Abstract
Background and Aims The evolution of complex rooting systems during the Devonian had significant impacts on global terrestrial ecosystems and the evolution of plant body plans. However, detailed understanding of the pathways of root evolution and the architecture of early rooting systems is currently lacking. We describe the architecture and resolve the structural homology of the rooting system of an Early Devonian basal lycophyte. Insights gained from these fossils are used to address lycophyte root evolution and homology.
Methods Plant fossils are preserved as carbonaceous compressions at Cottonwood Canyon (Wyoming), in the Lochkovian–Pragian (∼411 Ma; Early Devonian) Beartooth Butte Formation. We analysed 177 rock specimens and documented morphology, cuticular anatomy and structural relationships, as well as stratigraphic position and taphonomic conditions.
Key Results The rooting system of the Cottonwood Canyon lycophyte is composed of modified stems that bear fine, dichotomously branching lateral roots. These modified stems, referred to as root-bearing axes, are produced at branching points of the above-ground shoot system. Root-bearing axes preserved in growth position exhibit evidence of positive gravitropism, whereas the lateral roots extend horizontally. Consistent recurrence of these features in successive populations of the plant preserved in situ demonstrates that they represent constitutive structural traits and not opportunistic responses of a flexible developmental programme.
Conclusions This is the oldest direct evidence for a rooting system preserved in growth position. These rooting systems, which can be traced to a parent plant, include some of the earliest roots known to date and demonstrate that substantial plant–substrate interactions were under way by Early Devonian time. The morphological relationships between stems, root-bearing axes and roots corroborate evidence that positive gravitropism and root identity were evolutionarily uncoupled in lycophytes, and challenge the hypothesis that roots evolved from branches of the above-ground axial system, suggesting instead that lycophyte roots arose as a novel organ.
Keywords: Devonian, root evolution, rooting system, lycophyte, fossil, Wyoming
INTRODUCTION
Rooting systems are a fundamental component of modern plant body plans, responsible for anchorage and uptake of water and nutrients from the substrate. The evolution of rooting systems transformed terrestrial ecosystems by influencing soil weathering processes and nutrient cycling at a global scale (Algeo and Scheckler, 1998; Berner and Kothavala, 2001; Bergman et al., 2004; Taylor et al., 2009). Rooting systems of modern plants exhibit a wide range of morphological diversity, and consist of roots, modified shoots or both. Roots are recognized as axial organs with radial symmetry, endogenous origin, root hairs and a root cap (Raven and Edwards, 2001; Kenrick and Strullu-Derrien, 2014). Throughout this article the term root is used in this strict sense and is not applied to other organs that have a rooting function. In many plants the rooting system consists only of roots, but in numerous seed-free plants and angiosperms modified stems called rhizomes also play a key role in anchoring the plant, and often also produce roots. Other plants lack roots entirely. For instance, the lycophyte Isoetes has a rooting system consisting of a highly modified shoot whose appendages (‘rootlets’) are homologous to leaves (Rothwell and Erwin, 1985; Raven and Edwards, 2001; Rothwell et al., 2014), whereas the rooting system of Psilotum consists of rhizoid-bearing undifferentiated axes (Gifford and Foster, 1989).
While organs that can be recognized as roots are ubiquitous among vascular plant lineages, not all of these organs are homologous. Roots evolved independently at least twice in the two major clades of vascular plants – the lycophytes and euphyllophytes (sensu Kenrick and Crane, 1997; Raven and Edwards, 2001; Boyce, 2005; Seago and Fernando, 2013; Kenrick and Strullu-Derrien, 2014). In these groups, an independent origin of roots is inferred from the fossil record and developmental differences. Lycophyte and euphyllophyte stem groups lacked roots (although they were not entirely devoid of other types of rooting structures; Kenrick, 2002; Kenrick and Strullu-Derrien, 2014), and so the most parsimonious hypothesis is that the common ancestor of the two clades was also rootless (Friedman et al., 2004; Boyce, 2005). Developmental differences also support independent origins: euphyllophyte roots branch endogenously from cells of the pericycle or endodermis, whereas lycophyte roots branch apically through division of the meristem (Gifford and Foster, 1989; Friedman et al., 2004). However, despite the evidence indicating that they are not homologous, lycophyte and euphyllophyte roots share defining characters, listed above, that distinguish them from other organs.
Although the independent origin of roots in lycophytes and euphyllophytes is widely accepted, fundamental questions on how roots evolved within these lineages remain unanswered. For instance, what is the homology of these organs – were they derived from the undifferentiated axes of the tracheophyte stem groups, or did they arise as novel organs? Lycophyte roots appear earlier in the fossil record than those of euphyllophytes (Boyce, 2005; Raven and Edwards, 2001) and several authors have suggested that they evolved from aerial stems or axes (Stewart and Rothwell, 1993; Gensel and Berry, 2001; Gensel et al., 2001; Seago and Fernando, 2013). This hypothesis stems from the prevalence among extinct lycophytes of rooting systems derived from branches of the above-ground axial system. Such occurrences are documented in basal lycophyte stem groups (zosterophylls, Asteroxylon) and isoetalean lycophytes (Kidston and Lang, 1920; Rothwell and Erwin, 1985; Gensel et al., 2001; Hao et al., 2010). Attempts to address such questions on root evolution are frustrated by the rarity of well-preserved rooting systems in the fossil record, especially in early vascular plants. Moreover, although some extensive early rooting systems have been documented (Elick et al., 1998; Poschmann and Gossmann, 2014) their parent plants are not known.
Here we document the rooting system of a basal lycophyte from the Early Devonian (late Lochkovian – early Pragian; approx. 411 Ma) Beartooth Butte Formation exposure at Cottonwood Canyon, in Wyoming. Both the above-ground shoot system and the rooting system of this plant (hereafter, Cottonwood Canyon lycophyte) are preserved in situ, providing rare insights into the growth habit and overall architecture of the rooting system, as well as the structural homology of these rooting organs. This is the oldest occurrence of a rooting system preserved in growth position, in connection with a parent plant. We discuss it in the context of the current understanding of the fossil record and morphology of roots, to address questions on lycophyte root evolution and homology.
MATERIAL AND METHODS
The lycophyte fossils that form the basis of this study were recovered from the Beartooth Butte Formation at Cottonwood Canyon, Wyoming (Big Horn Co., 44°51′51″N, 108°02′46″W). There, plant fossils occur in dense autochthonous and parautochthonous assemblages and in situ preservation is frequent. The lycophyte is preserved as coalified to oxidized compressions and impressions.
Throughout northern Wyoming and southern Montana, the Beartooth Butte Formation displays geometries reminiscent of channel-fill deposits, characterized by long narrow bodies of sediment that are lenticular in cross-section. The deposits consist primarily of dolomitized siltstone and shale with dolomitized sandstone interbeds (Sandberg, 1961, 1967; Caruso and Tomescu, 2012). The unit contains fish, eurypterid and terrestrial plant fossils, as well as encrusting microconchid lophophorates (Dorf, 1933, 1934; Schultes and Dorf, 1938; Denison, 1956; Elliot and Johnson, 1997; Tetlie, 2007; Lamsdell and Legg, 2010; Lamsdell and Selden, 2013; Caruso and Tomescu, 2012). Based on its geometry and fossil content, the unit has been interpreted as fresh- and brackish-water deposits of estuarine to fluvial environments (Dorf, 1934; Denison, 1956; Sandberg, 1961), an interpretation corroborated by limited isotope data (Fiorillo, 2000). Palynological analyses by D. C. McGregor (reported by Tanner, 1983) suggest a late Lochkovian – early Pragian age for the plant fossil layers at Cottonwood Canyon, independently confirmed by fish biostratigraphy (Elliot and Johnson, 1997).
