Abstract
Proponents of personalized medicine predict that genetic information will provide pivotal perspectives for the prevention and management of complex disorders. Personalized medicine differs from traditional Western medicine, in that it focuses on more complex disorders that require mechanistic disease modeling and outcome simulation by integrating genomic risk, environmental stressors, and biomarkers as indicators of disease state. This information could be useful to guide targeted therapy and prevent pathologic outcomes. However, gaps exist in the process of linking the pieces together; currently, genetic data are seldom used to assist physicians in clinical decision making. With rapid growth in genetic data and the requirements for new paradigms for complex disorders comes the need to train professionals to understand and manage the impact of genetic information on patients within these clinical settings. Here we describe the challenges, controversies, and opportunities for genetics and genetic counselors in managing complex disorders and discuss the rationale for modifications in genetic counselor training and function. We conclude that a major paradigm shift is underway and a compelling functional, ethical, and financial argument can be made for employing properly trained genetic counselors to be strategically positioned within the health-care industries that are responsible for managing complex disorders.
INTRODUCTION
Since the Human Genome Project was completed in 2003, the volume of genetic information linked to human disease expanded exponentially.1 Development of new technologies, particularly next-generation sequencing and increased computer capacity, facilitates the continual generation and widening accessibility of genomic information. This new wealth of information provides opportunities to advance medical care, improve health outcomes, and cut costs through more effective management of complex conditions.
The term “personalized medicine” has been used in the literature with a number of different definitions.2 Here, we define personalized medicine as an alternative paradigm to the current form of Western Medicine that was built on the Germ Theory of Disease following the Flexner Report of 1910.3, 4 The Germ Theory assumes that each disease or syndrome has a single dominant factor, such as a germ. This approach is valuable for a wide spectrum of diseases—but not others. Personalized medicine assumes that a disease can be caused by multiple factors, where any one factor is neither necessary nor sufficient to cause disease. It recognizes gene x environment, gene x gene, and other more complex risk combinations and interactions, making genetics a vital component of personalized medicine. Personalized medicine is needed when multiple etiologies cause the same syndrome, when the same syndrome has multiple outcomes, and when response to therapies is unpredictable based on the disease signs and symptoms alone.4 Predictive disease modeling in personalized medicine requires the integration of multiple types of information—genomic, environmental, physiologic, and disease biomarkers—to manage health and prevent disease.4 The goal of the Germ Theory paradigm is to diagnose established disorders and treat them. The goal of personalized medicine is to predict the outcomes of complex disorders and prevent them.
Personalized medicine requires a greater understanding of the complex relationships between genetics, environment, physiology, and variant responses to stress or injury than current medical practice requires. The enormous amount of medical information in some health-care systems (“big data”) is being tapped to provide insight and direction. However, the origin, structure, and quality of the data limit its utility. Furthermore, most other hospitals are not equipped to handle this wealth of information, and solutions are needed for efficient storage and access.5 Finally, interpretation and application of this data by physicians for specific patients continues to be the greatest challenge.
Pharmacogenetics illustrates one area where genetic information informs clinical practice.6 Pharmacogenetic algorithms link pathogenic variants in drug-metabolizing genes to predicted changes in the concentration of active drugs in target tissue that could then assist physicians in choosing drug dosing and monitoring approaches. Generally, the public supports genetic testing for pharmacogenetic variants to manage their therapeutic treatment.7
Test cases such as pharmacogenetic analysis illustrate the feasibility of integrating genetic testing into clinical practice. These early successes and the emergence of the personalized medicine paradigm provide pathways for further expansion of genetic testing and management of more complex disorders. New information and interpretation of genetic variants continues to accumulate at a rapid pace. Genetic tests are rapidly reducing in price and being offered directly to consumers, making genetic information increasingly more affordable and available. New approaches and algorithms need to be established for the expanded use of personalized medicine in the coming years. Furthermore, understanding the psychosocial consequences of genetic information is vital to crafting an ethically responsible paradigm.
Genetic counselors have intense training and experience in the psychosocial implications of genetic testing, as well as proven adaptability in the field. However, a personalized medicine model for the treatment of complex diseases requires alterations to genetic counseling education as preparation for this shift in medicine. How will genetics professionals be trained to understand the implications of this new paradigm and apply it to patients on a case-by-case basis so that all aspects of health and quality of life are maximized?
