Abstract
The basic theory of protein precipitation by addition of ammonium sulfate is presented and the most common applications are listed, Tables are provided for calculating the appropriate amount of ammonium sulfate to add to a particular protein solution.
Key terms for indexing: Ammonium sulfate, ammonium sulfate tables, protein concentration, protein purification
BASIC THEORY
The solubility of globular proteins increases upon the addition of salt (<0.15 M), an effect termed salting-in. At higher salt concentrations, protein solubility usually decreases, leading to precipitation; this effect is termed salting-out ((Green and Hughes, 1955). Salts that reduce the solubility of proteins also tend to enhance the stability of the native conformation. In contrast, salting-in ions are usually denaturants.
The mechanism of salting-out is based on preferential solvation due to exclusion of the cosolvent (salt) from the layer of water closely associated with the surface of the protein (hydration layer). The hydration layer, typically 0.3 to 0.4 g water per gram protein (Rupley et al., 1983), plays a critical role in maintaining solubility and the correctly folded native conformation. There are three main protein-water interactions: ion hydration between charged side chains (e.g., Asp, Glu, Lys), hydrogen bonding between polar groups and water (e.g., Ser, Thr, Tyr, and the main chain of all residues), and hydrophobic hydration (Val, Ile, Leu, Phe). In hydrophobic hydration, the configurational freedom of water molecules is reduced in the proximity of apolar residues. This ordering of water molecules results in a loss of entropy and is thus energetically unfavorable. When salt is added to the solution, the surface tension of the water increases, resulting in increased hydrophobic interaction between protein and water. The protein responds to this situation by decreasing its surface area in an attempt to minimize contact with the solvent—as manifested by folding (the folded conformation is more compact than the unfolded one) and then self-association leading to precipitation. Both folding and precipitation free up bound water, increasing the entropy of the system and making these processes energetically favorable. Timasheff and his colleagues provide a detailed discussion of these complex effects (e.g., Kita et al., 1994; Timasheff and Arakawa, 1997).
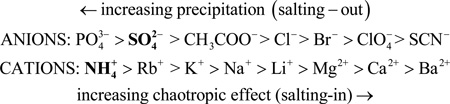
It should be mentioned that the increase in surface tension of water by salt follows the well-known Hofmeister series, shown below (see Parsegian, 1995, and references therein). Hence, as an approximation, those salts that favor salting-out raise the surface tension of water the highest. As (NH4)2SO4 has much a higher solubility than any of the phosphate salts, it is the reagent of choice for salting-out.
TIPS AND GUIDELINES
With solid ammonium sulfate, a mortar and pestle can be used to break up any lumps.
Use analytical grade as lower grade material is often contaminated with heavy metals.
Addition of ammonium sulfate acidifies the solution so use at least a 50 mM HEPES or Tris buffer etc., 5mM EDTA can also be included.
Add solid ammonium sulfate slowly with gentle stirring; allow to dissolve before adding more solid, try to prevent foaming.
On-line calculators can be accessed to conveniently determine the amounts of solid ammonium sulfate required to reach a given saturation. For example, EnCor Biotechnology Inc., has an on-line calculator based on the equations given in this appendix: http://www.encorbio.com/protocols/AM-SO4.htm.
Ammonium sulfate solution, 4.1M saturated at 25 °C can be purchased from Sigma-Aldrich and other suppliers.
Note: In the literature sulfate is often referred to by UK spelling: sulphate.
COMMON APPLICATION
Concentration of Proteins
Because precipitation is due to reduced solubility and not denaturation, pelleted protein can be readily resolubilized using standard buffers. After concentration, the protein is well suited for gel filtration (unit 8.3) whereby the buffer can be exchanged and the remaining ammonium sulfate removed. Alternately, the protein can be dissolved in a nonprecipitating concentration of (NH4)2SO4 (e.g., 1 M) and then applied to a hydrophobic interaction matrix (unit 8.4).
