Abstract
Background and Aims Senescence is the process of losing fitness when growing old, and is shaped by the trade-off between maintenance and reproduction that makes reproduction more unsure and maintenance more costly with age. In repeatedly reproducing plants, reductions in growth and fertility are signs of senescence. Disturbance, however, provides an opportunity to reset the ageing clock and consequently potentially ameliorate senescence.
Methods To test the effects of disturbance on traits closely related to fitness and thus to senescence, a long-term garden experiment was established with two short-lived perennial congeners, Barbarea vulgaris and Barbarea stricta, that differ in their ability to resprout after injury. In the experiment, five damage treatments were applied to plants in four different phenophases.
Key Results It was found that damage to the plant body significantly prolonged life span in B. vulgaris but decreased whole-life seed production in both species. High concentration of seed production in one growing season characterized short life spans. Both more severe damage and a more advanced phenological phase at the time of damage caused reproduction to be spread over more than one growing season and equalized per-season seed production. In terms of seed quality, average weight of a single seed decreased and seed germination rate increased with age regardless of damage.
Conclusions Although disturbance is able to reset the ageing clock of plants, it is so harmful to plant fitness that resprouting serves, at best, only to alleviate slightly the signs of senescence. Thus, in terms of whole-life seed production, injured plants were not more successful than uninjured ones in the two studied species. Indeed, in these species, injury only slightly postponed or decelerated senescence and did not cause effective rejuvenation.
Keywords: Ageing clock, Barbarea vulgaris, Barbarea stricta, disturbance, fitness, injury, life span, phenophase, seed germination, seed number, seed weight
INTRODUCTION
Senescence in general is a term referring to the process of losing fitness when growing old (Lacey et al., 2003; Van Dijk, 2009; Morales and Munné-Bosch, 2015). In plants, in contrast to the majority of animals, life-long existence of undifferentiated tissues and the modular body arrangement are two main characteristics that can influence the manifestation of senescence at the individual level (Harper, 1977; Munné-Bosch, 2015). Thus, it has been hypothesized that plants can escape senescence through replacement of old modules (i.e. in clonal plants), resist it by having durable organs (e.g. in trees) or preclude it by programmed death after reproduction, i.e. in monocarps (Thomas, 2013). Only sparse information has been accumulated about the senescence of plant individuals (Munné-Bosh, 2008), although it is believed that senescence is being eliminated by natural selection (Baudisch et al., 2013; Salguero-Gómez et al., 2013; Munné-Bosch, 2015). However, senescence is controlled by trade-offs between maintenance and reproduction, with the increased cost of maintenance in old age progressively tilting the balance in favour of reproduction (Kirkwood, 1977). Therefore, we can expect that plants should be mortal and die abruptly after they achieve their maximum life span reproductive output. In plants that reproduce repeatedly, reduction of growth and fertility, and thus decreased fitness, are seen as signs of senescence (Lacey et al., 2003; Van Dijk, 2009), although we can expect differences in this pattern due to morphological constraints (Klimešová et al., 2014, Munné-Bosch, 2014).
There have been rather few studies of senescence in individual plant species. In the vegetatively reproducing plant Lemna minor, a strong age-related decline in survival, reproduction and offspring quality was found (Barks and Laird, 2015). Salguero-Gómez and Casper (2010) showed that plants of the perennial herb Plantago lanceolata typically exhibit a decline in size over a period of 3 years prior to death, accompanied by lower inflorescence production. Müller et al. (2014), studying the perennial shrub Cistus albidus grown in controlled conditions, found that older plants had lower numbers of flowers and seeds, lighter seeds and higher rates of embryo abortion in mature seeds. In the monocarpic perennial herb Cardiocrinum cordatum, it was found that survival as well as the probability of reaching the next life stage decreased after 6 years of age and that growth in plant size occurred until flowering; thus, loss in – or even lack of change in – plant size would lead to death in the subsequent season (Araki et al., 2010). Seed production was shown to decrease with age in Beta vulgaris ssp. maritima, a species with variable life span (Van Dijk, 2009). On the other hand, no signs of senescence were found in grafted shoots from old trees of Pinus sylvestris; they grew as fast as those from young trees and produced the same number of germinable seeds (Mencuccini et al., 2014). Similarly, growth and fecundity did not decrease with age in the herb Borderea pyrenaica (Garcia et al., 2011). From the above-mentioned examples, it follows that the manifestation of signs of senescence not only is life history specific but also varies among species having the same life history (Davison et al., 2014; Munné-Bosch 2015).
Programmed death after a maximized reproductive event is typical for monocarpic plants and has been documented many times (Thomas, 2013). We know, however, that numerous monocarps have variable life cycles responding to environmental clues and that they can change to short-lived polycarpic plants (Klimešová et al., 2007). The trigger for such a monocarpy/polycarpy switch is usually disturbance, and therefore cutting or trimming provides an opportunity to reset the ageing clock (Salguero-Gómez and Casper, 2010). The reason for the reset of life history stage is the fact that severe injury hinders the investment of all storage compounds towards a single, large reproduction event (Sosnová and Klimešová, 2013; Thomas, 2013), and, after such a reset, seeding can instead take place over multiple seasons. The mechanism by which this occurs is that the dominant apical meristem, borne by the single dominant shoot typical of the monocarpic plant body, is destroyed and thus the main source of hormones ensuring apical dominance and the main carbohydrate sink is replaced by several shoots differing in size, which compete for carbohydrates. Differences in auxin flow (Davies and Gan, 2012) and reserve acquisition among the regenerated shoots then lead to variability in shoot development and therefore to the spread of reproduction over several seasons (Sosnová and Klimešová, 2013).
According to Davies and Gan (2012), senescence can be modulated by carbohydrate sink size; however, global shifts in hormonal and nutrient balance triggered by flowering are probably major factors controlling senescence after flowering in monocarps (Sklensky and Davies, 2011). Perennial plants differ from monocarps by not investing all their resources in one reproductive event, and monocarpic plants that lose the main carbohydrate sink behave similarly. Their ability to recover and change strategy in respect to senescence might be affected by the phenological phase in which the damage occurs, because total reallocation toward generative structures in monocarps is triggered at the same time as flowering (Davies and Gan, 2012). Apart from the effect of phenology, injury severity can have an effect on further growth of monocarpic plants (Huhta et al., 2003) due to the reduction of apical dominance. A monocarpic plant that has lost part of a dominant shoot will retain apical dominance and thus the main carbohydrate sink to a higher degree than a plant losing the whole shoot and forced to resprout from a root fragment (Martínková et al., 2008, 2015; Bartušková and Klimešová, 2010). Therefore, we could expect that after injury, a monocarpic plant will have a tendency to prolong its life and spread its reproduction over several, more equal reproductive events as is typical for a perennial plant. This effect would increase with severity of injury and decrease with ontogenetic stage of a plant at the time of injury.
To examine the effect on senescence-related traits, and thus on plant fitness, of resetting the ageing clock reset via disturbance, we established a 5-year garden experiment with two short-lived Barbarea species (Barbarea vulgaris and Barbarea stricta) that differ in their ability to resprout after injury. In this experiment, we applied five levels of disturbance severity to plants at four different phenophases. The experimental design fully reflects natural conditions since both Barbarea species occur in unpredictable, frequently disturbed habitats. Injury survival, seed production, seed viability and life span of plants were followed over several years. We specifically tested the following hypotheses: (a) severe injury to the plant body causes prolongation of plant life span and also the spread of reproduction over more growing seasons; (b) injury increases whole-life fecundity; (c) life span is negatively correlated with both the number of seeds formed during the first reproductive season and the concentration of seed production in one growing season; (d) differences in reproduction between the first reproductive season and reproduction in subsequent years diminish with increasing injury severity and increase with advancing phenology of injured plants; and (e) germination rate and seed weight change with the maternal plant’s age and differ between injured and intact plants.