THE TRANSITION TO PERSONALIZED MEDICINE
Personalized medicine is a new system that will require widespread genetic testing and integration of genetic data, with other clinical information, into new practice models. The transition from traditional Western medicine to personalized medicine is wrought with questions regarding timing, data collection, genomic methods, data management, the ethics of managing unexpected results, and cost. Paradigm shifts are inherently difficult, requiring a crisis to force abandonment of old ways (when necessary) with a shift to a new way of thinking. Furthermore, this shift in medical paradigms requires a transformation in both medical practice patterns and the education of health professionals. Nevertheless, we believe the benefits of improved quality of life with a much lower cost of care are enormous.
The challenge with a paradigm shift from our traditional approach to a personalized medicine approach is that the shift is only partial—it applies only to complex disorders. Indeed, the historic work in Mendelian genetics remains relevant and useful. Complex genetics, the interaction between multiple genes and environmental factors, is only just beginning to be addressed in a medical context. Major functional challenges include the cost of testing, reimbursement for testing, access of test results to practicing physicians, and ability of practicing physicians to comprehend and apply the results. When whole-genome sequencing (WGS) is performed in research, patients do not receive these results in ways that directly impact their care. Along with others, we believe that WGS is the cornerstone of personalized medicine.8, 9 Therefore, this paradigm shift requires the careful integration of more widespread genomic sequencing, with results accessible to physicians and qualified health-care workers who are trained to use it. The solution to these barriers remains complex. But when WGS becomes a standard resource, two very important questions must be addressed: how can this massive amount of information be used in specific cases to optimize care? How should the ethical barriers to its implementation be addressed?
Ethical controversies
A personalized medicine paradigm requires the transformation of many practice patterns to guard against potential ethical gray zones and violations. Ethical standards must be addressed and operationalized to protect both patients and health professionals from the unexpected or unintentional consequences of genetic testing. Certainly, this effort will require widespread awareness of the Genetic Information Nondiscrimination Act of 2008 (GINA) and other laws in place to protect individuals receiving genetic testing.10 However, more challenging ethical dilemmas arise related to informed consent, the return of results, testing of minors, and health disparities in complex disorders where individual variants resulting in a complex disease are, by definition, harmless, but where, within the same genome, life-changing Mendelian disease-associated variants lurk. These examples represent a partial list of the ethical issues associated with personalized medicine. Here, we address two of these ethical concepts—informed consent and return of results—and discuss the important questions that need to be answered in a clinical context.
Informed consent
Informed consent is the process by which individuals are given adequate information to make a decision to consent to an intervention or procedure. It is a critical process to protect patients, and it conveys community standards for ethical conduct. In 2013, the American College of Medical Genetics and Genomcis (ACMG) released a policy statement regarding informed consent for genome and exome sequencing, providing a number of points to consider. Included among these points is the position that counseling should be performed by either a medical geneticist or affiliated genetic counselor before exome/genome sequencing.11
In the context of more widespread adoption of personalized medicine, there is competition between the feasibility of counseling large numbers of individuals and the adequacy of such counseling. Currently, there are not enough genetic counselors or medical geneticists to address this challenge of widespread exome–genome sequencing with the current counseling model. Pre-test counseling for WGS can be lengthy, requiring as much as 2–3 h.12 Educational videos and interactive technologies may be one avenue to help overcome this obstacle. Such technologies do not dismiss the necessity of the genetic counselor, but rather relieve the burden of patient load and increase efficiency by reducing time spent one-on-one counseling of every detail. Furthermore, adjusting the return of results to only include pharmacogenetic variants and common low-risk variants for complex diseases may expedite and simplify this process to promote individualized patient care.
Incorporation of technology-assisted distance genetic counseling into the delivery model facilitates access to genetic services for patients in remote locations and when face-to-face genetic counseling is not feasible.13, 14 Technologies include telegenetics and internet-based services. The benefits of internet-based counseling are great and include access to a remote patient with the ability to counsel multiple family members from different locations at once. However, issues of privacy, security, identity verification, technology failure, and miscommunication arise when patients are counseled through this approach. Further investigation is needed, but successes with this delivery model have already been reported.14, 15 Although a potentially useful approach, caution must be taken with internet-based counseling to protect patient health information, provide an adequate counseling environment and ensure patient understanding.
Continual improvement in the understanding and clinical utility of genetic information makes it impossible to be certain how it will be used to optimize patient care in the future.16 Furthermore, the massive amount of information that is currently discussed in WGS counseling can be overwhelming, creating difficulty in comprehension and retention for actionable Mendelian conditions—which is even more extensive and complicated for complex disorders. These challenges are not unique to personalized medicine, but are further complicated by its complexity.