This study used 177 rock specimens held in collections curated at the US National Museum of Natural History – Smithsonian Institution (USNM), the Denver Museum of Nature and Science (DMNH), the Field Museum of Natural History (FMNH), the University of Kansas Biodiversity Institute (KU) and Humboldt State University (HPH). The specimens originate from a 1·5- to 1·8 -m-thick section and, within it, from locations along the outcrop no more than 10 m apart. Although not all specimens were collected by us, we know they all originate from the same stratigraphic level because accessibility issues in the field, at Cottonwood Canyon, preclude collection of specimens from other levels and locations.
High-resolution images obtained in parallel with direct examination of collection specimens were compiled in a database and used for morphological and morphometric analyses. When needed for resolution of morphological detail, selected specimens were obtained on loan from KU and NMNH.
Specimens of the Cottonwood Canyon lycophyte were assigned by Tanner (1983) to Drepanophycus devonicus. However, examination of museum specimens and of our own recently excavated material indicates that while the plant is indeed a drepanophycalean lycophyte, assignment to D. devonicus should be re-evaluated. Whole-plant reconstruction and taxonomic revision of this lycophyte involve much additional information and discussion of Cottonwood Canyon fossils, and are therefore not addressed here.
Fragments of cuticle were removed using cellulose acetate peels taken from the surface of rock specimens. Peels were placed in 0·5 % hydrochloric acid to dissolve residual minerals before mounting on slides using Eukitt (O. Kindler, Freiburg, Germany). No clearing of cuticles was necessary.
The material was imaged using a Nikon Coolpix E8800 digital camera mounted on a Nikon Eclipse E400 microscope, an Olympus DP73 digital camera mounted on an Olympus SZX16 dissecting microscope, an LG G4 cell phone camera and an Epson Perfection 4490 Photo scanner. Measurements were obtained using ImageJ (National Institutes of Health, Bethesda, MD, USA). Images were processed using Pixelmator 3.4 (Pixelmator Team, London, UK).
RESULTS
Body plan
The body plan of the Cottonwood Canyon lycophyte consists of three distinct types of axial organ found in physical connection: robust leafy stems, smaller leafless axes and fine, dichotomously branching lateral appendages borne exclusively on the leafless axes. We interpret these lateral appendages as roots, as discussed below, and for clarity we hereafter refer to them as such. The leafless axes subtending the roots are referred to as root-bearing axes.
The leafy stems are up to 34 mm wide (mean 15 mm; n = 567) and bear loose helices of small triangular leaves (1–7 mm) (Fig. 1). A narrow central vascular strand (stele) (1–2 mm) is conspicuous in most specimens (Fig. 1). The leafless root-bearing axes are much narrower on average than stems (2–8 mm wide; mean 4·4 mm; n = 452) and possess a thin central stele (0·5–1·5 mm) (Fig. 2). These axes are produced by branching of the leafy stems, described in detail below. Segments of root-bearing axes range from 3 mm to 13 cm in length, and the combined length of all the segments used in this study is approx. 14 m.
Fig. 1.
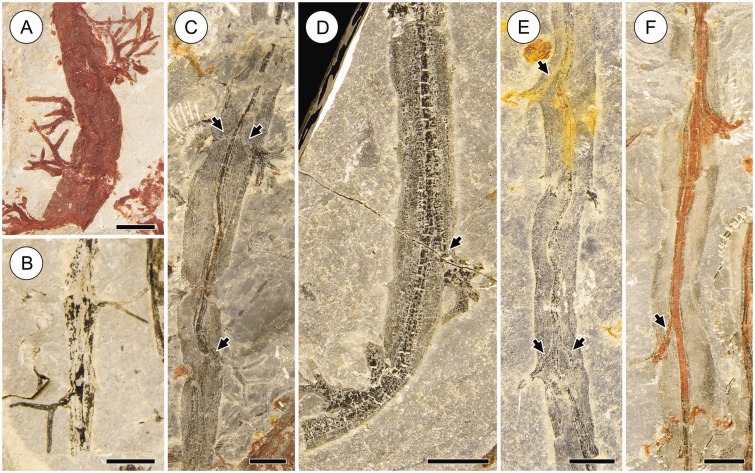
Leafy stems of the Cottonwood Canyon lycophyte. (A) Leafy stem and smaller root-bearing axis. Note narrow central stele of leafy stem (arrow) and bases of root tufts on root-bearing axis (arrowheads). Scale bar = 20 mm. HPH 229. (B) Stem mat consisting of numerous leafy stems in shale layer. Although the vertical dimension is not shown, this layer hosts many interwoven stems separated by millimetre to submillimetre shale laminae over a thickness of several centimetres. Scale bar = 40 mm. KU D1806. (C) Leafy stem. Note central stele (arrow) and leaves with visible veins (arrowheads). Scale bar = 20 mm. FMNH PP15972.
Fig. 2.
Root arrangement and variable preservation of root-bearing axes and attached roots in the Cottonwood Canyon lycophyte. Root tufts range from intact (A, B) to completely absent due to poor preservation or to the fact that they are covered by the rock matrix (F). The tips of some otherwise well-preserved roots are not seen because they depart into the rock matrix (C, D). In other specimens only the bases of root tufts are preserved (E), or roots are not preserved at all and their position is indicated only by root traces in the cortex of subtending axes (F; arrow); note similar root vascular traces in the cortex of other root-bearing axes (C, D; arrows). Preservation of the root-bearing axes ranges from coalified (B, D) to fully oxidized (A). (C), (E) and (F) show partial oxidation of the stele. Note alternate and opposite root arrangement on the same axis in (C) and (E). Scale bars = 5 mm. Specimens: KU D1813 (A), KU D1618b (B), KU D1804 (C), KU D876b (D), KU D1281b (E), KU D1166a (F).
Lateral roots, occurring exclusively on root-bearing axes, have a narrow central vascular strand that is continuous with that of the subtending axis (Figs 2 and 5A). Root arrangement along axes ranges from alternate to opposite (Fig. 2), with root tufts spaced from 2 to 40 mm apart (average distance 11 mm; n = 161). Segments that lack roots can be recognized as root-bearing axes by their size, the absence of leaves, the conspicuous narrow central stele and, where preserved, characteristic epidermal anatomy (described below). When root preservation is incomplete, the position of roots along the axis is revealed by protruding root bases (Fig. 2E, F) or by the presence of root vascular traces in the cortex. Root vascular traces exhibit a characteristic pattern, with divergence angles <90° (Fig. 2E, F). While root-bearing axes are leafless overall, their bases can bear sparse reduced leaves near their divergence from leafy stems (Figs 3A and 7B). Because of this, root-bearing axes that do not show attachment to leafy stems, but have sparse minute leaves, probably represent basal regions (Fig. 7H).
Fig. 5.
Comparison of roots attached to root-bearing axes of the Cottonwood Canyon lycophyte (A, C) with those detached from or not visibly attached to axes (B, D). The close similarity in morphology and branching architecture indicates that the isolated root tufts belong to the Cottonwood Canyon lycophyte. Scale bars = 5 mm. Specimens: KU D1804 (A), KU D1631a (B), KU D1618b (C), KU D1631b (D).
Fig. 3.
