Abstract
Significance: The demand for wound care therapies is increasing. New wound care products and devices are marketed at a dizzying rate. Practitioners must make informed decisions about the use of medical devices for wound healing therapy. This paper provides updated evidence and recommendations based on a review of recent publications.
Recent Advances: The published literature on the use of medical devices for wound healing continues to support the use of hyperbaric oxygen therapy, negative pressure wound therapy, and most recently electrical stimulation.
Critical Issue: To inform wound healing practitioners of the evidence for or against the use of medical devices for wound healing. This information will aid the practitioner in deciding which technology should be accepted or rejected for clinical use.
Future Directions: To produce high quality, randomized controlled trials or acquire outcome-based registry databases to further test and improve the knowledge base as it relates to the use of medical devices in wound care.

George A. Perdrizet, MD, PhD, FACS
Scope and Significance
This monograph aims to provide the wound care practitioner with a succinct and updated evaluation of popular nonpharmacologic wound therapies.
Translational Relevance
Techniques reviewed here have ample basic science evidence to support potential application to the clinic. While this is supportive only through the completion of well-designed controlled clinical trials should a new therapy become widely accepted into practice.
Clinical Relevance
Practitioners are routinely exposed to an array of wound healing devices. It is difficult to weigh the potential benefits and risks related to a particular technique and wound type. The practitioner must be up to date on the level of evidence and class of recommendation related to these devices so as to maximize patient benefit and resource utilization.
Background
Methodology
The grading of evidence and recommendations used for this monograph follows that adopted by the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines in 2013.1 Briefly, the level of evidence is rated A, B, or C based on the precision of the supporting study design (A=multiple populations studied with randomized controlled trials [RCTs] or meta-analyses, B=limited populations with single RCT or nonrandomized studies, C=limited populations, case reports, or consensus opinion). The class of recommendation is based on the size of the treatment effect, weighing relative benefits versus risks (I=the treatment should be used, IIa=it is reasonable to use the treatment, IIb=the treatment may be considered, III=not recommended as there is a risk of harm and no potential benefit). The recent literature was searched using the PubMed search engine from calendar years 2005 to 2015.
Discussion
Hyperbaric oxygen therapy
Hyperbaric oxygen therapy (HBOT) is defined as exposure to 100% oxygen at greater than 1 atmosphere of pressure (>760 mmHg). HBOT plays an adjunctive role in the management of acute and chronic wounds. The potential risks of HBOT are relatively small and contraindications are rare.2 Exposure to increased barometric pressure inside a hyperbaric chamber increases the oxygen content dissolved in plasma. The dissolved oxygen is the metabolically active fraction of oxygen that can now penetrate tissues compromised by chronic or acute inflammation, tissue edema, and microvascular thrombosis or rarefaction. There are multiple mechanisms of action of HBOT, including reduction of leukocyte adhesion to vascular endothelium and increase in tissue levels of nitric oxide, hypoxia inducible factor-1, and vascular endothelial growth factor.3 The induction of neoangiogenesis and vasculogenesis is observed and is related to the stimulation of bone marrow-derived progenitor cells.4 HBOT has antimicrobial effects and increases intracellular leukocyte killing by the oxygen-dependent peroxidase system.5 To benefit wound healing, daily treatments range from 20 to 60 sessions. Typical doses and durations range from 2.0 to 3.0 atmospheres absolute for a total of 60–120 min. Rigorous comparison of dose responses and treatment algorithms is lacking.
Undersea and hyperbaric medicine society-approved indications6
Enhancement of healing in selected problem wounds: diabetic foot ulcer
Wounds that fail to progress through the normal phases of healing demonstrate significant tissue hypoxia due to poor local perfusion.7,8 The diabetic foot ulcer (DFU) has become a major focus of HBOT and research. Several RCTs report favorable effects on DFU healing.9,10 Centers for Medicaid and Medicare Services (CMS) cover HBOT for DFUs that have failed 30 days of standard wound care and show evidence of deep soft-tissue infection, osteomyelitis, or gangrene (Wagner Grades 3,4,5) (Level/Class: A/I).