Return of results
Both incidental findings and unclear variants are inevitable outcomes of WGS. Whole-exome sequencing alone reveals an average of 20,000 variants, and the exome only comprises 1–3% of the genome.17 The sheer amount of information that can be obtained from WGS reduces the feasibility of the “right not to know”. How can patients possibly be counseled on and make anticipatory decisions about every possible result, particularly when it is not possible to anticipate every way that these results will impact an individual's care? There has been much discussion in recent years about a “duty to warn” patients about medically actionable diseases. ACMG has recommended that mutations in 57 genes associated with medically actionable conditions be automatically screened for by whole exome sequencing/WGS.18 This recommendation has created controversy surrounding the right not to know and patient autonomy for these conditions.16, 19, 20 Furthermore, this recommendation has not been broadly accepted, possibly due to the cost and practical inconvenience of including a genetic counselor in the clinical workflow. In contrast to ACMG's position, the European Society of Human Genetics recommends that these “unsolicited findings” be avoided.21
Additional difficulty comes from the inescapable and overwhelming amount of variants of unclear significance obtained from WGS. How can we update every individual when a variant of unclear significance becomes reclassified as a mutation or disease risk polymorphism? Novel approaches must be developed and vetted to address these issues, especially as medical information becomes more widely available on the Internet and through the influences of social media. One approach is to focus testing on gene panels that only target subsets of genes. Another approach is to separate the “technical” report from the “clinical” report, so that only a pre-determined set of genetic loci are reviewed and linked with the clinical context, and this report would then be delivered to the patient. This approach avoids the need to manage information about variants in genes that are irrelevant to the clinical question being asked, especially in a clinic focused on specific complex disorders where broad knowledge about many other genetic syndromes is unavailable.
The other major challenge for personalized medicine is generating useful clinical reports that provide diagnostic accuracy and clinical guidance. A useful clinical report for a complex disorder must include the integration and parsing of multiple genes and genotypes within the context of a complex clinical scenario. It is the purview of pathologists to interpret genotypes in Mendelian disorders and tumors because they specialize in looking at affected tissue samples under the microscope to determine the molecular phenotype. But for complex clinical disorders there may be limited or no pathology, and when there is tissue pathology, the findings may be independent of the etiology (e.g., inflammation and scarring for no clear reason). This problem is epitomized in functional disorders, when organ function is abnormal, whereas the tissue appearance is completely normal. What is needed to generate a useful clinical report for a complex clinical disorder is a new type of physician with both clinical experience and understanding of genetics who can provide diagnostic insights and clinical guidance. Thus, in addition to generating a technical report of an accurate genotyping process, a new approach to interpreting the genotypes from a person with a complex disorder must be developed and may not require a pathologist. A shift in medical education will prepare new physicians with understanding of the utility of genetics in managing complex disorders, and curriculum revisions to address this educational need have already begun to be discussed.22, 23 For current physicians that manage complex disorders, continuing medical education programs on genetics and personalized medicine will be critical for this transition.
CLINICAL UTILITY
Now that we have access to this genetic information, we must answer how it will be used to improve patient care in the future. Such decisions will require a continuation of the massive effort to effectively classify the pathogenicity of genetic variants.24 Furthermore, sorting this information into priority categories for genetic counseling and for the involvement of other appropriate providers will be critical for a cost-effective model of personalized medicine. This will be a matter of turning a continuous spectrum of risk into clear categorical levels while recognizing the individuality of each patient. Already, models for this concept have been proposed. A clinically oriented binning system has been suggested by Berg et al.,25 which places incidental information into the following bins: (1) medically actionable; (2A–C) low, medium, and high risk; and (3) all other loci with unknown clinical implications. A binning system would allow providers and genetic counselors to make standards on which bins should be returned to the patient and how they will be counseled. This approach must be robust, however, because it must be multiplied when multiple loci are considered and when variants are pathologic in some disorders and not others.