MATERIALS AND METHODS
Study species
Barbarea vulgaris R. Br. and Barbarea stricta ANDRZ. (Brassicaceae) are common European species. Barbarea vulgaris prefers anthropogenic habitats, e.g. arable land, ruderal habitats, roadside ditches and railway banks, while Barbarea stricta occurs in more natural, moist habitats, i.e. pond banks and river alluvia (Dvořák, 1992) that are also affected by disturbance. Both species are short-lived perennial herbs reproducing once or, under certain conditions, repeatedly (MacDonald and Cavers, 1991; Dvořák, 1992; J. Martínková, pers. obs.). During the first year of life, the plants remain vegetative, and rosettes overwinter to the next season. Seed production is initiated during the second year of life. In the beginning of the second year, vegetative rosettes transform into reproductive stems, losing their rosette leaves. Both species are capable of root sprouting, as adventitious buds may develop on the main root or on root branches that lie near the soil surface, and a number of adventitious rosettes or stems may arise from these buds (Klimešová et al., 2007). Root-sprouting ability is forced by damage of above-ground biomass (Klimešová and Martínková, 2004). Barbarea vulgaris and B. stricta differ in their degree of root sprouting, with B. vulgaris regenerating from root more vigorously than B. stricta (J. Martínková, pers. obs.).
Experiment
Seeds for the experiment were collected during 2003. The seeds originated from South Bohemian natural populations: 15 populations for B. vulgaris and three for B. stricta. To minimize seed-origin effects, the seeds from different populations were mixed, separately for each species. During the winter, seeds were kept in laboratory conditions without stratification.
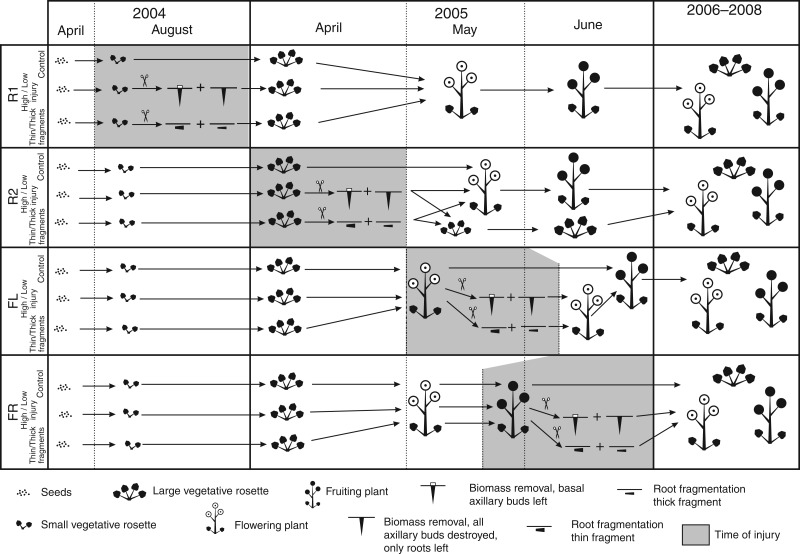
In the spring of 2004, 2·5 L containers were filled with a 2:3 garden substrate:sand mixture and five seeds per container were sowed for each species separately. Containers were kept outdoors in the experimental garden during the whole experiment and positioned randomly. One week after seedling emergence, the number of seedlings was reduced to only one per container. Plants were fertilized regularly with standard NPK solution and watered when necessary. Containers were randomly assigned to ten groups (30 replicates in each) for each species according to the damage to be applied at a particular life phase. The first group represented plants slightly injured in the first-year rosette phase (low at R1). The second group comprised plants severely injured in the first-year rosette phase (high at R1). The third group represented plants slightly injured in the second-year rosette phase (low at R2). The fourth group comprised plants severely injured in the second-year rosette phase (high at R2). Similarly, the fifth, sixth, seventh and eighth groups represented plants slightly or severely injured in the flowering or fruiting phases during the second year of life (low at FL, high at FL, low at FR and high at FR, respectively). Slight injury constituted removal of all above-ground biomass of a plant except for the basal axillary bud. To inflict severe injury, we would leave only the roots intact while destroying all axillary buds along with the above-ground biomass. The ninth group of plants was not injured and served as a control treatment, while the tenth group served as a source of root fragments.
The plants of the two R1 groups were injured in 2004; injury to other plants was applied in 2005 (Table 1; Fig. 1). At the same times that plants in injury treatments were injured (once in 2004 and three occasions in 2005), root fragments were obtained from the tenth plant group (Table 1; Fig. 1). Each plant served as the source of two fragments, both of 6 cm length but of different diameters and from different positions in the root system. The thick fragments were cut from the topmost part of the main root, directly under the hypocotyl. The thin fragments were cut from the first lateral root immediately beyond its branching from the main root (Table 1). After cutting, each root fragment was immediately placed horizontally into a 2·5 L container filled with a 2:3 substrate:sand mixture. The containers were placed in an experimental garden, regularly fertilized with NPK fertilizer and watered when necessary.
Table 1.
Overview of the set-up of the experiment on Barbarea vulgaris and Barbarea stricta, including a description of damage types applied, list of phenological phases at which plants were injured and dates of injuries
| Phenophase | Date of injury or cutting | Damage | Description |
|---|---|---|---|
| Control | 2 April 2004 (sowing) | Control | Plants without injury |
| R1: first-year rosette | 18 August 2004 | Low injury | First-year rosettes slightly injured* |
| High injury | First-year rosettes severely injured† | ||
| Thick fragment | Root fragments from the main root cut during the first-year rosette phase | ||
| Thin fragment | Root fragments from the first branch cut during the first-year rosette phase | ||
| R2: second-year rosette | 6 April 2005 | Low injury | Second-year rosettes slightly injured* |
| High injury | Second-year rosettes severely injured† | ||
| Thick fragment | Root fragments from the main root cut during the second-year rosette phase | ||
| Thin fragment | Root fragments from the first branch cut during the second-year rosette phase | ||
| FL: flowering | 20 May 2005 | Low injury | Flowering plants slightly injured* |
| High injury | Flowering plants severely injured† | ||
| Thick fragment | Root fragments from the main root cut during the flowering phase | ||
| Thin fragment | Root fragments from the first branch cut during the flowering phase | ||
| FR: fruiting | 28 June 2005 | Low injury | Fruiting plants slightly injured* |
| High injury | Fruiting plants severely injured† | ||
| Thick fragment | Root fragments from the main root cut during the fruiting phase | ||
| Thin fragment | Root fragments from the first branch cut during the fruiting phase |
All plants were grown from seed sown on 2 April 2004.
*Slightly injured: above-ground biomass removal but basal axillary buds left in place.
†Severely injured: removal of above-ground biomass and all axillary buds, leaving only roots in place; thus, regeneration only from the roots is possible.
Fig. 1.
Time diagram of the experiment on Barbarea vulgaris and Barbarea stricta in the years 2004–2008. Five types of injury were applied to plants during four phenological phases. Descriptions of damage types and phenological phases along with abbreviations are presented in Table 1.
For all plants, the following characteristics were recorded or calculated during the years 2004–2008: whether the plant regenerated after injury; each season that it reproduced; and, for each reproductive season, the total number of seeds produced, the average weight of one seed, the seed germination rate and the number of viable seeds produced. The description and schedule of treatments are summarized in Table 1.
During May 2009, the experiment was terminated because the majority of plants had died and the rest were so weak that a high probability of death without reproduction was obvious.
Seed trapping and germination tests
Seed collection and germination tests were done every year of the experiment except the first year because neither species started reproducing until the second year of their lives. To trap all seeds produced by each plant, reproducing plants were wrapped in light white cloth after all their flowering had finished. For each reproducing plant, the total weight of all trapped seeds was measured after seed maturation. Then, for each plant, the average weight of one seed was calculated based on three replicates of 30 seeds, and the total number of seeds for the plant would be estimated based on the total weight of its trapped seeds.
In the autumn following each reproductive season, germination tests were done in the standardized conditions of a growth chamber (15 vs. 8 h and 23 °C vs. 15 °C for ‘day’ vs. ‘night’ regimes, respectively). For each reproducing plant, three replicates of 30 seeds were placed on wet sand in Petri dishes. Over the next 21 d, the number of germinated seeds would be recorded.