Protein Purification
Practical details of selective precipitation are presented in unit 4.5, and an example in the purification of interleukin 1β is given in unit 6.2 where the protein is fractionated between ~50 – 77% saturation. Low molecular weight proteins, like interleukin-1β, as a rule require higher salt concentration for precipitation than larger molecular proteins, for example, large multiprotein complexes can often be salted out with < 20% saturation. Another example (classic) is the precipitation of IgG from blood sera. The addition of 40 – 45% ammounium sulfate precipitates IgG which can be further purified by anion exchange chromatography. Salt precipitation has been widely used to fractionate membrane proteins (Schagger, 1994). Due to bound lipid and/or detergents, ammonium sulfate precipitates have lower density than protein-only precipitates. During centrifugation, these precipitates will often float to the top of tube rather than pelleting; the use of swing-out rotors is recommended. Crystallization is a traditional method of protein purification. Jakoby (1971) describes a general method that involves extracting (NH4)2SO4-precipitated protein with successively dilute (NH4)2SO4 solutions at low temperature. Although there are several methods for removing contaminating nucleic acids from protein solutions including, for example, addition of 0.1% (w.v) polyethyleneimine, a simple and effective approach is to apply the protein to a small anion exchange column equilibriated with 0.4M ammonium sulfate, where the nucleic acids binds to the column and the protein is collected in the flow-through.
Folding and Stabilization of Protein Structure
As mentioned above, (NH4)2SO4 and other neutral salts stabilize proteins by preferential solvation (Timasheff and Arakawa, 1997). Proteins are often stored in (NH4)2SO4, which inhibits bacterial growth and contaminating protease activities. Protein unfolded by denaturants such as urea can be pushed into native conformations by the addition of (NH4)2SO4 (Mitchinson and Pain, 1986). A practical application is the folding of recombinant proteins. For example, HIV-1 Rev expressed in E. coli was solubilized using urea, purified by ion-exchange chromatography in the presence of urea, then folded by the addition of 0.5 to 1.0 M (NH4)2SO4 (Wingfield et al., 1991).
Basic Calculations
Basic definitions
Percentage (%) saturation concentration of (NH4)2SO4 in solution as % of maximum solubility at the given temperature. For example, at 0°C, a 100% saturated solution is 3.9 M.
Specific volume (sp. vol.) volume occupied by 1 g of (NH4)2SO4 (ml/g) = inverse of density. At 0°C, if 706.8 g of (NH4)2SO4 is added to 1 L of water the volume = 1000 ml + volume occupied by the salt (706.8 × 0.5281 ml) = total volume of 1373.26 ml. The molarity = 3.9 M.
Calculating quantities of (NH4)2SO4 to be added
By weight. The following equation is used to calculate the weight of solid (NH4)2SO4 to be added to 1 liter of solution of initial concentration S1 to produce final saturation S2:
where:
Gsat = grams of (NH4)2SO4 contained in 1 liter of saturated solution. For example, at 0°C, Gsat = 515.35 (see Table A.3F.1).
Table A.3F.1.
| Temperature (°C) | 0 | 10 | 20 | 25 |
|---|---|---|---|---|
| (NH4)2SO4 (g) added to 1 liter of water to give saturated solution | 706.8 | 730.5 | 755.8 | 766.8 |
| (NH4)2SO4 (g) per liter saturated solution | 515.35 | 524.60 | 536.49 | 541.80 |
| Molarity of saturated solution | 3.90 | 3.97 | 4.06 | 4.10 |
| Density (g/ml) | 1.2428 | 1.2436 | 1.2447 | 1.2450 |
| Specific volume in saturated solution (ml/g) | 0.5281 | 0.5357 | 0.5414 | 0.5435 |
Molecular weight of (NH4)2SO4 = 132.14.
Adapted from Dawson et al. (1986).
S1 and S2 are fractions of complete saturation; for example, a 20% saturation is expressed as 0.2.
For example, P = 0.2722 and 0.2945 at 0°C and 25°C, respectively.
By volume. The following equation is used to calculate volume of saturated (NH4)2SO4 solution to be added to 100 ml of solution to increase saturation from S1 to S2:
For example, to raise 100 ml of 0.2 saturated solution to 0.70 saturation:
Hence, 166.66 ml of saturated solution is added to 100 ml of 20% saturated solution to give 266.66 ml of 70% saturated solution.
AMMONIUM SULFATE TABLES
The tables shown are taken from Wood (1976). Table A.3F.2 gives the weight of (NH4)2SO4 to be added to a solution to obtain the desired concentration. Table A.3F.3 gives the volume of a 3.8 M solution to add to obtain a desired concentration. Tables A.3F.4 and A.3F.5 give the final volumes after the addition of the solid salt or a 3.8 M solution, respectively. The concentration of (NH4)2SO4 is expressed in molarity (corresponding % saturation is indicated in Table A.3F.2). The data is valid for solutions at 0°C, and the variation of specific volume with concentration is taken into account. For a table referring to solutions at 25°C, see Green and Hughes (1955).