The goal of personalized medicine is to use large amounts of information to improve health outcomes, thereby cutting costs in the long-run. It is currently limited by the lack of resources and framework to effectively integrate genetic variants into the care and treatment of complex diseases. In isolation, many common risk alleles have little phenotypic relevance, particularly given that they are also present in individuals without disease. However, allele combinations in the presence of environmental factors have stronger implications for disease phenotype and course. For most complex disease loci, modeling of interacting risk factors and simulating the effects under different treatment approaches will be critical for producing relevant information for clinicians, particularly as genomic information alone has limited utility in this context. With the rapid advancements being made in complex disease genetics, raw data should quickly be turning into information with the capacity to reduce inappropriate test and treatment prescriptions, identify targeted treatments, predict uncommon complications, and identify at-risk individuals who will benefit from cheaper, preventative measures. Concurrently, this rapid advancement in genomics is likely to result shortage of professionals who are capable of interpreting and using genetic information. As the demand for genetic guidance expands into primary care offices, we believe that genetic counselors should have a major role in managing the influx of genetic information in both the clinical and laboratory settings. We arrived at this conclusion based on the history of the genetic counseling profession and on the proven capability of genetic counselors to adapt and advance with the state of the field. However, with growing demands for genetic counselors, even the genetic counseling profession will likely be stretched to fill needed positions in the United States.
GENETIC COUNSELING FOR MENDELIAN DISEASES
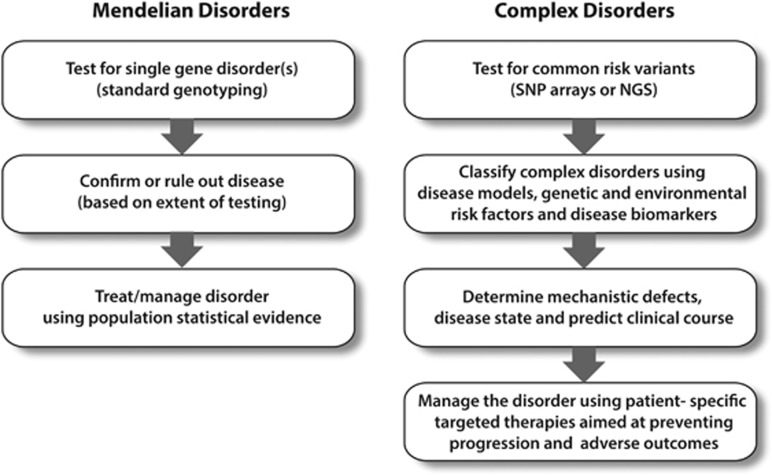
Genetic counseling for Mendelian disease is a critical component of genetic counseling practice. Examples of single-gene gastrointestinal diseases for which genetic counseling is well established include familial adenomatous polyposis, Lynch syndrome, and cystic fibrosis. Genetic testing for familial adenomatous polyposis and cystic fibrosis is straightforward, where sequencing of a single gene typically confirms or rules out a diagnosis (Figure 1). Lynch syndrome exemplifies how tumor genetics can inform germline genetic testing. To assess a patient for Lynch syndrome and familial adenomatous polyposis, genetic counselors acquire a family and personal history of cancer/polyps for a holistic view of the family and pattern of inheritance. Specific criteria have been established to inform genetic testing for these conditions, and genetic counselors are trained to determine the appropriate testing approach. In this situation, a genetic counselor will discuss the test and its implications, review genes/chromosomes, and facilitate the testing process if the patient consents. Once the testing is completed, the genetic counselor will review the results with the physician and patient and coordinate appropriate care. If testing comes back negative, it may be appropriate to reflex testing to another gene or panel of genes. Owing to the impact of genetic diseases on family members, genetic counselors can provide patients with resources and aid in discussing this information with at-risk family members.
Figure 1.
Schematic for evaluating Mendelian and complex disorders. Genetic testing for Mendelian disorders is a well-established discipline with the ultimate goal of identifying, confirming, or ruling out a highly penetrant genetic disorder. In complex disorders, genetic information using single-nucleotide polymorphism (SNP) arrays or next generation sequencing (NGS) panels are combined with other risk factors and markers within a disease model to classify patients based on disease mechanism. The actionable result(s) lead to better management of the patient based on targeted therapies and avoiding adverse outcomes.
Many genetic tests are currently available for gastrointestinal diseases, including gastrointestinal cancer syndromes (e.g., Lynch syndrome, familial adenomatous polyposis, hereditary diffuse gastric cancer), hereditary pancreatitis, cystic fibrosis, and Hirschprung disease (isolated or syndromic), among others. Appropriate testing may involve single-gene testing, a panel of genes, or site-specific testing when a familial mutation has already been identified. Reflex testing to a panel or other gene may be appropriate in cases where testing of the suspected gene is negative for a pathogenic mutation. Panel testing is appropriate when multiple genes and/or diseases are suspicious but cannot be ruled out. Available testing is constantly changing, and information on currently available genetic tests in available through the NCBI Genetic Testing Registry (http://www.ncbi.nlm.nih.gov/gtr/).