Calculation of temporal concentration of seed production
For each plant, temporal concentration of seed production was represented by the ratio of the maximum number of seeds that it produced in one season to its whole-life seed production. Therefore, a temporal concentration of seed production equal to 1 would mean that the plant reproduced only once. Lower values of the ratio would mean that seed production was spread over more than one growing seasons. This calculation was performed separately for all seeds produced and for viable seeds only.
Statistical analysis
The response of survival probability and selected plant characteristics to damage type, plant phenophase at the time of injury and species identity were evaluated using generalized linear models (GLMs) with the assumed error distribution employed depending on the response variable analysed: binomial distribution for survival probability; Poisson distribution with explicitly modelled overdispersion for numbers of seeds (both total and viable); and plant life span. The effects of damage type and plant phenophase were assessed using a crossed model term that included both main effects and their interaction. To compare the responses of the species, we compared a model that included (in addition to damage type and phenophase effects) only the main effect of species with another model that was extended by including interaction terms between species and the two experimentally manipulated variables.
To describe changes in seed weight and germination rate over an individual’s lifetime, we used a mixed linear effect model (LMM) with log-transformed seed weight and arcsin-transformed germination rate, and with plant individual identity as a random-effect predictor. The full models included not only the main effect of time (year), but also its interactions with damage type and phenophase. As in the case of the GLMs, we also tested the difference between the species by comparing models with and without interactions of the species with other explanatory variables (damage type, phenophase and time). To compare the effects of temporal concentration of seed production on the life span of the plants under different experimental conditions, we replaced a fully factorial model with a model in which the temporal concentration effect was nested within a combination of phenology stage and damage type.
Finally, to evaluate the effect of seed production in their first productive season on the survival of individuals, we used a Cox proportional hazard model.
Hypothesis testing on fitted models was complemented – for selected models – by estimating the sizes of the effects for significant model terms. Toward this aim, we calculated 95 % confidence intervals (CIs) for regression coefficient estimates.
All calculations were done in program R, version 3·0 (R Core Team, 2013); LMMs were fitted using the ‘nlme’ package (Pinheiro and Bates, 2000); Cox proportional hazard models were fitted using the ‘survival’ package (Therneau and Grambsch, 2000); post-hoc tests and planned contrast tests were performed using the ‘multcomp’ package (Bretz et al., 2011).
RESULTS
Survival after injury
Barbarea vulgaris and B. stricta did not differ significantly in the effects of damage and phenophase upon survival (Table 2; Fig. 2A). When examining species separately, a post-hoc test did not find any significant difference in the proportions of regenerated individuals among damage types or among phenophases at the time of injury in B. vulgaris (Table 2; Fig. 2A). On the other hand, for B. stricta, plants subjected to low or high injury had higher proportions of regenerated individuals than did plants originating from root fragments, and plants injured as rosettes had a higher proportion of regenerated individuals than plants injured during their reproductive phases (Table 2; Fig. 2A).
Table 2.
Summary of fitted models for characteristics obtained from the injury experiment with Barbarea vulgaris and Barbarea stricta
| Overall tests | Tests of B. vulgaris | Tests of B. stricta | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proportion of regenerated individuals (Fig. 3A) | ||||||||||||
| d.f. = 15, χ2 = 16·23, n.s. | d.f. | χ2 | P | d.f. | χ2 | P | ||||||
| Damage | 3 | 74·38 | ** | Damage | 3 | 372·81 | *** | |||||
| Phenophase | 3 | 64·69 | * | Phenophase | 3 | 299·66 | *** | |||||
| Damage × Phenophase | 9 | 59·32 | n.s. | Damage × Phenophase | 9 | 249·64 | *** | |||||
| d.f. | F | P | d.f. | F | P | d.f. | F | P | ||||
| Life span (Fig. 3B) | ||||||||||||
| d.f. = (16, 659), F = 16·84*** | ||||||||||||
| Species | 1 | 199·14 | *** | Damage | 4 | 9·29 | *** | Damage | 4 | 16·69 | *** | |
| Phenophase | 4 | 8·42 | *** | Phenophase | 3 | 3·40 | * | Phenophase | 3 | 15·41 | *** | |
| Damage | 3 | 14·81 | *** | Damage × Phenophase | 9 | 3·06 | ** | Damage × Phenophase | 9 | 11·62 | *** | |
| Species × Phenophase | 4 | 14·64 | *** | |||||||||
| Species × Damage | 3 | 24·55 | *** | z-value | P | z-value | P | |||||
| Phenophase × Damage | 9 | 8·52 | *** | Control vs. damaged plants | –5·81 | *** | Control vs. damaged plants | 0·63 | n.s. | |||
| Species × Phenophase × Damage | 9 | 12·71 | *** | |||||||||
| d.f. | F | P | d.f. | F | P | d.f. | F | P | ||||
| No. of all seeds (Fig. 3C) | ||||||||||||
| d.f. = (16, 659), F = 8·10*** | ||||||||||||
| Species | 1 | 129·94 | *** | Damage | 4 | 9·93 | *** | Damage | 4 | 19·42 | *** | |
| Phenophase | 4 | 40·71 | *** | Phenophase | 3 | 10·68 | *** | Phenophase | 3 | 35·71 | *** | |
| Damage | 3 | 5·44 | ** | Damage × Phenophase | 9 | 1·96 | * | Damage × Phenophase | 9 | 5·75 | *** | |
| Species × Phenophase | 4 | 12·19 | *** | |||||||||
| Species × Damage | 3 | 8·91 | *** | z-value | P | z-value | P | |||||
| Phenophase × Damage | 9 | 1·58 | n.s. | Control vs. damaged plants | 5·26 | *** | Control vs. damaged plants | 5·88 | *** | |||
| Species × Phenophase × Damage | 9 | 6·07 | *** | |||||||||
| d.f. | F | P | d.f. | F | P | d.f. | F | P | ||||
| No. of viable seeds (Fig. 3D) | ||||||||||||
| d.f. = (7, 677), F = 3·73*** | ||||||||||||
| Species | 1 | 511·49 | *** | Damage | 4 | 5·85 | *** | Damage | 4 | 6·08 | *** | |
| Phenophase | 4 | 13·55 | *** | Phenophase | 3 | 6·97 | *** | Phenophase | 3 | 16·28 | *** | |
| Damage | 3 | 6·40 | *** | Damage × Phenophase | 9 | 1·26 | n.s | Damage × Phenophase | 9 | 1·48 | n.s. | |
| Species × Phenophase | 4 | 5·81 | *** | |||||||||
| Species × Damage | 3 | 0·97 | n.s. | z-value | P | z-value | P | |||||
| Control vs. damaged plants | 3·59 | *** | Control vs. damaged plants | 3·20 | ** | |||||||
In the first column, both species are tested together; in the second and third columns, B. vulgaris and B. stricta, respectively, are tested separately. Effects of phenophase, damage type and species were tested.
Overall tests represent the tests of differences between the two species in each species’ combined effects of phenophase and damage type.
Degrees of freedom (d.f.), F-statistic or z-value and significance range are shown.
*P < 0·05; **P < 0·01; ***P < 0·00; n.s, non-significant.
