Abstract
Both bone morphogenetic protein 2 (BMP2) and the wingless-type MMTV integration site (WNT)/β-catenin signalling pathway play important roles in odontoblast differentiation and dentinogenesis. Cross-talk between BMP2 and WNT/β-catenin in osteoblast differentiation and bone formation has been identified. However, the roles and mechanisms of the canonical WNT pathway in the regulation of BMP2 in dental pulp injury and repair remain largely unknown. Here, we demonstrate that BMP2 promotes the differentiation of human dental pulp cells (HDPCs) by activating WNT/β-catenin signalling, which is further mediated by p38 mitogen-activated protein kinase (MAPK) in vitro. BMP2 stimulation upregulated the expression of β-catenin in HDPCs, which was abolished by SB203580 but not by Noggin or LDN193189. Furthermore, BMP2 enhanced cell differentiation, which was not fully inhibited by Noggin or LDN193189. Instead, SB203580 partially blocked BMP2-induced β-catenin expression and cell differentiation. Taken together, these data suggest a possible mechanism by which the elevation of β-catenin resulting from BMP2 stimulation is mediated by the p38 MAPK pathway, which sheds light on the molecular mechanisms of BMP2-mediated pulp reparative dentin formation.
Keywords: human dental pulp cells, bone morphogenetic protein 2, β-catenin, cell differentiation, p38
Introduction
Dental pulp is a specialized connective tissue that maintains dentin homeostasis, sensation, nutrition and defence. In clinically relevant insults due to trauma or infection, pulp has some ability to form reparative dentin. The differentiation of dental pulp cells probably plays a key role in homeostasis and pulp/dentin regeneration.1 Dental pulp cells can differentiate into odontoblast-like cells, which are thought to be responsible for secreting the dentin matrix and forming reparative dentin, making it possible to retain the vitality of dental pulp by clinical therapies.2,3,4,5,6 A variety of factors participate in the regulation of dental pulp cell differentiation, including specific proteins and cytokines such as bone morphogenetic protein (BMP), dentin sialophosphoprotein (DSPP) and dentin matrix protein 1 (DMP1).7
BMPs are multi-functional growth factors that belong to the transforming growth factor β (TGF-β) superfamily. BMP2 has been found to play important roles in the regulation of odontoblast differentiation,8 with canonical BMP signalling involving Smad1/5 (ref. 9) or Smad4 (ref. 10) and non-canonical BMP signalling involving the c-Jun N-terminal kinase (JNK) pathway.11 Preclinical and clinical studies have shown that BMP2 can be utilized in various therapeutic interventions, including cases of bone defects, non-union fractures, spinal fusion, osteoporosis and root canal surgery.12,13,14,15 Previous in vitro and in vivo experiments demonstrated that odontoblasts are capable of producing and secreting BMP2 (ref. 16) and that BMP2 can promote odontoblast differentiation and dental pulp cell differentiation.8,17,18
Similar to the BMP pathway, the WNT/β-catenin signalling pathway also regulates the differentiation and proliferation of odontoblasts.19,20 However, despite previous work on the cross-talk between WNT/β-catenin and BMP in osteogenic differentiation and bone formation,21 hypothetical cross-talk between WNT/β-catenin and BMP in the differentiation of dental pulp cells has not been illustrated. BMP stimulates osteoblast differentiation and bone formation in vitro and in vivo,22,23,24 and BMP2 and β-catenin have a synergistic effect on osteoblast differentiation in vitro,21,25 as BMP2 enhances the protein level of the non-phosphorylated β-catenin in osteoblasts.26 In contrast, canonical WNT signalling negatively regulates the odontoblast-like differentiation of dental pulp stem cells27 and the cementoblast-like differentiation of dental follicle cells.28 Activated WNT/β-catenin is not sufficient to induce osteogenic differentiation and bone formation unless other stimulatory signals are present, BMP2 in particular.21 Similar to their promotion of osteogenic differentiation, members of the BMP and WNT families also mediate reciprocal interactions between epithelial and mesenchymal tissues and regulate tooth initiation and morphogenesis, including odontoblast differentiation and dentin formation.20
Recent advances have suggested possible cross-talk among other major signalling pathways, including mitogen-activated protein kinase (MAPK) pathways with the WNT/β-catenin signalling pathway;29,30,31,32 e.g., the p38 MAPK pathway regulates canonical WNT/β-catenin signalling via inactivation of glycogen synthase kinase-3β (GSK3β).33 In mesenchymal stem cells (MSCs), WNT4-mediated activation of p38 MAPK was found to be crucial for enhancing the osteogenic differentiation of MSCs.34 Similarly, in C3H10T1/2 mesenchymal cells, WNT3a induces transient activation of p38 signalling, which regulates alkaline phosphatase (ALPase) activity and matrix mineralization, suggesting an important role for p38 MAPK in the development of mesenchymal cells into osteoprogenitors.31 Another study showed that BMP2 promotes chondrogenesis by activating p38 MAPK, which in turn downregulates β-catenin signalling in chick wing bud mesenchymal cells.35 These findings showed that p38 MAPK and β-catenin are both involved in the effect of BMP2, but the underlying mechanisms remain controversial. In the present study, we tested the hypothesis that there is a cross-talk mechanism between BMP2/p38 and β-catenin that acts on the differentiation of human dental pulp cells (HDPCs). We found that BMP2 promoted the differentiation of HDPCs through the p38 MAPK signalling pathway, in addition to the canonical WNT/β-catenin pathway.
Materials and Methods
Isolation and cultivation of human dental pulp cells
The entire study was performed according to an informed protocol approved by the West China Hospital of Stomatology of Sichuan University Committee on Human Research. Fully erupted, non-carious human third molars were collected from patients (19–25 years old) who had given their written consent. HDPCs were isolated and cultured via the tissue-explant method.36 Upon confluence, HDPCs that had migrated from the tissue were collected and sub-cultured. Experiments were carried out using HDPCs between the third and fourth passages.