This genetic counseling process involves family and medical history interpretation, patient education, and non-directive decision facilitation. Genetic counselors are trained to address the physical, mental, social, and emotional impacts of genetic conditions, acting as advocates for their patients. Their skills also include calculating recurrence risks, determining appropriate testing, and identifying risks to other family members. These are skills that have already been demonstrated to be transferrable into the realm of complex diseases. However, as with other historical shifts within the profession, the process must adapt to fit the technology, data interpretation, and objectives.
GENOTYPE INTERPRETATION AND GENETIC COUNSELING IN COMPLEX DISEASES
A new and rising need exists for genetic counselors and medical geneticists in chronic, complex diseases that will have a different role than “traditional” counseling. This need reflects rapid advancements in the scope and cost of genetic sequencing, the knowledge of how genetics contributes to complex and chronic diseases such as pancreatitis, and the enormous complexity of genome science in medicine today. Combinations of common genetic variants that, together, cause organ dysfunction or alter responses to injury can be identified early and result in the “actionable result” of anticipating complications and selecting optimal management strategies to minimize the impact of the disease risk (Figure 1).
Complex diseases such as pancreatitis differ from Mendelian diseases in that no single genetic locus defines etiology. In fact, over a dozen genes and/or environmental factors contribute to pancreatitis in different ways in different people although the clinicopathologic disease features and outcomes may be identical. For example, alcohol is an established environmental risk factor for pancreatitis. However, the risk for alcohol-related pancreatitis is modified by genetic variants. The CLDN2 gene encodes Claudin-2, a protein that regulates tight junctions and is expressed in the pancreatic ducts and islet cells.26 The X-linked CLDN2 risk allele interacts with alcohol to increase the risk for alcohol-related pancreatitis.27
Inflammatory bowel disease, liver cirrhosis, asthma, and chronic renal diseases illustrate other complex disorders where analysis of the genome may provide guidance and better management. The complexity of the genotypes and uncertainty of clinical course or optimal interventions makes genetic counseling of complex diseases substantially more difficult than Mendelian diseases. In addition to standard patient education by genetic counselors about genes, chromosomes, and inheritance, conversations must also include explanations of interactions between genes and environmental factors. Thus, some major knowledge gaps and poorly defined interdisciplinary roles and interactions exist in complex diseases and complicate the genetic counseling process. Although genetic information cannot be changed with current technology, physiologic and environmental factors can be improved through behavior modification and pharmacology. In the example of chronic pancreatitis, modeling of risk loci and environmental risk factors, such as smoking and alcohol use, will provide beneficial information on etiology, appropriate treatment options, and risks for complications such as diabetes mellitus and pancreatic adenocarcinoma on a case-by-case basis. Such information will inform surveillance, treatment, and prevention in at-risk family members. For more complex conditions, such as inflammatory bowel disease, where the number of cell types and complexity of environment is orders of magnitude higher than chronic pancreatitis, the challenge is greater, but the problem is solvable.
Evolving roles for genetic counselors
As the field of genetics becomes more complex it may be necessary for genetic counselors to become specialized. This specialization may follow physician specialization, which is divided by organ systems such as gastroenterology, hepatology, cardiology, pulmonary, etc. Within these systems there are both rare Mendelian disorders and common complex disorders that should be addressed. Qualified genetic counselors must be able to address the psychosocial issues associated with complex diagnosis, prognosis, reproductive planning, and risk to family members for both simple and complex disorders. But there are likely greater needs and opportunities for future genetic counselors who specialize along with physician specialists.
The greater needs that genetic counselors should be addressing in complex diseases include choosing the appropriate genetic testing approach such as single gene, sequencing panels, WGS, etc., the costs and benefits of testing, the interpretation of negative results, and the follow-up steps needed based on the results. Furthermore, genetic counselors will be important for the management of unknown, rare, minor risk, and major risk variants in complex diseases, as well as identifying which findings represent new insights that will strengthen medical decision making.28 If this role for genetic counselors improves care and saves money, then their positions must be supported by the organizations that benefit from this effort.