Table A.3F.2.
Grams of Ammonium Sulfate to Add to 1 Liter of Solution at 0°C
| Percent saturation |
Initial molarity |
Final molarity |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.00 | 0.20 | 0.40 | 0.60 | 0.80 | 1.00 | 1.20 | 1.40 | 1.60 | 1.80 | 2.00 | 2.20 | 2.40 | 2.60 | 2.80 | 3.00 | 3.20 | 3.40 | 3.60 | 3.80 | 3.90 | ||
| 0.0 | 0.00 | 0.00 | 26.7 | 54.0 | 81.9 | 111 | 140 | 170 | 202 | 234 | 267 | 302 | 338 | 375 | 413 | 453 | 495 | 539 | 585 | 632 | 682 | 707 |
| 5.1 | 0.20 | 0.00 | 27.0 | 54.7 | 83.0 | 112 | 142 | 173 | 205 | 238 | 272 | 308 | 344 | 383 | 422 | 464 | 507 | 552 | 599 | 649 | 673 | |
| 10.3 | 0.40 | 0.00 | 27.4 | 55.4 | 84.2 | 114 | 144 | 176 | 209 | 243 | 278 | 314 | 352 | 391 | 432 | 475 | 519 | 566 | 615 | 639 | ||
| 15.4 | 0.60 | 0.00 | 27.7 | 56.2 | 85.5 | 116 | 147 | 179 | 213 | 247 | 283 | 321 | 359 | 400 | 442 | 486 | 533 | 581 | 605 | |||
| 20.5 | 0.80 | 0.00 | 28.1 | 57.1 | 87.0 | 118 | 150 | 183 | 217 | 252 | 289 | 328 | 368 | 409 | 453 | 499 | 546 | 570 | ||||
| 25.7 | 1.00 | 0.00 | 28.6 | 58.1 | 88.5 | 120 | 153 | 186 | 221 | 258 | 296 | 335 | 376 | 420 | 465 | 512 | 535 | |||||
| 30.8 | 1.20 | 0.00 | 29.1 | 59.1 | 90.2 | 122 | 156 | 190 | 226 | 264 | 303 | 343 | 386 | 430 | 477 | 499 | ||||||
| 35.9 | 1.40 | 0.00 | 29.6 | 60.2 | 91.9 | 125 | 159 | 194 | 231 | 270 | 310 | 351 | 395 | 441 | 464 | |||||||
| 41.1 | 1.60 | 0.00 | 30.2 | 61.4 | 93.7 | 127 | 162 | 199 | 236 | 276 | 317 | 360 | 405 | 428 | ||||||||
| 46.2 | 1.80 | 0.00 | 30.7 | 62.6 | 95.7 | 130 | 166 | 203 | 242 | 282 | 325 | 369 | 391 | |||||||||
| 51.3 | 2.00 | 0.00 | 31.3 | 63.9 | 97.7 | 133 | 170 | 208 | 248 | 290 | 333 | 355 | ||||||||||
| 56.5 | 2.20 | 0.00 | 32.0 | 65.2 | 99.8 | 136 | 174 | 213 | 254 | 297 | 318 | |||||||||||
| 61.6 | 2.40 | 0.00 | 32.7 | 66.7 | 102 | 139 | 178 | 218 | 260 | 281 | ||||||||||||
| 66.8 | 2.60 | 0.00 | 33.4 | 68.2 | 104 | 142 | 182 | 224 | 244 | |||||||||||||
| 71.9 | 2.80 | 0.00 | 34.2 | 69.8 | 107 | 146 | 187 | 207 | ||||||||||||||
| 77.0 | 3.00 | 0.00 | 35.0 | 71.5 | 110 | 150 | 169 | |||||||||||||||
| 82.2 | 3.20 | 0.00 | 35.8 | 73.2 | 112 | 132 | ||||||||||||||||
| 87.3 | 3.40 | 0.00 | 36.7 | 75.0 | 94.0 | |||||||||||||||||
| 92.4 | 3.60 | 0.00 | 37.6 | 56.1 | ||||||||||||||||||
| 97.6 | 3.80 | 0.00 | 18.1 | |||||||||||||||||||
| 100.0 | 3.90 | 0.00 | ||||||||||||||||||||
Table A.3.F.3.