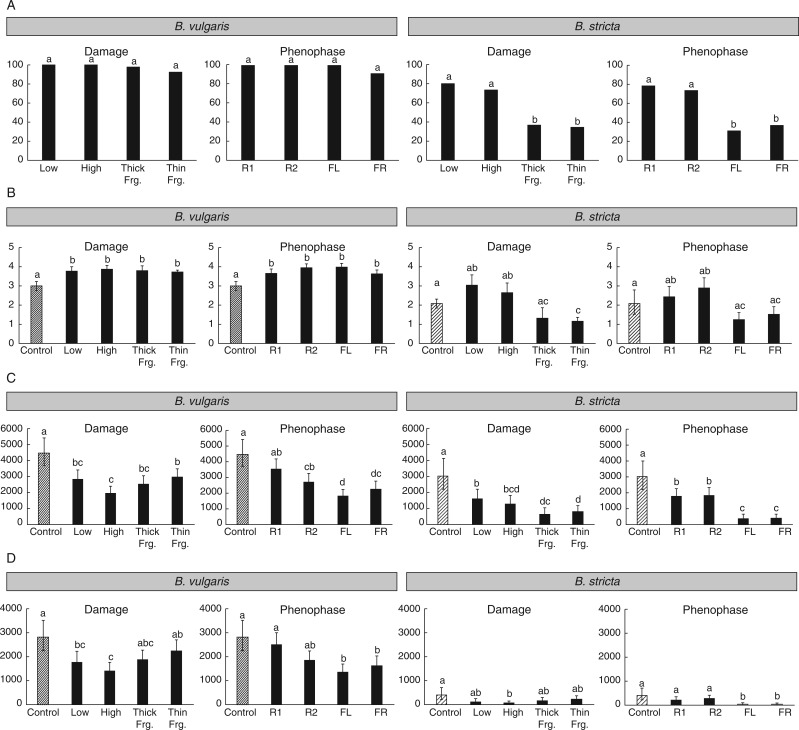
Fig. 2.
Box diagrams of the characteristics obtained from the experiment on Barbarea vulgaris and Barbarea stricta for individual injury treatments and phenological phases at which the injury was inflicted. (A) Proportion of regenerated individuals (%). (B) Life span (years). (C) Total number of seeds. (D) Number of viable seeds. Descriptions of damage types and phenological phases along with abbreviations are presented in Table 1. Means and 95 % confidence intervals are plotted for B, C and D; means are shown for A. Results of post-hoc tests are shown above the columns. ‘Thick Frg.’ and ‘Thin Frg.’ represent thick and thin fragment treatments, respectively.
Life span
Barbarea vulgaris and B. stricta differed significantly in their life spans, and their life spans were significantly influenced by both damage type and the phenophase during which the plant was injured (Table 2; Fig. 2B). Plants of B. vulgaris lived longer than plants of B. stricta without respect to injury. Damaged plants of B. vulgaris lived significantly longer than uninjured plants, however, and no difference in life span was found among damage types or among phenophases in this species if the control treatment was excluded (Fig. 2B). On the other hand, plants of B. stricta subjected to low or high injury had longer life spans than plants that originated from roots fragments, and plants injured as rosettes had a longer life span than plants injured during reproductive phases (Fig. 2B).
Whole-life seed production
Number of all seeds
Barbarea vulgaris and B. stricta differed in the responses their seed production showed to both phenophase at the time of injury and damage severity. Production of seeds was higher in B. vulgaris than in B. stricta, without respect to injury (Table 2; Fig. 2C). In both species, total seed production was significantly greater for control plants compared with damaged plants, and those injured during rosette phases had greater seed production than those injured during reproductive phases (Table 2; Fig. 2C).
Number of viable seeds
Differences between B. vulgaris and B. stricta were much larger in the number of viable seeds than in the number of all seeds (Table 2; Fig. 2D). In B. vulgaris, the number of viable seeds did not differ between control plants and plants that originated from roots fragments (Table 2; Fig. 2D). In B. stricta, no significant effect of damage type was found when control plants were excluded (Table 2; Fig. 2D). No difference in the number of viable seeds between control plants and plants injured during rosette stages was found in either species (Table 2; Fig. 2D).
Temporal concentration of seed production in relation to life span
Barbarea vulgaris and B. stricta always flowered and fruited in the second season after germination (control plants) or injury (other treatments) and then reproduced each subsequent year of their lives. Life spans of both species were significantly influenced by the temporal concentration of seed production (Table 3). For both species, when considering all seeds, post-hoc tests showed significant negative relationships between temporal concentration of seed production and life span only for control plants and plants injured during rosette phases (Table 3). However, when considering only viable seeds, a similar negative relationship between concentration of seed production and lifespan was found in control and uninjured plants of B. vulgaris and those plants injured at rosette phases (Table 3B). In B. stricta, in contrast, neither the interaction of temporal concentration of seed production and damage nor the interaction of temporal concentration of seed production and phenophase at time of injury significantly influenced life span (Table 3). No significant positive relationship between temporal concentration of seed production and life span was found in either species.
Table 3.
Summary of models describing the effects of two calculated characteristics on the lifespans of Barbarea vulgaris and Barbarea stricta
|
B. vulgaris |
B. stricta | |||||||
|---|---|---|---|---|---|---|---|---|
| Temporal concentration of all seed production (TSconc) | ||||||||
| d.f. | F | P | d.f. | F | P | |||
| TSconc | 1 | 10·15 | ** | TSconc | 1 | 23·05 | *** | |
| Phenophase | 4 | 51·07 | *** | Phenophase | 4 | 37·72 | *** | |
| Damage | 3 | 4·58 | ** | Damage | 3 | 1·33 | n.s. | |
| TSconc × Phenophase | 4 | 20·93 | *** | TSconc ×phenophase | 4 | 4·87 | *** | |
| TSconc × Damage | 3 | 2·25 | n.s. | TSconc × damage | 3 | 2·91 | * | |
| Phenophase × Damage | 9 | 3·17 | ** | Phenophase × damage | 7 | 0·22 | n.s. | |
| TSconc × Phenophase × Damage | 9 | 1·48 | n.s. | TSconc × phenophase × damage | 5 | 2·04 | n.s. | |
| Post-hoc tests | Lower CI | Upper CI | Post-hoc tests | Lower CI | Upper CI | |||
| Control | –1·486 | –0·978 | Low injury at R1 | –1·864 | –0·818 | |||
| Low injury at R1 | –1·673 | –0·759 | Thick fragment at R1 | –1·016 | –0·097 | |||
| High injury at R1 | –1·392 | –0·048 | Thin fragment at R1 | –2·146 | –0·879 | |||
| Thick fragment at R1 | –0·887 | –0·124 | Thick fragment at R2 | –1·428 | –0·152 | |||
| Low injury at R2 | –0·785 | –0·198 | Thick fragment at FL | –1·975 | –0·098 | |||
| Temporal concentration of viable seed production (VSconc) | ||||||||
| d.f. | F | P | d.f. | F | P | |||
| VSconc | 1 | 8·83 | ** | VSconc | 1 | 7·42 | ** | |
| Phenophase | 4 | 46·85 | *** | Phenophase | 4 | 16·11 | *** | |
| Damage | 3 | 4·55 | ** | Damage | 3 | 0·68 | n.s. | |
| VSconc × Phenophase | 4 | 15·95 | *** | VSconc × Phenophase | 4 | 1·26 | n.s. | |
| VSconc × Damage | 3 | 2·93 | * | VSconc × Damage | 3 | 0·75 | n.s. | |
| Phenophase × Damage | 9 | 2·72 | ** | Phenophase × Damage | 7 | 0·10 | n.s. | |
| VSconc × Phenophase × Damage | 9 | 1·74 | n.s. | VSconc × Phenophase × damage | 5 | 0·27 | n.s. | |
| Post-hoc tests | Lower CI | Upper CI | Post-hoc tests | Lower CI | Upper CI | |||
| Control | –1·454 | –0·906 | No significant relationship found | |||||
| Low injury at R1 | –1·579 | –0·674 | ||||||
| Thick fragment at R1 | –1·113 | –0·202 | ||||||
| Low injury at R2 | –1·012 | –0·283 | ||||||
Results for B. vulgaris are shown in the first column and results for B. stricta in the second column.
TSconc = the ratio of maximal seed production within one season to whole-life seed production; VSconc = the ratio of maximal viable seed production within one season to whole-life viable seed production.
Degrees of freedom (d.f.), F-statistic and significance are shown.
*P < 0·05; **P < 0·01; ***P < 0·00; n.s., non-significant.
Results of post-hoc tests are shown for significant model effects only; lower CI and upper CI define the 95 % confidence interval.
Descriptions of damage types and phenological phases (and their abbreviations) are in Table 1.