Medical specialists focusing on complex diseases are usually not genetics experts, and the explosion of new genetic findings in various medical fields makes it challenging, if not impossible to remain up to date on available tests, interpretation of results and implications to the patient in terms of psychosocial issues, right to know and right to not know potentially life-altering uncommon or unexpected findings. In addition, given the centrality of genetics in complex diseases, there are new ethical challenges for informed consent and disclosure of results. Thus, recognition of a need for more trained genetics professionals parallels expanded genetic information in common, complex, and multisystem diseases.29
If the lack of trained professionals is holding back the application of genetics in complex disease, could genetic counselors fill this growing need? Genetic counselors are already trained to handle genomic information, and these competences are outlined in the 2013 revised practice-based competencies by the Accreditation Council for Genetic Counseling (see Practice Based Competencies at http://gceducation.org/Pages/Standards.aspx). Competencies include, understanding personalized genomic medicine and the ability to communicate genomic information to clients.30, 31, 32 How can the application of genetic–environmental interactions be applied to this counseling model? Although simple risk factors such as smoking and alcohol are well established in many chronic diseases, how can subtle nuances, such as those that affect biochemical pathways over time, be incorporated into personalized medicine?
In addition to traditional genetic counseling, and subspecialization with practice groups, genetic counselors are being used in “non-traditional” roles. Laboratory genetic counselors facilitate communication between health-care professionals and the laboratory to ensure that genetic testing is used wisely and correctly. This moves the genetic counselor out of the clinic to serve as a remote consultant. The benefits of adapting genetic counselors to this role include improved care above no counselor at all and significantly reduced costs of inappropriate testing.33, 34 Genetic counselors employed by direct-to-consumer genetic testing companies provide education and risk interpretation for consumers.35 Genetic counselors participate in addressing preventable complex diseases that involve risk calculation and counseling on the influence of both genes and environment on disease development, such as type 2 diabetes,36 or kidney disease.37 The risk counseling framework for preventable polygenic diseases suggested by Waxler et al.36 aligns with potential, but debated, roles for genetic counselors in preventative genomic medicine.38 O'Daniel39 has discussed the utility of genetic counselors in genomic predictive medicine and agreed that genetic counselors are poised to help bridge the gap between genomics and primary care. Mills and Haga40 discuss other adaptations that should be incorporated into the genomic counseling model, especially the use of motivational and more directive approaches. Therefore, although discussions of this new counseling model are already occurring, there is much progress to be made in outlining a clear, standard framework for genetic counseling in this new era of genomic medicine.
In the future, genetic counselors must demonstrate a significant cost–benefit relationship to providers in a physician practice by offering unique skills and serving as effective physician-extenders. Their role is cost effective in terms of patient education, counseling on “high-risk” variants, such as mutations in the BRCA1 and BRCA2 genes, which cause Hereditary Breast and Ovarian Cancer syndrome, and judicially recommending appropriate testing. However, common genetic risk variants may not require advanced and specialized counseling from a genetic counselor, particularly when there are not enough genetic counselors to fill this role. In counseling for common risk variants for conditions with evidence of disease activity, the conversation will likely center more around the natural history of a particular complex disease, lifestyle changes, and treatment/preventative options rather than inheritance, risk for family members, and identification of appropriate testing. Skills such as understanding of basic physiology, motivational interviewing, and forming an action plan with the patient will be important for complex disease counseling. Perhaps, other health-care professionals with supplemental training in genetics and counseling, such as nurses, could help fill this need under the supervision of genetic counselors.
Currently, genetic counseling training programs equip genetic counselors with the tools to adapt to new information in the field of human genetics and apply it to their patients. Training on Mendelian genetics and counseling will continue to be vital to the profession. However, should the training have a greater focus on guiding specialty physician groups on appropriate ordering and interpretation of complex genotypes for personalized medicine? Is the cost of inappropriate ordering and interpretation of genetic information by various physician groups and the lost opportunity to provide optimal care more than the cost of genetic counselors acting as personalized genome managers? These issues must be addressed by curriculum committees who recognize the need for training in emerging areas and reducing time spent on pre-requisite concepts. In addition, preparation for diverse job markets enhances the value of the graduates. Education must extend to both hospital and other health-care professionals on the value of genetics counselors and personalized medicine. A successful shift to a new paradigm will benefit all stakeholders.
