Abstract
Oncolytic vesicular stomatitis virus (VSV) has potent antitumor activity but some cancer cells are resistant to VSV killing, either constitutively or due to type I interferon (IFN) inducing an antiviral state in the cells. Here, we evaluated VSV oncolysis of a panel of human head and neck cancer cells and showed that VSV resistance in SCC25 and SCC15 cells could be reversed with Janus kinase (JAK) 1/2 inhibitors (JAK inhibitor I and ruxolitinib). Pre-treatment of cells with JAK1/2 inhibitors before or in conjunction with VSV enhanced viral infection, spread and progeny yield (100- to 1000-fold increase). In contrast, inhibitors of histone deacetylase (LBH589), phosphatidylinositol 3-kinase (GDC-0941, LY294002), mammalian target of rapamycin (rapamycin) or signal transducer and activator of transcription 3 (STAT3 inhibitor VII) were ineffective. Compared with VSV-sensitive SW579 cells, IFNα/β responsive antiviral genes (IRF-9, IRF-7, OAS1 but not MxA) are constitutively expressed in SCC25 cells. Pretreatment with JAK inhibitors reduced mRNA levels of these genes, increasing VSV expression in the cells. Interestingly, 1 h of drug exposure was sufficient to reverse SCC25 resistance to VSV and was still effective if virus was added 24 h later. Overall, we show here that JAK inhibitor I and ruxolitinib (Jakafi) can reverse resistance to VSV, supporting the rationale to incorporate JAK1/2 inhibitors in future VSV virotherapy trials.
Keywords: oncolytic VSV, SCCHN, JAK inhibitor I, ruxolitinib, interferon, antiviral genes
INTRODUCTION
Vesicular stomatitis virus (VSV) has a broad tropism and uses the recently identified receptor from the low-density lipoprotein family to infect cells.1 Vesicular stomatitis virus has several features that make it attractive as an oncolytic virotherapy agent for cancer.2 The virus is highly lytic, has fast replication kinetics and a large burst size. Importantly, the majority of the population does not have pre-existing antibodies to VSV that could otherwise reduce the therapeutic activity of systemic VSV administration.3 The natural hosts of VSV include cattle, horses and pigs.4,5 The animals recover from the infection but VSV infection of livestock is associated with significant disease, including vesicular lesions around the mouth, hoofs and teats, and loss of beef and milk production.6 Human infections with VSV through laboratory work or working with affected animals have occurred, and typically results in a mild fever from which the infected individual recovers with no incidents. When injected experimentally into the brains of research rodents, wild-type VSV is neurovirulent and causes acute infection of the central nervous system, resulting in neurological signs including ataxia and hind limb paralysis.7
To attenuate VSV (Indiana strain) for therapeutic use as a vaccine or an oncolytic agent in cancer therapy, additional transcription units, truncations in the cytoplasmic tail of the glycoprotein, microRNA targeting and insertion of picornaviral internal ribosome entry sites have been used successfully to attenuate the neurotoxicity of recombinant VSV.8–11 Furthermore, the virus is exquisitely sensitive to the antiviral effects of type I interferon (IFN) and two popular engineering platforms involve IFN production by the infected cell. These strategies include mutation or deletion of the methionine residue 51 in the matrix (ΔM51) protein of VSV such that M is no longer able to inhibit the nuclear export of cellular mRNA (for example, IFN) or insertion of an additional transcription unit encoding IFN-β (VSV-IFNβ).12–14 Both VSV-ΔM51 and VSV-hIFNβ have been used successfully to treat a variety of murine and human cancers.2,3 In particular, VSV-hIFNβ is being developed for clinical testing against hepatocellular carcinoma and myeloma.15,16 The safety of the virus was evaluated in mice, rats and rhesus macaques toxicology and pharmacology studies. When injected directly into the liver of rhesus macaques under CT guidance, the maximal feasible dose of 1010 TCID50 was well tolerated with no adverse clinical symptoms and no significant hematological or chemical changes.15 VSV-hIFNβ is currently undergoing testing in a Phase I dose escalation trial (seven dose levels, 105–108 TCID50) where patients with sorafenib refractory or intolerant hepatocellular carcinoma will receive a single intratumoral injection of VSV-hIFNβ into a target lesion (principal investigator: Mitesh Borad, Mayo Clinic Arizona, Clinical Trials.gov identifier: NCT01628640).
In general, cancer cells have defective IFN signaling pathways and are susceptible to oncolysis by the IFN-inducing viruses, VSV-ΔM51 or VSV-hIFNβ.17,18 In contrast, non-transformed cells have intact IFN signaling pathways and respond to VSV infection with production of type I IFN, and induction of an antiviral state that inhibits viral amplification in the infected cell and viral spread into neighboring cells. Each IFN receptor (IFNAR1 and IFNAR2) subunit binds constitutively to a single specific member of the Janus kinase (JAK) family: IFNAR1 to tyrosine kinase 2 (TYK2) and IFNAR2 to JAK1.19 Type I IFN binding induces the phosphorylation of JAK1, TYK2, intracellular tyrosine residues of each receptor chain and signal transducers and activators of transcription (STATs). Activated STATs dimerize, dissociate from the receptor and together with IRF-9 (ISGF3 complex) translocate to the nucleus to induce the expression of more than 300 IFN-stimulated genes (ISGs).20–22 The four main effector pathways of IFN-mediated antiviral response are the Mx GTPase pathway, the 2′5′-oligoadenylate-synthetase-directed ribonuclease L pathway, the protein kinase R (PKR) pathway and the ISG15 ubiquitin-lie pathway.19 These pathways serve to inhibit viral transcription, degrade viral RNA, inhibit translation and modify protein function to limit the spread and infection of the virus.
In this report, we evaluated the oncolytic activities of VSV-green fluorescent protein (GFP) and VSV-ΔM51-GFP in a panel of human head and neck squamous cell carcinoma (HNSCC) cell lines and showed that some cell lines were highly sensitive to VSV oncolysis, whereas others were resistant to VSV. Previous studies have investigated pharmacological strategies to improve VSV oncolysis in resistant cells by incorporating drugs and inhibitors of IFN-associated signaling or apoptotic pathways. For example, histone deacetylase (HDAC) inhibitors and JAK inhibitors increased VSV replication in otherwise resistant human prostate PC3 cancer cells.23,24 Rapamycin, a highly specific inhibitor of the mammalian target of rapamycin (mTOR), increased VSV oncolysis of human and rat glioblastoma cells.25 To overcome VSV resistance and oncolysis in HNSCC, we evaluated a panel of inhibitors of JAK1/2, HDAC, phosphatidylinositol 3-kinase (PI3K), mTOR or signal transducer and activator of transcription 3 (STAT3) and showed that JAK inhibitors and not the others can reverse VSV resistance in HNSCC cells. Importantly, Jakafi (ruxolitinib), which was recently approved for clinical use in myleoproliferative disorders, was effective, making it feasible to consider incorporating this drug into VSV virotherapy.
