Abstract
The potential of maraviroc (MVC), a small-molecule CCR5 antagonist, as a candidate to prevent HIV-1 sexual transmission by oral or topical dosing has not yet been completely established. Using relevant cellular and mucosal tissue explant models, we show partial antiviral activity of MVC when tested in multiple preclinical dosing strategies.
The predominant transmission of R5 tropic HIV-1 isolates through sexual intercourse1,2 makes CCR5 an appealing target to prevent viral entry in mucosal target cells. Maraviroc (MVC) is the first antiretroviral (ARV) small-molecule CCR5 inhibitor3 to have been included in highly active antiretroviral therapy (HAART) and is currently being considered in the field of prevention as a candidate for oral pre-exposure prophylaxis (PrEP) or for topical application as a microbicide. Formulated as a microbicide gel, MVC has shown promising pharmacological results in humans and nonhuman primates (NHPs)4,5 and efficacy in NHPs when tested as a vaginal gel.6,7
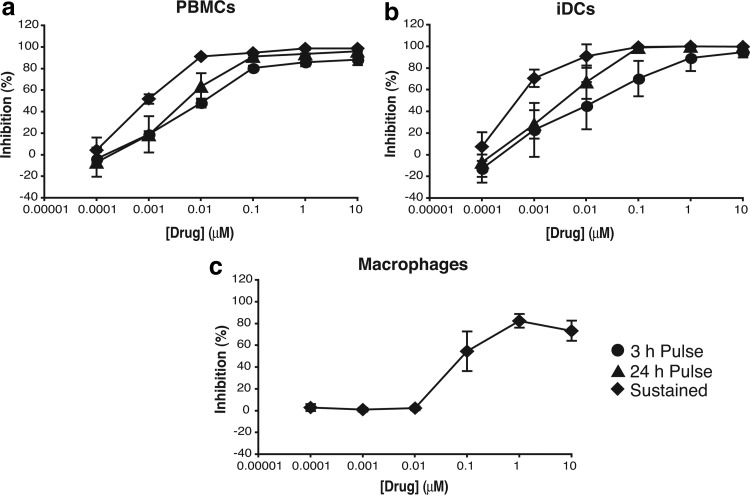
To model different potential prevention dosing conditions, we tested drug efficacy before viral challenge (3-h drug exposure), before and after challenge (24-h drug exposure), or continuous drug exposure mimicking sustained release. We first assessed the activity of MVC with these dosing conditions in activated peripheral blood mononuclear cells (PBMCs) and other cellular models for preclinical evaluation in a mucosal environment, including immature dendritic cells (iDCs) and monocyte-derived macrophages (MDMs).8 We observed different susceptibility to infection in the three models with higher levels of p24 in culture supernatant after 14 days of culture in PBMCs (33.29 ± 1.43 ng/ml) compared with MDMs (12.77 ± 0.73 ng/ml) and iDCs (1.27 ± 0.60 ng/ml).
With all three drug exposure times, a dose–response curve was obtained against HIV-1 BaL in activated PBMCs (Fig. 1a) and MVC was able to reach an IC50 at low and decreasing nanomolar concentrations with increasing dosing times (9.93 ± 1.68 nM with a 3-h exposure, 5.00 ± 1.38 nM with a 24-h exposure, and 0.94 ± 0.12 nM after continuous exposure). The IC50 of MVC in monocyte-derived iDCs against HIV-1 BaL was in the nanomolar range as shown in PBMCs. The antiviral potency of MVC increased with longer drug exposure times, resulting in a reduction of the IC50 value from 23.96 ± 26.08 nM with a 3-h exposure to 3.37 ± 2.21 nM with a 24-h exposure and 0.47 ± 0.14 nM with continuous exposure.
FIG. 1.