Delayed radiation tissue injuries/late effects of radiation therapy
Delayed radionecrosis of skin is an adverse event of radiation therapy, which results in painful fibrotic wounds that do not heal with standard care. Late effects of radiation therapy cause obliterative endarteritis leading to tissue hypoxia and fibrosis. The foundational studies by Dr. R. Marx have been substantiated by more recent data.11–14 This beneficial effect was dependent on good surgical and medical care in conjunction with HBOT. HBOT does not replace the basic need for timely surgical debridement and antimicrobial therapy (Level/Class: B/IIa).
Crush injuries and skeletal muscle-compartment syndrome
Surgical and traumatic wounds generally heal with standard wound care. In compromised hosts, severe infection or situations of increased tissue tension, normal healing mechanisms fail due to tissue hypoxia. An acute ischemia–reperfusion cascade generates an overwhelming inflammatory response leading to capillary leak, tissue edema, and microvascular thrombosis. Timely HBOT counteracts this cascade by penetration of dissolved oxygen into the damaged tissues. Evidence consists of many uncontrolled clinical reports, two small RCTs, and one evidence-based review showing benefits.15–17 HBOT does not replace the need for surgical decompression for acute compartment syndromes (Level/Class: B/IIa).
Necrotizing soft-tissue infections
There is a wide array of necrotizing soft-tissue infections. These life- and limb-threatening conditions require major surgical debridement and have severe impairments in wound healing. The pathophysiology includes local tissue hypoxia secondary to severe edema, inflammation, and microvascular thrombosis. The cornerstones of therapy include wide surgical debridement and antibiotic therapy. Adjunctive timely HBOT helps control infection and reduce tissue loss. There are hundreds of positive case reports, multiple nonrandomized clinical trials, and one meta-analysis suggesting that HBOT is associated with a significant reduction in mortality and morbidity.18,19 A recent analysis of the University Health Consortium database demonstrated a significant survival benefit for the subset of patients with the most extreme illness severity. A mortality rate of 4% was reported for the HBOT-treated group versus 23% for those not receiving HBOT, p<0.0120 (Level/Class: B/IIb).
Thermal burns
Thermal burn wounds are characterized by a marked inflammatory response and microvascular damage that lead to severe tissue edema, hypoxia, and vulnerability to invasive infection. An impaired leukocyte function further increases the risk for burn wound infection and sepsis. Human studies ranging from case series to RCTs have supported the benefit of HBOT in burn wound management. Cianci et al. have reported a significant reduction in the length of hospital stay and need for surgery21 (Level/Class: B/IIb).
Compromised flaps and grafts
Surgical flap and graft compromise is initiated by acute ischemia–reperfusion injury and leads to a robust acute inflammatory response, tissue edema, increased mechanical tension, and impairment in microcirculation. These factors lead to worsening of tissue hypoxia and acidosis and threaten flap survival. HBOT plays a limited role, if any, in the support of split-thickness skin grafts (STSGs). There are currently no RCTs testing the effect of HBOT on full-thickness skin grafts or for myocutaneous flap survival in humans. The use of HBOT is supported by small case series and animal work22 (Level/Class: C/IIb).
