Abstract
Proximity-driven metalation has been extensively exploited to achieve reactivity and selectivity in C–H bond activation. Despite the substantial improvement in developing more efficient and practical directing groups, their stoichiometric installation and removal limit efficiency and often applicability as well. Herein, we report the development of an amino acid reagent that reversibly reacts with aldehydes and ketones in situ via imine formation to serve as a transient directing group for activation of inert C–H bonds. Arylation of a wide range of aldehydes and ketones at the β- or γ-positions proceeds in the presence of a Pd catalyst and a catalytic amount of amino acid. The feasibility of achieving enantioselective C–H activation reactions using a chiral amino acid as the transient directing group is also demonstrated.
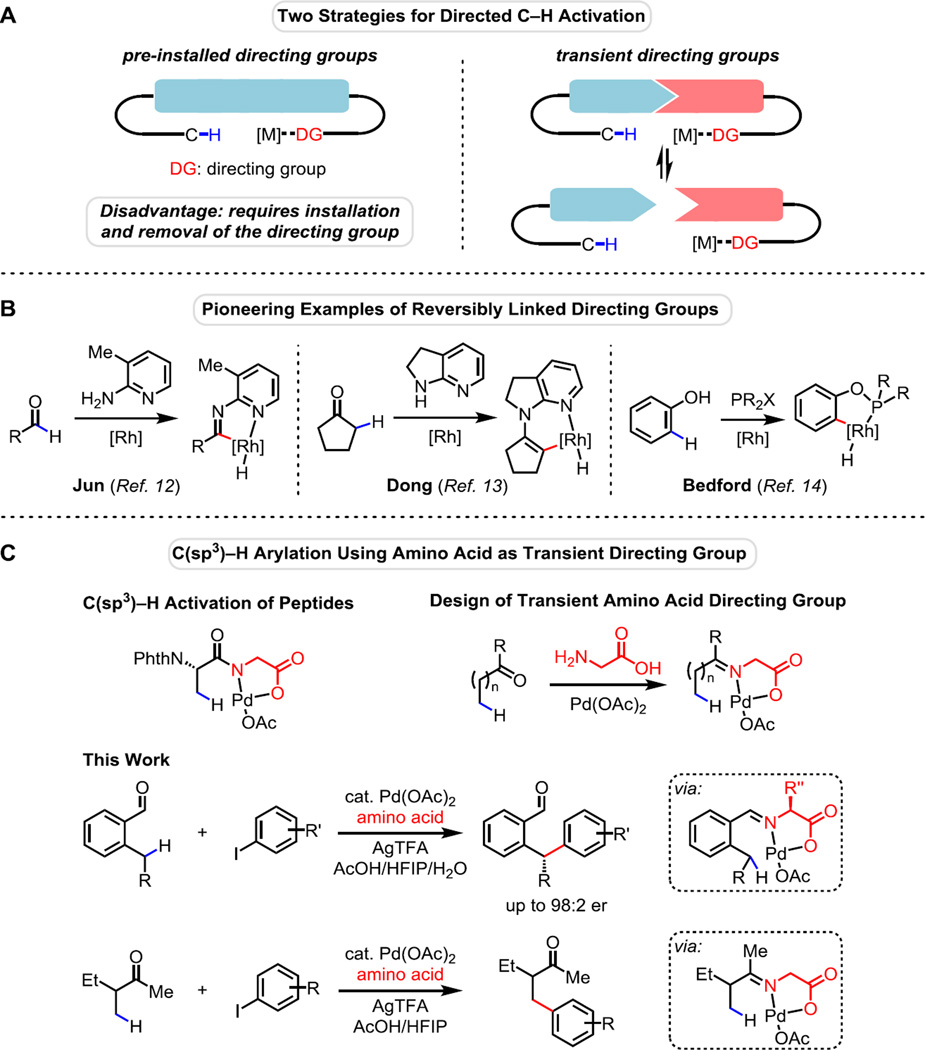
Precoordination of metal with functional groups in substrates has been extensively exploited to control selectivity and promote reactivity in metal-catalyzed or -mediated reactions (1–5). The same approach has been successfully implemented in directed carbon–hydrogen (C–H) activation reactions (6–11). However, the covalent installation and removal of directing groups is a major drawback for synthetic applications. First, an additional two steps must be added to the synthetic sequence. Second, the conditions for installation or removal of the directing groups are sometimes incompatible with other functional groups present in advanced synthetic intermediates. It is therefore highly desirable to devise a functionally tolerant reagent that can be reversibly linked to the substrate and serve as a directing group. Upon C–H activation and subsequent functionalization, this reagent would dissociate from the product and transiently link to another substrate molecule so that only a catalytic quantity of the directing group would be needed (Fig. 1A). This approach has been successfully implemented in Rh(I)-catalyzed C(sp2)–H activation reactions in a number of pioneering examples. Jun et al. reported the use of 2-amino pyridine as a transient directing group for Rh-catalyzed activation of aldehydic C–H bonds (12) (Fig. 1B). Recently, using a related strategy, Mo and Dong reported a Rh-catalyzed α-alkylation of ketones via a vinyl C–H activation step, featuring an enamine intermediate with a pyridine moiety as the transient directing group (13). Bedford et al. developed a Rh-catalyzed ortho-arylation through reversible in situ transesterification of catalytic amounts of phosphinite ligands with the phenol substrate (14). The strategy of using catalytic directing groups has also been employed by Lightburn et al. (15) and Grünanger and Breit (16) to achieve selectivity in Rh-catalyzed hydroformylation reactions.
Fig. 1. C–H activation using transient directing groups.