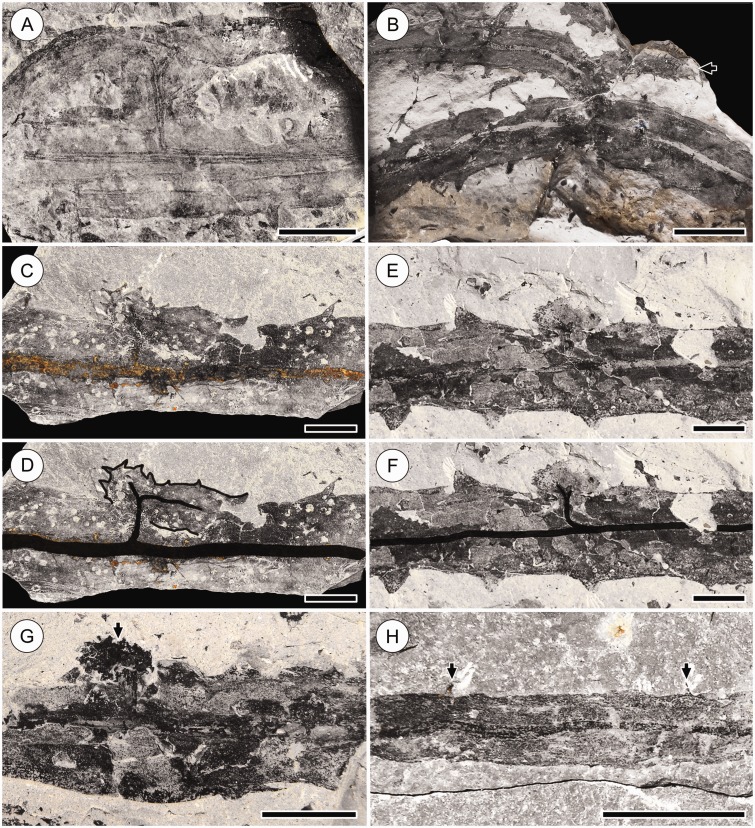
Root-bearing axes produced by K-branching in the Cottonwood Canyon lycophyte. (A) Part (top) and counterpart (bottom) of specimen showing a leafy stem with a root-bearing axis diverging from it. Note impression of the stele of the root-bearing axis in the counterpart (white arrow) demonstrating continuity between the vasculature of the main stem and root-bearing axis. The root-bearing axis has several reduced leaves at the base (black arrowheads) and four root tufts (black arrows). It is unclear if the other arm of the K-branch is present, but the curvature of the stele in the main stem (white arrowhead) suggests the arm, if developed, may overlap the main stem. Scale bar = 10 mm. HPH 356. (B) Leafy stem with two K-branches (white arrows). The steles of the main stem, K-branches and root traces are oxidized, revealing the details of branching. One K-branch (at left) produces one arm, which is cut off at the edge of the rock specimen, and a leafy bud (black arrowhead). The other K-branch (at right) produces one arm that is cut off at the edge of the rock specimen and a root-bearing axis, which is folded over itself (at asterisk). Five root tufts (black arrows) are present along this root-bearing axis; note that root traces diverge away from the base of the axis, in the direction of its apex (top). Images of the partially preserved portions of the part (D1812a) and counterpart (D1812b) of this specimen have been merged digitally (boxed area, at right) to show continuity between the root-bearing axis and the main stem. Scale bar = 10 mm. KU D1812.
Fig. 7.
Different degrees of development of K-branches of the Cottonwood Canyon lycophyte. (A) Fully developed K-branch. Note the thick vascular trace diverging from the stele of main stem at 90° angle. Scale bar = 10 mm. KU D1039a. (B) Fully developed K-branch producing a leafy stem and root-bearing axis (arrow). Note the smaller size and reduced leaves of the root-bearing axis; no roots are present on this segment of the axis. Scale bar = 20 mm. FMNH PP15959. (C, D) K-branch consisting of one fully developed arm and a leafy bud; in (D) parts of the specimen are traced for clarity. Note vascularization of the bud and fully developed arm. Scale bar = 10 mm. KU D932b. (E, F) Leafy bud; in (F) the vasculature is traced for clarity. Note the wide, squat shape of the bud and bifurcation of its vascular strand, indicating presence of two adjacent apices. Scale bar = 10 mm. KU D1471b. (G) Leafy bud exhibiting characteristic wide, squat shape and a slight central furrow (arrow) indicating presence of two adjacent apices. Scale bar = 10 mm. DMNH 29591. (H) Root-bearing axis with reduced leaves (arrows). Basal segments of root-bearing axes bear reduced leaves, as seen in (B) and Fig. 3A. Scale bar = 10 mm. KU D1281a.
Root morphology was documented based on 615 root tufts observed on 310 root-bearing axes. The roots are fine (0·4–0·7 mm thick) and branch dichotomously (Fig. 4). Darker regions observed occasionally at their tips may represent root caps (Fig. 4A–C). No root hairs were observed on the roots. This could be due to the delicate nature and low preservation potential of root hairs, or to the rarity of preserved root tips in the fossil material (root hairs are only found immediately behind the root apex). Alternatively, the Cottonwood Canyon lycophyte may not have produced root hairs.
Fig. 4.
Roots of the Cottonwood Canyon lycophyte. (A–C) Roots with darkened apices that may represent root caps (arrows). Part (B) and counterpart (C) of specimen are shown to demonstrate that all three roots have darkened apices. (D–G) Roots showing various degrees and patterns of branching. Note the thin central stele clearly visible in (D), and the dense branching at the base of roots in (F), which have a tufted appearance. (F) and (G) illustrate root tufts diverging in opposite directions, in a horizontal plane, from vertical root-bearing axes (asterisks) exposed in cross-section on the bedding plane. Scale bars = 5 mm. Specimens: HPH 27 (A), KU D872a (B), KU D872b (C), KU D1616b (D), KU D1625h (E), KU D1153 (FG), KU D1814 (G).
The branching architecture of the roots is widely variable. Dense branching close to the root base results in a tufted habit wherein numerous roots seem to arise from a thick base up to 3 mm wide (Fig. 4F, see also Fig. 3B). In other instances, more widely and regularly spaced dichotomies produce roots with thinner bases (<2 mm) (Figs 2B and 4D, G). These two situations are end-points of a continuous series, as seen, for example, in roots that show dense branching proximally and greater distance between branches distally (Fig. 4E). The largest root tufts observed extend as far as 20 mm laterally from the subtending axes and have up to five orders of branching. Seemingly isolated root tufts that are found without clear attachment to root-bearing axes can be recognized as belonging to the Cottonwood Canyon lycophyte by their size, thin stele and characteristic branching architecture (Fig. 5).
Leafy stems and root-bearing axes are distinguished by their epidermal anatomy. In leafy stems, epidermal cells are isodiametric to slightly elongate longitudinally, 37–66 × 46–96 µm, and stomata are abundant (Fig. 6A–D). In contrast, epidermal cells of root-bearing axes are narrow and elongate-rectangular, 22–31 × 95–155 µm (Fig. 6F, H). When present on root-bearing axes, stomata are similar to those of the leafy stems but are sparse and irregularly distributed (Fig. 6E). No cuticle could be recovered from roots and their epidermal anatomy is unknown (Fig. 6G).
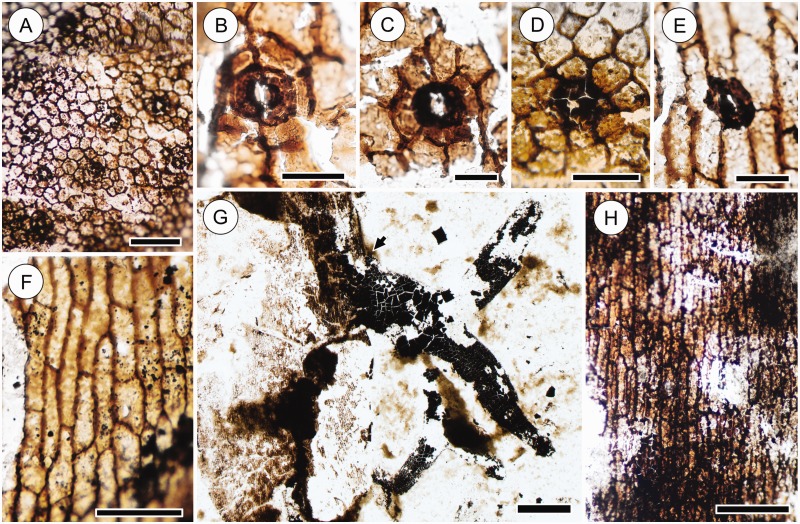
Fig. 6.