Alizarin red staining
To induce mineralization, HDPCs were plated in 48-well plates (Falcon, Franklin Lakes, NJ, USA) at an initial density of 5 × 103 cells per well and cultured at 37 °C with 5% CO2 for 1 day. The HDPCs were pretreated with 200 ng·mL−1 recombinant human protein dickkopf-1 (rhDKK1) (an inhibitor of the canonical WNT pathway; R&D Systems, Minneapolis, MN, USA) for 24 h, or 30 μmol·L−1 SB203580 (an inhibitor of the p38 MAPK signalling pathway; Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 2 h, or 1 μmol·L−1 LDN193189 (an inhibitor of the canonical BMP pathway; Sigma-Aldrich Corporation, St. Louis, MO, USA) for 1 h, or 2 μmol·L−1 XAV939 (an inhibitor of the canonical WNT pathway; Sigma-Aldrich, St. Louis, MO, USA) for 1 h then stimulated with 30 ng·mL−1 rhBMP2 (R&D Systems, Minneapolis, MN, USA) in osteogenic medium containing high-glucose Dulbecco minimum essential medium (DMEM) with 10% fetal bovine serum (FBS), 50 mg·L−1 ascorbic acid (Sigma Chemical, St. Louis, MO, USA), 10 mmol·L−1 β-glycerol phosphate (Sigma-Aldrich, St. Louis, MO, USA), 10 nmol·L−1 dexamethasone (Sigma-Aldrich, St. Louis, MO, USA), 2 mmol·L−1 L-glutamine (Sigma-Aldrich, St. Louis, MO, USA), 100 U·mL−1 penicillin and 100 µg·mL−1 streptomycin (GIBCO-BRL, Life Technologies, Grand Island, NY, USA). After 10 days of culture, the HDPCs were fixed with 4% paraformaldehyde (PFA) for 10 min at room temperature, then washed with phosphate-buffered saline (PBS) for 3 × 3 min and stained for calcium nodules using 2% Alizarin red S (ScienCell Research Laboratory, Carlsbad, CA, USA) for 15 min, after which the washing process was repeated with ddH2O. The mineral nodules that formed were photographed and counted under an inverted microscope. Each experiment was repeated 3 times.
Alkaline phosphatase staining
In accord with the design of the Alizarin red staining, HDPCs were cultured in 48-well plates (Falcon, Franklin Lakes, NJ, USA) in their usual medium at a density of 5 × 104 cells per well for 1 day, then in osteogenic medium for 4 days. Then, ALPase staining was performed following the manufacturer's instructions. Briefly, HDPCs were washed with PBST (Tween-20 0.05% (V/V) in PBS), fixed with fix solution for 2 min, washed with PBST again, treated with AP solution for 7–10 min and washed with PBS, after which pictures were taken under a stereomicroscope for whole well or a microscope for magnifying one.
Real-time polymerase chain reaction
Total RNA was extracted, with TRIzol (Invitrogen, Carlsbad, CA, USA) from HDPCs that had been pretreated with either 200 ng·mL−1 rhDKK1 for 24 h or 30 µmol·L−1 SB203580 for 2 h and cultured with medium containing 30 ng·mL−1 rhBMP2 for 24 h. The concentration and purity of the total RNA were determined with a Nanodrop (Nanodrop Technology, Wilmington, USA). Total RNA (1 μg) was reverse transcribed using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). The primer sequences are as follows: Lef1 forword AACATGGTGGAAAACGAAGC; reverse GGGTTGGCAGTGATTGTCTT and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forword ACAACTTTGGTATCGTGGAAGG; reverse GCCATCACGCCACAGTTTC. Real-time polymerase chain reaction (RT-PCR) analysis of the mRNA was performed using the SYBR green (Invitrogen, Carlsbad, CA, USA) PCR method according to the manufacturer's instructions. The mean cycle threshold value (Ct) obtained from triplicate samples was used to calculate gene expression levels. The PCR products were normalized to the level of GAPDH for each reaction. Relative gene expression levels are presented as the mean ± standard deviation from three independent experiments.
Western blot analysis
HDPCs were grown to 90% confluence and pretreated with different inhibitors, including 500 ng·mL−1 rhNoggin (inhibitor of the BMP pathway; R&D Systems, Minneapolis, MN, USA) for 24 h, 1 µmol·L−1 LDN193189 for 1 h, 30 µmol·L−1 SB203580 for 2 h, and 200 ng·mL−1rhDKK1 for 24 h, then stimulated with 30 ng·mL−1 rhBMP2 for 15, 30, 60 and 120 min or 30, 60, 120 and 240 min.
Total proteins, cytoplasmic proteins and nuclear proteins were obtained using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Thermo Scientific, Wilmington, NC, USA). The protein concentration was determined with a BCA protein assay kit (Pierce, Rockford, IL, USA). Total cytoplasmic (10 µg) or nuclear (5 µg) proteins were separated via sodium dodecyl sulfate polyacrylamide gel electropheresis (sodium dodecyl sulfate polyacrylamide gel electropheresis (SDS-PAGE) and then transferred to nitrocellulose membrane blots. The blots were blocked with bovine serum albumin (BSA) blocking buffer in triethanolamine buffered saline (TBS) and Tween-20 (Boston BioProducts, Boston, MA, USA) for 1 h, then rinsed with TBST and incubated overnight at 4 °C with primary antibodies including anti-GAPDH diluted 1:10 000 (Sigma-Aldrich, St. Louis, MO, USA), anti-GAPDH diluted 1:1 000 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-β-catenin diluted 1:1 000 (Cell Signaling Technology, MA, USA), anti-β-catenin (E247) diluted 1:5 000 (Abcam, Cambridge, MA, USA), anti-Histone3 diluted 1:1 000 (Cell Signaling Technology, Beverly, MA, USA), anti-p-Smad1/5/8 and anti-Smad1/5/8 diluted 1:1 000 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-p-p38 and anti-p38 diluted 1:1 000 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), or anti-α-tubulin diluted 1:1 000 (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Then, the blots were incubated with the IRDye 800CW antibody diluted 1:10 000 (LI-COR Biosciences, Lincoln, NE, USA) for 1 h at room temperature and rinsed with TBST. The signal was detected using IR fluorescence & Odyssey (LI-COR Biosciences, Lincoln, NE, USA). The expression of GAPDH, Histone 3 or α-tubulin was used as a control. Finally, we determined the ratio of the target protein to the reference protein. The obtained ratios are presented as the mean ± standard deviation from three independent experiments.