(a) PBMCs were isolated from multidonor buffy coats from healthy HIV-seronegative donors by centrifugation onto Ficoll-Hypaque, mitogen stimulated as previously described,27 and maintained in RPMI 1640 medium containing 10% FCS, 2 mM l-glutamine, antibiotics (100 U of penicillin/ml, 100 μg of streptomycin/ml), and 100 U of IL-2/ml. (b) iDCs were grown from PBMC-derived monocytes cultured for 6 days in complete RPMI medium supplemented with 1000 U/ml GM-CSF and 500 U/ml IL-4 (R&D Systems). iDCs were phenotypically characterized by staining with anti-CD40, anti-CD80, anti-CD86, anti-CD83, anti-CD209, anti-CD123, and anti-CD11c (BD Pharmingen). (c) MDMs were obtained from buffy coat-derived PBMCs by adherence and matured for 5–7 days by culture in RPMI 1640 plus 10% human serum (HS) and 20 ng/ml of GM-CSF (R&D Systems). Cells were exposed to serial dilutions of MVC for 1 h before addition of HIV-1 BaL at 2,000 TCID50/ml. After 2 h of incubation, cells were washed and cultured for 14 days in the absence (3-h exposure, ●) or presence of MVC for a total of 24 h (24-h exposure, ▲) or the duration of the assay (continuous exposure, ♦). The concentrations of p24 in harvested supernatants were quantified by ELISA. The percentage of inhibition was normalized relative to the p24 values obtained for cells not exposed to virus (0% infectivity) and for cells infected with virus in the absence of compound (100% infectivity). Data are means ± standard deviations from three independent experiments performed in triplicate. FCS, fetal calf serum; GM-CSF, granulocyte-macrophage colony-stimulating factor; iDCs, immature dendritic cells; IL, interleukin; MDM, monocyte-derived macrophage; MVC, maraviroc; PBMC, peripheral blood mononuclear cell.
When iDCs were pre-exposed to MVC for 3 h, only partial inhibition was observed, failing to reach 95% inhibition even at the highest drug concentration tested (10 μM) (Fig. 1b). The plateau of maximum inhibition was increased with longer drug exposure, 24 h, and continuous exposure and was reached at lower concentrations with IC95 values of 68.44 ± 48.85 nM and 20.91 ± 23.42 nM, respectively.
The level of CCR5 expressed on the surface of MDMs has been described to increase when generated in the presence of granulocyte-macrophage colony-stimulating factor and to be higher than that measured in monocyte-derived DCs.9 The expression of higher levels of CCR5 on the cell surface requires higher doses of MVC to prevent HIV-1 entry10 (C. Herrera and R.J. Shattock, unpublished data); hence, we assessed the potency of MVC in MDMs with the more active exposure duration mimicking sustained release. With continuous exposure during 14 days, MVC was only able to reach ∼80% of inhibition at 10 μM, the highest concentration tested against HIV-1 BaL (Fig. 1c). The IC50 value in these conditions was 112.23 ± 67.45 nM.
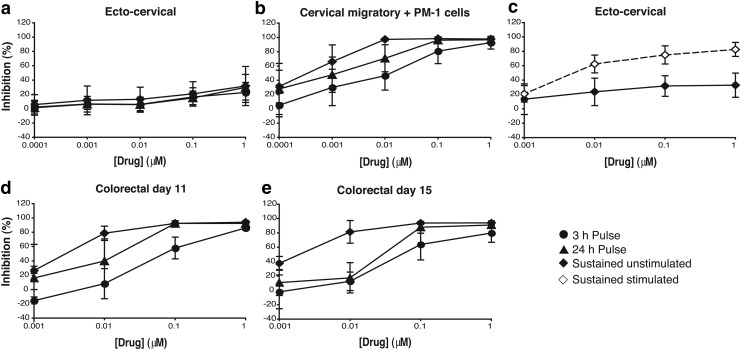
Based on the results obtained with monocyte-derived cells, we then titrated the activity of MVC in mucosal tissue explants, which better model the environment of the portal of viral entry during sexual transmission. Surgically resected ectocervical and colorectal tissues were used to assess the activity of the drug in the female reproductive compartment and in the sigmoidal tract, respectively, using the same three dosing times tested in the cellular models (3-h exposure, 24-h exposure, and continuous exposure).
Interestingly, no significant antiviral activity was observed for MVC with any of the dosing strategies in ectocervical tissue even at the highest concentration of MVC tested (Fig. 2a). However, dose–response curves for MVC (Fig. 2b) were obtained when ectocervical migratory cells, which have been characterized as DCs,11 were harvested from MVC-treated and HIV-1-challenged tissue and cocultured with CD4+ T PM-1 cells. This model mimics the potential cell-associated transmission of HIV-1 from virus-exposed iDCs to uninfected CD4+ T cells that occurs during the local expansion of infection following establishment of the initial foci of infection in mucosal tissues.12,13
FIG. 2.