Relationship between seed production in the first reproductive season and subsequent probability of plant death
Both measures of seed production during the first reproductive season showed significant correlations with the probability of subsequent plant death. In the case of all seeds, the probability that a plant would die during the following growing season was higher for plants that produced a greater number of seeds, and this relationship was stronger for B. vulgaris than for B. stricta (Table 4). In the case of viable seeds, the number of seeds produced during the first reproductive season was also positively correlated with the probability of plant death during the next growing season; however, this correlation was weaker for B. vulgaris. However, for uninjured B. stricta, the relationship was opposite to the overall pattern, as for these plants production of viable seeds during the first reproductive season was negatively correlated with probability of death during the following season (Table 4).
Table 4.
Summary of the models describing the effects of the first reproductive output on the probability of plant death in the following growing season in Barbarea vulgaris and Barbarea stricta
| d.f. |
χ2 | P | Estimated effect size | Exp (coef) | |
|---|---|---|---|---|---|
| No. of all seeds | |||||
| Species | 1 | 26·86 | *** | Damage_YES | 0·236 |
| Damage | 1 | 106·68 | *** | Species_vulgaris | 0·350 |
| Seed.First | 1 | 6·09 | * | Seed.First | 1·001 |
| Species × Damage | 1 | 1·23 | n.s. | Species_vulgaris × Seed.First | 1·002 |
| Species × Seed.First | 1 | 4·45 | * | ||
| Damage × Seed.First | 1 | 2·40 | n.s. | ||
| Species × Damage × Seed.First | 1 | 0·03 | n.s. | ||
| No. of viable seeds | |||||
| Species | 1 | 26·86 | *** | Species_vulgaris | 0·308 |
| Damage | 1 | 106·68 | *** | Damage_YES | 0·142 |
| Viable.First | 1 | 0·95 | n.s. | Species_stricta × Damage_NO × Viable.First | 0·999 |
| Species ×Damage | 1 | 1·06 | n.s. | Species_vulgaris*damage_NO × Viable.First | 1·001 |
| Species × Viable.First | 1 | 17·76 | *** | Species_stricta × Damage_YES × Viable.First | 1·003 |
| Damage × Viable.First | 1 | 1·31 | n.s. | species_vulgaris × Damage_YES*Viable.First | 1·001 |
| Species × Damage × Viable.First | 1 | 15·23 | *** |
Reproductive output was expressed as total number of seeds produced during the first reproductive season (Seed.First) and the number of viable seeds produced during the first reproductive season (Viable.First). The effects of species, damage, first reproductive output, and their interactions on plant death probability were tested.
Degrees of freedom (d.f.), χ2 value, and significance are shown.
*P < 0·05; **P < 0·01; ***P < 0·00; n.s., non-significant.
Effect sizes for significant terms of a Cox proportional hazard model are shown as Exp(coef), representing the multiplicative effect of the predictor on the basal hazard rate.
Seed characteristics
Weight of one seed
In B. vulgaris, the average weight of a seed was significantly influenced by plant age and also by age–damage and age–phenophase interactions (Table 5). However, there was no overall difference in seed weight between control and damaged plants; moreover, the difference between control and damaged plants in decrease of seed weight with age was not significant (Table 5). In plants injured in the first-year rosette (R1) phenophase, seed weight decreased with age irrespective of damage type (Table 5; Fig. 3A), but seeds produced by plants subjected to low or high damage treatment were somewhat lighter (Table 6). Similarly, for R2 plants, there was no interaction between time and damage, but the effect of plant age was relatively weaker and the effect of damage stronger when compared with R1 plants (Table 5; Fig. 3A). This was mainly due to a larger drop in seed weight for plants in the high injury treatment (Table 6). In the FL phenophase, there was a different pattern from that for the rosette stages: the main effect of damage was not significant, but its interaction with age was strong (Table 5; Fig. 3A). Post-hoc tests showed that the decrease in seed weight with age was larger for plants that originated from root fragments than for plants with low or high damage treatments (Table 6). For FR plants, the decrease of seed weight was not affected by damage type at all.
Table 5.
Summary of statistical models examining effects of time (plant age) and experimentally manipulated factors on weight of one seed and seed germination rate of Barbarea vulgaris and Barbarea stricta
|
Weight of one seed (g ) d.f. = 27, χ2 = 89·18*** (Fig. 3A) |
Seed germination (%) d.f.= 27, χ2 = 55·14 ** (Fig. 3B) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
B. vulgaris |
B. stricta |
B. vulgaris |
B. stricta |
||||||||||||
| d.f. | χ2 | P | d.f. | χ2 | P | d.f. | χ2 | P | d.f. | χ2 | P | ||||
| Full model | Time | 1 | 187·27 | *** | 1 | 1·53 | n.s. | Full model | Time | 1 | 190·92 | *** | 1 | 78·52 | *** |
| Phenophase | 3 | 16·91 | ** | 3 | 7·13 | n.s. | Phenophase | 3 | 11·18 | * | 3 | 5·44 | n.s. | ||
| Damage | 4 | 17·37 | *** | 4 | 2·24 | n.s. | Damage | 4 | 1·79 | n.s. | 4 | 28·84 | *** | ||
| Phenophase × Damage | 9 | 18·16 | * | 7 | 20·01 | ** | Phenophase × Damage | 9 | 8·49 | n.s. | 7 | 9·13 | n.s. | ||
| Time × Damage | 4 | 13·69 | ** | – | – | – | Time × Damage | – | – | – | 4 | 19·05 | *** | ||
| Time × Phenophase | 3 | 8·63 | * | – | – | – | Time × Shenophase | 4 | 27·97 | *** | – | – | – | ||
| Time × Damage × Phenophase | 9 | 24·59 | ** | – | – | – | Time × Damage × Phenophase | – | – | – | – | – | – | ||
| Control vs damaged plants | Time | 1 | 188·40 | *** | 1 | 2·70 | n.s. | Control vs damaged plants | Time | 1 | 186·63 | *** | 1 | 70·95 | *** |
| Damage | 1 | 3·11 | n.s. | 1 | 0·61 | n.s. | Damage | 1 | 0·11 | n.s. | 1 | 0·44 | n.s. | ||
| Time × Damage | 1 | 2·99 | n.s. | 1 | 4·32 | * | Time × Damage | 1 | 10·38 | * | 1 | 8·03 | ** | ||
| Partial model for R1 | Time | 1 | 107·32 | *** | 1 | 3·97 | * | Partial model for R1 | Time | 1 | 46·07 | *** | Phenophase not significant, so analysing data separately by phenophase not constructive | ||
| Damage | 3 | 8·95 | * | 3 | 6·58 | n.s. | Damage | 3 | 1·33 | n.s. | |||||
| Time × Damage | 3 | 6·25 | n.s. | 3 | 34·39 | *** | Time × Damage | 3 | 0·71 | n.s. | |||||
| Partial model for R2 | Time | 1 | 17·09 | *** | 1 | 7·06 | ** | Partial model for R2 | Time | 1 | 97·92 | *** | |||
| Damage | 3 | 14·32 | ** | 3 | 1·12 | n.s. | Damage | 3 | 4·77 | n.s. | |||||
| Time × Damage | 3 | 5·48 | n.s. | 3 | 2·49 | n.s. | Time × Damage | 3 | 1·50 | n.s. | |||||
| Partial model for FL | Time | 1 | 38·13 | *** | 1 | 4·29 | * | Partial model for FL | Time | 1 | 23·37 | *** | |||
| Damage | 3 | 1·76 | n.s. | 2 | 0·19 | n.s | Damage | 3 | 3·58 | n.s. | |||||
| Time × Damage | 3 | 17·38 | *** | 1 | 0·30 | n.s. | Time × Damage | 3 | 9·27 | * | |||||
| Partial model for FR | Time | 1 | 20·58 | *** | 1 | 1·53 | n.s. | Partial model for FR | Time | 1 | 81·01 | *** | |||
| Damage | 3 | 0·83 | n.s. | 2 | 4·41 | n.s. | Damage | 3 | 3·39 | n.s. | |||||
| Time × Damage | 3 | 2·64 | n.s. | 1 | 3·97 | * | Time × Damage | 3 | 1·07 | n.s. | |||||
In addition to the full model, separate models were constructed for individual phenological phases and for comparison of control vs. damaged plants.
Degrees of freedom (d.f.), χ2 statistic, and significance values are shown.
