Abstract
Background:
Primary axillary hyperhidrosis (PAH) is a chronic idiopathic disorder causing major stress in patients. Among the common therapies for PAH, only surgical interventions have proven feasible as a permanent solution.
Objective and Aim:
The aim of this study was to evaluate the efficacy and safety of fractional microneedle radiofrequency (FMR) as an alternative permanent treatment for PAH with long-term follow-up.
Materials and Methods:
This was a single-blind, sham-controlled comparative study. Twenty-five patients with severe PAH were provided three treatments of FMR at 3-week intervals (the treatment group), and a control group was provided the sham treatment. Clinical efficacy was evaluated using the hyperhidrosis disease severity scale (HDSS) at baseline and the end of the study, as well as during the 1 year follow-up phase.
Results:
HDSS demonstrated significant improvement after treatment in the treatment group compared to the sham control. The mean (±standard deviation) of HDSS in the group being treated with radiofrequency was 2.50 (±0.88) after 1 year follow-up, and that of the control group was 3.38 (±0.49; P < 0.001). Follow-up results show that there were 10 patients (41.6%) with no relapse and 11 patients (45.9%) with relapse after 1 year. There was a significant correlation between HDSS changes in relapse and body mass index (BMI) (P = 0.03).
Conclusion:
Treatment of PAH with FMR is a safe and noninvasive procedure with a positive therapeutic effect on HDSS. It is recommended, however, that sessions of FMR be repeated after 1 year, particularly in overweight patients with high BMIs.
Clinical Trial Registration:
IRCT2013111915455N1.
Level of Evidences:
Level II-1.
Keywords: Fractionated microneedle radiofrequency, hyperhidrosis disease severity scale, primary axillary hyperhidrosis
What was known?
Primary axillary hyperhidrosis is a chronic idiopathic disorder characterized by uncontrollable excessive sweating without a recognizable cause.
Introduction
Primary axillary hyperhidrosis (PAH) is a chronic idiopathic disorder characterized by uncontrollable excessive sweating without a recognizable cause. Profuse sweating that accompanies PAH can result in skin maceration and secondary microbial infections.[1,2] Treatment options for PAH include topical aluminum salts, systemic anticholinergics, various laser devices, and surgical interventions. The main limitations of the above-mentioned options are financial constraints, and transient results, such as those are observed with botulinum toxin therapy.[2] In recent years, despite the potential risks, including scarring, burning and symptom recurrence, surgical interventions present the most effective options.[3,4] A handful of studies evaluating radiofrequency (RF) energy in the treatment of PAH have suggested that newer bipolar RF devices are effective in reducing the amount of sweating.[5,6] The combination of RF and fractional microneedle technology, termed fractional microneedle radiofrequency (FMR), creates an effective method with a better safety profile for treating various dermatologic conditions.[7] Several variables, including temperature and humidity, seasonal conditions, and physiological and psychological conditions can affect the accurate assessment of patient sweating.[8] Axillary hyperhidrosis represents a good model to perform placebo-controlled comparative studies, in which each patient acts as his/her own control, to clinically evaluate novel treatments for this disease. The aim of this study was to evaluate the efficacy and safety of FMR as an alternative, permanent treatment for PAH with long-term follow-up.
Materials and Methods
Subjects
This was a single-blind, right-left comparative study in which each patient acted as his/her own control. This study included 25 subjects with PAH, defined as having a score of 3 or 4 on a hyperhidrosis disease severity scale (HDSS). The exclusion criteria were as follows a history of surgery for PAH, history of botulinum toxin therapy within the previous 6 months, history of pacemaker implantation, active infection in the treatment area, the tendency for keloid formation, pregnancy or intention to become pregnant, or breastfeeding. To be included in the study, all patients had to have been unresponsive to any conventional treatments. The Ethics Committee of Isfahan University of Medical Sciences approved this study and written informed consent was obtained from all patients. The Clinical Trial Registration was IRCT2013111915455N1.
