptibilities of human Mycoplasmas and Ureaplasmas to a new investigational ketolide, CEM-101. Antimicrob Agents Chemother (2009) 53:2139–41. 10.1128/AAC.00090-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Furfaro LL, Spiller OB, Keelan JA, Payne MS. In vitro activity of solithromycin and its metabolites, CEM-214 and N-acetyl-CEM-101, against 100 clinical Ureaplasma spp. isolates compared with azithromycin. Int J Antimicrob Agents (2015) 46:319–24. 10.1016/j.ijantimicag.2015.04.015 [DOI] [PubMed] [Google Scholar]
- 76.McGhee P, Clark C, Kosowska-Shick KM, Nagai K, Dewasse B, Beachel L, et al. In vitro activity of CEM-101 against Streptococcus pneumoniae and Streptococcus pyogenes with defined macrolide resistance mechanisms. Antimicrob Agents Chemother (2010) 54:230–8. 10.1128/AAC.01123-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roblin PM, Kohlhoff SA, Parker C, Hammerschlag MR. In vitro activity of CEM-101, a new fluoroketolide antibiotic, against Chlamydia trachomatis and Chlamydia (Chlamydophila) pneumoniae. Antimicrob Agents Chemother (2010) 54:1358–9. 10.1128/AAC.01343-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oldach D, Clark K, Schranz J, Das A, Craft JC, Scott D, et al. Randomized, double-blind, multicenter phase 2 study comparing the efficacy and safety of oral solithromycin (CEM-101) to those of oral levofloxacin in the treatment of patients with community-acquired bacterial pneumonia. Antimicrob Agents Chemother (2013) 57:2526–34. 10.1128/AAC.00197-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hook EW, III, Golden M, Jamieson BD, Dixon PB, Harbison HS, Lowens S, et al. A phase 2 trial of oral solithromycin 1200 mg or 1000 mg as single-dose oral therapy for uncomplicated gonorrhea. Clin Infect Dis (2015) 61:1043–8. 10.1093/cid/civ478 [DOI] [PubMed] [Google Scholar]
- 80.Jamieson BD, Ciric S, Fernandes P. Safety and pharmacokinetics of solithromycin in subjects with hepatic impairment. Antimicrob Agents Chemother (2015) 59:4379–86. 10.1128/AAC.04652-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Keelan JA, Pugazhenthi K. Trans-placental passage and anti-inflammatory effects of solithromycin in the human placenta. Placenta (2014) 35:1043–8. 10.1016/j.placenta.2014.09.009 [DOI] [PubMed] [Google Scholar]
- 82.Keelan JA, Kemp MW, Payne MS, Johnson D, Stock SJ, Saito M, et al. Maternal administration of solithromycin, a new, potent, broad-spectrum fluoroketolide antibiotic, achieves fetal and intra-amniotic antimicrobial protection in a pregnant sheep model. Antimicrob Agents Chemother (2014) 58:447–54. 10.1128/AAC.01743-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bahat Dinur A, Koren G, Matok I, Wiznitzer A, Uziel E, Gorodischer R, et al. Fetal safety of macrolides. Antimicrob Agents Chemother (2013) 57:3307–11. 10.1128/AAC.01691-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Berard A, Sheehy O, Zhao JP, Nordeng H. Use of macrolides during pregnancy and the risk of birth defects: a population-based study. Pharmacoepidemiol Drug Saf (2015) 24(12):1241–8. 10.1002/pds.3900 [DOI] [PubMed] [Google Scholar]
- 85.Lund M, Pasternak B, Davidsen RB, Feenstra B, Krogh C, Diaz LJ, et al. Use of macrolides in mother and child and risk of infantile hypertrophic pyloric stenosis: nationwide cohort study. BMJ (2014) 348:g1908. 10.1136/bmj.g1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eberly MD, Eide MB, Thompson JL, Nylund CM. Azithromycin in early infancy and pyloric stenosis. Pediatrics (2015) 135:483–8. 10.1542/peds.2014-2026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.de Vries F. The difficulty in evaluating all findings in study on use of macrolides in mother and child and risk of infantile hypertrophic pyloric stenosis. BMJ (2014) 349:g5201. 10.1136/bmj.g5201 [DOI] [PubMed] [Google Scholar]