SUMMARY AND CONCLUSIONS
Developments on our understanding of the human genome have pinpointed the importance of common, low independent risk genetic variants in complex diseases, resulting in high-risk combinations, requiring a shift from the Germ Theory paradigm to a personalized medicine paradigm of disease modeling and treatment simulation. Personalized medicine does not replace important work on Mendelian disease, making the paradigm shift only partial. Widespread incorporation of genetic information into the health-care system requires careful integration of both genetic and environmental risk into care models. Furthermore, gaps still exist in the knowledge and process of delivering new concepts and medical guidance in the context of this risk information. This paradigm shift also presents an emergent need for professionals with training in genetics to assist physicians that treat complex disorders. We identify the genetic counseling profession as an ideal resource in this new field. However, the training and positioning of the genetic counselor in the delivery system must be optimized to ensure a successful and ethically sound paradigm shift. We believe that a successful paradigm shift to personalized medicine is a critical step in guiding physician decisions to improve care and quality of life for individuals with complex diseases.
Study Highlights

Guarantor of the article: David C. Whitcomb, MD, PhD.
Specific author contributions: C.A.S. and D.C.W. contributed equally to the planning and drafting of the manuscript and approved of the final document.
Financial support: This work was supported by the Wayne Fusaro Pancreatic Cancer Research Fund (CAS), and the Division of Gastroenterology, Hepatology and Nutrition, Department of Medicine, University of Pittsburgh.
Potential competing interests: None.
References
- Naidoo N, Pawitan Y, Soong R et al. Human genetics and genomics a decade after the release of the draft sequence of the human genome. Hum Genomics 2011; 5: 577–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleidgen S, Klingler C, Bertram T et al. What is personalized medicine: sharpening a vague term based on a systematic literature review. BMC Med Ethics 2013; 14: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flexner A. Medical Education in the United States and Canada: A Report to the Carnegie Foundation for the Advancement of Teaching Bulletin No 4 Carnegie Foundation for the Advancement of Teaching: New York, NY, 1910. [PMC free article] [PubMed] [Google Scholar]
- Whitcomb DC. What is personalized medicine and what should it replace? Nat Rev Gastroenterol Hepatol 2012; 9: 418–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merelli I, Perez-Sanchez H, Gesing S et al. Managing, analysing, and integrating big data in medical bioinformatics: open problems and future perspectives. Biomed Res Int 2014; 2014: 134023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abul-Husn NS, Owusu Obeng A, Sanderson SC et al. Implementation and utilization of genetic testing in personalized medicine. Pharmgenomics Pers Med 2014; 7: 227–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga SB, Tindall G, O'Daniel JM. Public perspectives about pharmacogenetic testing and managing ancillary findings. Genet Test Mol Biomarkers 2012; 16: 193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzker ML. Sequencing technologies - the next generation. Nat Rev Genet 2010; 11: 31–46. [DOI] [PubMed] [Google Scholar]
- Dixon-Salazar TJ, Silhavy JL, Udpa N et al. Exome sequencing can improve diagnosis and alter patient management. Science translational medicine 2012; 4: 138ra78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genetic Information Nondiscrimination Act of 2008. PubL 110–233, 122 Stat 881 2008. [PubMed]
- Directors ABo. Points to consider for informed consent for genome/exome sequencing. Genet Med 2013; 15: 748–749. [DOI] [PubMed] [Google Scholar]
- Tabor HK, Stock J, Brazg T et al. Informed consent for whole genome sequencing: a qualitative analysis of participant expectations and perceptions of risks, benefits, and harms. Am J Med Genet Pt A 2012; 158A: 1310–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SA, Marvin ML, Riley BD et al. Identification of genetic counseling service delivery models in practice: a report from the NSGC Service Delivery Model Task Force. J Genet Couns 2013; 22: 411–421. [DOI] [PubMed] [Google Scholar]
- Meropol NJ, Daly MB, Vig HS et al. Delivery of Internet-based cancer genetic counselling services to patients' homes: a feasibility study. J Telemed Telecare 2011; 17: 36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan AH, Datta SK, Skinner CS et al. Randomized trial of telegenetics vs. in-person cancer genetic counseling: cost, patient satisfaction and attendance. J Genet Couns 2015. [e-pub ahead of print]. [DOI] [PMC free article] [PubMed]