Cell immunofluorescence staining
HDPCs were grown on glass cover slips to approximately 30%∼50% confluence in regular medium following serum starvation for 2 h, and they were then treated with 30 ng·mL−1 rhBMP2 for 6 h (for SB203580, the cells were pre-incubated with 30 µmol·L−1 SB203580 for 1 h before stimulation with rhBMP2). Then, the covered cells were fixed with 4% PFA for 15 min at room temperature. The cells were permeabilized in 0.5% Triton X for 5 min and blocked in blocking buffer (1% BSA/4% goat serum) for 30 min at room temperature. All subsequent incubations were carried out in a humid light-tight box. The specimens were incubated overnight with an Alexa Fluor 555 conjugated primary anti-β-catenin antibody (Cell Signaling Technology, Beverly, MA, USA) diluted 1:50 in 1% BSA, followed by incubation with diamidino-phenyl-indole (DAPI; Roche Molecular Biochemicals, Mannheim, Germany) working solution (1 µg·mL−1) directly at room temperature for 3 min. Finally, the specimens were mounted in buffered glycerol and examined under a fluorescence microscope. Fluorescence microscopy images were obtained using an Axioplan epifluorescence microscope (Carl Zeiss, Jena, Germany) with a ×20 or ×40 objective lens.
TOPflash dual luciferase reporter assay
Cells were transfected with 6 µg of the TOPflash reporter plasmid (Millipore, Billerica, MA, USA) and 20 ng of the pGL4-TK plasmid (Promega, Madison, WI, USA) using the Neon Transfection System (Invitrogen, Carlsbad, CA, USA). An inhibitor pretreatment and BMP2 stimulation were added 24 h after transfection. Then, after an additional 24 h, cell extracts were prepared and assayed sequentially for firefly and Renilla luciferase activity. Firefly luciferase readings were normalized against Renilla luciferase.
Statistical analysis
Statistical analysis was performed using one-way analysis of variance (ANOVA), followed by a post hoc procedure (Bonferroni analysis). A P-value of less than 0.05 was considered significant.
Results
Canonical WNT signalling is activated by BMP2 stimulation in HDPCs
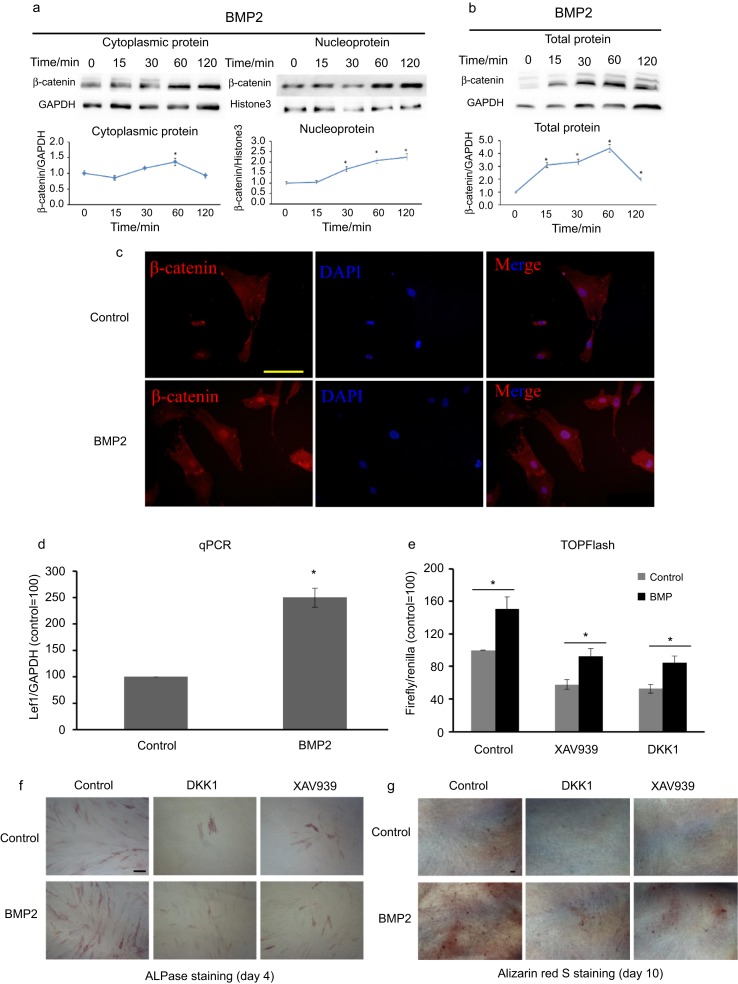
To elucidate the relationship between BMP2 and canonical WNT signalling, the rhBMP2 (30 ng·mL−1) was added to the culture medium of HDPCs, and the expression of β-catenin was detected by Western blot analysis. BMP2 stimulation promoted cytoplasmic, nuclear (Figure 1a) and total protein (Figure 1b) expression of β-catenin (P< 0.05). Further detection via immunofluorescence staining showed that stimulation of HDPCs with BMP2 for 6 h promoted nuclear translocation of β-catenin (Figure 1c). Moreover, the expression of lymphoid enhancer-binding factor 1 (Lef1) mRNA was increased by stimulation with BMP2 for 24 h (Figure 1d). HDPCs were transfected with a TOPflash reporter, and it was found that BMP2 stimulation significantly increased the obtained TOPflash values, indicating activation of canonical WNT signalling (Figure 1e). When we used DKK1 or XAV939 to block canonical WNT signalling, BMP2 still increased the TOPflash reporter values and promoted HDPC differentiation, as shown through ALPase and Alizarin red S staining (Figure 1f and 1g). These data demonstrate that BMP2 can activate canonical WNT signalling but in a dissimilar manner to a WNT ligand.