Negative Pressure Wound Therapy
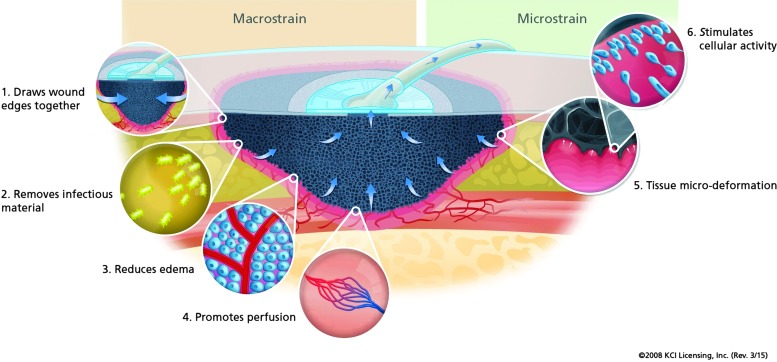
Negative pressure wound therapy (NPWT) provides multiple unique mechanisms to aid wound healing. Primary mechanisms of action are due to physical effects on tissues (Fig. 1). Macrodeformation causes a reduction in wound volume, and microdeformation induces changes in cytoskeletal structure, enhances cell-to-cell interactions, and stimulates cell division.23 Another effect is the removal of excess tissue edema and inflammatory exudate. Perfusion about the wound bed margins may be enhanced or reduced and is a subject of debate.24
Figure 1.
Mechanical mechanisms of negative pressure wound therapy. Provided by KCI Corp. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
NPWT-chronic wounds
Several RCTs have been reported. Fifty-four patients were randomized to NPWT versus gauze therapy. The rate of wound area reduction was significantly greater in the NPWT group (3.8% per day vs. 1.7% per day, p<0.05).25 Another study allocated 65 patients to NPWT versus modern dressings.26 No significant difference in time to complete granulation. A third study included leg ulcers in hospitalized patients. Mean time to complete healing with NPWT was 29 versus 45 days for controls (p=0.0001)27 (Level/Class: A/IIb).
NPWT-diabetic foot ulcer
There are more than 10 prospective clinical trials which include DFUs, only a few are randomized and controlled. Two relatively large RCTs evaluated NPWT in DFUs and showed benefits. The first study addressed partial foot amputations.28 Wound healing was greater in the NPWT group at 56% versus 39% for controls, p=0.04. Time to achieve the wound bed preparation decreased from 84 days for controls to 42 days for NPWT, p=0.02. The second trial showed a statistically significant increase in the number of wounds healed with NWPT (42%) compared to moist dressings (28%).29 A significant decrease in time to complete wound closure was reported in the NPWT group (median 96 days) versus median not reached by 16 weeks for controls (Level/Class: A/I).
NPWT–pressure ulcer
There are three reports on Stage III and IV pressure ulcers (PUs). A nonrandomized controlled trial comparing NPWT to conventional dressings showed a significant reduction in wound size with NPWT.30 Another RCT that compared NPWT to modern dressings reported a reduction in the mean ulcer volume from 42% in controls to 52% for NPWT, not statistically significant (p=0.46).31 Finally, a prospective observational study reported by the Department of Veterans Affairs found no significant effect on healing of Stage III/IV PU.32 Given the small size of these trials and conflicting results, no recommendations can be made (Level/Class: B/None).
NPWT-split-thickness skin graft
NPWT provides stable fixation of STSG. The NPWT dressing immobilizes the graft, reducing shear motion and fluid accumulation beneath the graft. Two well-designed and implemented RCTs (total n=124 grafts) reported a statistically significant improvement in graft take and decreased time to healing33,34 (Level/Class: A/IIa).
Ultrasound Therapy
The Food and Drug Association (FDA) classifies US devices as Class II medical devices and has cleared several devices for wound therapy. A review of the individual devices is beyond the scope of this presentation.† Typical therapeutic US frequencies fall in the range of high-frequency ultrasound (HFU, 0.7–3.3 MHz) or low-frequency ultrasound (LFU 20–40 kHz). The depth of penetration ranges from 2 to 5 cm. Therapeutic US has both thermal and nonthermal effects. The absorption of HFU can produce thermal effects. Nonthermal effects are produced by LFU and are related to principals of cavitation, standing waves, acoustic streaming, and microstreaming.35 US provides direct physical stimulation of cells and stimulates repair and healing processes.36 The nonthermal effects of US are used for wound debridement and may have additive bactericidal properties.37 The selection of exposure time and intensity level can significantly alter therapeutic effects. Most devices provide LFU (nonthermal, 20–40 kHz) and are used for wound debridement.38 No standard prototypical algorithm has been reported in the literature.