- 88.Ludvigsson JF, Lundholm C, Ortqvist AK, Almqvist C. No association between macrolide treatment in infancy and later pyloric stenosis in Sweden. Eur J Epidemiol (2015). 10.1007/s10654-015-0114-6 [DOI] [PubMed] [Google Scholar]
- 89.Kallen BA, Otterblad Olausson P, Danielsson BR. Is erythromycin therapy teratogenic in humans? Reprod Toxicol (2005) 20:209–14. 10.1016/j.reprotox.2005.01.010 [DOI] [PubMed] [Google Scholar]
- 90.Crider KS, Cleves MA, Reefhuis J, Berry RJ, Hobbs CA, Hu DJ. Antibacterial medication use during pregnancy and risk of birth defects: National Birth Defects Prevention Study. Arch Pediatr Adolesc Med (2009) 163:978–85. 10.1001/archpediatrics.2009.188 [DOI] [PubMed] [Google Scholar]
- 91.Lin KJ, Mitchell AA, Yau WP, Louik C, Hernandez-Diaz S. Safety of macrolides during pregnancy. Am J Obstet Gynecol (2013) 208(221):e221–8. 10.1016/j.ajog.2012.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li M, Wang M, Donovan SM. Early development of the gut microbiome and immune-mediated childhood disorders. Semin Reprod Med (2014) 32:74–86. 10.1055/s-0033-1361825 [DOI] [PubMed] [Google Scholar]
- 93.Mueller NT, Bakacs E, Combellick J, Grigoryan Z, Dominguez-Bello MG. The infant microbiome development: mom matters. Trends Mol Med (2015) 21:109–17. 10.1016/j.molmed.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Prince AL, Antony KM, Ma J, Aagaard KM. The microbiome and development: a mother’s perspective. Semin Reprod Med (2014) 32:14–22. 10.1055/s-0033-1361818 [DOI] [PubMed] [Google Scholar]
- 95.Faa G, Gerosa C, Fanni D, Nemolato S, van Eyken P, Fanos V. Factors influencing the development of a personal tailored microbiota in the neonate, with particular emphasis on antibiotic therapy. J Matern Fetal Neonatal Med (2013) 26(Suppl 2):35–43. 10.3109/14767058.2013.829700 [DOI] [PubMed] [Google Scholar]
- 96.Azad MB, Bridgman SL, Becker AB, Kozyrskyj AL. Infant antibiotic exposure and the development of childhood overweight and central adiposity. Int J Obes (Lond) (2014) 38:1290–8. 10.1038/ijo.2014.119 [DOI] [PubMed] [Google Scholar]
- 97.Azad MB, Konya T, Persaud RR, Guttman DS, Chari RS, Field CJ, et al. Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: a prospective cohort study. Br J Obstet Gynaecol (2015). 10.1111/1471-0528.13601 [DOI] [PubMed] [Google Scholar]
- 98.Lapin B, Piorkowski J, Ownby D, Freels S, Chavez N, Hernandez E, et al. Relationship between prenatal antibiotic use and asthma in at-risk children. Ann Allergy Asthma Immunol (2015) 114:203–7. 10.1016/j.anai.2014.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cox LM, Blaser MJ. Antibiotics in early life and obesity. Nat Rev Endocrinol (2015) 11:182–90. 10.1038/nrendo.2014.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Metsälä J, Lundqvist A, Virta LJ, Kaila M, Gissler M, Virtanen SM. Prenatal and post-natal exposure to antibiotics and risk of asthma in childhood. Clin Exp Allergy (2015) 45:137–45. 10.1111/cea.12356 [DOI] [PubMed] [Google Scholar]
- 101.Ng PY, Ireland DJ, Keelan JA. Drugs to block cytokine signaling for the prevention and treatment of inflammation-induced preterm birth. Front Immunol (2015) 6:166. 10.3389/fimmu.2015.00166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Keelan JA. Pharmacological inhibition of inflammatory pathways for the prevention of preterm birth. J Reprod Immunol (2011) 88:176–84. 10.1016/j.jri.2010.11.003 [DOI] [PubMed] [Google Scholar]
- 103.Ireland DJ, Kemp MW, Miura Y, Saito M, Newnham JP, Keelan JA. Intra-amniotic pharmacological blockade of inflammatory signalling pathways in an ovine chorioamnionitis model. Mol Hum Reprod (2015) 21:479–89. 10.1093/molehr/gav005 [DOI] [PubMed] [Google Scholar]