(A) Two strategies for directed C–H Activation. (B) Pioneering examples of reversibly linked directing groups. (C) C(sp3)–H arylation using amino acid as a transient directing group.
In conjunction with our efforts to develop Pd-catalyzed C(sp3)–H functionalizations (17, 18), we have extensively investigated the feasibility of Pd(II)-catalyzed C(sp3)–H activation of aldehydes and ketones using a wide range of potential transient directing groups including those previously developed for Rh(I) catalysts. Unfortunately, the resultant Pd(II)-complexes bound to the bidentate iminopyridine or iminooxazoline are unreactive toward cleavage of sp3 C–H bonds under various conditions. The development of mono-protected amino acid ligands (19, 20) and the recent use of amino acids as bidentate directing groups in C–H functionalizations of peptides (21) led us to speculate that amino acids could serve as a suitable transient directing group. We reasoned that the amino acid could be reversibly tethered to an aldehyde or ketone substrate via an imine linkage under suitable conditions. The imine moiety and the carboxylate could form a bidentate directing group in a similar manner to that operative in our dipeptide chemistry to enable subsequent C–H functionalization (Fig. 1C).
We chose arylation of 2-methylbenzaldehyde 1a with 4-iodoanisole as the model reaction for optimization. Benzaldehyde derivatives are known to readily form imine linkages with amino acids (22). After extensive experimentation, we found that the desired C(sp3)–H selective mono-arylation product 2a could be observed in 18% NMR yield when the reaction was conducted in hexafluoroisopropyl alcohol (HFIP) with 10 mol% Pd(OAc)2, 40 mol% glycine and 1.5 equiv. of silver trifluoroacetate (see Table S1, entry 5). To our delight, the use of acetic acid as the solvent improved the yield to 52%, albeit with significant decomposition of the starting material (Table S1, entry 6). We speculated that the low mass balance stemmed from a rate mismatch between the imine formation step and the following C–H cleavage step, leading to decomposition of the accumulated imine species. Therefore, we reasoned that the addition of water would reduce the concentration of the imine intermediate and prevent the decomposition during the reaction. Indeed, when a 9:1 mixture of AcOH and H2O was used as the solvent, we were able to achieve 93% conversion and 71% NMR yield, along with formation of the diarylated product in 11% yield (Table S1, entry 8). By switching the limiting reagent to the aryl iodide, the desired mono-arylated product was obtained exclusively in 81% NMR yield (Table S1, entry 10). We also evaluated a variety of amino acids and found that the side chain of the amino acid does not have a significant effect on the efficiency of this transformation (Table S1, entries 10 to 13). As expected, N-protected glycine led to a complete loss of reactivity, consistent with the hypothesis that imine formation is crucial for enabling C–H activation (Table S1, entry 14).