MATERIALS AND METHODS
Cell lines, drugs and viruses
HNSCC cell lines SW579, FaDu, SCC15 and SCC25 (American Type Culture Collection, Manassas, VA, USA) were maintained in medium as recommended by American Type Culture Collection. TG101348 (SAR302503) and Ruxolitinib (INCB018424) were purchased from Selleckchem (Houston, TX, USA), JAK Inhibitor I was from EMD Millipore (Billerica, MA, USA). VSV-GFP (VSV-expressing GFP; Indiana strain) and VSV-ΔM51-GFP were kindly provided, respectively, by Glen Barber (University of Miami) and John Bell (Ottawa Hospital Research Institute). Viruses were propagated on Vero African green monkey kidney cells and titered by TCID50 titrations on Vero cells as previously described.26
Virus infection and replication assay
Cells were plated and treated with or without inhibitors. After 24 h of incubation (or otherwise stated), cells were infected with VSV-GFP or VSV-ΔM51-GFP at multiplicity of infection (MOI) of 0.5. The supernatants were harvested 24, 48 and 72 h post infection and stored at −80 °C until the time of analysis. Viral progeny was determined by TCID50 assay on Vero cells.
VSV cell killing assay
HNSCC (3 × 104) cells were plated in 96-well plates and treated with or without inhibitors diluted in reduced serum media, Opti-MEM (Life Technologies, Carlsbad, CA, USA). Following 24 h of incubation with drugs or media, cells were infected with VSV-GFP or VSV-ΔM51-GFP at MOI of 0.5. Forty-eight hours post infection, media were removed and the cells were washed with PBS, and 100 μl of fresh media with 20 μl per well of Cell Titer 96 AQueous One Solution Cell Proliferation Assay (MTS; Promega, Madison, WI, USA) were added. The plates were read 1 h later at 490 nm using an ELISA plate reader (TECAN Infinite M200Pro, Tecan, San Jose, CA, USA).
RNA isolation and reverse transcription-PCR
Total RNA was extracted from 106 cells of each treatment using RNeasy Plus mini kit (QIAGEN, Valencia, CA, USA). One microgram of total RNA was used as template for reverse transcription using Superscript III 1st strand synthesis supermix (Life Technologies). Semi-quantitative PCR analysis was performed using the following primer sets: IRF-9 sense: 5′-CAAGCAGGACTTCCGGGAGG-3′ and IRF-9 antisense: 5′-CTTCCTGTGGCTCAGGGCTG-3′;27 IRF-7 sense: 5′-TGGTCCTGGTGAAGCTGGAA-3′ and IRF-7 antisense: 5′-GATGTCGTCATAGAGGCTGTTGG-3′;28 OAS1 sense: 5′-TCGGACGGTCTTGGAATTAGTC-3′ and OAS1 antisense: 5′-TAATTCAGCCAGGCCTCAGC-3′;29 MxA sense: 5′-GCTACACACCGTGACGGATATGG-3′ and MxA antisense: 5′-CGAGCTGGATTGGAAAGCCC-3′;27 PKR sense: 5′-GCCTTTTCATCCAAATGGAATTC-3′ and PKR antisense: 5′-GAAATCTGTTCTGGGCTCATG-3′; VSV-N sense: 5′-TGATAGTACCGGAGGATTGACGAC-3′,30 VSV-N antisense: 5′-CCTTGCAGTGACATGACTGCTCTT-3′;15 GAPDH sense: 5′-GGTATCGTGGAAGGACTCATGAC-3′ and GAPDH antisense: 5′-ATGCCAGTGAGCTTCCCGTTCAGC′3′.31 Semi-quantitative PCR conditions were optimized to obtain reproducible and reliable amplification within the logarithmic phase of the reaction. Amplification for IRF9, 2′–5′ OAS, PKR and GAPDH, involved denaturation 94 °C for 3 min, primer annealing at 55 °C for 45 s, and extension at 72 °C for 45 min, 27 cycles; for IRF7, denaturation at 94 °C for 3 min, primer annealing 58 °C for 45 s, an extension at 72 °C, 29 cycles; for VSV-N, denaturation at 94 °C for 3 min, primer annealing at 60 °C for 30 s, an extension at 72 °C for 30 s, 29 cycles; for MxA, denaturation at 94 °C for 3 min, primer annealing at 50 °C for 45 s, an extension at 72 °C for 45 s, 29 cycles.
Western blot analysis
Total protein was extracted from 106 cells using 200 μl RIPA buffer (25 mm Tris-HCL (pH 7.6), 150 mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS) complemented with protease/phosphatase inhibitors cocktail (Cell Signaling Technology, Danvers, MA, USA). Total protein was quantified with Pierce BCA protein assay kit (Thermo Scientific, Waltham, MA, USA) using bovine serum albumin as standard. Protein extracts representing 10 μg of the total protein were separated on 10% SDS-polyacrylamide gel electrophoresis and electrophoretically transferred onto nitrocellulose membranes. The membranes were blocked in 5% of bovine serum albumin in Tris-buffered saline-Tween overnight, incubated with primary antibodies polyclonal rabbit α-JAK1 (sc-277, Santa Cruz, Dallas, TX, USA), rabbit polyclonal α-STAT1 (ab2415, Abcam, Cambridge, MA, USA), rabbit monoclonal α-STAT1 phospho, which binds to residues surrounding serine 727 (ps727, Epitomics, Burlingame, CA, USA), polyclonal rabbit α-VSV15 or rabbit polyclonal α-Actin (sc-1616, Santa Cruz, Dallas, TX, USA) washed three times with TBS-Tween, incubated with secondary antibody goat α-rabbit IgG (H + L) conjugated with horse radish peroxidase (PI1000, Vector Labs, Burlingame, CA, USA). Signal was developed using Pierce ECL western blotting substrate kit (Thermo Scientific).