Figure 1.
BMP2 stimulation activates the WNT/β-catenin signalling pathway and promotes differentiation partly via the canonical WNT pathway in HDPCs. (a, b) HDPCs were starved for 2 h and then stimulated with 30 ng·mL −1 rhBMP2 for the indicated times. BMP2 significantly upregulates the expression of β-catenin in the cytoplasm, nucleolus and whole-cell lysates of HDPCs. GAPDH and Histone3 were used as normalization controls. (c) After stimulation with 30 ng·mL−1 rhBMP2 for 6 h, immunofluorescence staining for β-catenin (red) showed that BMP2 promotes nuclear translocation of β-catenin in HDPCs. GAPDH was used as the normalization control. Bar=50 μm. (d) BMP2 significantly upregulates the expression of Lef1 mRNA at 24 h. (e) Luciferase activity is upregulated by treatment with 30 ng·mL−1 rhBMP2 for 24 h after co-transfection of HDPCs with the TOPflash and PGL4-TK plasmids using Neon Transfection Systems. (f, g) HDPCs were cultured in osteogenic medium with or without 20 ng·mL−1 rhBMP2 for 4 days or 10 days, after which ALPase and Alizarin red S staining was performed. Following rhDKK1 or XAV939 treatment, BMP2 still promotes TOPflash luciferase activity and cell differentiation. Bar = 100 μm in f; Bar = 200 μm in g. *Significant differences (P < .05) versus the control. ALPase, alkaline phosphatase; BMP, bone morphogenetic protein; DAPI, diamidino-phenyl-indole; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HDPC, human dental pulp cell; Lef, lymphoid enhancer-binding factor; qPCR, quantitative polymerase chain reaction.
BMP2 activates Smad signalling and p38 signalling in HDPCs
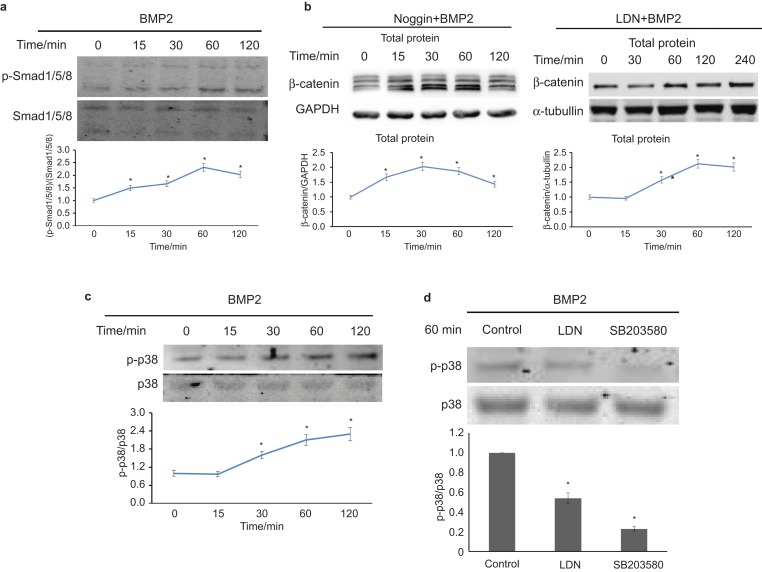
The above results suggest that BMP2 can activate canonical WNT signalling and promote HDPC differentiation partly through canonical WNT signalling, but the mechanism whereby BMP2 enhances the expression and nuclear translocation of β-catenin is unclear. Considering that β-catenin can significantly enhance the differentiation of dental pulp cells37 and that BMP2 mediates cell differentiation in most cell types through canonical BMP signalling,13 we assumed that BMP2 also upregulates the expression of β-catenin via the canonical BMP pathway.
BMP2 can upregulate the expression of p-Smad1/5/8 (Figure 2a). However, when we used LDN193189 or rhNoggin to block canonical BMP signalling, the expression of β-catenin was still upregulated by rhBMP2 stimulation (Figure 2b). Another pathway, p38 MAPK signalling, has been studied in conjunction with WNT/β-catenin signalling in the regulation of cell differentiation.31 When we attempted to study the role of non-canonical BMP signalling, we found that BMP2-activated p38 signalling (Figure 2c), and when we blocked canonical BMP signalling with LDN193189, the expression of p-p38 was downregulated (Figure 2d). These results suggest a possible role of p38 MAPK in the BMP2/β-catenin signalling pathway.
Figure 2.
BMP2 activates canonical BMP and non-canonical p38 MAPK signalling. (a) Starvation for 2 h and treatment with 30 ng·mL−1 rhBMP2 upregulates the expression of p-smad1/5/8 in HDPCs, as determined via western blot analysis. (b) rhNoggin (500 ng·mL−1) pretreatment for 12 h or LDN193189 (1 µmol·L−1) pretreatment for 1 h, followed by 30 ng·mL−1 BMP2 stimulation, promotes the expression of β-catenin in whole-cell lysates. (c) As shown in a, BMP2 upregulates the expression of p-p38 in HDPCs. (d) Starvation for 2 h and pretreatment with 1 µmol·L−1 LDN193189 or 30 µmol·L−1 SB203580 for 1 h before 30 ng·mL−1 rhBMP2 stimulation. Both LDN and SB treatment can downregulate the BMP2-induced expression of p-p38. ALPase, alkaline phosphatase; BMP, bone morphogenetic protein; DAPI, diamidino-phenyl-indole; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HDPC, human dental pulp cell; p-Smad1/5/8, phosphor-Smad1/5/8; p-p38, phosphor-p38.