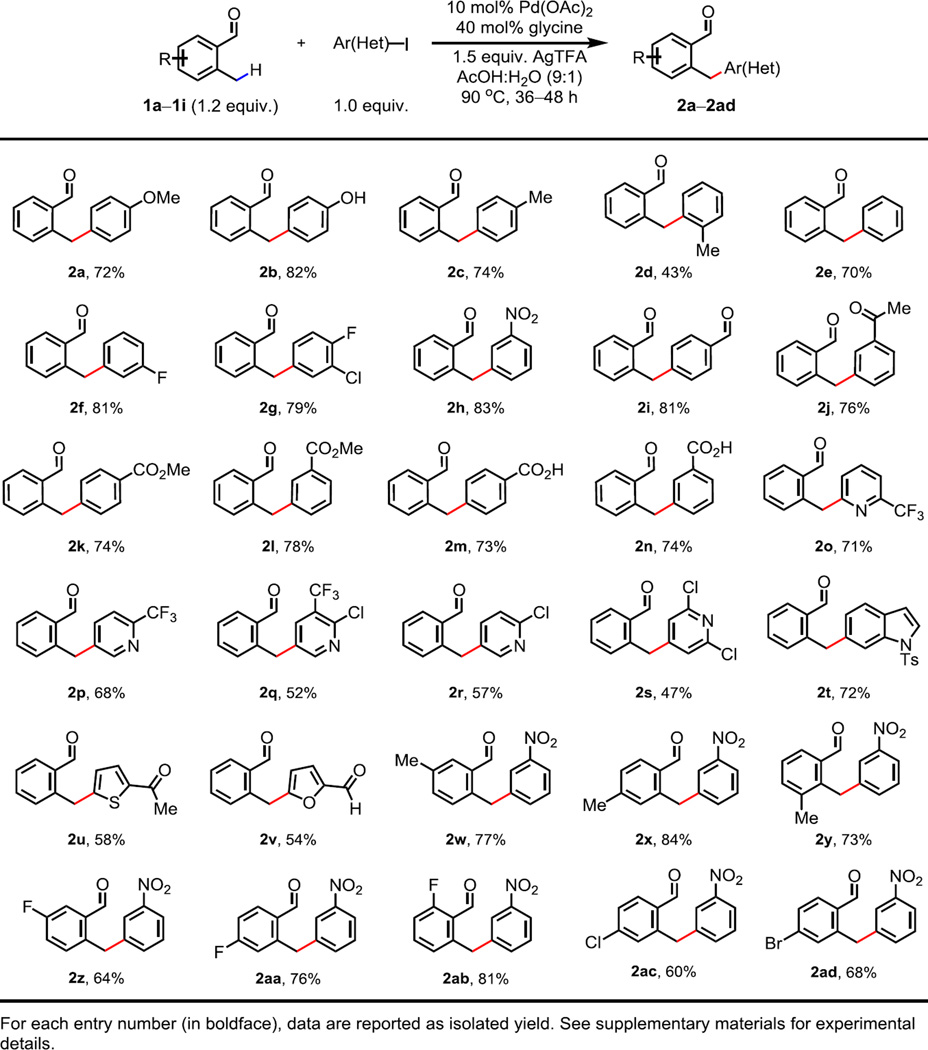
With the optimal conditions in hand, the scope of C–H arylation was examined (Fig. 2). We were delighted to observe that the arylation of 2-methylbenzaldehyde 1a proceeded with a wide range of aryl iodides in good yields. Both electron-donating (2a to 2c) and electron-withdrawing (2f to 2n) substituents at either para- or meta-position of the aryl iodide are well tolerated (72–83% yield). Use of a sterically hindered ortho-substituted aryl iodide led to a reduced yield (2d, 43%). The reaction can accommodate a range of functional groups, including halides (F and Br), nitro groups, aldehydes, ketones, esters, and even free carboxylic acid groups. More importantly, this reaction is compatible with heteroaryl iodides as coupling partners (2o to 2v), overriding the strong coordination of heteroatoms to the metal catalyst. This feature renders the reaction particularly useful for the synthesis of biologically active compounds bearing heterocycles. Lastly, benzaldehydes with various ortho-, meta- and para-substituents were effectively arylated with 1-iodo-3-nitrobenzene, providing the corresponding products in good yields under the standard conditions (2w to 2ad). The analogous ketone substrate 2’-methylacetophenone gave the arylated product in low yield, presumably due to the fact that ketimine is more difficult to form.