Treatment protocol
Three sessions of FMR treatment carried by using a novel applicator (INFINI TM; Lutronic, Goyang, Korea) at 3-week intervals of one side; the other side functioned as a sham control when the device was on standby mode. The applicator of the INFINI consists of rows of 49 insulated microneedles over an area of 10 mm that sent 1 MHz of RF current in a fractional manner that extends below the surface of the skin. The starch iodine test was used to outline the area of excessive sweating. For this procedure, iodine solution was applied to a dry surface on the skin and then corn starch was sprinkled over the area. The patients were asked to sit for 15 min in a room at 23°C. Starch and iodine interact in the presence of sweat leaving blue-black sediment. After marking the hyperhidrotic area accompanied starch-iodine test, all subjects were pretreated by a topical eutectic mixture of 2.5% lidocaine hydrochloric acid and 2.5% prilocaine (EMLA; Astra-Zeneca, Sodertalje, Sweden) under occlusion 45 min prior to FMR treatment to induce anesthesia. The skin was cleansed with alcohol and allowed to dry completely. The target areas were treated with a total of three passes. The treatment included the following parameters: (1) A depth of 2–3 mm; (2) a time range of 120–180 ms, (3) an energy level of 6–10, with a small range (±10%) of energy and time settings determining the optimal parameters for the population being treated. Ice packs were applied during treatment and for 10 min after FMR therapy to reduce heat damage to the epidermis. At the end of treatment, the treated area was covered with sterile vaseline gauze. The use of topical zinc oxide several times daily for a few days after each treatment session was recommended to promote local healing. All patients were subjected at baseline and posttreatment after 3, 6, and 9 weeks, as well as 3 months.
Outcome assessments
Photographic documentation of starch-iodine test (Canon power shot G12, Cannon components Inc., Japan) for each patient before and after the course of treatment was done to compare and evaluate the clinical response by means of the area of discoloration. HDSS assessments were performed for the right and left axillae to measure the disease severity of PAH on every visit and 1 year after the last treatment.[4] Table 1 provides the definition of each of the scores [Table 1].
Table 1.
Hyperhidrosis disease severity scale and their clinical definitions

The primary overall efficacy measure was the percentage of subjects whose HDSS scores were reduced from 3 or 4 at baseline to 1 or 2 on follow-up visits. Responders were defined as subjects reporting an HDSS score of 1 or 2. In addition, the relapse condition was evaluated after 1 year and correlations between the HDSS changes and some demographic parameters were determined.
To evaluate side effects, in addition to close dermatological examinations, patients were instructed to report experiencing any side effects including pruritus, scaling, crusting, compensatory hyperhidrosis and postinflammatory pigmentation, pain in the area of the FMR, erythema, swelling, reduction in muscular force in the hand, dysesthesia and/or paraesthesia in the arm and forearm etc. Side effects were categorized from Grade 1 (minor) to Grade 3 (severe).
Statistical analysis
SPSS V.22 (SPSS Inc., II, USA) was used for data analysis and the mean and standard deviation were used to describe the descriptive statistics. A general linear model with repeated measures was used to determine the clinical efficacy of the procedure. Statistical significance was defined as P < 0.05.
Results
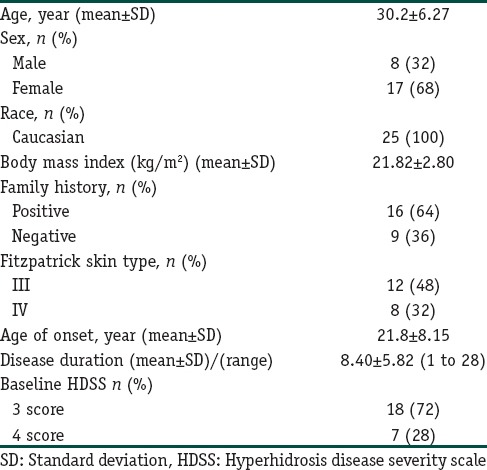
The demographic information and baseline sweat assessment values are summarized in Table 2.
Table 2.
The demographic information of patients
Twenty-four of the 25 enrolled subjects completed the study. One patient declined further treatment because of dysesthesia development on the arm. General linear model analysis showed that with the intervention of meaningful treatment (P < 0.001), the HDSS mean decreased in the treatment group compared to the control [Figure 1].
Figure 1.