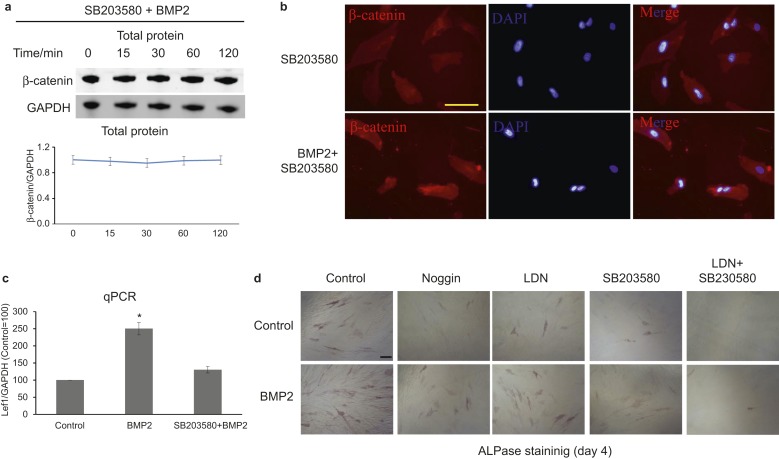
Blockade of p38 signalling can inhibit BMP2-induced activation of WNT/β-catenin signalling and cell differentiation
To study the role of p38 signalling in BMP2-induced WNT/β-catenin signalling, we blocked p38 signalling using SB203580, and interestingly, the expression of β-catenin was not observed to change after BMP2 stimulation (Figure 3a). Further experiments showed that SB203580 treatment blocked BMP2-induced β-catenin translocation (Figure 3b) and Lef1 mRNA expression (Figure 3c). Regarding HDPC differentiation, ALPase staining showed that blockade of canonical BMP or p38 signalling partially inhibited BMP2-induced differentiation, whereas the combined use of LDN193189 and SB203580 completely inhibited BMP2-induced differentiation (Figure 3d).
Figure 3.
Blockade of the p38 signalling pathway can reduce the BMP2-induced activation of WNT/β-catenin signalling and cell differentiation. (a) After starvation for 2 h, pretreatment with 30 µmol·L−1 SB203580 for 2 h, and then stimulation of HDPCs with 30 ng·mL−1 rhBMP2 for various time periods; SB203580 treatment blocks the activation of β-catenin by BMP2. (b) After 6 h, immunofluorescence staining showed that SB203580 treatment reduces the translocation of β-catenin by BMP. Bar = 50 μm. (c) After 24 h, qPCR showed that SB203580 treatment reduces the expression of lef1 mRNA. (d) Treatment with Noggin, LDN or SB partially blocks BMP2-induced HDPC differentiation, whereas combined treatment with LDN and SB was able to completely block BMP2-induced HDPC differentiation. Bar = 100 μm. ALPase, alkaline phosphatase; BMP, bone morphogenetic protein; DAPI, diamidino-phenyl-indole; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HDPC, human dental pulp cell; qPCR, quantitative polymerase chain reaction.
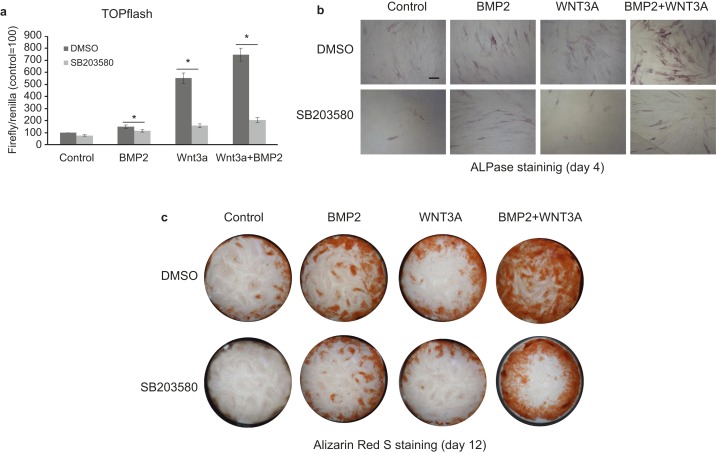
p38 MAPK signalling plays a role in both BMP2- and WNT3a-induced β-catenin signalling activation and cell differentiation
To confirm the role of p38 signalling in the WNT/β-catenin pathway, we further used WNT3a to activate WNT/β-catenin and combined the effects of Wnt3a and BMP2 on cell differentiation. BMP2 and WNT3a were each able to activate WNT/β-catenin signalling, showing synergistic effects on WNT/β-catenin and cell differentiation (Figure 4a–4c). An inhibitor of the p38 pathway attenuated the effects of WNT3a, BMP2 and WNT3a plus BMP2, demonstrating the role of p38 signalling in the regulation of WNT/β-catenin signalling. BMP2 induces β-catenin expression and cell differentiation in HDPCs partly through the p38 MAPK pathway.
Figure 4.
p38 MAPK signalling plays a role in BMP2-WNT3a-induced β-catenin signalling activation and cell differentiation. (a) A TOPflash dual luciferase assay reveals that SB203580 treatment can attenuate the effects of WNT3a, BMP2, or even BMP2 together with WNT3a. (b and c) ALPase staining and ARS staining reveal a synergistic effect of BMP2 and WNT3a on HDPC differentiation, and SB203580 treatment can attenuate the function of BMP2, even in the presence of WNT3a. BMP2 induces β-catenin expression and cell differentiation in HDPCs partly through the p38 MAPK pathway. Bar = 100 μm in b. ALPase, alkaline phosphatase; BMP, bone morphogenetic protein; DMSO, dimethyl sulfoxide; HDPC, human dental pulp cell.
Discussion
Our findings showed that BMP2 activated β-catenin signalling in HDPCs and induced the differentiation of these cells. Notably, BMP2 stimulation activated p38 MAPK signalling, and p38 MAPK activity was required for BMP2-induced β-catenin signalling in and differentiation of HDPCs.