Fig. 2. Palladium-catalyzed benzylic C(sp3)–H arylation of aldehydes.
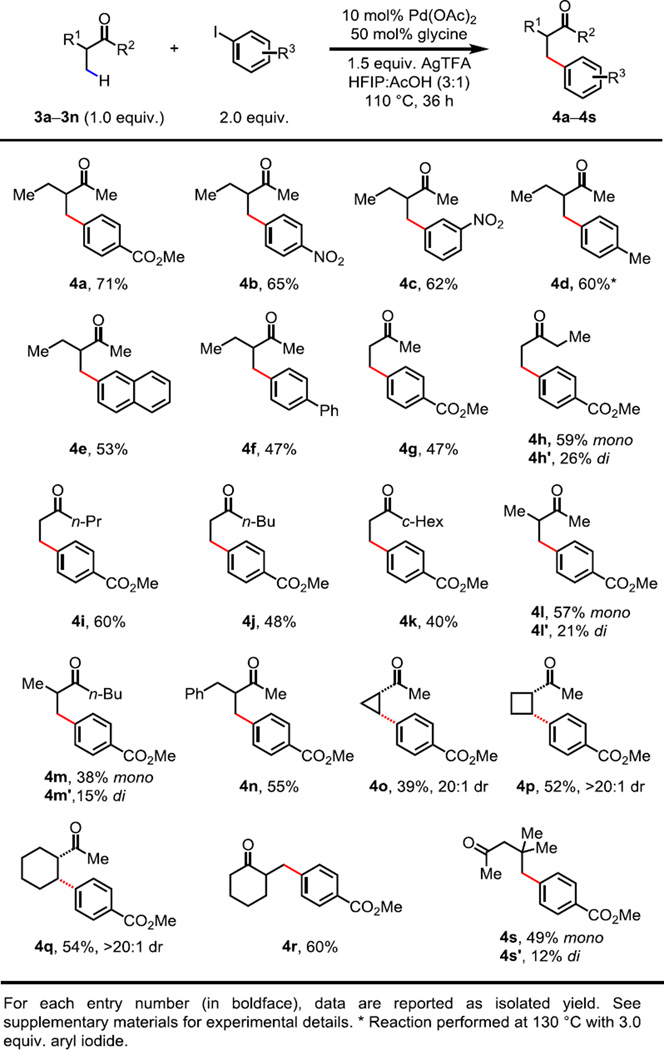
We next investigated whether the same strategy could be applied to aliphatic ketone substrates, which are versatile and widely used synthetic intermediates (23). While β-arylation of ketones via reaction pathways other than β-metal insertion has been limited to the substrates without α-substitution (24, 25), directed C(sp3)–H activation of ketones has only been studied through the intermediacy of preformed oximes (9, 26, 27). We were aware that direct arylation of ketones using a transient directing group would face several challenges: First, in situ formation of ketimines is generally less favored than that of aldimines. Second, one of the ketimine E/Z-isomers may not be reactive if the orientation of the imino acid directing group is not suitable for directing palladium in proximity to the target C–H bond. Third, tautomerization of imines to enamines may occur as a competing pathway, which would generate an ineffective directing group. With these considerations in mind, we further optimized the reaction conditions for arylation of ketones. Although the initial investigation with 3-methyl-2-pentanone 3a provided <2% desired product under the optimal conditions for aldehydes (Table S2, entry 1), we found that by using 50 mol% glycine and a 3:1 mixture of HFIP/AcOH as the solvent, the yield of β-arylation product 4a could be significantly improved to 71% (Table S2, entry 7). A variety of amino acids with side chains, however, were inferior and led to diminished yields (Table S2, entries 9–11).