RESULTS
VSV resistance of HNSCC can be overcame by JAK1/2 inhibitors Human HNSCC cancer cell lines, SW579, FaDu, SCC15 and SCC25, were infected with VSV-GFP at MOI of 0.01, 0.1 or 1.0. Forty-eight hours post infection, cell viability was measured by MTS cell proliferation assay. The HNSCC cell lines were highly sensitive to VSV oncolysis (80% cell death at MOI of 0.01) except for SCC25 that was constitutively resistant to VSV-GFP killing, showing less than 10% cell death at MOI of 1.0 (Figure 1a). Despite increasing the MOI to 10, only 40% of SCC25 cell killing was achieved (data not shown).
Figure 1.
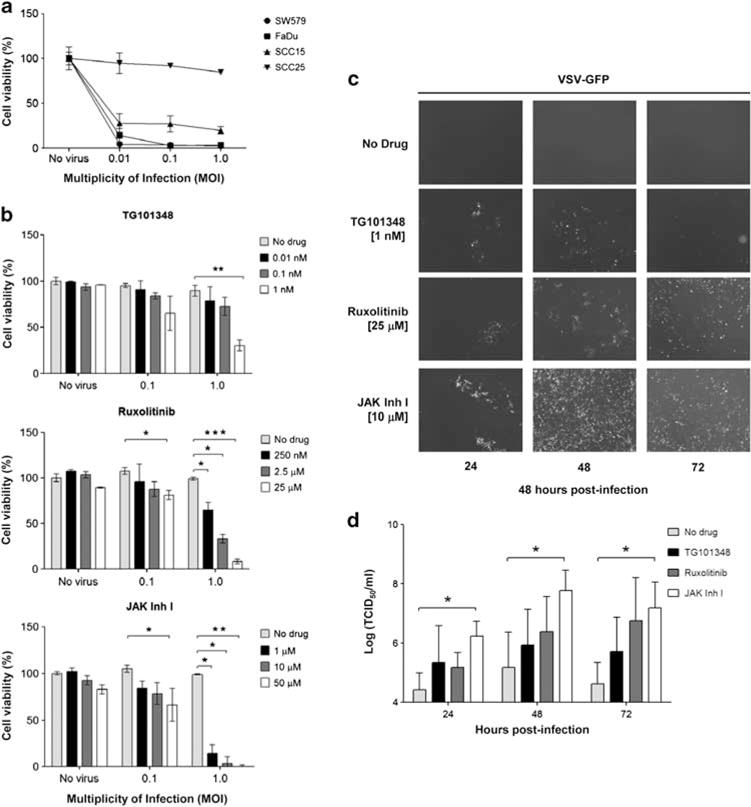
Janus kinase (JAK) 1/2 inhibitors increase vesicular stomatitis virus-green fluorescent protein (VSV-GFP) susceptibility in SCC25 cells. (a) SW579, FaDu, SCC15, SCC25 cell lines were analyzed for VSV-GFP-induced cytotoxicity by MTS cell viability assay. (b) SCC25 was treated with JAK1/2 inhibitors 24 h before VSV. Cells were infected with VSV-GFP and cell viability was determined 48 h later. (c) Representative photographs of VSV infection in treated cells with and without JAK1/2 inhibitors. (d) Viral progeny from b was measured at different times post infection by TCID50 assay. Average of three replicates (mean ± s.d.). *P ≤ 0.05, **P ≤ 0.001, ***P ≤ 0.0001.
To identify drugs that could increase the effectiveness of VSV killing, SCC25 cells were pretreated for 24 h with a panel of inhibitors of various signaling pathways at three different concentrations (Table 1). The concentrations were chosen so that drug alone did not induce significant cell death (data not shown). Cells were then infected with VSV-GFP (MOI of 0.1 and 1.0) and cell viability was measured 48 h later. Broad-spectrum HDAC inhibitor (LBH-589), mTOR inhibitor (rapamycin), PI3K inhibitors (GDC-0941, LY294002) and STAT 3 inhibitor (STAT3 inhibitor VII) did not improve VSV infection or oncolysis in the SCC25 cell line (Table 1). In contrast, JAK2 inhibitor (TG101348), JAK1/2 inhibitor (ruxolitinib) and JAK family inhibitor (JAK Inhibitor I) significantly increased the cytotoxicity and infectivity of VSV-GFP on SCC25 (Figures 1b and c). At the highest drug and VSV concentrations tested, the amount of cell killing increased from 10 to 70% with TG101348, to 90% with ruxolitinib and to more than 99% with JAK Inhibitor I (Figure 1b). There was minimal cell killing with drug alone in the absence of VSV at the concentrations used.
Table 1.
Panel of drugs tested in conjunction with VSV infection in SCC25 cells
| Drugs | Concentrations used in this study | Targets | Enhance VSV oncolysis? |
|---|---|---|---|
| LBH589 (Panobinostat, NVP-LBH589) | 5 nM, 50 nM, 250 nM | Broad-spectrum HDAC inhibitor41,42 | No |
| GDC-0941 | 0.2 μM, 1μM, 5μM | PI3K inhibitor against p110α43 | No |
| LY294002 | 2μM, 10μM, 50μM | PI3K inhibitor: competitive inhibitor for ATP-binding site44,45 | No |
| STAT3 Inhibitor VII | 20 nM, 0.1 μM, 0.5 μM | Cellular JAK1/JAK2/TYK2/STAT3 activation46 | No |
| Rapamycin | 25 nM, 0.25 μM, 2.5 μM | Binds to immunophilin FKBP12 to inhibit the activity of FRAP/mTOR,47 | No |
| TG101348 (SAR302503) | 0.01 nM, 0.1 nM, 1 nM | ATP-competitive JAK2 inhibitor48,49 | Yes, + (70% kill) |
| Jakafi (INCB018424) | 250 nM, 2.5 μM, 25 μM | JAK family inhibitor for JAK1, JAK2 and JAK350 | Yes, + + (90% kill) |
| JAK inhibitor I (P6, DBI, 2-(1,1-dimethylethyl)-9-fluoro-3,6-dihydro-7H-bens51-imiaz [4,5-f]isoquinolin-7-one, Pyridone 6) | 1 μM, 10 μM, 50 μM | JAK family inhibitor for JAK1, JAK2 and JAK3, and TYK232,33 | Yes, + + + (>99% kill) |
Abbreviations: FRAP, FKBP-12-rapamycin associated protein; HDAC, histone deacetylase; mTOR, mammalian target of rapamycin; PI3K, phosphatidylinositol-3-kinase; STAT, signal transducer and activator of transcription; TYK2, tyrosine kinase 2; VSV, vesicular stomatitis virus.