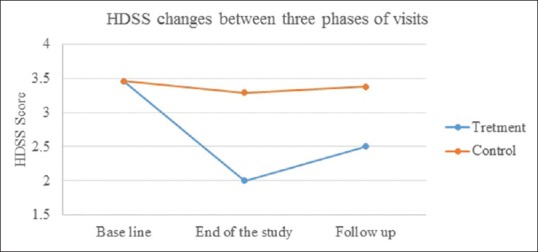
Hyperhidrosis disease severity scale comparison between two groups in three phases of visits
Comparisons of the HDSS at baseline, end of the study, and follow-up phase in the FMR-treated group demonstrated a significant response at the end of treatment (P < 0.001) and that the HDSS decreased significantly in comparison with that at the end of the study visit and follow-up visit (P < 0.001). In the control group, the difference between the 3 times remained unchanged until the last follow-up session [Table 3].
Table 3.
The mean±SD of hyperhidrosis disease severity scale changes between three phases of visits

The follow-up results show that there were 10 patients (41.6%) with no relapse after 1 year, 11 patients (45.9%) with relapse, and 3 patients (12.5%) with no treatment response. There were no significant correlations between HDSS changes and sex (P = 0.649), age (P = 0.255), age at onset (P = 0.816), and duration of disease (P = 0.926) but there was a significant correlation between HDSS change and body mass index (BMI) (P = 0.033).
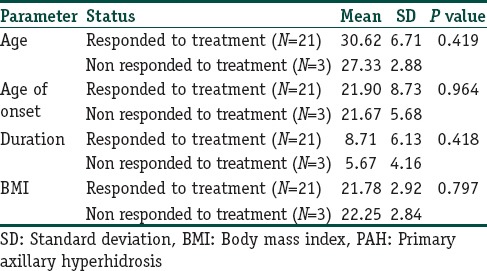
In patients who were nonresponder to the treatment, there were not any significant differences between ages (P = 0.419), age of onset (P = 0.964), duration of disease (P = 0.0418), BMI (P = 0.797), and family history (P = 0.266) [Table 4].
Table 4.
The relation between ages, age of onset, duration of disease, BMI and respond to the treatment in patients with PAH
No major permanent adverse effects, such as scarring or ulceration, were experienced with the FMR treatment. In most patients, temporary side effects (e.g., swelling, pain, and redness) were observed in the affected areas. The reported Grade 1 events showed that a large number of subjects experienced mild procedure effects that typically lasted a few days to a week. The most common side effects were erythema (68% of the subjects) and pin point bleeding (56% of the subjects). One subject experienced transient tingling and numbness of the left arm after the procedure; the prognosis from the consulting neurologist was good, and the subject showed spontaneous improvement 2 months after treatment. No major permanent adverse effects were reported at long-term follow-up.
Discussion
The results of our recent study showed the efficacy and safety of FMR for the treatment of PAH in short-period.[7,9,10,11] The clinical findings of this study at long-term follow-up suggest that FMR is an effective and safe option to reduce sweating in PAH patients.