Epidermal anatomy of leafy stems (A–D) and root-bearing axes (E–H) of the Cottonwood Canyon lycophyte. (A) Leafy stem epidermis showing isodiametric epidermal cells and numerous stomata. Scale bar = 200 µm. HPH 333. (B–D) Details of stomata on leafy stems. Note that epidermal cells surrounding guard cells range from smaller than (B) to similar in size as regular epidermal cells (C, D). Also note variation in the size of epidermal cells surrounding the stoma in (C). Scale bars = 50 µm. HPH 76 (B, C), HPH 333 (D). (E) Detail of one of the rare stomata on a root-bearing axis. Note similarity in size and shape to the leafy stem stomata in (B–D). Scale bar = 50 µm. HPH 330. (F) Epidermis of root-bearing axis showing elongate epidermal cells. Note the absence of stomata. Scale bar = 100 µm. HPH 330. (G, H) Film pull of cuticle of a root-bearing axis at the point of the attachment of a root. Note that whereas the cuticle of the root-bearing axis preserves cellular patterns (H), the root consists of thick carbonaceous material. This sharp transition between the cellular pattern of the axis and the lack thereof in the root (arrow) is interpreted as a lack of cuticle in the root. Scale bar = 1 mm (G). Scale bar = 200 µm (H). HPH 330.
Stomata on leafy stems are surrounded by six or seven epidermal cells. The size and shape of these cells are variable, ranging from cells that have the appearance of subsidiary cells to cells that look the same as ordinary epidermal cells (Fig. 6B–D). The rare stomata found on root-bearing axes are surrounded by elongate cells characteristic of the epidermis of these axes (Fig. 6E). Owing to the variability of stem stomata, and to the clear lack of subsidiary cells on root-bearing axes, stomata of the Cottonwood Canyon lycophyte are interpreted as anomocytic.
Branching
Branching of the plant consists exclusively of K-branches. These are produced only on leafy stems; branching has never been observed in the root-bearing axes, despite the large sample analysed. K-branching characterizes several early lycophytes (e.g. Drepanophycus, Zosterophyllum) and consists of two closely spaced dichotomies, the second of which produces two branches (hereafter referred to as ‘arms’) that diverge at wide angles, resulting in a K-shaped morphology (Fig. 7A, B). In the Cottonwood Canyon lycophyte, each K-branch generates a leafy stem and a root-bearing axis (Figs 3 and 7B). All K-branches are vascularized by a thick vascular strand, which diverges from the stele of the main stem at approximately 90° and bifurcates to supply the two arms of the K-branch (Figs 3B and 7).
K-branches were observed in several stages of development. Some occur as lateral buds, which are K-branch primordia. The buds are squat and covered in small leaves (Fig. 7E–G). A central furrow and apical bifurcation of the vascular strand observed in some of the buds indicates the presence of two apical meristems corresponding to early stages of the second dichotomy (Fig. 7E–G). Later developmental stages are represented by K-branches in which only one arm is developed and bears a dormant bud at its base (Fig. 7C, D), and complete K-branches exhibiting full development of both arms (Fig. 7A, B).
In situ preservation and downward growth of root-bearing axes
The Cottonwood Canyon lycophyte is frequently preserved in situ, forming thick layers of plant material. In situ preservation is supported by several observations. First, the lycophyte forms extensive, dense mats of interwoven stems in finely laminated shales devoid of other plant taxa (Fig. 1B). Second, organic detritus indicative of transport is absent from these stem mat layers. Third, the lycophyte shoot systems and attached root-bearing axes are very well preserved. Finally, the stem mat layers recur stratigraphically, alternating with hard-cemented siltstones (Fig. 9A) that contain transported fragments of plant material, fish plates and other organic detritus (Fig. 8A). The stratigraphic recurrence of these stem mat layers, which lack other organic detritus, supports their interpretation as in situ, as it is unlikely that well-sorted plant material was consistently deposited in the same location repeatedly through time.
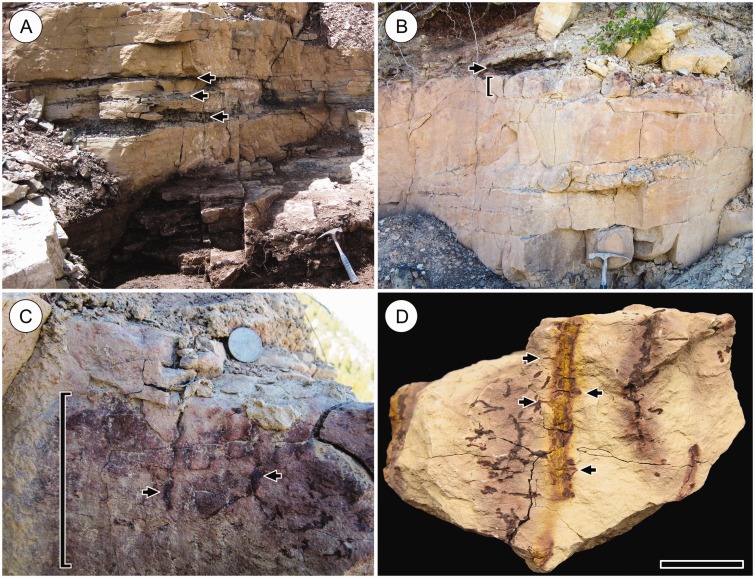
Fig. 9.
Outcrop at Cottonwood Canyon. (A) Stratigraphy of layers containing in situ specimens of the Cottonwood Canyon lycophyte. Darker shale layers containing in situ stem mats (arrows) alternate with lighter, thicker siltstone layers, in which downward-growing root-bearing axes are preserved (see Fig. 8). Hammer head = 17 cm across. (B) Uppermost stem mat layer (arrow) overlying a thick siltstone layer. The approx. 12-cm-thick zone at the top of this siltstone layer (bracket) contains numerous vertical in situ root-bearing axes and exhibits much more pronounced oxidation mottling than the layers below, reflecting pedogenic effects of rooting structures. (C) Detail of rooting zone of siltstone layer in (B) showing exposed oxidized root-bearing axes (arrows) and pronounced oxidation mottling. Quarter dollar coin is shown for scale. Brackets in (B) and (C) indicate the same thickness of rock. (D) Vertical root-bearing axes in rock specimen originating from rooting zone in (C). Note bases of diverging roots (arrows). Scale bar = 20 mm. HPH 310.
Fig. 8.
Vertical in situ root-bearing axes of the Cottonwood Canyon lycophyte; all specimens shown in original stratigraphic orientation. (A) Oblique view of rock specimen showing both vertical face (top) and lower horizontal face (bottom). In situ root-bearing axes preserved on the vertical face (top box) produce roots that diverge onto lower horizontal face (bottom box); upper box detailed in (B) and lower box in (C). Note abundant fragmented organic material on the lower horizontal face of the rock specimen. Scale bar = 10 mm. HPH 210. (B) Vertical face of rock specimen in (A) with two root-bearing axes that cross bedding planes. Note the thin central stele in both axes (arrows) and numerous lateral roots (arrowheads) diverging from the axis at right. Diverging roots are compressed on horizontal planes, but diverge with a downward curvature before becoming horizontal (e.g. at white arrowhead). One root tuft (not visible in this plane) diverges from the axis where it intersects the lower horizontal face of the rock specimen (detailed in C). Scale bar = 10 mm. (C) Horizontal face of rock specimen in (A) showing detail of root tuft connected to the vertical root-bearing axis shown in (B). Note branching of the root, continuity with the vertical axis (asterisk) and thin central stele of root (arrow). Scale bar = 2 mm. (D) Vertical face of rock specimen with three root-bearing axes that cross bedding planes. All three axes are compressed in the vertical plane of the rock face, which reveals their width. Note horizontally compressed roots diverging from the axes (arrowheads). Scale bar = 10 mm. DMNH 29594. (E) Root-bearing axes preserved in oblique growth position, with lateral roots compressed along the same plane. Note central stele of axis in the middle, detailed in (F). Scale bar = 10 mm. HPH 71. (F) Detail of axis and attached root in (E). Note the downward divergence of the root trace preserved in the cortex of the axis (arrow), indicating the direction of growth. Scale bar = 5 mm.