*P < 0·05; **P < 0·01; ***P < 0·00; n.s., non-significant.
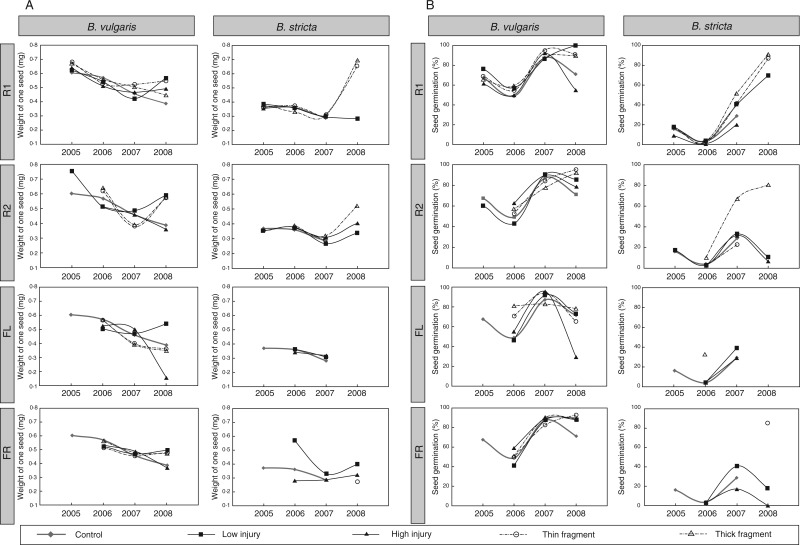
Fig. 3.
Time diagrams of the characteristics obtained from the experiment with Barbarea vulgaris and Barbarea stricta. (A) Average weight of one seed (mg). (B) Seed germination rate (%). Each part is divided into four rows corresponding to four different phenological phases at which injury to the plant body was inflicted and into two columns corresponding to species. Descriptions of damage types and phenological phases along with abbreviations are presented in Table 1. Means for individual injury treatments are shown in Fig. 2.
Table 6.
Sizes of significant effects in Barbarea vulgaris and Barbarea stricta of time (age) on average single-seed weight (g) estimated for individual damage types in models fitted separately for plants damaged at different phenophases
|
B. vulgaris |
B. stricta |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lower CI | Upper CI | Lower CI | Upper CI | ||||||
| R1 | Low injury | –0·180 | –0·014 | R1 | Thick Frg. | 0·018 | 0·120 | ||
| High injury | –0·161 | –0·017 | Thin Frg. | 0·016 | 0·111 | ||||
| R2 | High injury | –0·267 | –0·057 | R2 | No significant relationship | ||||
| FL | Low injury | –0·260 | –0·115 | FL | No significant relationship | ||||
| High injury | –0·278 | –0·125 | |||||||
| Thick Frg. | –0·308 | –0·159 | |||||||
| Thin Frg. | –0·298 | –0·142 | |||||||
| FR | No significant relationship | FR | No significant relationship | ||||||
Only significant effect sizes are shown; lower CI and upper CI represent the 95 % confidence interval for the effect size.
In B. stricta, there was no main effect of plant age, damage or phenophase, as only the interaction between damage and phenophase was significant (Table 5). This suggests no significant change of seed weight with age. Testing control plants separately from those whichwere damaged, seed weight decreased slightly with age for control plants, but not for damaged plants (Table 5: post-hoc effect for control plants, 95 % CI = –0·120 to –0·005; for injured plants, 95 % CI = –0·063 to 0·006). For R1 plants, seed weight increased with age for plants that originated from root fragments, but did not change for plants subjected to the low or high injury treatment (Table 6). Seed weight decreased with age for R2 and FL plants irrespective of the damage type (Table 5; Fig. 3A). For plants injured in the fruiting (FR) phenophase, seed weight did not change with age, nor did seed weight respond to damage (Table 5; Fig. 3A).
Seed germination
In B. vulgaris, the germination rate increased with plant age and was also affected by the stage at which the plant was damaged but not by the type of damage (Table 5). The germination rates differed between control and damaged plants (Table 5), and increased more slowly with age in control than in damaged plants (post-hoc effect for control plants, 95 % CI = 0·116–0·183; for injured plants, 95 % CI = 0·145–0·193). In R1, R2 and FR plants, germination increased with age, but no effect of damage type was found (Table 5; Fig. 3B). In FL plants, germination increased with age and was also weakly affected by damage type.
In B. stricta, the germination rates differed significantly among damage types but not among plant phenophases (Table 5). The germination rate generally increased with age, and damaged plants increased their germination rate over the years slightly more quickly than did the control plants (Table 5: post-hoc effect for damaged plants, 95 % CI = 0·100–0·249; for control plants, 95 % CI = 0·145–0·234). The germination rate generally increased for all treatments (Fig. 3B). Plants that originated from root fragments showed a more rapid increase in germination rate of their seeds over the years than did plants in the low or high damage treatment (post-hoc effects: low damage, 95 % CI = 0·087–0·244; high damage, 95 % CI = 0·023–0·237; thick fragment 95 % CI = 0·215–0·396; thin fragment, 95 % CI = (0·194–0·398).
DISCUSSION
Life span and survival after injury
Severe injury to the plant body caused prolongation of life and spread of reproduction to more growing seasons, in agreement with our expectations. However, even control plants did not behave as monocarps, as has already been mentioned in the literature (e.g. MacDonald and Cavers, 1991); nevertheless Barbarea species are still erroneously considered to be biennials. Despite this, injury caused postponement of reproduction and reset of the ageing clock (Salguero-Gómez and Casper, 2010). The life span of injured individuals, however, was only one season longer than that of uninjured plants and only in Barbarea vulgaris. In the case of Barbarea stricta, no significant prolongation of plant life was found, and, additionally, more severe injury during later phenological phases shortened the life span of B. stricta in comparison with control plants. The proportion of individuals that regenerated after injury was nearly 100 % in B. vulgaris; however, regeneration of B. stricta was significantly lower, especially for individuals severely injured at later phenological phases. These results probably reflect the habitat preferences of these species and thus their life strategies. Both species prefer disturbed habitats, but of different types. Barbarea vulgaris prefers man-made habitats (Dvořák, 1992) in which disturbance acts unpredictably and a dominant shoot is frequently removed or even roots are fragmented. In such habitats, the resprouting strategy is not restricted and allows the injured individual to finish its reproductive cycle (Sparrow and Bellingham, 2001; Vesk and Westoby, 2004). On the other hand, B. stricta occurs in wet or flooded areas (Dvořák, 1992) where severe injury resulting in loss of the main shoot’s apical dominance does not usually occur and resprouting is also restricted by anoxia (Sosnová and Klimešová, 2013). Therefore, B. stricta instead employs the seeding strategy (Grime, 2001), although some of its ability to resprout after severe injury seems to be inherited from a common ancestor. Injury does not serve as a tool to escape from senescence and effectively prolong life span in either Barbarea species since rejuvenation is probably restricted by resprouting abilities, more strongly in B. stricta.
Although we aimed to mimic natural conditions as much as possible and consider our disturbance regime realistic for the two studied species, the experiment was performed in pots without competition, probably affecting plant outputs (Poorter et al., 2012). In natural communities, weak, old plants would probably not reproduce or survive due to competition; however, competition is not usually high in the habitats of these two Barbarea species.
Whole-life fecundity
We expected that life span prolongation and increase in the number of reproductive seasons would increase whole-life fecundity, similarly to the previously described positive effect of herbivores on plant fitness (Lehtilä and Strauss, 1999; Agrawal, 2000). However, in our experiment, in all cases, whole-life fecundity was significantly lower than or at most equal to fecundity of uninjured plants. No overcompensation in terms of the number of seeds was found. Therefore, even the prolongation of life found in B. vulgaris (but not in B. stricta) did not increase the fitness of injured plants in comparison with uninjured ones, and thus the hypothesis that severe biomass removal can increase fitness of B. vulgaris proposed by Martínková et al. (2008) was not supported. In the tested Barbarea species, senescence expressed as declining seed production (Lacey et al., 2003; Van Dijk, 2009; Munné-Bosch, 2008) was not eliminated by the injury, but only decelerated.