We then explored the scope of ketones and aryl iodides under the new conditions. As illustrated in Fig. 3, a wide range of aryl iodides with electron-donating or electron-withdrawing substituents were well tolerated (4a to 4f). Moderate to good yields were achieved with a variety of linear, α-branched and cyclic ketones (4g to 4r). We found that this method can activate methylene C(sp3)–H bonds in cyclic substrates, providing the corresponding syn-products with excellent diastereoselectivity (4o to 4q). Furthermore, one example of γ-arylation was observed when β-C(sp3)–H bonds were absent in the substrate (4s). Aliphatic aldehydes, however, are not suitable substrates due to competing reaction pathways leading to the decomposition of the starting material under the current conditions.
Fig. 3. Palladium-catalyzed C(sp3)–H arylation of ketones.
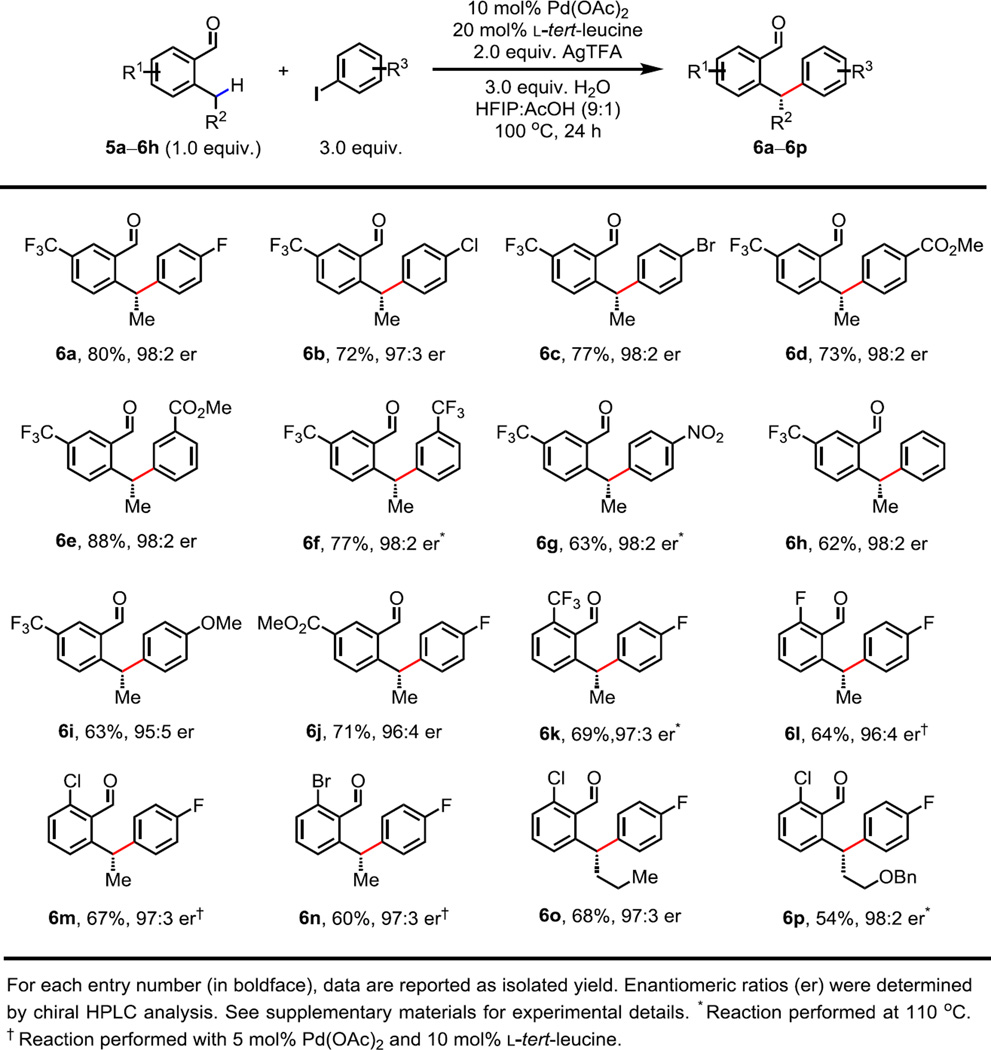
Considering that Pd-catalyzed enantioselective C–H insertion reactions remain a substantial challenge (19, 28–30), we wondered whether the enantioselective C–H arylation could be achieved by using a chiral reversible directing agent. Thus, benzaldehydes bearing methylene C(sp3)–H bonds were investigated in the presence of a chiral amino acid. This transformation is challenging due to the more sluggish C–H activation, as well as the requirement of discrimination between the two enantiotopic C–H bonds. On the basis of our proposed intermediate in Fig. 1C, we envisioned that the enantioselection could be realized through the steric interaction between the R group (R ≠ H) and the bulky side chain R” of the amino acid. Indeed, when 40 mol% l-valine (R” = isopropyl) was used instead of glycine, the arylation of 2-ethyl-5-(trifluoromethyl)benzaldehyde 5a provided the desired product 6a with 85:15 er (Table S3, entry 4). Encouraged by this outcome, we speculated that a more sterically encumbered amino acid should lead to superior enantioselectivity. When l-tert-leucine (R” = tert-butyl) was employed, excellent