(a–c) Ectocervical tissue was obtained from premenopausal females undergoing routine hysterectomies with nonmalignant pathology. Tissue was dissected into explants as previously described11 and incubated with serial dilutions of MVC for 1 h before virus (104 TCID50/ml) was added for 2 h. Explants were then washed four times with phosphate-buffered saline and cultured in fresh plates (a) or migratory cells were harvested at 24 h postinfection and cocultured with 40,000 PM-1 cells (b). Both culture models were kept for 15 days in RPMI 1640 medium containing 10% fetal bovine serum, 2 mM l-glutamine, and antibiotics (100 U of penicillin/ml, 100 μg of streptomycin/ml) in the absence (3-h exposure, ●) or presence of MVC for a total of 24 h (24-h exposure, ▲) or the duration of the assay (continuous exposure, ♦). (c) Stimulated ectocervical explants were treated with 100 U/ml IL-2 and 5 μg/ml PHA for 72 h before infection, with all cultures subsequently maintained in 100 U/ml IL-2. Stimulated (⋄) and non-stimulated (♦) explants were exposed continuously to MVC. (d, e) Colorectal tissue was obtained from patients undergoing rectocele repair and colectomy from colorectal cancer. Colorectal explants were maintained with Dulbecco's modified Eagle's medium containing 10% FCS, 2 mM l-glutamine, and antibiotics (100 U of penicillin/ml, 100 μg of streptomycin/ml, 80 μg of gentamicin/ml). Tissue explants were treated and exposed to virus as the ectocervical explants. After four washes, explants were transferred onto gelfoam rafts (Welbeck Pharmaceuticals) and cultured for 15 days in the absence (3-h exposure, ●) or presence of MVC for a total of 24 h (24-h exposure, ▲) or the duration of the assay (continuous exposure, ♦). Antiviral activity was assessed at days 11 (d) and 15 (e) of culture. For all tissues, ∼50% of the supernatants were harvested every 2–3 days and explants were refed with fresh media with or without MVC. For all assays, p24 concentrations in harvested supernatants were quantified by ELISA. The percentage of inhibition was normalized as described in Figure 1. Data are means ± standard deviations from three independent experiments performed in triplicate. All tissues were collected after receiving signed informed consent from all patients and under protocols approved by the Local Research Ethics Committee. All patients were HIV negative. PHA, phytohaemagglutinin.
Similar to the results obtained with iDCs, the potency of MVC increased with longer time of exposure, reaching higher levels of inhibition with 24-h and continuous exposure than with a 3-h exposure (Fig. 2b). This increase in activity was also reflected in a decrease of the IC50 value for MVC from 12.06 ± 7.36 nM with a 3-h exposure to 1.86 ± 0.87 nM with a 24-h exposure and to 0.49 ± 0.56 nM with continuous exposure. The IC50 values obtained for each dosing protocol were in the same range as those observed for the same time of exposure in iDCs.
To investigate if the lack of activity of MVC in ectocervical explants was due to the low number of activated CCR5+ cells in the female genital tract compared with the colorectal mucosa,14–16 we compared the antiviral activity of MVC in nonstimulated versus phytohaemagglutinin (PHA) and interleukin (IL)-2 pretreated tissue explants from the same donors.
As expected, the levels of p24 measured in culture supernatants of stimulated ectocervical tissue (1.69 ± 1.34 ng/ml) were greater than in nonstimulated tissue (0.54 ± 0.19 ng/ml). However, this increase was not observed in cocultures of cervical migratory cells with PM-1 cells (nonstimulated: 252.65 ± 241.14 ng/ml; and stimulated: 336.39 ± 218.52 ng/ml). With continuous exposure to MVC, a dose–response curve against HIV-1 BaL was observed with stimulated ectocervical tissue (Fig. 2c), reaching ∼80% of inhibition at the highest concentration of MVC tested. The IC50 value for MVC in these conditions was 4.97 ± 2.57 nM. Stimulation of ectocervical explants did not increase the inhibitory potency of MVC in the migratory trans-infection assays (data not shown).
CCR5 is a highly modulated G-protein-coupled receptor (GPCR) on the cell surface and can be expressed with different states of activation, as a monomer, as a homodimer, or can even form heterodimers with other GPCRs.17–19 These results suggest that the lack of antiviral activity of MVC in nonstimulated ectocervical tissue could be due not only to a low level of CCR5+ cells but also to their resting state where CCR5 may be expressed in different conformations to that on PHA/IL-2-stimulated cells.