Dental pulp at a given age exhibits an intrinsic reparative capacity that needs to be further explored in the context of tooth development and regeneration.1 Dental pulp cells cultured in vitro include undifferentiated cells with the ability to differentiate into odontoblasts and induce the formation of mineralization nodules.38,39,40 ALPase, which is widely found in various tissues and organs, is very important for tooth development and metabolism and shows functions related to odontoblast differentiation and dentin formation.41 ALP activity and the number of mineralized nodules can indirectly represent the mineralization capacity and differentiation ability of cells. In the present study, BMP2-stimulated HDPCs showed increased formation of mineralization nodules, ALPase activity, and Lef1 mRNA expression compared with control cells. These results indicated that BMP2 was able to promote the mineralization and differentiation of HDPCs, which is consistent with most previous studies indicating that BMP2 can enhance the ALPase activity of HDPCs in vitro8 and promote the formation of reparative dentin.17
Multiple signalling pathways participate in the process of dental pulp repair, and the BMP and WNT signalling pathways always co-exist during the process of tooth development and embryonic differentiation.42,43,44,45,46 Some authors have found that bone defects caused by a β-catenin mutation cannot be reversed by exogenous BMP2,47 whereas activated β-catenin induces osteoblast differentiation and participates in BMP2-mediated signal transduction,48 which indicates that β-catenin may be involved in the process of osteogenesis promotion by BMP2. Moreover, activation of the WNT/β-catenin pathway can suppress the BMP2-mediated differentiation of dental follicle cells, although the process of differentiation still requires endogenous β-catenin.43 It is generally thought that DKK1 inhibits the canonical WNT pathway through competitively binding to the membrane receptor LRP6.49,50 In C3H10T1/2 cells, BMP2 was found to induce osteogenic differentiation through the canonical WNT pathway, and when the canonical WNT pathway was inhibited by DKK1, BMP2-induced ALP activity was eliminated.25 The present study showed that the roles of BMP2 and β-catenin in the process of odontogenesis show great similarities to their roles in the process of osteogenesis, and we also found that the canonical WNT signalling pathway plays a vital role in the BMP2-mediated differentiation of HDPCs, with exogenous BMP2 increasing the expression of β-catenin and LEF1 mRNA and DKK1 treatment partially eliminating the promotion of HDPC differentiation by BMP2.
BMP signalling is regulated at different molecular levels, and BMP signalling can be blocked by extracellular antagonists such as Noggin, which binds BMP ligands such as BMP2, 4 and 7 and prevents their association with BMP receptors.13,51 Over-expression of Noggin in mature osteoblasts causes osteoporosis in mice.52,53 To explore how BMP2 activates the canonical WNT signalling pathway, we pretreated cells with Noggin to observe the relationship between canonical BMP/Smad signalling and WNT/β-catenin signalling. However, when Noggin or LDN was used to block the BMP/Smad signalling pathway, exogenous BMP2 was still able to increase the expression of β-catenin; therefore, we speculated that the BMP2 might active canonical WNT pathway in HDPCs might occur through another signalling pathway. Some researchers have reported that BMP2 can transmit BMP signals by acting together with XIAP, TAB1 and TAK1, which then activate the p38 MAPK pathway,54 and BMP2 can regulate the WNT pathway through the p38 MAPK pathway during cartilage development.35 Other authors have suggested that the p38 MAPK pathway could regulate the canonical WNT signalling pathway by inhibiting GSK3β.33 All of the available literature shows that BMP2 may activate WNT signalling through MAPK signalling or GSK3β activation. BMP signalling is activated when Smad1, a BMP-specific transcription factor, is phosphorylated by type I BMP receptors (Alks) and then phosphorylated by MAPK, resulting in the cytoplasmic retention of Smad1 and a subsequent decrease in its ability to transduce BMP signals. In the absence of WNT, GSK3β specifically phosphorylates the dually phosphorylated form of Smad1, leaving Smad1 susceptible to ubiquitination by Smurf1. Finally, the signalling capacity of Smad1 is terminated through proteasomal degradation.55 In the presence of WNTs, GSK3β is unavailable, and Smad1 remains active, increasing the duration of the BMP signal. Therefore, to investigate whether BMP2 activates the p38 MAPK pathway to regulate WNT/β-catenin signalling in HDPCs, we treated HDPCs with SB203580, an inhibitor of the p38 MAPK signalling pathway, and surprisingly, we found that both the BMP2-induced upregulation of the expression of β-catenin in total protein and its translocation to the nucleus were significantly inhibited. The BMP2-induced differentiation of HDPCs was also blocked by SB203580 treatment. These results confirmed our hypotheses that BMP2 promoted cell differentiation by activating the p38 MAPK pathway and the WNT canonical pathway in HDPCs, similar to a study in which hepatocyte growth factor (HGF) activation of the p38 MAPK pathway was found to induce the differentiation of human dental papilla cells by p38 MAPK pathway.56
In conclusion, our findings illustrate the role of BMP2 and WNT/β-catenin in the differentiation of and mineralization by HDPCs. p38 MAPK signalling was found to play a role in the process of BMP2-induced HDPC differentiation and WNT/β-catenin signalling activation. These findings indicate a potential mechanism by which BMP2 regulates WNT/β-catenin, thereby mediating cell differentiation and dentin formation in the pulp repair process.
Acknowledgments
This work was supported by the National Nature Science Foundation of China (grant nos. 81200759, 81070801 and 813220170), the Innovative Research Team of the Education Department of Sichuan Province (13TD0038), the Sichuan Province Science and Technology Support Program (2012SZ0034) and the Program of International Science and Technology Cooperation (2014DFA31990).