Vertical breaks in the siltstones that alternate with the stem mats reveal in situ root-bearing axes (Fig. 8). These axes are orientated vertically and extend downward from the stem mats at least 12 cm (Fig. 9B, C), crossing bedding planes (Fig. 8B, D). Like the horizontal root-bearing axes, these vertical axes have a thin central stele and lateral, dichotomously branching roots, which are typically compressed on horizontal bedding planes (Figs 8A, C and 4F, G). The arrangement and divergence of lateral roots from the vertical axes are identical to those of the horizontal root-bearing axes preserved on bedding planes.
The direction of growth of the vertical root-bearing axes is revealed by two types of observations on specimens from rock samples with known vertical orientation. First, in horizontal root-bearing axes, decurrent root traces diverge from the axis stele laterally and in the direction of the apex (Fig. 3B). In vertical root-bearing axes, root traces diverge laterally and downwards from the axis stele (Fig. 8F), and therefore these axes were growing downwards. Second, even in cases where the root traces are not visible through the cortex, the roots themselves diverge downwards at wide angles from the subtending axes before becoming horizontal (Fig. 8B), consistent with downward growth of the root-bearing axes. Together, these indicate that the growth trajectory of root-bearing axes was downward.
DISCUSSION
Conspecificity of organs
The conspecificity of specimens used in this study is supported by several lines of evidence. First, the frequent co-occurrence of in situ root-bearing axes and leafy stems in layers devoid of any other taxa provides strong circumstantial evidence for conspecificity. Second, where leafy stems and root-bearing axes co-occur, they exhibit identical preservation, such as comparable degrees of differential preservation of cortex and stele, or comparable degrees of oxidation. These similarities in preservation suggest that both types of organs have similar anatomy and chemistry, and thus support conspecificity. Third, despite being different in size, leafy stems and root-bearing axes do overlap morphologically. Both organs possess a narrow central stele that often has longitudinal striations potentially related to its anatomy. Moreover, some segments of root-bearing axis bear sparse minute leaves similar to those found on bases of K-branches (Figs 3A, 7B, H); these segments probably represent proximal fragments of root-bearing axes. Fourth, unequivocal evidence of conspecificity comes from specimens showing physical attachment of leafy stems and root-bearing axes (Fig. 3).
Homology of the root-bearing axes
Although the root-bearing axes grow downwards, several of their features indicate that they are modified stems and not roots. First, they are parts of K-branches, which arise from lateral buds that are consistently bisected into two identical leafy apices. This morphology indicates that the buds branch apically to produce the two K-branch arms. Thus, the root-bearing axes produced by K-branching arise exogenously, as products of shoot apical branching, and not endogenously like roots. Second, despite being predominantly leafless, root-bearing axes produce leaves early in their development. The axes originate as leafy buds (Fig. 7C–H) and, as a result, bear sparse reduced leaves at their base (Figs 3A and 7B) – no roots have ever been known to produce leaves. Finally, the presence of cuticle and stomata on the epidermis of root-bearing axes (Fig. 6E–H) is consistent with stem homology. Moreover, the thick cuticle comparable to that of the stems suggests that root-bearing axes were not involved in water absorption. Together, all these features are consistent with stem homology of the root-bearing axes, despite their positively gravitropic subterranean growth. This is not surprising, considering that the lycophyte clade includes one other instance of shoot homologues with positively gravitropic subterranean growth – the rhizomorphic clade (Rothwell and Erwin, 1985).
Root recognition in the fossil record – a conundrum
The roots of extant plants are traditionally defined and recognized based on a set of morpho-anatomical criteria: radial symmetry, endogenous origin, positive gravitropism, root hairs and a root cap (Gensel et al., 2001; Raven and Edwards, 2001; Kenrick and Strullu-Derrien, 2014). However, because recognizing these characters requires detailed anatomical and developmental data, some of these criteria are difficult, if not impossible, to apply to fossils (Lyon and Edwards, 1991; Edwards, 1994; Gensel et al., 2001). Further complicating the situation, some early vascular plants have rooting structures, derived from stems or axes, that are not roots (e.g. Zosterophyllum; Hao et al., 2010). As a result, most Early Devonian root-like structures have been treated cautiously, as none of them meets all of the root recognition criteria (e.g. Gensel et al., 2001).
Studies of the root fossil record are thus presented with a problem: on the one hand, criteria are necessary to ensure fossils provide accurate data for studying character evolution; on the other hand, the traditional root recognition criteria are not applicable to most fossils due to their mode of preservation. Consequently, for the purposes of identifying fossil roots, the traditional morpho-anatomical criteria for root recognition are usually impractical. To circumvent this problem, we propose a different approach that we apply to assess the homology of the roots of the Cottonwood Canyon lycophyte, which in the following discussion will be referred to as ‘lateral appendages’. This approach considers two aspects: (1) alternative potential homologies of the lateral appendages, i.e. could these appendages be stems, leaves or a novel organ type; and (2) additional characters that the lateral appendages share with roots.
Did the Cottonwood Canyon lycophyte have roots?
Stem homology of the lateral appendages of root-bearing axes is rejected because they do not bear leaves and are different from both types of stems produced by this plant (leafy stems and root-bearing axes) in size and mode of branching. Several characters of the lateral appendages are also inconsistent with leaf homology: irregular arrangement along the root-bearing axes, indeterminate growth and irregular branching. While appendages homologous to leaves and displaying indeterminate growth and irregular branching are known in the lycophyte clade (rhizomorphic lycopsids; Karrfalt, 1980; Rothwell and Erwin, 1985), those appendages have regular arrangement and undergo abscission, unlike the lateral appendages of the Cottonwood Canyon lycophyte.
If the lateral appendages are not stems or leaves, the only other alternatives are that they represent roots or a novel type of organ. The lateral appendages do share several features with roots. First, they are exclusively subterranean organs whose morphology is consistent with absorptive functions. Second, the lateral appendages lack a cuticle. Film pulls of root-bearing axes and their lateral appendages show that the cuticle, well preserved along the axis, ends abruptly at the base of the lateral appendage (Fig. 6G). This indicates the appendages had very thin cuticle, if any, which is a common feature of roots that are actively involved in water uptake (Esau, 1977). Third, some specimens have darkened apices, which may represent preserved root caps (Fig. 4A–C). Finally, these lateral appendages exhibit the same apical branching architecture that characterizes the roots of modern lycophytes such as Lycopodium and Selaginella (Eames, 1936).
Our approach demonstrates that although they do not fit traditional criteria for root recognition, the lateral appendages of the Cottonwood Canyon lycophyte are most similar to roots. However, despite sharing a set of developmental and functional traits by which they can be recognized, roots are not homologous across tracheophytes. Consequently, any discussion of morphological evolution that the appendages of the Cottonwood Canyon lycophyte can inform should be framed within the context of lycophyte root evolution. In this context, the appendages of the Cottonwood Canyon lycophyte are most similar to the roots of Lycopodium and Selaginella, which renders their alternative interpretation as a novel organ less parsimonious.
The rooting system – functional morphology
The Cottonwood Canyon lycophyte possesses a distinctively complex rooting system that consists of two types of organs – root-bearing axes and roots – that perform different functions (Fig. 10). The root-bearing axes have variable orientations, consistent with growth responses to external stimuli – in situ root-bearing axes are found growing downwards into the substrate as well as horizontally, both on the ground surface and in the substrate. The roots, by contrast, extend laterally from subtending axes up to only a couple of centimetres. They are delicate and probably, alone, insufficient for anchoring the plant, especially given the large size of the above-ground stems. Moreover, owing to their small size they were only capable of exploring small volumes of substrate. In contrast, the root-bearing axes are extensive and robust enough to anchor the plant and produce roots at various depths in the substrate, thus allowing access to larger volumes of underground resources. Together, these features indicate that in this plant the root-bearing axes, and not the roots, functioned as the primary organ responsible for anchorage and substrate exploration. The roots borne on these axes, while contributing minimally to anchorage, were the main absorptive organ.