We next assessed the antiviral activity of MVC in colorectal tissue explants. The titration of MVC with a 3-h exposure and after 15 days of culture resulted in a dose–response curve with an IC50 of 109.88 ± 84.51 nM and maximum inhibition below 80% (with an IC70 of 238.20 ±85.24 nM) (Fig. 2e). This plateau as well as the potency of MVC was progressively increased with longer periods of tissue exposure to the drug (Fig. 2e). The IC50 values decreased to 22.05 ± 13.75 nM after a 24-h exposure and to 2.18 ±1.05 nM with continuous exposure.
Interestingly, in this model, the maximum antiviral potency of MVC was more pronounced at an earlier time point in culture, day 11 (Fig. 2d), with a 3-h exposure, displaying a maximum level of inhibition above 85%, with an IC50 of 79.66 ± 50.95 nM and an IC70 of 162.76 ± 72.79 nM compared with the day 15 IC50 of 109.88 ± 84.51 nM and IC70 of 238.20 ± 85.24 nM described above. When comparing the levels of p24 in culture supernatant, as a result of a productive infection, there was an increase of p24 between days 11 (4.31 ± 2.48 ng/ml) and 15 (18.84 ± 5.74 ng/ml). Independently of the time point analyzed, day 11 or 15, the inhibitory concentrations observed during sustained exposure of colorectal tissue tended to be 1 log higher than those observed in ectocervical tissue.
Histological analysis has shown that colorectal explants remained viable for more than 10 days with progressive loss of architecture, but maintenance of CD4:CD8 T-cell ratios.20 There is currently a paucity of data regarding preservation of functions such as immune competence and mucus secretion for tissue explants; however, conservation of function has been described in other tissue explants.21 Validation of the explant model with respect to intracellular drug distribution is not yet available. However, in combination with other models, such as TZM-bl cells and blood derived-cells used in this study, it provides powerful preclinical information to identify efficacious prevention candidates.
The differential activity of MVC in the cellular and tissue explant models likely reflects heterogeneity in CCR5 conformation and/or expression.14–16 CCR5 is a dynamic receptor, where abundance and spatial distribution are modulated by binding of natural ligands and derivatives, inducing oligomerization and endocytosis,22,23 and by cellular activation, leading to enhanced expression.24,25 The binding of HIV gp120 to CCR5, and therefore susceptibility to infection, is critically affected by the coreceptor heterogeneity and conformation.22,26,27
Crystallographic studies17,28 have revealed that gp120 and MVC do not bind to the same structural determinants in CCR5, but that allosteric modulation of CCR5 by MVC can inhibit gp120 binding, preventing infection.17 However, MVC preferentially binds to higher energy conformations of CCR5,29 displaying differential anti-HIV activity against G-protein-coupled and uncoupled forms.30 The comparative lack of antiviral activity of MVC in nonstimulated ectocervical tissue compared with PHA/IL-2-activated cultures suggests that resting tissue expresses CCR5 in a conformation that may be refractory to MVC binding and/or allosteric modulation required to prevent HIV infection.17 This important observation warrants further investigation, but this falls beyond the scope of this current study. Furthermore, we cannot exclude that differences in the activity of drug transporters between the two mucosal compartments (rectal and cervical) might also impact on MVC activity31,32
The impact of differential states of target cell activation in specific mucosal tissues has also been observed for other PrEP agents such as reverse transcriptase inhibitors (RTIs). In NHPs, despite similar levels of drug found in blood, rectal, and lymphoid tissues, no protection was observed following rectal challenge due to higher levels of CD4 T-cell activation in rectal tissue compared with other compartments.33 In this study, cellular activation causes increase of intracellular nucleotides, which are the natural substrates of RT, therefore, competing with RTIs. While the mechanisms are distinct, differences in the activation status of HIV target cells within the different mucosal compartments (vaginal and rectal) likely impact on the activity of MVC through modulation of CCR5 expression and/or conformation as described above. These data highlight the need to take into account the activation status and target cell population within different tissue microenvironments when evaluating drugs for mucosal HIV prophylaxis.
The partial activity of MVC particularly in ectocervical tissue raises significant questions over its use as a stand-alone prevention modality. In fact, no protective activity has been observed when MVC has been tested as an oral PrEP candidate in a study in NHPs34 and in the first two clinical trials conducted to date.35,36 Furthermore, the duration of protection in NHPs following vaginal application was short.7 Nevertheless, the mechanism of action and pharmacological profile37 of MVC make this compound a good candidate for the design of an ARV combination-based prevention strategy, as reported in an NHP study where a combination of MVC with tenofovir, an RTI, was highly protective against rectal challenge when formulated as a gel microbicide.38 The ongoing oral PrEP clinical trial, HPTN 069/ACTG 5305 (NEXT-PrEP), evaluating double combinations of MVC, FTC, and TDF will be key to inform the potential of MVC in the field of prevention.