References
- Mao JJ, Robey PG, Prockop DJ. Stem cells in the face: tooth regeneration and beyond. Cell Stem Cell 2012; 11(3): 291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronthos S, Mankani M, Brahim J et al. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA 2000; 97(25): 13625–13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann LM, Lennon DP, Caplan AI. Cultured rat pulp cells have the potential to form bone, cartilage, and dentin in vivo//Davidovitch Z, Norton LA eds. Biological mechanisms of tooth movement and craniofacial adaptation. Boston: Harvard Society of the Advancement of Orthodontics, 1996: 7–10. [Google Scholar]
- Yildirim S, Fu SY, Kim K et al. Tooth regeneration: a revolution in stomatology and evolution in regenerative medicine. Int J Oral Sci 2011; 3(3): 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L, Ye L, Zhou XD. Mesenchymal stem cells and tooth engineering. Int J Oral Sci 2009; 1(1): 6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan AJ, Smith AJ. Stem cells and the dental pulp: potential roles in dentine regeneration and repair. Oral Dis 2007; 13(2): 151–157. [DOI] [PubMed] [Google Scholar]
- Smith AJ, Lesot H. Induction and regulation of crown dentinogenesis: embryonic events as a template for dental tissue repair? Crit Rev Oral Biol Med 2001; 12(5): 425–437. [DOI] [PubMed] [Google Scholar]
- Saito T, Ogawa M, Hata Y et al. Acceleration effect of human recombinant bone morphogenetic protein-2 on differentiation of human pulp cells into odontoblasts. J Endod 2004; 30(4): 205–208. [DOI] [PubMed] [Google Scholar]
- Qin W, Yang F, Deng R et al. Smad 1/5 is involved in bone morphogenetic protein-2-induced odontoblastic differentiation in human dental pulp cells. J Endod 2012; 38(1): 66–71. [DOI] [PubMed] [Google Scholar]
- Li J, Huang X, Xu X et al. SMAD4-mediated WNT signaling controls the fate of cranial neural crest cells during tooth morphogenesis. Development 2011; 138(10): 1977–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W, Liu P, Zhang R et al. JNK MAPK is involved in BMP2-induced odontoblastic differentiation of human dental pulp cells. Connect Tissue Res 2014; 55(3): 217–224. [DOI] [PubMed] [Google Scholar]
- Nakashima M. Bone morphogenetic proteins in dentin regeneration for potential use in endodontic therapy. Cytokine Growth Factor Rev 2005; 16(3): 369–376. [DOI] [PubMed] [Google Scholar]
- Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors 2004; 22(4): 233–241. [DOI] [PubMed] [Google Scholar]
- Nakashima M, Reddi AH. The application of bone morphogenetic proteins to dental tissue engineering. Nat Biotechnol 2003; 21(9): 1025–1032. [DOI] [PubMed] [Google Scholar]
- Gautschi OP, Frey SP, Zellweger R. Bone morphogenetic proteins in clinical applications. ANZ J Surg 2007; 77(8): 626–631. [DOI] [PubMed] [Google Scholar]
- Bègue-Kirn C, Smith AJ, Loriot M et al. Comparative analysis of TGF beta s, BMPs, IGF1, msxs, fibronectin, osteonectin and bone sialoprotein gene expression during normal and in vitro-induced odontoblast differentiation. Int J Dev Biol 1994; 38(3): 405–420. [PubMed] [Google Scholar]
- Iohara K, Nakashima M, Ito M et al. Dentin regeneration by dental pulp stem cell therapy with recombinant human bone morphogenetic protein 2. J Dent Res 2004; 83(8): 590–595. [DOI] [PubMed] [Google Scholar]
- Yang W, Harris MA, Cui Y et al. BMP2 is required for odontoblast differentiation and pulp vasculogenesis. J Dent Res 2012; 91(1): 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Lan Y, Baek JA et al. Wnt/beta-catenin signaling plays an essential role in activation of odontogenic mesenchyme during early tooth development. Dev Biol 2009; 334(1): 174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura M, Nemoto E, Sato MM et al. Role of the Wnt signaling pathway in bone and tooth. Front Biosci: Elite Ed 2010; 2: 1405–1413. [DOI] [PubMed] [Google Scholar]
- Mbalaviele G, Sheikh S, Stains JP et al. Beta-catenin and BMP2 synergize to promote osteoblast differentiation and new bone formation. J Cell Biochem 2005; 94(2): 403–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Harris MA, Rossini G et al. Bone morphogenetic protein 2 (BMP2) enhances BMP3, BMP4, and bone cell differentiation marker gene expression during the induction of mineralized bone matrix formation in cultures of fetal rat calvarial osteoblasts. Calcif Tissue Int 1997; 60(3): 283–290. [DOI] [PubMed] [Google Scholar]
- Chen D, Ji X, Harris MA et al. Differential roles for bone morphogenetic protein (BMP) receptor type IB and IA in differentiation and specification of mesenchymal precursor cells to osteoblast and adipocyte lineages. J Cell Biol 1998; 142(1): 295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Harris SE, Horn D et al. Bone morphogenetic protein receptor signaling is necessary for normal murine postnatal bone formation. J Cell Biol 2002; 157(6): 1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawadi G, Vayssière B, Dunn F et al. BMP2 controls alkaline phosphatase expression and osteoblast mineralization by a Wnt autocrine loop. J Bone Miner Res 2003; 18(10): 1842–1853. [DOI] [PubMed] [Google Scholar]
- Zhang M, Yan Y, Lim YB et al. BMP2 modulates beta-catenin signaling through stimulation of Lrp5 expression and inhibition of beta-TrCP expression in osteoblasts. J Cell Biochem 2009; 108(4): 896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller EL, Chang J, Wang CY. Wnt/beta-catenin inhibits dental pulp stem cell differentiation. J Dent Res 2008; 87(2): 126–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvério KG, Davidson KC, James RG et al. Wnt/β-catenin pathway regulates bone morphogenetic protein (BMP2)-mediated differentiation of dental follicle cells. J Periodont Res 2012; 47(3): 309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildesheim J, Salvador JM, Hollander MC et al. Casein kinase 2- and protein kinase A-regulated adenomatous polyposis coli and beta-catenin cellular localization is dependent on p38 MAPK. J Biol Chem 2005; 280(17): 17221–17226. [DOI] [PubMed] [Google Scholar]