The ratio of the number of all seeds and the number of viable seeds (see Fig. 2C, D) was not changed by injury in B. vulgaris. However, in B. stricta, the proportion of viable seeds to all seeds increased in injury treatments (see Fig. 2C, D). We presume that the seeds that are not able to germinate immediately after harvest are those that form the seed bank (Baskin and Baskin 1989) or have poor quality or aborted embryos (Müller, 2014). Variability in seed dormancy of B. vulgaris has already been reported (Taylorson, 1970). Therefore, plant flexibility in the proportion of actually germinable seeds can influence the number of new individuals that immediately restore a population after severe disturbance. Plants of B. stricta that survive severe biomass removal probably invest in immediate restoration of population rather than in the seed bank. On the other hand, B. vulgaris, with its higher resprouting efficiency, relies on its resprouting ability, and its seed bank formation is not influenced. However, this hypothesis should be tested by, for example, performing a tetrazolium test of seed viability (Porter et al., 1947) to assess the ratio of dormant seeds to seeds with aborted embryos.
First reproduction and temporal concentration of seed production
Short-lived species invest their stored reserves in a single reproductive event that is followed by senescence and death (Silvertown et al., 2001). We expected that injury to the plant body would cause seeding to be spread over more than one season due to the removal of apical dominance (Davies and Gan, 2012) and consequent formation of many adventitious shoots of various sizes (Bartušková and Klimešová, 2010). We also expected that life span would be negatively correlated with the number of seeds formed during the first reproductive season. Thus, the occurrence of greater seed production during the first reproductive season for plants that had been injured resulted in a shorter life span for two reasons. First, the number of seeds is related to the amount of reserves intended for seed production (Davies and Gan, 2012), and the more the plant invests into reproduction, the less it can invest in survival (Sosnová and Klimešová, 2009). Secondly, the high investment in reproduction is tied to the existence of a dominant shoot having strong apical dominance that hinders further resprouting – in contrast to what would be the behaviour of numerous equivalent shoots. Thus, due to both exhaustion of reserves and suppression of additional sprouting, senescence is inevitable. Indeed, in our experiment, we found no positive relationship between the number of seeds formed during the first reproductive season and plant life span. Similarly, a higher concentration of seed production in any vegetative season causes a shorter life span. However, negative effects on life span from both first reproductive output and reproduction concentration disappear with advancing phenological phase at the time of injury and with removal of all axillary buds. Therefore, disturbance causes equalization of subsequent reproductive events, and signs of senescence are somewhet alleviated in Barbarea subjected to it, similarly to the case of Cistus albidus, reported by Müller et al. (2014).
Injury severity and phenophase at the time of injury
Regrowth after injury and subsequent reproduction depend on the amount of available reserves (Banta et al., 2010; Latzel et al., 2014). The amount of available reserves is closely related to the severity of injury and also to the phenological phase during which the plant is injured. A less advanced life history stage at the time of injury and a larger remaining plant body can provide higher amounts of stored reserves for resprouting (Martínková et al., 2008, 2015; Sosnová et al., 2014). Therefore, more severely injured plants and plants injured during reproduction will form weaker adventitious shoots without strict dominance, in contrast to less severely injured vegetative rosettes in which one adventitious shoot will dominate. It is thus probable that signs of senescence will be less obvious in plants that have suffered more severe injury at later phenological phases. Correspondingly, we found that the difference between first reproduction and reproduction in subsequent years diminished with both injury severity and progressive life stages at which the plants were injured. Moreover, whole-life production of viable seeds in severely injured plants is comparable with whole-life production of viable seeds in uninjured plants. Senescence in the studied species is not markedly influenced by either the severity of injury or its timing, but it is generally alleviated more upon suffering more severe disturbance in later phenological phases.
Seed weight and germination rate
Although Lanner and Connor (2001) did not find an effect of age on seed weight and viability, a decrease in seed weight with plant age can be seen as a sign of senescence (Brutovská et al., 2013), and is closely related to plant fitness. In our experiment, we found a distinct decrease in seed weight with age. Because seed weight decreases with age, decreasing germinability with age can be expected (Sánchez et al., 2012; Müller et al., 2014). Contrary to such an expectation, in our experiment, the germination rate increased with age in all cases and more quickly for injured than for uninjured plants, in B. stricta much more strongly than in B. vulgaris. The decrease in seed weight with age was not different between injured and uninjured plants of B. vulgaris; however, in B. stricta, a decrease in seed weight with age was found for uninjured plants. Therefore, it seems that in B. vulgaris injury to the plant body causes different investment in seed mass and does not alleviate signs of senescence in terms of seed weight. However, in B. stricta an opposite effect was found. It has already been discussed that B. stricta is probably able to influence formation of its seed bank in response to severe disturbance, and our above-mentioned findings regarding its seed weight and germinability suggest that it is probably via these two variables that this is accomplished. Barbarea stricta reacts to disturbance by changes in seed characteristics, but B. vulgaris reacts to disturbance by resprouting, with its seed characteristics not influenced to such a great extent.
CONCLUSION
In a 5-year long garden experiment, it was found that disturbance prolongs life span and multiplies the number of reproductive seasons in B. vulgaris. However, the majority of injured plants had a life span only one season longer than control plants. Moreover, disturbance decreased whole-life seed production of both B. vulgaris and B. stricta. Resprouting rescues injured plants and, even if it leads to undercompensation in seed production in comparison with uninjured plants, it does allow substantial seed production and the opportunity to pass this ability on to the following generations. In B. vulgaris and B. stricta, senescence is either slightly postponed or decelerated by injury, or is unaffected by it, and thus injury does not cause effective rejuvenation. However, B. stricta subjected to severe removal of above-ground biomass showed increases both in the number of germinable seeds and in seed weight, suggesting that this species invests more in immediate germination than in seed bank maintenance when it faces destructive disturbance.
Our results imply that short-lived plants subjected to disturbance may postpone senescence and that this effect is species specific. However, further research is needed to be able to generalize the results to all herbs, including perennials.
ACKNOWLEDGEMENTS
This study was supported by the Grant Agency of the Czech Republic (The Centre of Excellence PLADIAS 14-36079G). P.Š. was supported by project GAJU 04-142/2010/P. We are indebted to Jonathan Rosenthal for his linguistic help.