US-DFU and venous leg ulcer
Ennis et al. reported a multicenter double-blinded RCT of US therapy for the treatment of chronic DFU. US did not achieve a statistically significant improvement in wound closure rates.39 Watson and Kang'ombe assessed the clinical effectiveness of weekly delivery of low-dose HFU for treatment of venous leg ulcer (VLU) in a multicenter RCT. No difference in time to complete healing or evidence of a difference in rates of recurrence of healed ulcers (17/31 with US vs. 14/31 with standard care) was observed. Moreover, they reported significantly more adverse events in the US group.40 The Wound Healing Society Guidelines (2006) did not evaluate the use of US for DFUs or PUs. However, the WHS did assign Level I to its lack of statistically significant effect on healing VLUs (Guideline #7b.5) and Level III C for arterial insufficiency ulcers (Guideline #6. A.1).41 CMS reviewed US therapy and failed to cover this service. The National Institute for Health and Clinical Excellence guidance concluded that the relative effectiveness remains insufficient at this time to support routine use42 (Level/Class: C/III).
Electrical Stimulation‡
A natural electrical gradient exists between the outer surface of intact skin (positive charge) and the subepidermal tissues (negative charge) and measures ∼40–100 mV.43 Any breach in the epidermal barrier leads to a loss of polarity and a mild electrical current then flows outward from the wound creating what has been termed “a current of injury.”44 The electrical current generated by wounding has been shown to stimulate multiple cell types within the wound environment. Electrical stimulation (eStim) is one type of electrical therapy. Many additional forms of electrical therapies exist such as functional electric stimulation, neuromuscular electric stimulation, peripheral nerve stimulation, and electromagnetic therapy, and are not addressed here. The FDA clearance classifies eStim devices as Class II medical devices. eStim applies an electrical current across the wound bed using one positively charged electrode (cathode, aka active electrode) and one negatively charged electrode (anode, aka grounding electrode). The dose range of electricity commonly used is a low-intensity (continuous or pulsed) microcurrent and can be up to 1 mA.45 The delivery of energy can be varied based on wave forms, frequencies, pulse duration, and polarity. eStim is a single clinical term; however, there exists a multitude of varied currents and dosages.§ A prototypical algorithm reported in the literature for the acceleration of chronic wound healing is low-intensity current (direct or pulsed, 0.2–0.8 mA) delivered for 2 h twice or thrice per day, 5 days/week for 5–9 weeks.
Evidence
Numerous publications exist describing the use of eStim in multiple clinical settings, including orthopedics, physical therapy and rehabilitation medicine, pain management, and wound healing. These papers address the effects of eStim on cells and tissues in both animals and humans.46,47 Several systematic reviews of human clinical trials arrived at similar conclusions: There is a clinical benefit for eStim treatment of wounds in humans.48–50 Wound diagnoses tested include DFU, VLU, and PU. Each has small, randomized controlled clinical trials that show some degree of benefit. The Wound Healing Society Guidelines assigns Level I evidence for the use of eStim therapy in VLU (#7b.4), PU (#7b.2) and DFU (#7.7)41 CMS accepts eStim as a covered service (Level/Class: Level A/Class IIa).
Thermal Therapy
Warming wounds by direct noncontact radiant heat or by indirect global warming has been employed for years in an effort to restore normothermia to the wound bed. Due to the lack of evidence of benefit in multiple RCTs, CMS discontinued coverage and the manufacturer withdrew the only wound warming device (WarmUp®) from the US market in 2002. There is insufficient evidence to grade or recommend thermal therapy at this time (Level/Class: None/not done).