Cell killing at multiplicity of infection 1.0 with the highest drug concentration tested is denoted for drugs showing positive effect on VSV oncolysis.
To further understand the effect of JAK inhibitors on VSV-induced killing of SCC25, cells were treated with the respective inhibitors at indicated concentrations for 24 h before infection by VSV-GFP (MOI of 0.5). Viral spread, as indicated by GFP expression, was significantly increased in treated cells compared with cells that did not receive drug treatment (Figure 1c). By 72 h, more than 90% of cells were GFP positive (infected) or were non-viable (loss of GFP expression). The impact of JAK inhibitors treatment on VSV-GFP progeny production in SCC25 cells was also analyzed (Figure 1d). Conditioned media from VSV-infected cells were harvested at 24, 48 and 72 h post infection and viral progeny was measured by TCID50 assay on Vero cells. Virus progeny production was increased significantly when cells were pretreated with JAK1/2 inhibitors (Figure 1d). JAK inhibitor I was the most potent and significantly increased viral progeny yield by 100- to 1000-fold.
JAK1/2 inhibitors increases cancer cell killing by VSV-ΔM51-GFP
In contrast to VSV-GFP, VSV-ΔM51-GFP is attenuated by deletion of methionine 51 in the matrix protein and induces production of IFN in infected cells, limiting viral spread in cells with functional IFN signaling pathways. As such, the virus is tolerated to higher levels when injected intranasally into Balb/c mice12 VSV-ΔM51-GFP efficiently infects FaDu and SW579, whereas SCC25 is highly resistant and SCC15 show intermediate resistance. Resistance of SCC25 and SCC15 to VSV-ΔM51-GFP infection and oncolysis can be overcome by pretreatment with JAK inhibitor I or ruxolitinib (Figures 2b and c). Pre-treatment of VSV-ΔM51-GFP-infected SCC25 and SCC15 cells with JAK inhibitors also resulted in a significant 100- to 500-fold increase (P < 0.05) in production of viral progeny compared with infected cells that received no inhibitors (Figure 2d).
Figure 2.
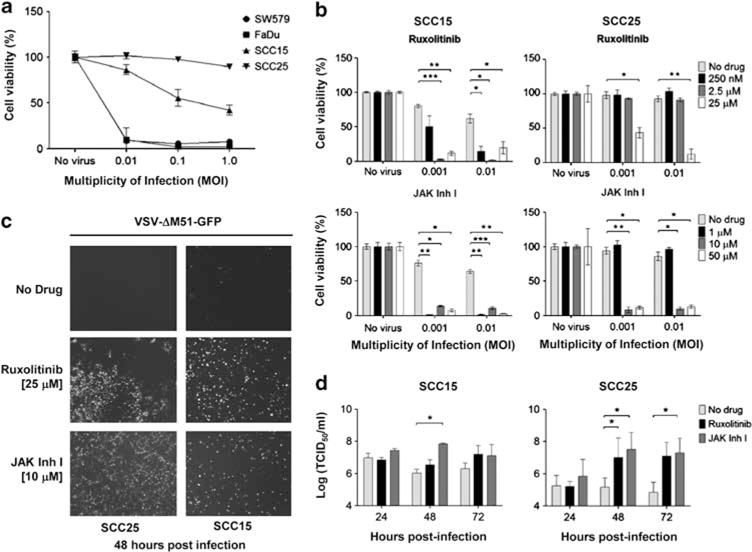
Janus kinase (JAK1/2) inhibitors increased vesicular stomatitis virus-ΔM51-green fluorescent protein (VSV-ΔM51-GFP) infection in SCC25 and SCC15 cells. (a) SW579, FaDu, SCC15, SCC25 were analyzed for VSV-ΔM51-GFP-induced cytotoxicity by MTS cell viability assay. (b) Cells were treated with JAK1/2 inhibitors 24 h before VSV and cell viability was determined 48 h later. (c) Representative photographs of VSV infection in treated cells. (d) Viral progeny produced by infected SCC15 and SCC25 cells (TCID50 assay). Average of three replicates (mean ± s.d.). *P ≤ 0.05, **P ≤ 0.001, ***P ≤ 0.0001.
JAK1/2 inhibitors decrease IFN-α/β inducible genes and increase VSV expression
To understand why SCC25 is constitutively resistant to VSV and how JAK inhibitors facilitate VSV infection in the HNSCC cells, reverse transcription-PCR for IFN responsive genes was performed. VSV-permissive SW579 and VSV-resistant SCC25 were treated with ruxolitinib (25 μm) or JAK inhibitor I (10 μM) for 24 h after which the cells were infected with VSVs (MOI of 0.5) or mock infected. Twenty-four hours later, cellular RNA was harvested and semi-quantitative reverse transcription-PCR for representative genes that have pivotal roles in virus detection (IFN regulatory factor, IRF-7), activation of type I IFN production (IRF-9) and limiting viral replication (IFN-α/β inducible antiviral genes MxA, OAS1, PKR). As shown in Figure 3a, IRF7, IRF9, OAS1 but not MxA, are constitutively expressed in SCC25 and not in SW579. Exposure of SCC25 to IFNα did not further increase transcription of these genes. Treatment of SCC25 cells with ruxolitinib and JAK inhibitor I for 24 h reduced constitutive expression of IRF7, IRF9 and OAS1 (Figure 3a). PKR mRNA level in SCC25 was not affected by inhibitor treatment (Figure 3a). Importantly, mRNA levels of VSV nucleocapsid were higher in SCC25 cells pretreated with JAK inhibitors at 24 h post infection than cells without drug treatment (Figure 3a). In contrast, IRF-7, IRF-9 or OAS1 is not constitutively expressed in VSV-permissive SW579 cells but IRF9 and OAS1 were upregulated by IFNα treatment or exposure to VSV-ΔM51-GFP. Ruxolitinib and JAK inhibitor I reduced the activation level of IRF-9 and OAS1 in IFNα-treated SW579.
Figure 3.