Acknowledgments
The authors thank Mr. Haggar, Mr. Melville, and the Colorectal Surgery Team, St. George's Hospital, London, for their assistance in obtaining human colorectal tissue. The authors are grateful to St. George's Hospital, London, for the donation of cervical tissue. This work was funded by IPM and the National Institutes of Health (grant no. U19 AI076982).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Grivel JC, Shattock RJ, Margolis LB: Selective transmission of R5 HIV-1 variants: Where is the gatekeeper? J Transl Med 2011;9(Suppl 1):S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salazar-Gonzalez JF, Salazar MG, Keele BF, et al. : Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J Exp Med 2009;206:1273–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dorr P, Westby M, Dobbs S, et al. : Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob Agents Chemother 2005;49:4721–4732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dumond JB, Patterson KB, Pecha AL, et al. : Maraviroc concentrates in the cervicovaginal fluid and vaginal tissue of HIV-negative women. J Acquir Immune Defic Syndr 2009;51:546–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forbes CJ, Lowry D, Geer L, et al. : Non-aqueous silicone elastomer gels as a vaginal microbicide delivery system for the HIV-1 entry inhibitor maraviroc. J Control Release 2011;156:161–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malcolm RK, Forbes CJ, Geer L, et al. : Pharmacokinetics and efficacy of a vaginally administered maraviroc gel in rhesus macaques. J Antimicrob Chemother 2013;68:678–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veazey RS, Ketas TJ, Dufour J, et al. : Protection of rhesus macaques from vaginal infection by vaginally delivered maraviroc, an inhibitor of HIV-1 entry via the CCR5 co-receptor. J Infect Dis 2010;202:739–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herrera C, Shattock RJ: Candidate microbicides and their mechanisms of action. Curr Top Microbiol Immunol 2014;383:1–25 [DOI] [PubMed] [Google Scholar]
- 9.Lee B, Sharron M, Montaner LJ, Weissman D, Doms RW: Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proc Natl Acad Sci U S A 1999;96:5215–5220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacqmin P, McFadyen L, Wade JR: A receptor theory-based semimechanistic PD model for the CCR5 noncompetitive antagonist maraviroc. Br J Clin Pharmacol 2008;65(Suppl 1):95–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu Q, Frank I, Williams V, et al. : Blockade of attachment and fusion receptors inhibits HIV-1 infection of human cervical tissue. J Exp Med 2004;199:1065–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haase AT: Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu Rev Med 2011;62:127–139 [DOI] [PubMed] [Google Scholar]
- 13.Shattock RJ, Rosenberg Z: Microbicides: Topical Prevention against HIV. Cold Spring Harb Perspect Med 2012;2:a007385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anton PA, Elliott J, Poles MA, et al. : Enhanced levels of functional HIV-1 co-receptors on human mucosal T cells demonstrated using intestinal biopsy tissue. AIDS 2000;14:1761–1765 [DOI] [PubMed] [Google Scholar]
- 15.Lapenta C, Boirivant M, Marini M, et al. : Human intestinal lamina propria lymphocytes are naturally permissive to HIV-1 infection. Eur J Immunol 1999;29:1202–1208 [DOI] [PubMed] [Google Scholar]
- 16.Poles MA, Elliott J, Taing P, Anton PA, Chen IS: A preponderance of CCR5(+) CXCR4(+) mononuclear cells enhances gastrointestinal mucosal susceptibility to human immunodeficiency virus type 1 infection. J Virol 2001;75:8390–8399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Perez J, Rueda P, Alcami J, et al. : Allosteric model of maraviroc binding to CC chemokine receptor 5 (CCR5). J Biol Chem 2011;286:33409–33421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Perez J, Rueda P, Staropoli I, et al. : New insights into the mechanisms whereby low molecular weight CCR5 ligands inhibit HIV-1 infection. J Biol Chem 2011;286:4978–4990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mellado M, Rodriguez-Frade JM, Vila-Coro AJ, et al. : Chemokine receptor homo- or heterodimerization activates distinct signaling pathways. EMBO J 2001;20:2497–2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fletcher PS, Elliott J, Grivel JC, et al. : Ex vivo culture of human colorectal tissue for the evaluation of candidate microbicides. AIDS 2006;20:1237–1245 [DOI] [PubMed] [Google Scholar]