- Pinxten W, Howard HC. Ethical issues raised by whole genome sequencing. Best Pract Res Clin Gastroenterol 2014; 28: 269–279. [DOI] [PubMed] [Google Scholar]
- Frebourg T. The challenge for the next generation of medical geneticists. Hum Mutat 2014; 35: 909–911. [DOI] [PubMed] [Google Scholar]
- Green RC, Berg JS, Grody WW et al. ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet Med 2013; 15: 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SM, Annas GJ, Elias S. Point-counterpoint. Patient autonomy and incidental findings in clinical genomics. Science 2013; 340: 1049–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormond KE, Cho MK. Translating personalized medicine using new genetic technologies in clinical practice: the ethical issues. Pers Med 2014; 11: 211–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van El CG, Cornel MC, Borry P et al. Whole-genome sequencing in health care: recommendations of the European Society of Human Genetics. Eur J Hum Genet 2013; 21: 580–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmer LA, Waggoner DJ. Professional medical education and genomics. Annu Rev Genomics Hum Genet 2014; 15: 507–516. [DOI] [PubMed] [Google Scholar]
- Katsanis SH, Dungan JR, Gilliss CL et al. Educating future providers of personalized medicine. N C Med J 2013; 74: 491–492. [PubMed] [Google Scholar]
- Dewey FE, Grove ME, Pan C et al. Clinical interpretation and implications of whole-genome sequencing. JAMA 2014; 311: 1035–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg JS, Khoury MJ, Evans JP. Deploying whole genome sequencing in clinical practice and public health: meeting the challenge one bin at a time. Genet Med 2011; 13: 499–504. [DOI] [PubMed] [Google Scholar]
- Aung PP, Mitani Y, Sanada Y et al. Differential expression of claudin-2 in normal human tissues and gastrointestinal carcinomas. Virchows Arch 2006; 448: 428–434. [DOI] [PubMed] [Google Scholar]
- Whitcomb DC, Larusch J, Krasinskas AM et al. Common genetic variants in the CLDN2 and PRSS1-PRSS2 loci alter risk for alcohol-related and sporadic pancreatitis. Nat Genet 2012; 44: 1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machini K, Douglas J, Braxton A et al. Genetic counselors' views and experiences with the clinical integration of genome sequencing. J Genet Couns 2014; 23: 496–505. [DOI] [PubMed] [Google Scholar]
- Radford C, Prince A, Lewis K et al. Factors which impact the delivery of genetic risk assessment services focused on inherited cancer genomics: expanding the role and reach of certified genetics professionals. J Genet Couns 2013; 23: 522–530. [DOI] [PubMed] [Google Scholar]
- Hooker GW, Ormond KE, Sweet K et al. Teaching genomic counseling: preparing the genetic counseling workforce for the genomic era. J Genet Couns 2014; 23: 445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Profato J, Gordon ES, Dixon S et al. Assessing the integration of genomic medicine in genetic counseling training programs. J Genet Couns 2014; 23: 679–688. [DOI] [PubMed] [Google Scholar]
- Wicklund C, Trepanier A. Adapting genetic counseling training to the genomic era: more an evolution than a revolution. J Genet Couns 2014; 23: 452–454. [DOI] [PubMed] [Google Scholar]
- Kotzer KE, Riley JD, Conta JH et al. Genetic testing utilization and the role of the laboratory genetic counselor. Clin Chim Acta 2014; 427: 193–195. [DOI] [PubMed] [Google Scholar]
- Zetzsche LH, Kotzer KE, Wain KE. Looking back and moving forward: an historical perspective from laboratory genetic counselors. J Genet Couns 2014; 23: 363–370. [DOI] [PubMed] [Google Scholar]
- Harris A, Kelly SE, Wyatt S. Counseling customers: emerging roles for genetic counselors in the direct-to-consumer genetic testing market. J Genet Couns 2013; 22: 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxler JL, O'Brien KE, Delahanty LM et al. Genetic counseling as a tool for type 2 diabetes prevention: a genetic counseling framework for common polygenetic disorders. J Genet Couns 2012; 21: 684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witasp A, Nordfors L, Carrero JJ et al. Genetic studies in chronic kidney disease: interpretation and clinical applicability. J Nephrol 2012; 25: 851–864. [DOI] [PubMed] [Google Scholar]
- Clarke A, Thirlaway K. 'Genomic counseling'? Genetic counseling in the genomic era. Genome Med 2011; 3: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Daniel JM. The prospect of genome-guided preventive medicine: a need and opportunity for genetic counselors. J Genet Couns 2010; 19: 315–327. [DOI] [PubMed] [Google Scholar]
- Mills R, Haga SB. Genomic counseling: next generation counseling. J Genet Couns 2013; 23: 689–692. [DOI] [PMC free article] [PubMed] [Google Scholar]