Milliliters of a 3.8 M Ammonium Sulfate Solution to Add to 1 Liter of Solution at 0°C
| Initial molarity |
Final molarity |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.00 | 0.20 | 0.40 | 0.60 | 0.80 | 1.00 | 1.20 | 1.40 | 1.60 | 1.80 | 2.00 | 2.20 | 2.40 | 2.60 | 2.80 | 3.00 | 3.20 | 3.40 | |
| 0.00 | 0.00 | 55.3 | 117 | 185 | 263 | 351 | 452 | 570 | 709 | 875 | 1077 | 1330 | 1655 | 2088 | 2693 | 3600 | 5111 | 8134 |
| 0.20 | 0.00 | 58.4 | 124 | 197 | 281 | 377 | 489 | 621 | 779 | 972 | 1213 | 1522 | 1933 | 2508 | 3371 | 4809 | 7684 | |
| 0.40 | 0.00 | 61.9 | 132 | 211 | 302 | 408 | 534 | 683 | 866 | 1094 | 1387 | 1777 | 2322 | 3140 | 4503 | 7228 | ||
| 0.60 | 0.00 | 65.9 | 141 | 227 | 327 | 446 | 587 | 760 | 975 | 1252 | 1620 | 2135 | 2907 | 4194 | 6768 | |||
| 0.80 | 0.00 | 70.5 | 152 | 246 | 357 | 490 | 652 | 855 | 1115 | 1462 | 1946 | 2673 | 3884 | 6305 | ||||
| 1.00 | 0.00 | 75.9 | 164 | 268 | 393 | 545 | 735 | 978 | 1303 | 1756 | 2437 | 3570 | 5837 | |||||
| 1.20 | 0.00 | 82.3 | 179 | 295 | 437 | 613 | 840 | 1143 | 1565 | 2199 | 3255 | 5366 | ||||||
| 1.40 | 0.00 | 89.7 | 197 | 328 | 492 | 702 | 981 | 1372 | 1959 | 2936 | 4891 | |||||||
| 1.60 | 0.00 | 98.7 | 219 | 369 | 562 | 819 | 1179 | 1718 | 2616 | 4412 | ||||||||
| 1.80 | 0.00 | 110 | 247 | 423 | 657 | 984 | 1475 | 2294 | 3931 | |||||||||
| 2.00 | 0.00 | 124 | 282 | 494 | 789 | 1232 | 1971 | 3447 | ||||||||||
| 2.20 | 0.00 | 141 | 330 | 593 | 988 | 1645 | 2961 | |||||||||||
| 2.40 | 0.00 | 165 | 396 | 742 | 1319 | 2472 | ||||||||||||
| 2.60 | 0.00 | 198 | 496 | 991 | 1981 | |||||||||||||
| 2.80 | 0.00 | 248 | 662 | 1489 | ||||||||||||||
| 3.00 | 0.00 | 332 | 994 | |||||||||||||||
| 3.20 | 0.00 | 498 | ||||||||||||||||
| 3.40 | 0.00 | |||||||||||||||||
Table A.3.F.4.