Fig. 10.
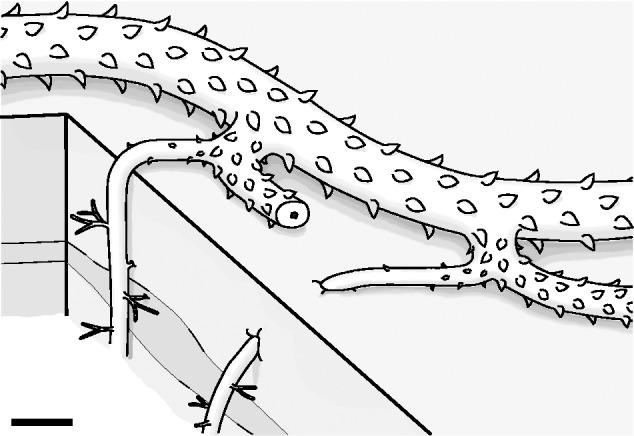
Cottonwood Canyon lycophyte – reconstruction of the rooting system. Two K-branches diverge from a leafy stem, each of which gives rise to a new leafy stem and a root-bearing axis. Root-bearing axes bear reduced leaves along the base and grow into the substrate. Growth orientations differ between the two root-bearing axes illustrated, consistent with orientations observed in in situ specimens. Lateral roots diverge horizontally from root-bearing axes and exhibit variable arrangement along the axes. Scale bar = 20 mm.
The degree of functional complexity of this rooting system provides direct evidence that by Pragian time, early vascular plants had evolved mechanisms to actively explore their substrate utilizing complex foraging behaviours. In the outcrop at Cottonwood Canyon we observe multiple layers of in situ plant populations, represented by stem mats, which produced rooting systems that penetrate and are preserved in the underlying layers (Fig. 9A, B). The highest densities of rooting structures are seen below the uppermost plant population observed, where numerous in situ root-bearing axes are exposed in the rock profile (Fig. 9B–D).
The effects on rock texture and structure associated with pedogenic-type interactions of these rooting strucures are not immediately apparent in fresh exposures of the layers containing the in situ root-bearing axes. However, weathered profiles reveal conspicouous differences between those layers and the sediment below the depth of root penetration (Fig. 9B–D). Together with the stratigraphic recurrence (Fig. 9) and density of the rooting structures, this demonstrates that substantial plant–substrate interactions were under way by Early Devonian time.
Root gravitropism in lycophytes
The root tufts of the Cottonwood Canyon lycophyte are consistently found spreading along horizontal planes from subtending in situ root-bearing axes, whether the latter are horizontal or vertical. This indicates that the roots may have lacked a positive gravitropic response, suggesting that in this plant root identity was developmentally uncoupled from positive gravitropism. Instead, direct evidence for the presence of a gravitropic response is provided by the downward growth documented in root-bearing axes. Consistent recurrence of downward orientated in situ axes bearing horizontally spreading roots in successive plant populations demonstrates that these growth patterns represent constitutive structural traits and not opportunistic responses of a flexible developmental programme.
Furthermore, the origin of these axes as branches of a shoot system suggests that in lycophytes the positive gravitropic response mechanism evolved first in specialized stems and not in roots. Bolstering this idea, in some Zosterophyllum species that lack roots altogether, branching produces axes with inferred downward growth (Walton, 1964; Gensel et al., 2001; Hao et al., 2007, 2010), consistent with positive gravitropism. This raises the possibility that positive gravitropism was not among the signal transduction programmes of the earliest lycophyte roots and that it was co-opted as a root developmental pathway later, within this clade.
K-branching and lycophyte root evolution
Detailed understanding of lycophyte root evolution is lacking at present. The early occurrence of lycophyte rooting structures in the fossil record and the overall diversity of rooting structures within the clade have inspired scenarios of root evolution. One hypothesis that has been put forth is that lycophyte roots evolved from K-branches in which one of the arms developed as a rooting organ and eventually became a root (Gensel and Berry, 2001; Gensel et al., 2001). The morphology of the Cottonwood Canyon lycophyte challenges this hypothesis and suggests an alternative hypothesis for the origin of roots among lycophytes. In this plant, K-branching does not produce roots and instead produces a modified stem (the root-bearing axis) that anchors the plant. It is this modified stem that produces lateral roots, which have no direct developmental relationship with K-branching. Consequently, the roots of the Cottonwood Canyon lycophyte show neither serial nor positional homology with the products of K-branching: roots are not borne on stems in the position of K-branches, nor are they produced directly by K-branching in the postion of root-bearing axes, and there are no other appendages on the root-bearing axes to which the root tufts could be homologous. These morphological relationships illustrated by the Cottonwood Canyon lycophyte indicate that in this plant, and possibly in lycophytes in general, roots are not derived from K-branches. This suggests strongly that lycophyte roots may have arisen as a novel organ type.
Early lycophyte rooting systems and the oldest roots
By the end of the Devonian, plant rooting systems were extensive, morphologically complex and taxonomically diverse, as documented in progymnosperms (Archaeopteris), arborescent lycopsids (Cyclostigma, Protostigmaria, Leptophloeum, Otzinachsonia) and cladoxylopsids (Eospermatopteris, Lorophyton, Metacladophyton tetraxylum, Calamophyton) (Jennings et al., 1983; Fairon-Demaret and Li, 1993; Stewart and Rothwell, 1993; Weng and Geng, 1997; Algeo and Scheckler, 1998; Cressler and Pfefferkorn, 2005; Giesen and Berry, 2013; Prestianni and Gess, 2014). However, comparatively little is known about their Early Devonian precursors, and in many cases either these early rooting systems or the plants that produced them are poorly understood.
Lycophyte rooting systems appear much earlier in the fossil record than those of euphyllophytes, for which the earliest are known from the Middle Devonian (Calamophyton; Giesen and Berry, 2013). The earliest lycophyte rooting systems are seen in Lochkovian–Pragian Zosterophyllum, whose K-branches produce axes with inferred downward growth (Hao et al., 2010). The rooting structures of Early Devonian lycophytes span a broad range of morphologies derived from either (1) branches formed by K-branching or (2) structures comparable to lateral adventitious roots (e.g. Edwards, 1994; Raven and Edwards, 2001; Gensel et al., 2001; Kenrick, 2002). Rooting structures derived from K-branching are produced on the simple axes of Zosterophyllum (Walton, 1964; Hao et al., 2007, 2010), Bathurstia (Kotyk and Basinger, 2000; Gensel et al., 2001) and Sawdonia (Rayner, 1983). Leafless axes interpreted as rooting organs are also produced by K-branching of stems of Drepanophycus spinaeformis (Gensel et al., 2001) and, possibly, D. devonicus (Schweitzer and Giesen, 1980) and the Middle Devonian D. minor (Xu et al., 2013). Although such structures derived from K-branching may have functioned as rooting organs, they do not represent roots because they are branches of simple axes or stems, produced by apical dichotomy.
Lateral organs that, in our opinion, more closely resemble adventitious roots have been documented from several taxa. These organs occur as smooth or sinuous lateral axes seen in Crenaticaulis, Bathurstia and Drepanophycus (Gensel et al., 2001), or tufts of fine branching axes documented in Drepanophycus (Schweitzer and Giesen, 1980; Li and Edwards, 1995; Gensel et al., 2001), Taeniocrada (Schweitzer, 1980), Hsua (Li, 1992) and Crenaticaulis (Gensel et al., 2001). Asteroxylon mackiei also produced leafless rhizomatous axes and fine root-like structures with inferred positive gravitropism (Kidston and Lang, 1920, 1921). The oldest of these Early Devonian structures are known from the Pragian Bathurstia and Drepanophycus, reported from Bathurst Island by Kotyk and Basinger (2000) and Gensel et al. (2001). Although these Pragian records may represent roots, they have been referred to tentatively as rooting structures due to the absence of diagnostic characters such as endogenous origin and a root cap (e.g. Gensel et al., 2001).