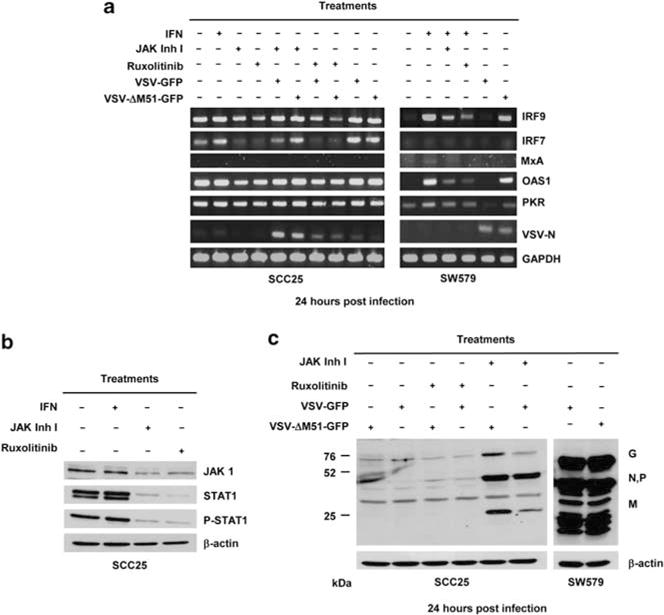
Treatment with Janus kinase (JAK) 1/2 inhibitors decreased interferon (IFN)-α/β inducible genes and increased vesicular stomatitis virus (VSV) gene expression. VSV-resistant SCC25 and VSV-permissive SW579 cells were treated with JAK1/2 inhibitors 24 h before VSV-green fluorescent protein (GFP) and VSV-ΔM51-GFP infection. (a) Semi-quantitative reverse transcription-PCR for VSV-N, IRF7, IRF9, PKR, MxA and OAS1. Immunoblot showing (b) expression of JAK1, signal transducer and activator of transcription 1 (STAT1) and phosphorated-STAT1 or (c) VSV proteins (glycoprotein G, nucleocapsid N, phosphoprotein P, and matrix M) under different treatment conditions.
JAK1/2 inhibitors decreased JAK1 and STAT1 protein expression and increased VSV expression in SCC25
JAK1/2 inhibitors bind to the ATP-binding site of JAK1 and JAK2 thus preventing activation of JAKs and the subsequent activation of proteins in the JAK1/STAT1 signaling pathway.32,33 To determine the effect of JAK inhibitors on JAK1 and STAT1 protein expression in SCC25 cells, immunoblotting was performed. The cells were treated with IFNα (500 U) or with ruxolitinib (25 μM) or JAK inhibitor I (10 μm) for 24 h. As shown in Figure 3b, SCC25 cells express JAK1 and STAT1 proteins and STAT1 is constitutively active (phosphorylated pSTAT1). IFN treatment did not further increase the expression of JAK, STAT1 and pSTAT1 proteins (Figure 3b). Treatment of cells with JAK1/2 inhibitors reduced the constitutive expression of JAK1, STAT1 and pSTAT1 proteins. Another cohort of SCC25 cells was infected with VSV-GFP or VSV-ΔM51-GFP. It is apparent from the immunoblot that JAK inhibitor I was more potent than ruxolitinib at improving VSV expression in the infected SCC25 cells (Figure 3c). As expected, VSV expression was robust in the permissive SW579 cells.
One hour of drug pretreatment is sufficient to improve VSV oncolysis
All the above studies used 24 h of inhibitor treatment before exposure of cells to VSV. Here, the timing required for drug exposure was further investigated. SCC25 cells were incubated with JAK1/2 inhibitors for 1, 3 or 24 h, after which the drug was removed and VSV-GFP or VSV-ΔM51-GFP was added (MOI of 0.5). As shown in Figure 4a, a relatively short exposure time of 1 or 3 h to the inhibitors rendered SCC25 cells susceptible to VSV oncolysis. There was no significant difference in cell killing between the 1, 3 or 24 h treatment groups at the respective drug and virus concentrations.
Figure 4.
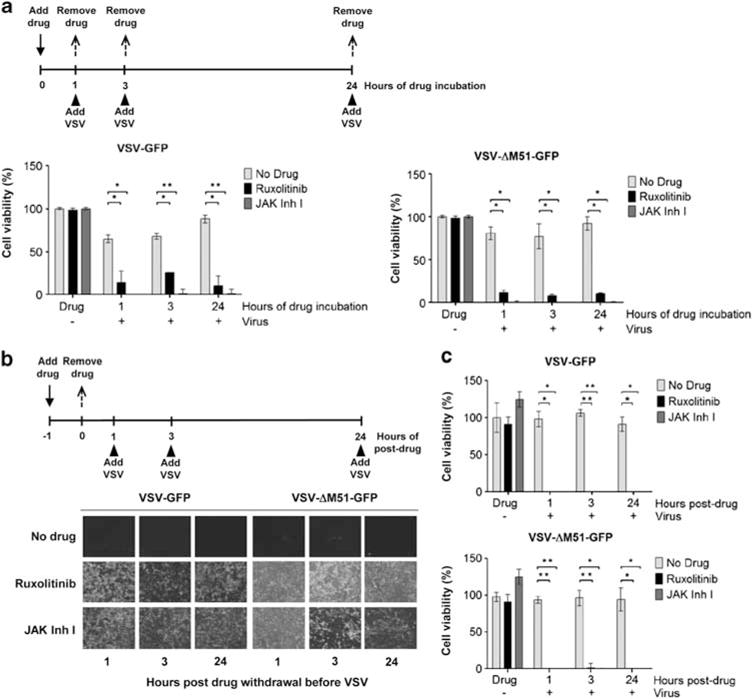
The effect of drug exposure times on vesicular stomatitis virus (VSV) infection and oncolysis. (a) SCC25 cells were incubated with Janus kinase (JAK) inhibitor I or ruxolitinib for 1, 3 or 24 h, after which the inhibitor was removed and cell infected with VSV-green fluorescent protein (GFP) or VSV-ΔM51-GFP. Cell viability was determined 48 h post virus infection. (b) SCC25 cells were incubated with inhibitors for 1 h after which the drug was removed and virus added 1, 3 or 24 h later. Representative photographs of cells are shown and (c) cell viability was determined at 48 h post virus infection. Average of three replicates (mean ± s.d.). *P ≤ 0.05, **P ≤ 0.001.
To determine the durability of the reversal of VSV resistance post inhibitor treatment, SCC25 cells were exposed to JAK Inhibitors for 1 h, after which the drug was removed and VSV was added 1, 3 or 24 h later (Figure 4b). Comparable GFP expression and cytotoxicity were observed regardless if the cells were infected with viruses 1, 3 or 24 h post inhibitor removal (Figure 4b). Cell proliferation assays showed that SCC25 cells could be infected and killed more than 99% by VSV-GFP and VSV-ΔM51-GFP even after the drugs had been removed 24 h ago (Figure 4c).