Final Volume in Millimeters After Addition of Solid Ammonium Sulfate to 1 Liter of Solution at 0°C
| Initial molarity |
Final molarity |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.00 | 0.20 | 0.40 | 0.60 | 0.80 | 1.00 | 1.20 | 1.40 | 1.60 | 1.80 | 2.00 | 2.20 | 2.40 | 2.60 | 2.80 | 3.00 | 3.20 | 3.40 | 3.60 | 3.80 | 3.90 | |
| 0.00 | 1000 | 1010 | 1023 | 1033 | 1046 | 1060 | 1074 | 1090 | 1106 | 1123 | 1142 | 1161 | 1181 | 1203 | 1225 | 1249 | 1275 | 1301 | 1329 | 1359 | 1373 |
| 0.20 | 1000 | 1011 | 1023 | 1035 | 1049 | 1063 | 1079 | 1095 | 1112 | 1130 | 1149 | 1169 | 1191 | 1213 | 1237 | 1262 | 1288 | 1316 | 1345 | 1359 | |
| 0.40 | 1000 | 1012 | 1024 | 1037 | 1052 | 1067 | 1083 | 1100 | 1118 | 1137 | 1157 | 1178 | 1200 | 1223 | 1248 | 1274 | 1301 | 1330 | 1344 | ||
| 0.60 | 1000 | 1012 | 1025 | 1039 | 1054 | 1070 | 1087 | 1105 | 1124 | 1143 | 1164 | 1186 | 1209 | 1233 | 1259 | 1286 | 1315 | 1329 | |||
| 0.80 | 1000 | 1013 | 1027 | 1042 | 1057 | 1074 | 1091 | 1110 | 1129 | 1150 | 1172 | 1194 | 1218 | 1244 | 1271 | 1299 | 1313 | ||||
| 1.00 | 1000 | 1014 | 1028 | 1044 | 1060 | 1077 | 1096 | 1115 | 1135 | 1156 | 1179 | 1203 | 1228 | 1254 | 1282 | 1296 | |||||
| 1.20 | 1000 | 1014 | 1030 | 1046 | 1063 | 1081 | 1100 | 1120 | 1141 | 1163 | 1187 | 1211 | 1237 | 1265 | 1278 | ||||||
| 1.40 | 1000 | 1015 | 1031 | 1048 | 1065 | 1084 | 1104 | 1125 | 1147 | 1170 | 1194 | 1220 | 1247 | 1260 | |||||||
| 1.60 | 1000 | 1016 | 1032 | 1050 | 1068 | 1088 | 1108 | 1130 | 1152 | 1176 | 1202 | 1228 | 1242 | ||||||||
| 1.80 | 1000 | 1016 | 1033 | 1052 | 1071 | 1091 | 1112 | 1135 | 1158 | 1183 | 1209 | 1222 | |||||||||
| 2.00 | 1000 | 1017 | 1035 | 1054 | 1073 | 1094 | 1116 | 1140 | 1164 | 1190 | 1203 | ||||||||||
| 2.20 | 1000 | 1018 | 1036 | 1056 | 1076 | 1098 | 1121 | 1145 | 1170 | 1183 | |||||||||||
| 2.40 | 1000 | 1018 | 1037 | 1058 | 1079 | 1101 | 1125 | 1150 | 1162 | ||||||||||||
| 2.60 | 1000 | 1019 | 1039 | 1060 | 1082 | 1105 | 1129 | 1142 | |||||||||||||
| 2.80 | 1000 | 1019 | 1040 | 1062 | 1085 | 1109 | 1121 | ||||||||||||||
| 3.00 | 1000 | 1020 | 1041 | 1064 | 1087 | 1099 | |||||||||||||||
| 3.20 | 1000 | 1021 | 1043 | 1066 | 1077 | ||||||||||||||||
| 3.40 | 1000 | 1022 | 1044 | 1055 | |||||||||||||||||
| 3.60 | 1000 | 1022 | 1033 | ||||||||||||||||||
| 3.80 | 1000 | 1011 | |||||||||||||||||||
| 3.90 | 1000 | ||||||||||||||||||||
Table A.3.F.5.
Final Volume in Millimeters After Addition of 3.8 M Ammonium Sulfate Solution to 1 Liter of Solution at 0°C
| Initial Molarity |
Final molarity |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.00 | 0.20 | 0.40 | 0.60 | 0.80 | 1.00 | 1.20 | 1.40 | 1.60 | 1.80 | 2.00 | 2.20 | 2.40 | 2.60 | 2.80 | 3.00 | 3.20 | 3.40 | |
| 0.00 | 1000 | 1051 | 1109 | 1174 | 1248 | 1333 | 1432 | 1547 | 1683 | 1847 | 2047 | 2298 | 2621 | 3051 | 3654 | 4560 | 6070 | 9091 |
| 0.20 | 1000 | 1055 | 1117 | 1187 | 1268 | 1362 | 1471 | 1601 | 1757 | 1947 | 2186 | 2493 | 2902 | 3476 | 4337 | 5773 | 8646 | |
| 0.40 | 1000 | 1059 | 1126 | 1202 | 1291 | 1394 | 1517 | 1665 | 1846 | 2072 | 2363 | 2751 | 3294 | 4111 | 5472 | 8196 | ||
| 0.60 | 1000 | 1063 | 1135 | 1219 | 1317 | 1433 | 1573 | 1743 | 1957 | 2232 | 2598 | 3112 | 3883 | 5168 | 7741 | |||