enantioselectivity (98:2 er) was achieved, albeit in only 30% NMR yield (Table S3, entry 5). Intriguingly, reducing the ratio of ligand/Pd from 4:1 to 2:1 resulted in a significantly enhanced yield (87%) without any erosion of enantioselectivity (Table S3, entry 6). Apparently, sterically bulky l-tert-leucine is less prone to imine formation, allowing the free form of the amino acid to coordinate with Pd(II) and deactivate the catalyst. Finally, the addition of 3 equiv. of water led to almost identical results (Table S3, entry 8). However, the presence of water was proven beneficial to other aldehyde substrates.
With the optimized conditions, the enantioselective arylation of 5a proceeded effectively with a wide range of aryl iodides bearing various functional groups at the para- or meta-position (Fig. 4, 6a to 6i). With respect to the scope of aldehydes, the reaction is compatible with ester (6j), trifluoromethyl (6k) and halogen substituents (6l to 6n). Halogen-substituted benzaldehydes exhibited higher reactivity, and the palladium catalyst loading could be reduced to 5 mol%. Moreover, the methylene C(sp3)–H bond on longer side chains could also be activated (6o and 6p), thus greatly expanding the scope of aldehydes.
Fig. 4. Palladium-catalyzed enantioselective benzylic C(sp3)–H arylation of aldehydes.
Supplementary Material
Acknowledgments
We gratefully acknowledge The Scripps Research Institute, the NIH (NIGMS, 2R01GM084019) for their financial support. F.-L. Z. thanks Wuhan University of Technology and T.-J. L. thanks Jiangsu Normal University for their financial support. J.-Q. Y. and F.-L. Z. conceived the concept. F.-L. Z. performed the arylation of primary C–H bonds. K. H. developed the enantioselective C–H arylation. T.-J. L. developed conditions for β-arylation of ketones. H. P. performed enantioselective C–H arylation. Metrical parameters for the structure of S2 are available free of charge from the Cambridge Crystallographic Data Centre under reference number CCDC 1434263.