DISCUSSION
In this study, we demonstrate that there is variability in the susceptibility of human HNSCC cells to oncolysis by VSV. Although FaDu and SW579 cells are highly permissive to VSV infection, SCC25 is highly resistant and SCC15 has intermediate resistance. Combining virus therapy with JAK inhibitor I or ruxolitinib (Jakafi) enhanced VSV infection, spread and replication in the resistant cells, resulting in significant cell death. JAK inhibitor I is an ATP competitive inhibitor of JAKs (http://www.emdmillipore.com) and displays potent inhibitory activity against JAK1 (IC50 = 15 nM for murine JAK1), JAK2 (IC50 = 1 nM), JAK3 (Ki = 5 nM) and TYK2 (IC50 = 1 nM). The IC50 of ruxolitinib is 3.3 nM for JAK1 and 2.8 nM for JAK2.33 In our study, JAK inhibitor I was more potent than ruxolitinib at blocking the IFN-inducible JAK1/STAT1 signaling pathway in HNSCC cells to yield a higher level of VSV expression, progeny yield and oncolysis of the infected SCC25 and SCC15 cells. Based on results from other drug/VSV combination studies, we also evaluated inhibitors of other pathways important in IFN or antiviral responses, including HDAC, PI3K, mTOR and STAT3 inhibitors. The roles of these proteins in antiviral responses are varied. STAT3 has an important role in IFN signaling,34 whereas HDAC activity, commonly correlated with transcriptional repression, is essential for transcriptional induction of ISGs.35 HDAC function is also required for gene induction driven by virus activation of IRF3, and essential for establishment of an antiviral response against VSV.35 Pretreatment of human prostate PC3 cancer cells with HDAC inhibitors, MS-275 or SAHA (Vorinostat), enhanced VSV oncolysis by blunting IFN response and inhibited the increase in IRF-7 and MxA mRNA levels post virus infection.23 The PI3K/mTOR/Akt pathway is important in coordinating innate immune defense and is an important regulator of type I IFN production via activation of IRF7.36 Combination therapy of VSV-ΔM51 with rapamycin (inhibitor of mTOR) reduced IFN production and significantly improved survival of rats bearing malignant glioma.25 However, our study shows that none of these inhibitors were effective at reversing VSV resistance in the HNSCC SCC15 or SCC25 cells.
The VSV-resistant SCC25 cells constitutively express high levels of IRF7, IRF9 and OAS1 mRNA. In contrast, these genes are not detectable in VSV-permissive SW579 cells where they are activated only after IFN or VSV treatment. Addition of JAK inhibitors reduced IRF7 and IRF9 mRNA only twofold in SCC25, whereas OAS1 mRNA levels showed no appreciable change. In contrast to the data on PC3 cancer cells where both HDAC and JAK inhibitors were effective at reversing VSV resistance, our data show that only JAK inhibitors were effective in the HNSCC cells.23,24 Interestingly, Carey et al. showed that besides constitutive expression of numerous antiviral gene products (for example, MxA, OAS1), VSV resistance in PC3 cells is also compounded by delays in VSV penetration (by 10–30 min), primary transcription and viral protein synthesis (by 6–8 h) relative to the VSV-permissive LNCaP cells. Clearly, there are numerous factors in play that contribute to relative resistance of different cell types to VSV oncolysis. Paglino and van den Pol37 performed a broad analysis on a panel of 13 sarcomas representing seven tumor types and showed that synovial sarcoma SW982 demonstrated remarkable resistance, even at a MOI of 100. These authors found that the basal pre-infection levels of ISGs, MxA and ISG-15 were aberrantly high in SW982 cells. JAK inhibitor I given 4 days pre-infection (but not coincident with VSV) reversed this antiviral state. Moerdyk-Schauwecker et al. showed that pancreatic cancer cells have substantial diversity in their susceptibility to VSV oncolysis, and constitutively express at least some ISGs, including the antiviral genes MxA and OAS.38 Treatment of cells for 48 h with JAK inhibitor I reduced MxA and OAS protein to undetectable levels in the uninfected cells.
Contrary to the above reports showing that 2–4 days of JAK inhibitor drug pre-treatment are needed to impact the VSV susceptibility of sarcoma and prostate cancer lines, VSV resistance in HNSCC cells can be reversed by pre-treatment of cells with inhibitors for 1 h. Interestingly, this inhibitory effect persists for 24 h after removal of the drug. This sustained effect is unexpected as JAK1/2 inhibitors bind reversibly to the ATP-binding site of JAK1 and JAK2 to prevent activation of JAKs and proteins in the Jak/Stat signaling pathway.32,33 However, the possibility that the inhibitors can be co-administered with VSV is very convenient to facilitate logistics of clinical trials. Although JAK inhibitor I is not being developed for clinical use, there are several JAK inhibitors under clinical testing for a variety of anti-cancer or anti-inflammatory applications.39 Ruxolitinb and Tofacitinib (JAK3, JAK1 inhibitor) were recently approved by the Food and Drug Administration, respectively, for the treatment of patients with intermediate or high-risk myelofibrosis or rheumatoid arthritis.40 As researchers continue to develop JAK inhibitors that are more specific, future studies could perhaps more accurately define the mechanism of virus resistance in cancer cells for improved oncolysis and therapeutic outcome.
Acknowledgments
We are grateful to funding support from Mayo Foundation.
Footnotes
CONFLICT OF INTEREST
SJR and KWP have financial interests in Omnis Pharma, an oncolytic VSV company.