Summary
Despite the existence of evidence-based reviews, guidelines, and recommendations, the interested wound care practitioner must be informed about the particular device and the patient population for which it is intended. The rigorous nature of RCTs provide scientific efficacy, however, the practitioner must understand the concept of generalizability as it applies to their patient population if effectiveness is to be attained. Finally, the general lack of reporting of adverse events associated with the devices included in this review mandates that adopters of these technologies put systems in place to adequately monitor adverse outcomes over time.
Disclaimer: The authors recognize and credit the existence of the multiple recent and extensive reviews covering each of the technologies included here. The aim of this monograph is to streamline this information to guide the busy clinician. Readers seeking more in depth information are directed to the usual resources, such as PubMed literature search, society-based guidelines, and systematic reviews of the literature.
Take-Home Messages.
Based on the current 2015 evidence, should I consider providing the following therapies to my patients?
YES for: HBOT, NPWT, and eStim
NO for: US and thermal therapy
Abbreviations and Acronyms
- CMS
Centers for Medicaid and Medicare Services
- DFU
diabetic foot ulcer
- eSTIM
electrical stimulation
- FDA
Food and Drug Association
- HBOT
hyperbaric oxygen therapy
- HFU
high-frequency ultrasound
- LFU
low-frequency ultrasound
- NPWT
negative pressure wound therapy
- PU
pressure ulcer
- RCT
randomized controlled trial
- STSG
split-thickness skin graft
- VLU
venous leg ulcer
Author Disclosure and Ghostwriting
C.A.A., M.A.H., and G.A.P. have no competing financial interests. G.A.P. has received funding for basic science research from OxyHeal Corp. (San Diego, CA). The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About The Authors
All authors provide wound care and hyperbaric therapies for the University of California San Diego Health System. Caesar A. Anderson, MD, MPH, is Associate Professor of Emergency Medicine and medical director for the Center for Wound Healing and Hyperbaric Medicine, Encinitas, CA. Marc A. Hare, MD, CWS, is Assistant Clinical Professor of Emergency Medicine and medical director for the Center for Wound Healing and Hyperbaric Medicine, Paradise Valley Hospital, National City, CA. George A. Perdrizet, MD, PhD, FACS, is Professor of Emergency Medicine, Endowed Chair in Hyperbaric Medicine Research and provides wound care services at the Wound Care Center at the Hillcrest Hospital, San Diego, CA.
Representative examples include: MIST Therapy System by Celleration, Inc., Sonica 185 by Soring, Inc., and Quostic Wound Therapy System by Arobella Medical, LLC.
Only electrical stimulation as a type of electric therapy will be addressed by this overview and is distinct from electromagnetic field therapy.
Direct current, pulsed current, alternating current, low-intensity direct current, low-intensity pulsed direct current, high- or low-voltage pulsed current, frequency rhythmic electrical modulation systems, biphasic and monophasic pulsed current, and others.