LITERATURE CITED
- Agrawal AA. 2000. Overcompensation of plants in response to herbivory and the by-product benefits of mutualism. Trends in Plant Science 5: 309–313. [DOI] [PubMed] [Google Scholar]
- Araki K, Shimatani K, Nishizawa M, Yoshizane T, Ohara M. 2010. Growth and survival patterns of Cardiocrinum cordatum var. glehnii (Liliaceae) based on a 13-year monitoring study: life history characteristics of a monocarpic perennial herb. Botany 88: 745–752. [Google Scholar]
- Banta JA, Stevens MHH, Pigliucci M. 2010. A comprehensive test of the ‘limiting resources’ framework applied to plant tolerance to apical meristem damage. Oikos 119: 359–369. [Google Scholar]
- Barks PM, Laird RA. 2015. Senescence in duckweed: age-related declines in survival, reproduction and offspring quality. Functional Ecology 29: 540–548. [Google Scholar]
- Bartušková A, Klimešová J. 2010. Reiteration in the short lived root-sprouting herb Rorippa palustris: does the origin of buds matter? Botany 88: 630–638. [Google Scholar]
- Baskin JM, Baskin CC. 1989. Physiology of dormancy and germination in relation to seed bank ecology In: Leck MA, Parker VT, Simpson RT, eds. Ecology of soil seed bank. London: Academic Press, 53–66. [Google Scholar]
- Baudisch A, Salguero-Gómez R, Jones OR, et al. 2013. The pace and shape of senescence in angiosperms. Journal of Ecology 101: 596–606. [Google Scholar]
- Bretz F, Hothorn ET, Westfall P. 2011. Multiple comparisons using R. Boca Raton: CRC Press. [Google Scholar]
- Brutovská E, Sámelová A, Dušička J, Mičieta K. 2013. Ageing of trees: application of general ageing theories. Ageing Research Reviews 12: 855–865. [DOI] [PubMed] [Google Scholar]
- Davies PJ, Gan S. 2012. Towards an integrated view of monocarpic plant senescence. Russian Journal of Plant Physiology 59: 467–478. [Google Scholar]
- Davison R, Boggs CL, Baudisch A. 2014. Resource allocation as a driver of senescence: life history tradeoffs produce age patterns of mortality. Journal of Theoretical Biology 360: 251–262. [DOI] [PubMed] [Google Scholar]
- Dvořák F. 1992. 14. Barbarea R. Br In: Hejný S, Slavík B, eds. Flora of the Czech Republic (Vol. 3). Praha: Academia, 72–76. [Google Scholar]
- Garcia MB, Dahlgren JP, Ehrlen J. 2011. No evidence of senescence in a 300-year-old mountain herb. Journal of Ecology 99: 1424–1430. [Google Scholar]
- Grime JP. 2001. Plant strategies, vegetation processes and ecosystem properties. Chichester: Wiley. [Google Scholar]
- Harper JL. 1977. Population biology of plants. London: Academic Press. [Google Scholar]
- Huhta AP, Hellstrom K, Rautio P, Tuomi J. 2003. Grazing tolerance of Gentianella amarella and other monocarpic herbs: why is tolerance highest at low damage levels? Plant Ecology 166: 49–61. [Google Scholar]
- Kirkwood TBL. 1977. Evolution of ageing. Nature 270: 301–304. [DOI] [PubMed] [Google Scholar]
- Klimešová J, Martínková J. 2004. Intermediate growth forms as a model for the study of plant clonality functioning: an example with root sprouters. Evolutionary Ecology 18: 669–681. [Google Scholar]
- Klimešová J, Sosnová M, Martínková J. 2007. Life-history variation in the short-lived herb Rorippa palustris: effect of germination date and injury timing. Plant Ecology 189: 237–246. [Google Scholar]
- Klimešová J, Nobis MP, Herben T. 2014. Senescence, ageing and death of the whole plant: morphological prerequisites and constraints of plant immortality. New Phytologist 206: 147–18. [DOI] [PubMed] [Google Scholar]
- Lacey PE, Roach DA, Herr D, Kincaid S, Perrott R. 2003. Multigenerational effects of flowering and fruiting phenology in Plantago lanceolata. Ecology 84: 2462–2475. [Google Scholar]
- Lanner RM, Connor KF. 2001. Does bristlecone pine senesce? Experimental Gerontology 36: 675–685. [DOI] [PubMed] [Google Scholar]
- Latzel V, Janeček Š, Hájek T, Klimešová J. 2014. Biomass and stored carbohydrate compensation after above-ground biomass removal in a perennial herb: does environmental productivity play a role? Folia Geobotanica 49: 17–29. [Google Scholar]
- Lehtilä K, Strauss SY. 1999. Effects of foliar herbivory on male and female reproductive traits of wild radish, Raphanus raphanistrum. Ecology 80: 116–124. [Google Scholar]
- MacDonald MA, Cavers PB. 1991. The biology of Canadian weeds. 97. Barbarea vulgaris R. Br. Canadian Journal of Plant Science 71: 149–166. [Google Scholar]
- Martínková J, Klimešová J, Mihulka S. 2008. Compensation of seed production after severe injury in the short-lived herb Barbarea vulgaris. Basic and Applied Ecology 1: 44–54. [Google Scholar]
- Martínková J, Klimešová J, Doležal J, Kolář F. 2015. Root sprouting in Knautia arvensis (Dipsacaceae): effects of polyploidy, soil origin and nutrient availability. Plant Ecology 216: 901–911. [Google Scholar]
- Mencuccini M, Onate M, Penuelas J, Rico L, Munné-Bosch S. 2014. No signs of meristem senescence in old Scots pine. Journal of Ecology 102: 555–565. [Google Scholar]
- Morales M, Munné-Bosch S. 2015. Secret of long life lies underground. New Phytologist 205: 463–467. [DOI] [PubMed] [Google Scholar]
- Müller M, Siles L, Cela J, Munné-Bosch S. 2014. Perennially young: seed production and quality in controlled and natural populations of Cistus albidus reveal compensatory mechanisms that prevent senescence in terms of seed yield and viability. Journal of Experimental Botany 65: 287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munné-Bosh S. 2008. Do perennials really senesce? Trends in Plant Science 13: 216–220. [DOI] [PubMed] [Google Scholar]
- Munné-Bosch S. 2014. Perennial roots to immortality. Plant Physiology 166: 720–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munné-Bosch S. 2015. Senescence: is it universal or not? Trends in Plant Science 20: 713–720. [DOI] [PubMed] [Google Scholar]
- Pinheiro JC, Bates DM. 2000. Mixed-effect models in S and S-PLUS. New York: Springer. [Google Scholar]
- Porter R, Durrell M, Romm H. 1947. The use of 2, 3, 5-triphenyl-tetrazoliumchloride as a measure of seed germinability. Plant Physiology 22: 149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorter H, Buhler J, van Dusschoten D, Climent J, Postma JA. 2012. Pot size matters: a meta-analysis of the effects of rooting volume on plant growth. Functional Plant Biology 39: 839–850. [DOI] [PubMed] [Google Scholar]
- R Core Team. 2013. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing, http://www.r-project.org [Google Scholar]
- Salguero-Gómez R, Casper BB. 2010. Keeping plant shrinkage in geographic loop. Journal of Ecology 98: 312–323. [Google Scholar]
- Salguero-Gómez R., Shefferson RP, Hutchings MJ. 2013. Plants do not count… or do they? New perspectives on the universality of senescence. Journal of Ecology 101: 545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez AM, Albert MJ, Rodríguez M, Escudero A. 2012. Extended flowering in a Mediterranean shrub: seasonal variability in seed quality and quantity. Flora 207: 821–827. [Google Scholar]
- Silvertown J, Franco M, Perez-Ishiwara R. 2001. Evolution of senescence in iteroparous perennial plants. Evolutionary Ecology Research 3: 393–412. [Google Scholar]
- Sklensky DE, Davies PJ. 2011. Resource partitioning to male and female flowers of Spinacia oleracea L. in relation to whole-plant monocarpic senescence. Journal of Experimental Botany 62: 4323–4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosnová M., Klimešová J. 2009. Life-history variation in the short-lived herb Rorippa palustris: the role of carbon storage. Acta Oecologica 35: 691–697. [Google Scholar]
- Sosnová M, Klimešová J. 2013. The effects of flooding and injury on vegetative regeneration from roots: a case study with Rorippa palustris. Plant Ecology 214: 999–1006. [Google Scholar]
- Sosnová M, Herben T, Martínková J, Bartušková A, Klimešová J. 2014. To resprout or not to resprout? Modelling population dynamics of a root-sprouting monocarpic plant under various disturbance regimes. Plant Ecology 215: 1245–1254. [Google Scholar]
- Sparrow AD, Bellingham PJ. 2001. More to resprouting than fire. Oikos 94: 195–197. [Google Scholar]
- Taylorson RB. 1970. Changes in dormancy and viability of weed seeds in soils. Weed Science 18: 265–269. [Google Scholar]
- Therneau TM, Grambsch PM. 2000. Modelling survival data: extending the Cox model. New York: Springer. [Google Scholar]
- Thomas H. 2013. Senescence, ageing and death of the whole plant. New Phytologist 197: 696–711. [DOI] [PubMed] [Google Scholar]
- Thomas H, Ougham HJ, Wagstaff C, Stead AD. 2003. Defining senescence and death. Journal of Experimental Botany 54: 1127–1132. [DOI] [PubMed] [Google Scholar]
- Van Dijk H. 2009. Ageing effects in an iteroparous plant species with a variable lifespan. Annals of Botany 104: 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesk PA, Westoby M. 2004. Sprouting ability across diverse disturbances and vegetation types worldwide. Journal of Ecology 92: 310–320. [Google Scholar]