With the exception of Zosterophyllum shengfengense (Hao et al., 2010), which preserves above-ground axes and attached rooting systems, the overall architecture of the early rooting systems discussed above is poorly understood. In many of these plants, rooting structures are seen only on rare specimens and are not preserved in situ. Conversely, the extensive Emsian rooting system documented by Elick et al. (1998) is one of few Early Devonian rooting systems preserved in growth position, but it cannot be attributed unequivocally to a known taxon. Thus, for these early rooting systems very limited data are available on their overall structure, morphological relationships with the rest of the plant or interactions with the substrate.
If we consider the fossil record of early rooting structures, the Cottonwood Canyon lycophyte stands out as a rare instance in which the above-ground shoot system and the underground rooting system are preserved together in growth position, and for which the structural homology of the rooting system is well resolved. The Cottonwood Canyon lycophyte is coeval with, if not older than, plants with root-like organs documented from Bathurst Island, and thus provides the earliest compelling evidence for roots. This corroborates evidence from other fossil localities, demonstrating that both roots and complex rooting systems had evolved in lycophytes by Pragian time. The morphological relationships between stems, root-bearing axes and roots of the Cottonwood Canyon lycophyte challenge the hypothesis that lycophyte roots evolved from branches of the above-ground axial system, suggesting instead that they arose as a novel organ. Details of the rooting system of the Cottonwood Canyon lycophyte provide a foundation for developing a comprehensive organismal concept for this plant, which will be essential for its inclusion in studies addressing plant phylogeny and morphological evolution. In more general terms, the data presented here offer insights into the structure of early lycophyte rooting systems and illustrate the kind of complex plant–substrate interactions that, in time, led to global changes by influencing soil weathering processes and nutrient cycling (Algeo and Scheckler, 1998; Berner and Kothavala, 2001; Bergman et al., 2004; Taylor et al., 2009).
ACKNOWLEDGEMENTS
We are indebted to Brent Breithaupt, US Bureau of Land Management Regional Paleontologist, for providing permits to collect at Cottonwood Canyon. We thank William DiMichele, Carol Hotton, Nathan Jud and Jonathan Wingerath (National Museum of Natural History – Smithsonian Institution); Thomas N. Taylor, Edith L. Taylor and Rudolph Serbet (University of Kansas); Kirk Johnson and Ian Miller (Denver Museum of Nature and Science); Ian Glasspool and Patrick Herendeen (Field Museum of Natural History) for access to collections and specimen loans. We also thank Christopher Steenbock, Joseph Caruso, Richard Tate, James Cornwell, Glenn Shelton, Allison Bronson, Ashley Ortiz, Hannah Barrett-Watson, Jeffery Barrett and Rachel Klassen for assistance in the field and lab. Peter Holterhoff provided assistance in the field and helpful information on stratigraphy and depositional environments. Comments from Gar Rothwell, Michael Mesler, Terry Henkel and three anonymous reviewers greatly improved the manuscript. This work was supported by graduate student research awards from the Botanical Society of America, Geological Society of America, Paleontological Society (James M. and Thomas J. M. Schopf Award), and Humboldt State University Department of Biological Sciences to K.K.S.M., and by grants from the Humboldt State University Office of Research and Graduate Studies, Humboldt State University Sponsored Programs Foundation, and the American Philosophical Society to A.M.F.T.
LITERATURE CITED
- Algeo TJ, Scheckler SE. 1998. Terrestrial–marine teleconnections in the Devonian: links between the evolution of land plants, weathering processes, and marine anoxic events. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 353: 113–130. [Google Scholar]
- Bergman NM, Lenton TM, Watson AJ. 2004. COPSE: a new model of biogeochemical cycling over Phanerozoic time. American Journal of Science 304: 397–437. [Google Scholar]
- Berner RA, Kothavala Z. 2001. GEOCARB III; a revised model of atmospheric CO2 over Phanerozoic time. American Journal of Science 301: 182–204. [Google Scholar]
- Boyce CK. 2005. The evolutionary history of roots and leaves. In: Holbrook NM, Zwieniecki MA, eds. Vascular transport in plants. Amsterdam: Elsevier, 479–499. [Google Scholar]
- Caruso JA, Tomescu AMF. 2012. Microconchid encrusters colonizing land plants: the earliest North American record from the Early Devonian of Wyoming, USA. Lethaia 45: 490–494. [Google Scholar]
- Cressler WL, III, Pfefferkorn HW. 2005. A Late Devonian isoetalean lycopsid, Otzinachsonia beerboweri, gen. et sp. nov., from north-central Pennsylvania, USA. American Journal of Botany 92: 1131–1140. [DOI] [PubMed] [Google Scholar]
- Denison RH. 1956. A review of the habitat of the earliest vertebrates. Fieldiana Geology 11: 357–457. [Google Scholar]
- Dorf E. 1933. A new occurrence of the oldest known terrestrial vegetation, from Beartooth Butte, Wyoming. Botanical Gazette 95: 240–257. [Google Scholar]
- Dorf E. 1934. Stratigraphy and paleontology of a new Devonian formation at Beartooth Butte, Wyoming. Journal of Geology 42: 720–737. [Google Scholar]
- Eames AJ. 1936. Morphology of vascular plants. Lower groups (Psilophytales to Filicales). New York: McGraw-Hill. [Google Scholar]
- Edwards D. 1994. Toward an understanding of pattern and process in the growth of early vascular plants. In: Ingram DS, Hudson A, eds. Shape and form in plants and fungi. London: Academic Press/Linnean Society of London, 39–59. [Google Scholar]
- Elick JM, Driese SG, Mora CI. 1998. Very large plant and root traces from the Early to Middle Devonian: implications for early terrestrial ecosystems and atmospheric p(CO2). Geology 26: 143–146. [Google Scholar]
- Elliot DK, Johnson HG. 1997. Use of vertebrates to solve biostratigraphic problems: examples from the Lower and Middle Devonian of Western North America. In: Klapper G, Murphy MA, Talent TJ, eds. Paleozoic sequence stratigraphy, biostratigraphy, and biogeography: studies in honor of J. Granville Johnson. Geological Society of America Special Paper; 321: 179–188. [Google Scholar]
- Esau K. 1977. Anatomy of seed plants, 4th edn New York: John Wiley & Sons. [Google Scholar]
- Fairon-Demaret M, Li C-S. 1993. Lorophyton goense gen. et sp. nov. from the Lower Givetian of Belgium and a discussion of the Middle Devonian Cladoxylopsida. Review of Palaeobotany and Palynology 77: 1–22. [Google Scholar]
- Fiorillo AR. 2000. The ancient environment of the Beartooth Butte Formation (Devonian) in Wyoming and Montana: combining paleontological inquiry with federal management needs. USDA Forest Service Proceedings RMRS-P-15 3: 160–167. [Google Scholar]
- Friedman WE, Moore RC, Purugganan MD. 2004. The evolution of plant development. American Journal of Botany 91: 1726–1741. [DOI] [PubMed] [Google Scholar]