References
- 1.Jacobs AK, Kushner FG, Ettinger SM, et al. ACCF/AHA clinical practice guideline methodology summit report: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;61:213–265. See page 234, Table 4.3 [DOI] [PubMed] [Google Scholar]
- 2.Neuman TS, Thom SR. Physiology and Medicine of Hyperbaric Oxygen Therapy. Philadelphia, PA: Saunders, 2008:Section V [Google Scholar]
- 3.Thom SR. Effects of hyperoxia on neutrophil adhesion. Undersea Hyperb Med 2004;31:123–131 [PubMed] [Google Scholar]
- 4.Thom SR, Bhopale VM, Velazquez OC, Goldstein LJ, Thom LH, Buerk DG. Stem cell mobilization by hyperbaric oxygen. Am J Physiol Heart Circ Physiol 2006;290:H1378–H1386 [DOI] [PubMed] [Google Scholar]
- 5.Almzaiel AJ, Billington R, Smerdon G, Moody AJ. Effects of hyperbaric oxygen treatment on antimicrobial function and apoptosis of differentiated HL-60 (neutrophil-like) cells. Life Sci 2013;93:125–131 [DOI] [PubMed] [Google Scholar]
- 6.Undersea and Hyperbaric Medical Society. Hyperbaric Oxygen Therapy Indications, 13th ed. The Hyperbaric Oxygen Therapy Committee Report. Weaver LK, ed. North Palm Beach, FL: Best Publishing, 2014 [Google Scholar]
- 7.Hunt TK, Twomey P, Zederfeldt B, Dunphy JE. Respiratory gas tensions and pH in healing wounds. Am J Surg 1967;114:302–307 [DOI] [PubMed] [Google Scholar]
- 8.Niinikoski JH. Clinical hyperbaric oxygen therapy, wound perfusion, and transcutaneous oximetry. World J Surg 2004;28:307–311 [DOI] [PubMed] [Google Scholar]
- 9.Duzgun AP, Satir HZ, Ozozan O, Saylam B, Kulah B, Coskun F. Effect of hyperbaric oxygen therapy on healing of diabetic foot ulcers. J Foot Ankle Surg 2008;47:515–519 [DOI] [PubMed] [Google Scholar]
- 10.Löndahl M, Katzman P, Nilsson A, Hammarlund C. Hyperbaric oxygen therapy facilitates healing of chronic foot ulcers in patients with diabetes. Diabetes Care 2010;33:998–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marx RE. Osteoradionecrosis: a new concept of its pathophysiology. J Oral Maxillofac Surg 1983;41:283–288 [DOI] [PubMed] [Google Scholar]
- 12.Marx RE. Radiation injury to tissue. In: Kindwall EP, ed. Hyperbaric Medicine Practice, 2nd ed. Flagstaff AZ: Best Publishing Co, 1999:678–723 [Google Scholar]
- 13.Westbury CB, Pearson A, Nerurkar A, et al. Hypoxia can be detected in irradiated normal human tissue: a study using the hypoxic marker pimonidazole hydrochloride. Br J Radiol 2007;80:934–938 [DOI] [PubMed] [Google Scholar]
- 14.Hampson NB, Holm JR, Wreford-Brown CE, Feldmeier J. Prospective assessment of outcomes in 411 patients treated with hyperbaric oxygen for chronic radiation tissue injury. Cancer 2012;118:3860–3868 [DOI] [PubMed] [Google Scholar]
- 15.Bouachour G, Cronier P, Gouello JP, Toulemonde JL, Talha A, Alquier P. Hyperbaric oxygen therapy in the management of crush injuries: a randomized double-blind placebo-controlled clinical trial. J Trauma 1996;41:333–339 [DOI] [PubMed] [Google Scholar]
- 16.Lindström T, Gullichsen E, Lertola K, Niinikoski J. Effects of hyperbaric oxygen therapy on perfusion parameters and transcutaneous oxygen measurements in patients with intramedullary nailed tibial shaft fractures. Undersea Hyperb Med 1998;25:87–91 [PubMed] [Google Scholar]
- 17.Garcia-Covarrubias L, McSwain NE, Jr., Van Meter K, Bell RM. Adjuvant hyperbaric oxygen therapy in the management of crush injury and traumatic ischemia: an evidence-based approach. Am Surg 2005;71:144–151 [PubMed] [Google Scholar]