A study by Solish et al. that used botulinum toxin for the treatment of PAH showed that at 4 and 12 weeks after treatment, the percentage of patients reaching an HDSS score of 1 or 2 was 85% and 90%, respectively.[6] Similarly, our results have demonstrated a significant decrease in HDSS in the treatment group compared to the sham control at the end of the follow-up visits. Although both interventions had similar efficacy, botulinum toxin injections are considered temporary, lasting only 3–6 months.[12]
Novel bipolar RF devices can destroy eccrine glands by a process of thermolysis at the interface of the deep dermis and subcutis while minimizing damage to the surrounding tissue.[9] The results demonstrate that microwave technology is well suited for targeting sweat glands while allowing for the protection of both upper skin layers and the structures beneath the subcutaneous fat.[13]
Hong et al. reported that microwave devices had long-term effects (12 months) on the treatment of PAH. In their study of 31 adults with PAH, at the 12-month follow-up visit, 90.3% had HDSS scores of 1 or 2 and 90.3% had a minimum 50% reduction in axillary sweat from baseline.[14]
In a large, multicenter, randomized, sham-controlled clinical trial by Glaser et al., 120 adult subjects were randomized to either a treatment group or a sham group. Patients were treated in two sessions spaced 2 weeks apart, with the primary efficacy measure as the percentage that had their HDSS scores reduced to 1 or 2. The results for the treatment group were as follows: 89% at 30-days posttreatment, 67% at 6-months, and 69% at 12-months. In the sham group, a significant difference was observed at 30-days and 6 months.[15] Similarly, in their study using a microwave device on Asian subjects, Lee et al. showed a 2-point drop or greater in HDSS in 83.3% of subjects (10/12 axillae) as measured at the 7-month follow-up.[16]
Our results suggest that HDSS scores increased in some cases after a 1 year follow-up, which indicates that repeated sessions of FMR therapy may be warranted. In addition, the significant correlations between HDSS score and BMI indicate that dermatologists should be aware of the potential for reduced effectiveness of FMR in patients with more amount of subcutaneous adiposity. The probable mechanism for this phenomenon is that, in these patients, the subcutaneous adipose hindered the delivery of heat to the sweat glands, which were not completely destroyed. Hence, repeated sessions are necessary to achieve successful treatment in overweight individuals.
In this study, 3 patients did not respond to the treatment. There were not any significant differences between ages, the age of onset, duration of disease, BMI, and family history. This phenomenon is probability related to genetic background and amount of subcutaneous adiposity in axilla area which lead to insufficient delivery of FMR. Further studies are needed for more information in this regard. Our results showed that the BMI is a significant factor in the durability of treatment. Hence, it is necessary to consider the BMI factor in this treatment method.
Among the side effects analyzed in this study, posttreatment inflammation was the notable problem; however, it spontaneously resolved within 2 months. All of the side effects, including pain and pruritus, however, frequent, were acceptable and temporary. Similarly, Kim et al. reported numbness and compensatory hyperhidrosis in the treatment of PAH with FMR in 1 and 2 patients, respectively.[17]
It is recommended that future studies compare FMR, a noninvasive treatment modality to permanent surgical interventions. FMR treatment appears to be a safe and effective treatment alternative for moderate to severe PAH. It is suggested that repeated sessions of FMR be considered to achieve a complete response. This advice is particularly relevant in the treatment of overweight patients.
Conclusion
Fractional microneedle radiofrequency (FMR) treatment appears to be a new safe and effective treatment alternative for moderate to severe primary axillary hyperhidrosis. It is suggested that repeated sessions of FMR be considered to achieve a complete response.
Financial support and sponsorship
This study was sponsored by Isfahan University of Medical Sciences, Isfahan, Iran.
Conflicts of interest
There are no conflicts of interest.
What is new?
Fractional microneedle radiofrequency (FMR) treatment appears to be a new safe and effective treatment alternative for moderate to severe primary axillary hyperhidrosis. It is suggested that repeated sessions of FMR be considered to achieve a complete response.