- Gensel PG, Berry CM. 2001. Early lycophyte evolution. American Fern Journal 91: 74–98. [Google Scholar]
- Gensel PG, Kotyk ME, Basinger JF. 2001. Morphology of above- and below-ground structures in Early Devonian (Pragian-Emsian) plants. In: Gensel PG, Edwards D, eds. Plants invade the land. New York: Columbia University Press, 83–102. [Google Scholar]
- Giesen P, Berry CM. 2013. Reconstruction and growth of the early tree Calamophyton (Pseudosporochnales, Cladoxylopsida) based on exceptionally complete specimens from Lindlar, Germany (Mid-Devonian): organic connection of Calamophyton branches and Duisbergia trunks. International Journal of Plant Sciences 174: 665–686. [Google Scholar]
- Gifford EM, Foster AS. 1989. Morphology and evolution of vascular plants, 3rd edn New York: W.H. Freeman. [Google Scholar]
- Hao S, Xue J, Liu Z, Wang D. 2007. Zosterophyllum Penhallow around the Silurian-Devonian boundary of northeastern Yunnan, China. International Journal of Plant Sciences 168: 447–489. [Google Scholar]
- Hao SG, Xue JZ, Guo DL, Wang DM. 2010. Earliest rooting system and root : shoot ratio from a new Zosterophyllum plant. New Phytologist 185: 217–225. [DOI] [PubMed] [Google Scholar]
- Jennings JR, Karrfalt EE, Rothwell GW. 1983. Structure and affinities of Protostigmaria eggertiana. American Journal of Botany 70: 963–974. [Google Scholar]
- Karrfalt EE. 1980. A further comparison of Isoetes roots and stigmarian appendages. Canadian Journal of Botany 58: 2318–2322. [Google Scholar]
- Kenrick P. 2002. The origin of roots. In: Waisel Y, Eshel A, Kafkafi U, eds. Plant roots. The hidden half. New York: Marcel Dekker, 1–13. [Google Scholar]
- Kenrick P, Crane PR. 1997. The origin and early diversification of land plants. Washington, DC: Smithsonian Institution Press. [Google Scholar]
- Kenrick P, Strullu-Derrien C. 2014. The origin and early evolution of roots. Plant Physiology 166: 570–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidston R, Lang WH. 1920. On Old Red Sandstone plants showing structure, from the Rhynie Chert Bed, Aberdeenshire. Part III. Asteroxylon mackiei, Kidston and Lang. Transactions of the Royal Society of Edinburgh 52: 643–680. [Google Scholar]
- Kidston R, Lang WH. 1921. On Old Red Sandstone plants showing structure, from the Rhynie Chert Bed, Aberdeenshire. Part IV. Restorations of the vascular cryptogams, and discussion of their bearing on the general morphology of the Pteridophyta and the origin of the organisation of land-plants. Transactions of the Royal Society of Edinburgh 52: 831–854. [Google Scholar]
- Kotyk ME, Basinger JF. 2000. The Early Devonian (Pragian) zosterophyll Bathurstia denticulata Hueber. Canadian Journal of Botany 78: 193–207. [Google Scholar]
- Lamsdell JC, Legg DA. 2010. An isolated pterygotid ramus (Chelicerata: Eurypterida) from the Devonian Beartooth Butte Formation, Wyoming. Journal of Paleontology 84: 1206–1208. [Google Scholar]
- Lamsdell JC, Selden PA. 2013. Babes in the wood – a unique window into sea scorpion ontogeny. BMC Evolutionary Biology 13: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-S. 1992. Hsua robusta, an Early Devonian plant from Yunnan Province, China and its bearing on some structures of early land plants. Review of Palaeobotany and Palynology 71: 121–147. [Google Scholar]
- Li C-S, Edwards D. 1995. A re-investigation of Halle’s Drepanophycus spinaeformis Goepp. from the Lower Devonian of Yunnan Province, southern China. Botanical Journal of the Linnean Society 118: 163–192. [Google Scholar]
- Lyon AG, Edwards D. 1991. The first zosterophyll from the Lower Devonian Rhynie Chert, Aberdeenshire. Transactions of the Royal Society of Edinburgh: Earth Sciences 82: 323–332. [Google Scholar]
- Poschmann M, Gossmann R. 2014. Wurzelstrukturen früher Gefäßpflanzen aus der Klerf-Formation (Unterdevon, höchstes Unter-Emsium) von Waxweiler (SW-Eifel, Rheinland-Pfalz, SW-Deutschland). Mainzer Naturwissenschaftliche Archiv 51: 11–22. [Google Scholar]
- Prestianni C, Gess RW. 2014. The rooting system of Leptophloeum Dawson: new material from the Upper Devonian, Famennian Witpoort Formation of South Africa. Review of Palaeobotany and Palynology 209: 35–40. [Google Scholar]
- Raven JA, Edwards D. 2001. Roots: evolutionary origins and biogeochemical significance. Journal of Experimental Botany 52: 381–401. [DOI] [PubMed] [Google Scholar]
- Rayner RJ. 1983. New observations on Sawdonia ornata from Scotland. Transactions of the Royal Society of Edinburgh: Earth Sciences 74: 79–93. [Google Scholar]
- Rothwell GW, Erwin DM. 1985. The rhizomorph apex of Paurodendron: implications for homologies among the rooting organs of Lycopsida. American Journal of Botany 72: 86–98. [Google Scholar]
- Rothwell GW, Wyatt SE, Tomescu AMF. 2014. Plant evolution at the interface of paleontology and developmental biology: an organism-centered paradigm. American Journal of Botany 101: 899–913. [DOI] [PubMed] [Google Scholar]
- Sandberg CA. 1961. Widespread Beartooth Butte Formation of Early Devonian age in Montana and Wyoming and its paleogeographic significance. Bulletin of the American Association of Petroleum Geologists 45: 1301–1309. [Google Scholar]
- Sandberg CA. 1967. Measured sections of Devonian rocks in northern Wyoming. Geological Survey of Wyoming Bulletin 52. Laramie, WY: University of Wyoming. [Google Scholar]
- Schultes RE, Dorf E. 1938. A sphenopsid from the Lower Devonian of Wyoming. Harvard University Botanical Museum Leaflets 7: 21–34. [Google Scholar]
- Schweitzer H-J. 1980. Die Gattungen Taeniocrada White und Sciadophyton Steinmann im Unterdevon des Rheinlandes. Bonner Palaobotanische Mitteilungen 5: 1–38. [Google Scholar]
- Schweitzer H-J, Giesen P. 1980. Uber Taeniophyton inopinatum, Protolycopodites devonicus und Cladoxylon scoparium aus dem Mitteldevon von Wuppertal. Palaeontographica B 173: 1–25. [Google Scholar]
- Seago JL, Jr, Fernando DD. 2013. Anatomical aspects of angiosperm root evolution. Annals of Botany 112: 223–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart WN, Rothwell GW. 1993. Paleobotany and the evolution of plants. Cambridge: Cambridge University Press. [Google Scholar]
- Tanner W. 1983. A fossil flora from the Beartooth Butte Formation of Wyoming. PhD thesis, Southern Illinois University, Carbondale. [Google Scholar]
- Taylor LL, Leake JR, Quirk J, Hardy K, Banwart SA, Beerling DJ. 2009. Biological weathering and the long-term carbon cycle: integrating mycorrhizal evolution and function into the current paradigm. Geobiology 7: 171–191. [DOI] [PubMed] [Google Scholar]
- Tetlie OE. 2007. Like father, like son? Not amongst the eurypterids (Chelicerata) from Beartooth Butte, Wyoming. Journal of Paleontology 81: 1423–1431. [Google Scholar]
- Walton J. 1964. On the morphology of Zosterophyllum and some other Early Devonian plants. Phytomorphology 14: 155–160. [Google Scholar]
- Weng Z, Geng B-Y. 1997. A new Middle Devonian plant: Metacladophyton tetraxylum gen. et sp. nov. Palaeontographica Abteilung B 243: 85–102. [Google Scholar]
- Xu H-H, Feng J, Jiang Q, Wang Y. 2013. Report of Drepanophycus Göppert (Lycopsida) from the Middle Devonian of Xinjiang, China. Journal of Systematics and Evolution 51: 765–772. [Google Scholar]