- 18.Escobar SJ, Slade JB, Jr., Hunt TK, Cianci P. Adjuvant hyperbaric oxygen therapy (HBO2) for treatment of necrotizing fasciitis reduces mortality and amputation rate. Undersea Hyperb Med 2005;32:437–443 [PubMed] [Google Scholar]
- 19.Jacoby I. Necrotizing soft tissue infections. Undersea Hyperb Med 2012;39:739–752 [PubMed] [Google Scholar]
- 20.Shaw JJ, Psoinos C, Emhoff TA, Shah SA, Santry HP. Not just full of hot air: hyperbaric oxygen therapy increases survival in cases of necrotizing soft tissue infections. Surg Infect (Larchmt) 2014;15:328–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cianci P, Slade JB, Jr., Sato RM, Faulkner J. Adjunctive hyperbaric oxygen therapy in the treatment of thermal burns. Undersea Hyperb Med 2013;40:89–108 [PubMed] [Google Scholar]
- 22.Friedman HI, Fitzmaurice M, Lefaivre JF, Vecchiolla T, Clarke D. An evidence-based appraisal of the use of hyperbaric oxygen on flaps and grafts. Plast Reconstr Surg 2006;117:175S–190S; discussion 191S–192S. Erratum in: Plast Reconstr Surg 2006;118:62e [DOI] [PubMed] [Google Scholar]
- 23.Scherer SS, Pietramaggiori G, Mathews JC, Prsa MJ, Huang S, Orgill DP. The mechanism of action of the vacuum-assisted closure device. Plast Reconstr Surg 2008;122:786–797 [DOI] [PubMed] [Google Scholar]
- 24.Kairinos N, Holmes WJ, Solomons M, Hudson DA, Kahn D. Does a zone of increased perfusion exist around negative-pressure dressings? Plast Reconstr Surg 2013;132:978–987 [DOI] [PubMed] [Google Scholar]
- 25.Mouës CM, Vos MC, van den Bemd GJ, Stijnen T, Hovius SE. Bacterial load in relation to vacuum-assisted closure wound therapy: a prospective randomized trial. Wound Repair Regen 2004;12:11–17 [DOI] [PubMed] [Google Scholar]
- 26.Braakenburg A, Obdeijn MC, Feitz R, van Rooij IA, van Griethuysen AJ, Klinkenbijl JH. The clinical efficacy and cost effectiveness of the vacuum-assisted closure technique in the management of acute and chronic wounds: a randomized controlled trial. Plast Reconstr Surg 2006;118:390–397; discussion 398–400 [DOI] [PubMed] [Google Scholar]
- 27.Vuerstaek JD, Vainas T, Wuite J, Nelemans P, Neumann MH, Veraart JC. State-of-the-art treatment of chronic leg ulcers: a randomized controlled trial comparing vacuum-assisted closure (V.A.C.) with modern wound dressings. J Vasc Surg 2006;44:1029–1037; discussion 1038 [DOI] [PubMed] [Google Scholar]
- 28.Armstrong D, Lavery L. Negative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomized controlled trial. Lancet 2005;366:1704–1710 [DOI] [PubMed] [Google Scholar]
- 29.Blume PA, Walters J, Payne W, Ayala J, Lantis J. Comparison of negative pressure wound therapy utilizing vacuum-assisted closure to advanced moist wound therapy in the treatment of diabetic foot ulcers: a multicenter, randomized controlled trial. Diabetes Care 2008;31:631–636 [DOI] [PubMed] [Google Scholar]
- 30.Srivastava RN, Dwivedi MK, Bhagat AK, Raj S, Agarwal R, Chandra A. A non-randomized, controlled clinical trial of an innovative device for negative pressure wound therapy of pressure ulcers in traumatic paraplegia patients. Int Wound J 2014. June 3 [Epub ahead of print]; DOI: 10.1111/iwj.12309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ford CN, Reinhard ER, Yeh D, Syrek D, De Las Morenas A, Bergman SB, et al. Interim analysis of a prospective, randomized trial of vacuum-assisted closure versus the healthpoint system in the management of pressure ulcers. Ann Plast Surg 2002;49:55–61 [DOI] [PubMed] [Google Scholar]
- 32.Ho CH, Powell HL, Collins JF, Bauman WA, Spungen AM. Poor nutrition is a relative contraindication to negative pressure wound therapy for pressure ulcers: preliminary observations in patients with spinal cord injury. Adv Skin Wound Care 2010;23:508–516 [DOI] [PubMed] [Google Scholar]