Footnotes
Supplementary Text
Tables S1 to S9
NMR Spectra
HPLC Spectra
X-ray Crystallographic Data
References (31)
References and Notes
- 1.Brown JM. Selectivity and mechanism in catalytic asymmetric synthesis. Chem. Soc. Rev. 1993;22:25–41. [Google Scholar]
- 2.Hoveyda AH, Evans DA, Fu GC. Substrate-directable chemical reactions. Chem. Rev. 1993;93:1307–1370. [Google Scholar]
- 3.Hartung CG, Snieckus V. In: Modern Arene Chemistry. Astruc D, editor. Weinheim, Germany: Wiley-VCH; 2004. pp. 330–367. [Google Scholar]
- 4.Johnson RA, Sharpless KB. In: Catalytic Asymmetric Synthesis. 2. Ojima I, editor. Weinheim, Germany: Wiley-VCH; 2005. pp. 231–280. [Google Scholar]
- 5.Li Z, Yamamoto H. Hydroxamic acids in asymmetric synthesis. Acc. Chem. Res. 2013;46:506–518. doi: 10.1021/ar300216r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X, Engle KM, Wang D-H, Yu J-Q. Palladium(II)-catalyzed C–H activation/C–C cross-coupling reactions: versatility and practicality. Angew. Chem. Int. Ed. 2009;48:5094–5115. doi: 10.1002/anie.200806273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daugulis O, Do H-Q, Shabashov D. Palladium- and copper-catalyzed arylation of carbon–hydrogen bonds. Acc. Chem. Res. 2009;42:1074–1086. doi: 10.1021/ar9000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ackermann L, Vicente R, Kapdi A. Transition-metal-catalyzed direct arylations of (hetero)arenes via C–H bond cleavage. Angew. Chem. Int Ed. 2009;48:9792–9826. doi: 10.1002/anie.200902996. [DOI] [PubMed] [Google Scholar]
- 9.Desai LV, Hull KL, Sanford MS. Palladium-catalyzed oxygenation of unactivated sp3 C–H bonds. J. Am. Chem. Soc. 2004;126:9542–9543. doi: 10.1021/ja046831c. [DOI] [PubMed] [Google Scholar]
- 10.Colby DA, Bergman RG, Ellman JA. Rhodium-catalyzed C–C bond formation via heteroatom-directed C–H bond activation. Chem. Rev. 2010;110:624–655. doi: 10.1021/cr900005n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wencel-Delord J, Dröge T, Liu F, Glorius F. Towards mild metal-catalyzed C–H bond activation. Chem. Soc. Rev. 2011;40:4740–4761. doi: 10.1039/c1cs15083a. [DOI] [PubMed] [Google Scholar]
- 12.Jun C-H, Lee H, Hong J-B. Chelation-assisted intermolecular hydroacylation: direct synthesis of ketone from aldehyde and 1-alkene. J. Org. Chem. 1997;62:1200–1201. [Google Scholar]
- 13.Mo F, Dong G. Regioselective ketone α-alkylation with simple olefins via dual activation. Science. 2014;345:68–72. doi: 10.1126/science.1254465. [DOI] [PubMed] [Google Scholar]
- 14.Bedford RB, Coles SJ, Hursthouse MB, Limmert ME. The catalytic intermolecular orthoarylation of phenols. Angew. Chem. Int. Ed. 2003;42:112–114. doi: 10.1002/anie.200390037. [DOI] [PubMed] [Google Scholar]
- 15.Lightburn TE, Dombrowski MT, Tan KL. Catalytic scaffolding ligands: an efficient strategy for directing reactions. J. Am. Chem. Soc. 2008;130:9210–9211. doi: 10.1021/ja803011d. [DOI] [PubMed] [Google Scholar]
- 16.Grünanger CU, Breit B. Branched-regioselective hydroformylation with catalytic amounts of a reversibly bound directing group. Angew. Chem. Int. Ed. 2008;47:7346–7349. doi: 10.1002/anie.200802296. [DOI] [PubMed] [Google Scholar]
- 17.Wang D-H, Wasa M, Giri R, Yu J-Q. Pd(II)-catalyzed cross-coupling of sp3 C–H bonds with sp2 and sp3 boronic acids using air as the oxidant. J. Am. Chem. Soc. 2008;130:7190–7191. doi: 10.1021/ja801355s. [DOI] [PubMed] [Google Scholar]