- 21.Grivel JC, Margolis L: Use of human tissue explants to study human infectious agents. Nat Protoc 2009;4:256–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colin P, Benureau Y, Staropoli I, et al. : HIV-1 exploits CCR5 conformational heterogeneity to escape inhibition by chemokines. Proc Natl Acad Sci U S A 2013;110:9475–9480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flegler AJ, Cianci GC, Hope TJ: CCR5 conformations are dynamic and modulated by localization, trafficking and G protein association. PLoS One 2014;9:e89056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gornalusse GG, Mummidi S, Gaitan AA, et al. : Epigenetic mechanisms, T-cell activation, and CCR5 genetics interact to regulate T-cell expression of CCR5, the major HIV-1 coreceptor. Proc Natl Acad Sci U S A 2015;112:E4762–E4771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mo H, Monard S, Pollack H, et al. : Expression patterns of the HIV type 1 coreceptors CCR5 and CXCR4 on CD4+ T cells and monocytes from cord and adult blood. AIDS Res Hum Retroviruses 1998;14:607–617 [DOI] [PubMed] [Google Scholar]
- 26.de Voux A, Chan MC, Folefoc AT, Madziva MT, Flanagan CA: Constitutively active CCR5 chemokine receptors differ in mediating HIV envelope-dependent fusion. PLoS One 2013;8:e54532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakano Y, Monde K, Terasawa H, et al. : Preferential recognition of monomeric CCR5 expressed in cultured cells by the HIV-1 envelope glycoprotein gp120 for the entry of R5 HIV-1. Virology 2014;452–453:117–124 [DOI] [PubMed] [Google Scholar]
- 28.Tan Q, Zhu Y, Li J, et al. : Structure of the CCR5 chemokine receptor-HIV entry inhibitor maraviroc complex. Science 2013;341:1387–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abrol R, Trzaskowski B, Goddard WA, 3rd, Nesterov A, Olave I, Irons C: Ligand- and mutation-induced conformational selection in the CCR5 chemokine G protein-coupled receptor. Proc Natl Acad Sci U S A 2014;111:13040–13045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berro R, Yasmeen A, Abrol R, et al. : Use of G-protein-coupled and -uncoupled CCR5 receptors by CCR5 inhibitor-resistant and -sensitive human immunodeficiency virus type 1 variants. J Virol 2013;87:6569–6581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hijazi K, Cuppone AM, Smith K, et al. : Expression of genes for drug transporters in the human female genital tract and modulatory effect of antiretroviral drugs. PLoS One 2015;10:e0131405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mukhopadhya I, Murray GI, Berry S, et al. : Drug transporter gene expression in human colorectal tissue and cell lines: Modulation with antiretrovirals for microbicide optimization. J Antimicrob Chemother 2016 [Epub ahead of print]. 2016;71:372–386 [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Lerma JG, Aung W, Cong ME, et al. : Natural substrate concentrations can modulate the prophylactic efficacy of nucleotide HIV reverse transcriptase inhibitors. J Virol 2011;85:6610–6617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Massud I, Aung W, Martin A, et al. : Lack of prophylactic efficacy of oral maraviroc in macaques despite high drug concentrations in rectal tissues. J Virol 2013;87:8952–8961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fox J, Tiraboschi JM, Herrera C, et al. : A Phase IV PrEP Study Reveals Limited Ex Vivo Potency of Oral Maraviroc Against HIV-1 [Abstract]. CROI, Seattle, WA, 2015 [Google Scholar]
- 36.Garcia E, Cabrera C, Coll J, et al. : Oral Single-Dose Maraviroc Does Not Prevent Ex Vivo HIV Infection of Rectal Mucosa in Healthy HIV-1–Negative Human Volunteers in Tissue Explants [Abstract]. CROI, Seattle, WA, 2015 [DOI] [PubMed] [Google Scholar]
- 37.Trezza CR, Kashuba AD: Pharmacokinetics of antiretrovirals in genital secretions and anatomic sites of HIV transmission: Implications for HIV prevention. Clin Pharmacokinet 2014;53:611–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dobard CW, Taylor A, Sharma S, et al. : Protection against rectal SHIV transmission in macaques by rectal specific-gel formulations of maraviroc and tenofovir. J Infect Dis 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]