- 33.Llanos S, Danilla S, Barraza C, Armijo E, Piñeros JL, Quintas M, et al. Effectiveness of negative pressure closure in the integration of split thickness skin grafts: a randomized, double-masked, controlled trial. Ann Surg 2006;244:700–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petkar K, Dhanraj P, Sreekar H. Vacuum closure as a skin-graft dressing: a comparison against conventional dressing. Eur J Plast Surg 2012;35:579–584 [Google Scholar]
- 35.Steven MO, Constatantin C, Seymour L, Carlisle R. Ultrasound enhanced drug delivery for cancer. Expert Opin Drug Deliv 2012;9:1525–1538 [DOI] [PubMed] [Google Scholar]
- 36.Leung MC, Ng GY, Yip KK. Effect of ultrasound on acute inflammation of transected medial collateral ligaments. Arch Phys Med Rehabil 2004;85:963–966 [DOI] [PubMed] [Google Scholar]
- 37.McCulloch JM, Kloth LC. Wound Healing: Evidence Based Management, 4th ed. Philladelphia, PA: F.A. Davis, 2010 [Google Scholar]
- 38.Johnson S. Low frequency ultrasound to manage chronic venous leg ulcers. Br J Nurs 2003;12:S14–S24 [DOI] [PubMed] [Google Scholar]
- 39.Ennis WJ, Formann P, Mozen N, Massey J, Conner-Kerr T, Meneses P. Ultrasound therapy for recalcitrant diabetic foot ulcers: results of a randomized, double blind, controlled, multicenter study. Ostomy Wound Manage 2005;51:24–39 [PubMed] [Google Scholar]
- 40.Watson JM, Kang'ombe AR. Use of weekly, low dose, high frequency ultrasound for hard to heal venous leg ulcers: the VenUS III randomized controlled trial. BMJ 2011;342:d1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.WHS Wound Care Guidelines. http://woundheal.org/Publications/WHS-Wound-Care-Guidelines.aspx (accessed February26, 2015)
- 42.National Institute for Health and Clinical Evidence (NICE). The MIST Therapy System for the Promotion of Wound Healing. London, United Kingdom: NICE, 2011 [Google Scholar]
- 43.Reid B, Zhao M. The electrical response to injury: molecular mechanisms and wound healing. Adv Wound Care 2014;3:184–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jaffe L, Vanable J., Jr. Electrical fields and wound healing. Clin Dermatol 1984;2:34–44 [DOI] [PubMed] [Google Scholar]
- 45.Kloth LC. Wound healing with conductive electrical stimulation: it's the dosage that counts. J Wound Technol 2009;6:30–37 [Google Scholar]
- 46.Thakral G, LaFontaine J, Najafi B, et al. Electrical stimulation to accelerate wound healing. Diabetic Foot Ankle 2013. September 16 [Epub ahead of print]; DOI: 10.3402/dfa.v4i0.22081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Houghton PE. Clinical trials involving biphasic pulsed current, micro-current, and/or low-intensity direct current. Adv in Wound Care 2014;3:166–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kawasaki L, Mushahwar VK, Ho C, et al. The mechanisms and efficacy of electrical stimulation for healing pressure ulcer: a systematic review. Wound Repair and Regen 2014;22:161–173 [DOI] [PubMed] [Google Scholar]
- 49.Martinez-Rodriquez A, Bello O, Fraiz M, Martnez-Bustelo S. The effect of alternating and biphasic currents on humans' wound healing: a literature review. Int J Dermatol 2013;52:1053–1062 [DOI] [PubMed] [Google Scholar]
- 50.Koel G, Houghton PE. Electrostimulation: current status, strength of evidence guidelines, and meta-analysis. Adv Wound Care (New Rochelle) 2014;3:118–126 [DOI] [PMC free article] [PubMed] [Google Scholar]