- 18.He J, Li S, Deng Y, Fu H, Laforteza BN, Spangler JE, Homs A, Yu J-Q. Ligand-controlled C(sp3)–H arylation and olefination in synthesis of unnatural chiral α–amino acids. Science. 2014;343:1216–1220. doi: 10.1126/science.1249198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi B-F, Maugel N, Zhang Y-H, Yu J-Q. PdII-catalyzed enantioselective activation of C(sp2)–H and C(sp3)–H bonds using monoprotected amino acids as chiral ligands. Angew. Chem. Int. Ed. 2008;47:4882–4886. doi: 10.1002/anie.200801030. [DOI] [PubMed] [Google Scholar]
- 20.Wang D-H, Engle KM, Shi B-F, Yu J-Q. Ligand-enabled reactivity and selectivity in a synthetically versatile aryl C–H olefination. Science. 2010;327:315–319. doi: 10.1126/science.1182512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gong W, Zhang G, Liu T, Giri R, Yu J-Q. Site-selective C(sp3)–H functionalization of di-, tri-, and tetrapeptides at the N-terminus. J. Am. Chem. Soc. 2014;136:16940–16946. doi: 10.1021/ja510233h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McIntire FC. Some Schiff bases of free amino acids. J. Am. Chem. Soc. 1947;69:1377–1380. [Google Scholar]
- 23.Larock RC. Comprehensive Organic Transformations. 2. New York: Wiley; 1999. pp. 1197–1620. [Google Scholar]
- 24.Pirnot MT, Rankic DA, Martin DBC, MacMillan DWC. Photoredox activation for the direct β-arylation of ketones and aldehydes. Science. 2013;339:1593–1596. doi: 10.1126/science.1232993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Z, Dong G. Catalytic direct β-arylation of simple ketones with aryl Iodides. J. Am. Chem. Soc. 2013;135:17747–17750. doi: 10.1021/ja410389a. [DOI] [PubMed] [Google Scholar]
- 26.Kang T, Kim Y, Lee D, Wang Z, Chang S. Iridium-catalyzed intermolecular amidation of sp3 C–H bonds: late-stage functionalization of an unactivated methyl group. J. Am. Chem. Soc. 2014;136:4141–4144. doi: 10.1021/ja501014b. [DOI] [PubMed] [Google Scholar]
- 27.Gao P, Guo W, Xue J, Zhao Y, Yuan Y, Xia Y, Shi Z. Iridium(III)-catalyzed direct arylation of C–H bonds with diaryliodonium salts. J. Am. Chem. Soc. 2015;137:12231–12240. doi: 10.1021/jacs.5b06758. [DOI] [PubMed] [Google Scholar]
- 28.Nakanishi M, Katayev D, Besnard C, Kündig EP. Fused indolines by palladium-catalyzed asymmetric C–C coupling involving an unactivated methylene group. Angew. Chem. Int. Ed. 2011;50:7438–7441. doi: 10.1002/anie.201102639. [DOI] [PubMed] [Google Scholar]
- 29.Anas S, Cordi A, Kagan HB. Enantioselective synthesis of 2-methyl indolines by palladium catalysed asymmetric C(sp3)–H/cyclisation. Chem. Commun. 2011;47:11483–11485. doi: 10.1039/c1cc14292e. [DOI] [PubMed] [Google Scholar]
- 30.Saget T, Lemouzy SJ, Cramer N. Chiral monodentate phosphines and bulky carboxylic acids: cooperative effects in palladium-catalyzed enantioselective C(sp3)–H functionalization. Angew. Chem. Int. Ed. 2012;51:2238–2242. doi: 10.1002/anie.201108511. [DOI] [PubMed] [Google Scholar]
- 31.Cahiez G, Lepifre F, Ramiandrasoa P. Manganese-catalyzed substitution of activated arylhalides (X = Cl, Br andF) and aryl ethers by organomagnesium reagents. Synthesis. 1999:2138–2144. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.