References
- 1.Finkelshtein D, Werman A, Novick D, Barak S, Rubinstein M. LDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virus. Proc Natl Acad Sci USA. 2013;110:7306–7311. doi: 10.1073/pnas.1214441110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30:658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hastie E, Grdzelishvili VZ. Vesicular stomatitis virus as a flexible platform for oncolytic virotherapy against cancer. J Gen Virol. 2012;93(Pt 12):2529–2545. doi: 10.1099/vir.0.046672-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reis JL, Jr, Rodriguez LL, Mead DG, Smoliga G, Brown CC. Lesion development and replication kinetics during early infection in cattle inoculated with Vesicular stomatitis New Jersey virus via scarification and black fly (Simulium vittatum) bite. Vet Pathol. 2011;48:547–557. doi: 10.1177/0300985810381247. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez LL. Emergence and re-emergence of vesicular stomatitis in the United States. Virus Res. 2002;85:211–219. doi: 10.1016/s0168-1702(02)00026-6. [DOI] [PubMed] [Google Scholar]
- 6.Roberts A, Buonocore L, Price R, Forman J, Rose JK. Attenuated vesicular stomatitis viruses as vaccine vectors. J Virol. 1999;73:3723–3732. doi: 10.1128/jvi.73.5.3723-3732.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bi Z, Barna M, Komatsu T, Reiss CS. Vesicular stomatitis virus infection of the central nervous system activates both innate and acquired immunity. J Virol. 1995;69:6466–6472. doi: 10.1128/jvi.69.10.6466-6472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clarke DK, Cooper D, Egan MA, Hendry RM, Parks CL, Udem SA. Recombinant vesicular stomatitis virus as an HIV-1 vaccine vector. Springer Semin Immunopathol. 2006;28:239–253. doi: 10.1007/s00281-006-0042-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Publicover J, Ramsburg E, Rose JK. Characterization of nonpathogenic, live, viral vaccine vectors inducing potent cellular immune responses. J Virol. 2004;78:9317–9324. doi: 10.1128/JVI.78.17.9317-9324.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelly EJ, Nace R, Barber GN, Russell SJ. Attenuation of vesicular stomatitis virus encephalitis through microRNA targeting. J Virol. 2010;84:1550–1562. doi: 10.1128/JVI.01788-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ammayappan A, Nace R, Peng KW, Russell SJ. Neuroattenuation of vesicular stomatitis virus through picornaviral internal ribosome entry sites. J Virol. 2013;87:3217–3228. doi: 10.1128/JVI.02984-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stojdl DF, Lichty BD, tenOever BR, Paterson JM, Power AT, Knowles S, et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4:263–275. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed M, McKenzie MO, Puckett S, Hojnacki M, Poliquin L, Lyles DS. Ability of the matrix protein of vesicular stomatitis virus to suppress beta interferon gene expression is genetically correlated with the inhibition of host RNA and protein synthesis. J Virol. 2003;77:4646–4657. doi: 10.1128/JVI.77.8.4646-4657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obuchi M, Fernandez M, Barber GN. Development of recombinant vesicular stomatitis viruses that exploit defects in host defense to augment specific oncolytic activity. J Virol. 2003;77:8843–8856. doi: 10.1128/JVI.77.16.8843-8856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jenks N, Myers R, Greiner SM, Thompson J, Mader EK, Greenslade A, et al. Safety studies on intrahepatic or intratumoral injection of oncolytic vesicular stomatitis virus expressing interferon-beta in rodents and nonhuman primates. Hum Gene Ther. 2010;21:451–462. doi: 10.1089/hum.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naik S, Nace R, Barber GN, Russell SJ. Potent systemic therapy of multiple myeloma utilizing oncolytic vesicular stomatitis virus coding for interferon-beta. Cancer Gene Ther. 2012;19:443–450. doi: 10.1038/cgt.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naik S, Russell SJ. Engineering oncolytic viruses to exploit tumor specific defects in innate immune signaling pathways. Expert Opin Biol Ther. 2009;9:1163–1176. doi: 10.1517/14712590903170653. [DOI] [PubMed] [Google Scholar]
- 18.Heiber JF, Barber GN. Evaluation of innate immune signaling pathways in transformed cells. Methods Mol Biol. 2012;797:217–238. doi: 10.1007/978-1-61779-340-0_15. [DOI] [PubMed] [Google Scholar]
- 19.Sadler AJ, Williams BR. Interferon-inducible antiviral effectors. Nat Rev Immunol. 2008;8:559–568. doi: 10.1038/nri2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 21.van Boxel-Dezaire AH, Rani MR, Stark GR. Complex modulation of cell type-specific signaling in response to type I interferons. Immunity. 2006;25:361–372. doi: 10.1016/j.immuni.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 22.de Veer MJ, Holko M, Frevel M, Walker E, Der S, Paranjape JM, et al. Functional classification of interferon-stimulated genes identified using microarrays. J Leukoc Biol. 2001;69:912–920. [PubMed] [Google Scholar]
- 23.Nguyen TL, Abdelbary H, Arguello M, Breitbach C, Leveille S, Diallo JS, et al. Chemical targeting of the innate antiviral response by histone deacetylase inhibitors renders refractory cancers sensitive to viral oncolysis. Proc Natl Acad Sci USA. 2008;105:14981–14986. doi: 10.1073/pnas.0803988105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carey BL, Ahmed M, Puckett S, Lyles DS. Early steps of the virus replication cycle are inhibited in prostate cancer cells resistant to oncolytic vesicular stomatitis virus. J Virol. 2008;82:12104–12115. doi: 10.1128/JVI.01508-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alain T, Lun X, Martineau Y, Sean P, Pulendran B, Petroulakis E, et al. Vesicular stomatitis virus oncolysis is potentiated by impairing mTORC1-dependent type I IFN production. Proc Natl Acad Sci USA. 2010;107:1576–1581. doi: 10.1073/pnas.0912344107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hadac EM, Peng KW, Nakamura T, Russell SJ. Reengineering paramyxovirus tropism. Virology. 2004;329:217–225. doi: 10.1016/j.virol.2004.08.036. [DOI] [PubMed] [Google Scholar]