- 104.Stinson LF, Ireland DJ, Kemp MW, Payne MS, Stock SJ, Newnham JP, et al. Effects of cytokine-suppressive anti-inflammatory drugs on inflammatory activation in ex vivo human and ovine fetal membranes. Reproduction (2014) 147:313–20. 10.1530/REP-13-0576 [DOI] [PubMed] [Google Scholar]
- 105.De Silva D, Mitchell MD, Keelan JA. Inhibition of choriodecidual cytokine production and inflammatory gene expression by selective I-kappaB kinase (IKK) inhibitors. Br J Pharmacol (2010) 160:1808–22. 10.1111/j.1476-5381.2010.00839.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kobayashi Y, Wada H, Rossios C, Takagi D, Charron C, Barnes PJ, et al. A novel macrolide/fluoroketolide, solithromycin (CEM-101), reverses corticosteroid insensitivity via phosphoinositide 3-kinase pathway inhibition. Br J Pharmacol (2013) 169:1024–34. 10.1111/bph.12187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kobayashi Y, Wada H, Rossios C, Takagi D, Higaki M, Mikura S, et al. A novel macrolide solithromycin exerts superior anti-inflammatory effect via NF-kappaB inhibition. J Pharmacol Exp Ther (2013) 345:76–84. 10.1124/jpet.112.200733 [DOI] [PubMed] [Google Scholar]
- 108.Cheung PS, Si EC, Hosseini K. Anti-inflammatory activity of azithromycin as measured by its NF-kappaB, inhibitory activity. Ocul Immunol Inflamm (2010) 18:32–7. 10.3109/09273940903359725 [DOI] [PubMed] [Google Scholar]
- 109.Desaki M, Okazaki H, Sunazuka T, Omura S, Yamamoto K, Takizawa H. Molecular mechanisms of anti-inflammatory action of erythromycin in human bronchial epithelial cells: possible role in the signaling pathway that regulates nuclear factor-kappaB activation. Antimicrob Agents Chemother (2004) 48:1581–5. 10.1128/AAC.48.5.1581-1585.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Desaki M, Takizawa H, Ohtoshi T, Kasama T, Kobayashi K, Sunazuka T, et al. Erythromycin suppresses nuclear factor-kappaB and activator protein-1 activation in human bronchial epithelial cells. Biochem Biophys Res Commun (2000) 267:124–8. 10.1006/bbrc.1999.1917 [DOI] [PubMed] [Google Scholar]
- 111.Ghartey J, Bastek JA, Brown AG, Anglim L, Elovitz MA. Women with preterm birth have a distinct cervicovaginal metabolome. Am J Obstet Gynecol (2015) 212:776.e1–12. 10.1016/j.ajog.2015.03.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hyman RW, Fukushima M, Jiang H, Fung E, Rand L, Johnson B, et al. Diversity of the vaginal microbiome correlates with preterm birth. Reprod Sci (2013) 21:32–40. 10.1177/1933719113488838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Prince AL, Antony KM, Chu DM, Aagaard KM. The microbiome, parturition, and timing of birth: more questions than answers. J Reprod Immunol (2014) 104–105:12–19. 10.1016/j.jri.2014.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Palacio M, Cobo T, Bosch J, Filella X, Navarro-Sastre A, Ribes A, et al. Cervical length and gestational age at admission as predictors of intra-amniotic inflammation in preterm labor with intact membranes. Ultrasound Obstet Gynecol (2009) 34:441–7. 10.1002/uog.6437 [DOI] [PubMed] [Google Scholar]
- 115.Holst RM, Jacobsson B, Hagberg H, Wennerholm UB. Cervical length in women in preterm labor with intact membranes: relationship to intra-amniotic inflammation/microbial invasion, cervical inflammation and preterm delivery. Ultrasound Obstet Gynecol (2006) 28:768–74. 10.1002/uog.3837 [DOI] [PubMed] [Google Scholar]
- 116.Hassan S, Romero R, Hendler I, Gomez R, Khalek N, Espinoza J, et al. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J Perinat Med (2006) 34:13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kenyon S, Boulvain M, Neilson JP. Antibiotics for preterm rupture of membranes. Cochrane Database Syst Rev (2013) 12:CD001058. 10.1002/14651858.CD001058.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]