| 0.80 | 1000 | 1068 | 1147 | 1239 | 1348 | 1479 | 1640 | 1841 | 2099 | 2444 | 2927 | 3652 | 4862 | 7282 | ||||
| 1.00 | 1000 | 1074 | 1160 | 1262 | 1385 | 1535 | 1723 | 1966 | 2289 | 2741 | 3420 | 4552 | 6818 | |||||
| 1.20 | 1000 | 1080 | 1176 | 1290 | 1430 | 1605 | 1831 | 2131 | 2553 | 3185 | 4240 | 6350 | ||||||
| 1.40 | 1000 | 1088 | 1194 | 1324 | 1486 | 1694 | 1973 | 2363 | 2948 | 3924 | 5878 | |||||||
| 1.60 | 1000 | 1097 | 1216 | 1365 | 1557 | 1813 | 2171 | 2709 | 3606 | 5402 | ||||||||
| 1.80 | 1000 | 1108 | 1244 | 1419 | 1652 | 1979 | 2469 | 3287 | 4922 | |||||||||
| 2.00 | 1000 | 1122 | 1280 | 1491 | 1785 | 2227 | 2965 | 4441 | ||||||||||
| 2.20 | 1000 | 1141 | 1328 | 1590 | 1984 | 2642 | 3956 | |||||||||||
| 2.40 | 1000 | 1164 | 1394 | 1740 | 2316 | 3469 | ||||||||||||
| 2.60 | 1000 | 1198 | 1494 | 1989 | 2979 | |||||||||||||
| 2.80 | 1000 | 1248 | 1661 | 2488 | ||||||||||||||
| 3.00 | 1000 | 1331 | 1994 | |||||||||||||||
| 3.20 | 1000 | 1498 | ||||||||||||||||
| 3.40 | 1000 | |||||||||||||||||
LITERATURE CITED
- Dawson RMC, Elliot DC, Elliot WH, Jones KM. Data for Biochemical Research. 3rd. Oxford: Oxford Science Publications, Clarendon Press; 1986. p. 537. [Google Scholar]
- Green AA, Hughes WL. Protein solubility on the basis of solubility in aqueous solutions of salts and organic solvents. Methods Enzymol. 1955;1:67–90. [Google Scholar]
- Jakoby WB. Crystallization as a purification technique. Methods Enzymol. 1971;22:248–252. [Google Scholar]
- Kita Y, Arakawa T, Lin T-Y, Timasheff S. Contribution of the surface free energy perturbations to protein-solvent interactions. Biochemistry. 1994;33:1517–1589. doi: 10.1021/bi00254a029. [DOI] [PubMed] [Google Scholar]
- Mitchinson C, Pain RH. The effect of sulphate and urea on the stability and reversible unfolding of β-lactamase from Staphylococcus aureus. J. Mol. Biol. 1986;184:331–342. doi: 10.1016/0022-2836(85)90384-5. [DOI] [PubMed] [Google Scholar]
- Parsegian VA. Hopes for Hofmeister. Nature. 1995;378:335–336. [Google Scholar]
- Rupley JA, Gratton E, Careri G. Water and globular proteins. Trend Biochem. Sci. 1983;8:18–22. [Google Scholar]
- Schagger H. Chromatographic techniques and basic operations in membrane protein purification. In: von Jagow G, Schagger H, editors. A Practical Guide to Membrane Protein Purification. San Diego: Academic Press; 1994. pp. 23–57. [Google Scholar]
- Timasheff SN, Arakawa T. membrane protein purification Stabilization of protein structure by solvents. In: Creighton TE, editor. Protein Structure: A Practical Approach. 2nd. Oxford: IRL Press at Oxford University Press; 1997. pp. 349–364. [Google Scholar]
- Wingfield PT, Stahl SJ, Payton MA, Vankatesan S, Misra M, Steven AC. HIV-1 Rev expressed in recombinant Escherichia coli: Purification, polymerization and conformational properties. Biochemistry. 1991;30:7527–7534. doi: 10.1021/bi00244a023. [DOI] [PubMed] [Google Scholar]
- Wood WI. Tables for the preparation of ammonium sulfate solutions. Anal. Biochem. 1976;73:250–257. doi: 10.1016/0003-2697(76)90165-2. [DOI] [PubMed] [Google Scholar]
KEY REFERENCE
- Wood 1976. See above.