- 27.Di Bona D, Cippitelli M, Fionda C, Camma C, Licata A, Santoni A, et al. Oxidative stress inhibits IFN-alpha-induced antiviral gene expression by blocking the JAK-STAT pathway. J Hepatol. 2006;45:271–279. doi: 10.1016/j.jhep.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 28.Izaguirre A, Barnes BJ, Amrute S, Yeow WS, Megjugorac N, Dai J, et al. Comparative analysis of IRF and IFN-alpha expression in human plasmacytoid and monocyte-derived dendritic cells. J Leukoc Biol. 2003;74:1125–1138. doi: 10.1189/jlb.0603255. [DOI] [PubMed] [Google Scholar]
- 29.Kawakubo K, Ohyashiki K, Ohyashiki JH, Shimamoto T, Fujimura T, Iwama H, et al. A possible correlation between interferon-stimulated gene expression and cytogenetic responses in chronic myelogenous leukemia patients treated with alpha-interferon. Jpn J Clin Oncol. 1996;26:59–64. doi: 10.1093/oxfordjournals.jjco.a023185. [DOI] [PubMed] [Google Scholar]
- 30.Peng G, Lei KJ, Jin W, Greenwell-Wild T, Wahl SM. Induction of APOBEC3 family proteins, a defensive maneuver underlying interferon-induced anti-HIV-1 activity. J Exp Med. 2006;203:41–46. doi: 10.1084/jem.20051512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hasegawa K, Nakamura T, Harvey M, Ikeda Y, Oberg A, Figini M, et al. The use of a tropism-modified measles virus in folate receptor-targeted virotherapy of ovarian cancer. Clin Cancer Res. 2006;12(20 Pt 1):6170–6178. doi: 10.1158/1078-0432.CCR-06-0992. [DOI] [PubMed] [Google Scholar]
- 32.Thompson JE, Cubbon RM, Cummings RT, Wicker LS, Frankshun R, Cunningham BR, et al. Photochemical preparation of a pyridone containing tetracycle: a Jak protein kinase inhibitor. Bioorg Med Chem Lett. 2002;12:1219–1223. doi: 10.1016/s0960-894x(02)00106-3. [DOI] [PubMed] [Google Scholar]
- 33.Mascarenhas J, Mughal TI, Verstovsek S. Biology and clinical management of myeloproliferative neoplasms and development of the JAK inhibitor ruxolitinib. Curr Med Chem. 2012;19:4399–4413. doi: 10.2174/092986712803251511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang CH, Murti A, Pfeffer LM. STAT3 complements defects in an interferon-resistant cell line: evidence for an essential role for STAT3 in interferon signaling and biological activities. Proc Natl Acad Sci USA. 1998;95:5568–5572. doi: 10.1073/pnas.95.10.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang HM, Paulson M, Holko M, Rice CM, Williams BR, Marie I, et al. Induction of interferon-stimulated gene expression and antiviral responses require protein deacetylase activity. Proc Natl Acad Sci USA. 2004;101:9578–9583. doi: 10.1073/pnas.0400567101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao W, Manicassamy S, Tang H, Kasturi SP, Pirani A, Murthy N, et al. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nat Immunol. 2008;9:1157–1164. doi: 10.1038/ni.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paglino JC, van den Pol AN. Vesicular stomatitis virus has extensive oncolytic activity against human sarcomas: rare resistance is overcome by blocking interferon pathways. J Virol. 2011;85:9346–9358. doi: 10.1128/JVI.00723-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moerdyk-Schauwecker M, Shah NR, Murphy AM, Hastie E, Mukherjee P, Grdzelishvili VZ. Resistance of pancreatic cancer cells to oncolytic vesicular stomatitis virus: role of type I interferon signaling. Virology. 2013;436:221–234. doi: 10.1016/j.virol.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kontzias A, Kotlyar A, Laurence A, Changelian P, O’Shea JJ. Jakinibs: a new class of kinase inhibitors in cancer and autoimmune disease. Curr Opin Pharmacol. 2012;12:464–470. doi: 10.1016/j.coph.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaddi K, Sarlis NJ, Gupta V. Ruxolitinib an oral JAK1 and JAK2 inhibitor, in myelofibrosis. Expert Opin Pharmacother. 2012;13:2397–2407. doi: 10.1517/14656566.2012.732998. [DOI] [PubMed] [Google Scholar]
- 41.Landreville S, Agapova OA, Matatall KA, Kneass ZT, Onken MD, Lee RS, et al. Histone deacetylase inhibitors induce growth arrest and differentiation in uveal melanoma. Clin Cancer Res. 2012;18:408–416. doi: 10.1158/1078-0432.CCR-11-0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lane AA, Chabner BA. Histone deacetylase inhibitors in cancer therapy. J Clin Oncol. 2009;27:5459–5468. doi: 10.1200/JCO.2009.22.1291. [DOI] [PubMed] [Google Scholar]
- 43.Folkes AJ, Ahmadi K, Alderton WK, Alix S, Baker SJ, Box G, et al. The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-t hieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J Med Chem. 2008;51:5522–5532. doi: 10.1021/jm800295d. [DOI] [PubMed] [Google Scholar]
- 44.Fransson S, Uv A, Eriksson H, Andersson MK, Wettergren Y, Bergo M, et al. p37delta is a new isoform of PI3K p110delta that increases cell proliferation and is over-expressed in tumors. Oncogene. 2012;31:3277–3286. doi: 10.1038/onc.2011.492. [DOI] [PubMed] [Google Scholar]
- 45.Young NR, Liu J, Pierce C, Wei TF, Grushko T, Olopade OI, et al. Molecular phenotype predicts sensitivity of squamous cell carcinoma of the head and neck to epidermal growth factor receptor inhibition. Mol Oncol. 2013;7:359–368. doi: 10.1016/j.molonc.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uehara Y, Mochizuki M, Matsuno K, Haino T, Asai A. Novel high-throughput screening system for identifying STAT3-SH2 antagonists. Biochem Biophys Res Commun. 2009;380:627–631. doi: 10.1016/j.bbrc.2009.01.137. [DOI] [PubMed] [Google Scholar]
- 47.Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, et al. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369:756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- 48.Pardanani A, Gotlib JR, Jamieson C, Cortes JE, Talpaz M, Stone RM, et al. Safety and efficacy of TG101348, a selective JAK2 inhibitor, in myelofibrosis. J Clin Oncol. 2011;29:789–796. doi: 10.1200/JCO.2010.32.8021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Geyer HL, Tibes R, Mesa RA. JAK2 inhibitors and their impact in myeloproliferative neoplasms. Hematology. 2012;17(Suppl 1):S129–S132. doi: 10.1179/102453312X13336169156375. [DOI] [PubMed] [Google Scholar]
- 50.Mascarenhas J, Hoffman R. Ruxolitinib: the first FDA approved therapy for the treatment of myelofibrosis. Clin Cancer Res. 2012;18:3008–3014. doi: 10.1158/1078-0432.CCR-11-3145. [DOI] [PubMed] [Google Scholar]
- 51.Ahmed M, Marino TR, Puckett S, Kock ND, Lyles DS. Immune response in the absence of neurovirulence in mice infected with m protein mutant vesicular stomatitis virus. J Virol. 2008;82:9273–9277. doi: 10.1128/JVI.00915-08. [DOI] [PMC free article] [PubMed] [Google Scholar]