- Bikkavilli RK, Feigin ME, Malbon CC. G alpha o mediates WNT-JNK signaling through dishevelled 1 and 3, RhoA family members, and MEKK 1 and 4 in mammalian cells. J Cell Sci 2008; 121(Pt 2): 234–245. [DOI] [PubMed] [Google Scholar]
- Caverzasio J, Manen D. Essential role of Wnt3a-mediated activation of mitogen-activated protein kinase p38 for the stimulation of alkaline phosphatase activity and matrix mineralization in C3H10T1/2 mesenchymal cells. Endocrinology 2007; 148(11): 5323–5330. [DOI] [PubMed] [Google Scholar]
- Keren A, Bengal E, Frank D. p38 MAP kinase regulates the expression of XMyf5 and affects distinct myogenic programs during Xenopus development. Dev Biol 2005; 288(1): 73–86. [DOI] [PubMed] [Google Scholar]
- Bikkavilli RK, Feigin ME, Malbon CC. p38 mitogen-activated protein kinase regulates canonical Wnt-beta-catenin signaling by inactivation of GSK3beta. J Cell Sci 2008; 121(Pt 21): 3598–3607. [DOI] [PubMed] [Google Scholar]
- Chang J, Sonoyama W, Wang Z et al. Noncanonical Wnt-4 signaling enhances bone regeneration of mesenchymal stem cells in craniofacial defects through activation of p38 MAPK. J Biol Chem 2007; 282(42): 30938–30948. [DOI] [PubMed] [Google Scholar]
- Jin EJ, Lee SY, Choi YA et al. BMP2-enhanced chondrogenesis involves p38 MAPK-mediated down-regulation of Wnt-7a pathway. Mol Cells 2006; 22(3): 353–359. [PubMed] [Google Scholar]
- Couble ML, Farges JC, Bleicher F et al. Odontoblast differentiation of human dental pulp cells in explant cultures. Calcif Tissue Int 2000; 66(2): 129–138. [DOI] [PubMed] [Google Scholar]
- Han N, Zheng Y, Li R et al. β-catenin enhances odontoblastic differentiation of dental pulp cells through activation of Runx2. PLoS One 2014; 9(2): e88890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima M, Nagasawa H, Yamada Y et al. Regulatory role of transforming growth factor-beta, bone morphogenetic protein-2, and protein-4 on gene expression of extracellular matrix proteins and differentiation of dental pulp cells. Dev Biol 1994; 162(1): 18–28. [DOI] [PubMed] [Google Scholar]
- Liu J, Jin T, Ritchie HH et al. In vitro differentiation and mineralization of human dental pulp cells induced by dentin extract. In Vitro Cell Dev Biol Anim 2005; 41(7): 232–238. [DOI] [PubMed] [Google Scholar]
- Zhang W, Walboomers XF, Wolke JG et al. Differentiation ability of rat postnatal dental pulp cells in vitro. Tissue Eng 2005; 11(3/4): 357–368. [DOI] [PubMed] [Google Scholar]
- Kido J, Ishida H, Nagata T et al. Effects of parathyroid hormone, 1,25-dihydroxyvitamin D3, and prostaglandin E2 on alkaline phosphatase activity in cultured dental pulp and gingiva cells of bovine calf. J Endod 1991; 17(4): 161–164. [DOI] [PubMed] [Google Scholar]
- Handrigan GR, Richman JM. A network of Wnt, hedgehog and BMP signaling pathways regulates tooth replacement in snakes. Dev Biol 2010; 348(1): 130–141. [DOI] [PubMed] [Google Scholar]
- Silvério KG, Davidson KC, James RG et al. Wnt/β-catenin pathway regulates bone morphogenetic protein (BMP2)-mediated differentiation of dental follicle cells. J Periodont Res 2012; 47(3): 309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya N, Ye L, Kobayashi T et al. BMP signaling negatively regulates bone mass through sclerostin by inhibiting the canonical Wnt pathway. Development 2008; 135(22): 3801–3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N, Song WX, Luo J et al. BMP9-induced osteogenic differentiation of mesenchymal progenitors requires functional canonical Wnt/beta-catenin signalling. J Cell Mol Med 2009; 13(8B): 2448–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima A, Katagiri T, Tamura M. Cross-talk between Wnt and bone morphogenetic protein 2 (BMP2) signaling in differentiation pathway of C2C12 myoblasts. J Biol Chem 2005; 280(45): 37660–37668. [DOI] [PubMed] [Google Scholar]
- Hill TP, Später D, Taketo MM et al. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell 2005; 8(5): 727–738. [DOI] [PubMed] [Google Scholar]
- Bain G, Müller T, Wang X et al. Activated beta-catenin induces osteoblast differentiation of C3H10T1/2 cells and participates in BMP2 mediated signal transduction. Biochem Biophys Res Commun 2003; 301(1): 84–91. [DOI] [PubMed] [Google Scholar]
- Semënov MV, Tamai K, Brott BK et al. Head inducer Dickkopf-1 is a ligand for Wnt coreceptor LRP6. Curr Biol 2001; 11(12): 951–961. [DOI] [PubMed] [Google Scholar]
- Mao B, Wu W, Li Y et al. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature 2001; 411(6835): 321–325. [DOI] [PubMed] [Google Scholar]
- Xiao YT, Xiang LX, Shao JZ. Bone morphogenetic protein. Biochem Biophys Res Commun 2007; 362(3): 550–553. [DOI] [PubMed] [Google Scholar]
- Wu XB, Li Y, Schneider A et al. Impaired osteoblastic differentiation, reduced bone formation, and severe osteoporosis in noggin-overexpressing mice. J Clin Invest 2003; 112(6): 924–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin RD, Du Z, Pereira RC et al. Skeletal overexpression of noggin results in osteopenia and reduced bone formation. Endocrinology 2003; 144(5): 1972–1978. [DOI] [PubMed] [Google Scholar]
- Hassel S, Schmitt S, Hartung A et al. Initiation of Smad-dependent and Smad-independent signaling via distinct BMP-receptor complexes. J Bone Joint Surg Am 2003; 85(Suppl 3): 44–51. [DOI] [PubMed] [Google Scholar]
- Rosen V. BMP2 signaling in bone development and repair. Cytokine Growth Factor Rev 2009; 20(5/6): 475–480. [DOI] [PubMed] [Google Scholar]
- Su Y, Xie W, Wang C et al. JNK/P38 mitogen-activated protein kinase used for hepatocyte growth factor-induced proliferation, differentiation, and migration in human dental papilla cells. J Endod 2012; 38(9): 1207–1213. [DOI] [PubMed] [Google Scholar]