References
- 1.Yadalla HK, Ambika H, Chawla S. A case of idiopathic unilateral circumscribed hyperhidrosis. Indian J Dermatol. 2013;58:163. doi: 10.4103/0019-5154.108102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naumann M, Lowe NJ. Botulinum toxin type A in treatment of bilateral primary axillary hyperhidrosis: Randomised, parallel group, double blind, placebo controlled trial. BMJ. 2001;323:596–9. doi: 10.1136/bmj.323.7313.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoorens I, Ongenae K. Primary focal hyperhidrosis: Current treatment options and a step-by-step approach. J Eur Acad Dermatol Venereol. 2012;26:1–8. doi: 10.1111/j.1468-3083.2011.04173.x. [DOI] [PubMed] [Google Scholar]
- 4.Rezende RM, Luz FB. Surgical treatment of axillary hyperhidrosis by suction-curettage of sweat glands. An Bras Dermatol. 2014;89:940–54. doi: 10.1590/abd1806-4841.20142873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nestor MS, Park H. Safety and efficacy of micro-focused ultrasound plus visualization for the treatment of axillary hyperhidrosis. J Clin Aesthet Dermatol. 2014;7:14–21. [PMC free article] [PubMed] [Google Scholar]
- 6.Solish N, Bertucci V, Dansereau A, Hong HC, Lynde C, Lupin M, et al. A comprehensive approach to the recognition, diagnosis, and severity-based treatment of focal hyperhidrosis: Recommendations of the Canadian Hyperhidrosis Advisory Committee. Dermatol Surg. 2007;33:908–23. doi: 10.1111/j.1524-4725.2007.33192.x. [DOI] [PubMed] [Google Scholar]
- 7.Fatemi Naeini F, Abtahi-Naeini B, Pourazizi M, Nilforoushzadeh MA, Mirmohammadkhani M. Fractionated microneedle radiofrequency for treatment of primary axillary hyperhidrosis: A sham control study. Australas J Dermatol. 2014. [Last accessed on 2015 Oct 01]. doi: 10.1111/ajd.12260. [Epub ahead of print] Availabe from: http://www.ncbi.nlm.nih.gov/pubmed/25496000 . [DOI] [PubMed]
- 8.Sampaio C, Costa J, Ferreira JJ. Clinical comparability of marketed formulations of botulinum toxin. Mov Disord. 2004;19(Suppl 8):S129–36. doi: 10.1002/mds.20066. [DOI] [PubMed] [Google Scholar]
- 9.Naeini FF, Saffaei A, Pourazizi M, Abtahi-Naeini B. Histopathological evidence of efficacy of microneedle radiofrequency for treatment of axillary hyperhidrosis. Indian J Dermatol Venereol Leprol. 2015;81:288–90. doi: 10.4103/0378-6323.154789. [DOI] [PubMed] [Google Scholar]
- 10.Fatemi Naeini F, Pourazizi M, Abtahi-Naeini B, Nilforoushzadeh MA, Najafian J. A novel option for treatment of primary axillary hyperhidrosis: Fractionated microneedle radiofrequency. J Postgrad Med. 2015;61:141–3. doi: 10.4103/0022-3859.153111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abtahi-Naeini B, Fatemi Naeini F, Adibi N, Pourazizi M. Quality of life in patients with primary axillary hyperhidrosis before and after treatment with fractionated microneedle radiofrequency. J Res Med Sci. 2015;20:631–5. doi: 10.4103/1735-1995.166196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heckmann M, Ceballos-Baumann AO, Plewig G. Hyperhidrosis Study Group. Botulinum toxin A for axillary hyperhidrosis (excessive sweating) N Engl J Med. 2001;344:488–93. doi: 10.1056/NEJM200102153440704. [DOI] [PubMed] [Google Scholar]
- 13.Johnson JE, O’Shaughnessy KF, Kim S. Microwave thermolysis of sweat glands. Lasers Surg Med. 2012;44:20–5. doi: 10.1002/lsm.21142. [DOI] [PubMed] [Google Scholar]
- 14.Hong HC, Lupin M, O’Shaughnessy KF. Clinical evaluation of a microwave device for treating axillary hyperhidrosis. Dermatol Surg. 2012;38:728–35. doi: 10.1111/j.1524-4725.2012.02375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glaser DA, Coleman WP, 3rd, Fan LK, Kaminer MS, Kilmer SL, Nossa R, et al. A randomized, blinded clinical evaluation of a novel microwave device for treating axillary hyperhidrosis: The dermatologic reduction in underarm perspiration study. Dermatol Surg. 2012;38:185–91. doi: 10.1111/j.1524-4725.2011.02250.x. [DOI] [PubMed] [Google Scholar]
- 16.Lee SJ, Chang KY, Suh DH, Song KY, Ryu HJ. The efficacy of a microwave device for treating axillary hyperhidrosis and osmidrosis in Asians: A preliminary study. J Cosmet Laser Ther. 2013;15:255–9. doi: 10.3109/14764172.2013.807114. [DOI] [PubMed] [Google Scholar]
- 17.Kim M, Shin JY, Lee J, Kim JY, Oh SH. Efficacy of fractional microneedle radiofrequency device in the treatment of primary axillary hyperhidrosis: A pilot study. Dermatology. 2013;227:243–9. doi: 10.1159/000354602. [DOI] [PubMed] [Google Scholar]


