Abstract
Electromyography is often used to infer the pattern of production of force by skeletal muscles. The interpretation of muscle function from the electromyogram (EMG) is challenged by the fact that factors such as type of muscle fiber, muscle length, and muscle velocity can all influence the relationship between electrical and mechanical activity of a muscle. Simultaneous measurements of EMG, muscle force, and fascicle length in hindlimb muscles of wild turkeys allow us to probe the quantitative link between force and EMG. We examined two features of the force–EMG relationship. First, we measured the relaxation electromechanical delay (r-EMD) as the time from the end of the EMG signal to time of the end of force. This delay varied with locomotor speed in the lateral gastrocnemius (LG); it was longer at slow walking speeds than for running. This variation in r-EMD was not explained by differences in muscle length trajectory, as the magnitude of r-EMD was not correlated with the velocity of shortening of the muscle during relaxation. We speculate that the longer relaxation times at slow walking speeds compared with running may reflect the longer time course of relaxation in slower muscles fibers. We also examined the relationship between magnitude of force and EMG across a range of walking and running speeds. We analyzed the force–EMG relationship during the swing phase separately from the force–EMG relationship during stance phase. During stance, force amplitude (average force) was linearly related to mean EMG amplitude (average EMG). Forces during swing phase were lower than predicted from the stance phase force–EMG relationship. The different force–EMG relationships during the stance and swing phases may reflect the contribution of passive structures to the development of force, or a nonlinear force–EMG relationship at low levels of muscle activity. Together the results suggest that any inference of force from EMG must be done cautiously when a broad range of activities is considered.
Introduction
In comparative biology, electromyography is arguably the most commonly used tool for investigating muscle function during locomotion. The electromyograph (EMG) has revealed details of the timing and magnitude of muscle activation for many muscles powering a variety of types of locomotion. An integrated analysis of EMG, kinematic, and anatomical information can provide an accurate picture of the function of individual muscles during movement (Goslow et al. 1981; Dial et al. 1988; Jayne and Lauder 1993; Gatesy 1997; Stern and Larson 2001). Most of our understanding of the role of individual muscles in movement is founded on such analysis.
The use of EMG as an indicator of the mechanical function of a muscle is challenged by the fact that the EMG reflects the electrical, not the mechanical, events of a contraction. It has long been recognized that any interpretation of EMG as a measure of the timing or magnitude of muscle force requires caution (Hof 1984; Basmajian and De Luca 1985). The timing of onset of force production relative to the onset of EMG, for example, depends on numerous steps in the excitation–contraction pathway. These include the time course of release of calcium from the sarcoplasmic reticulum, its binding to troponin, and the rates of cross-bridge formation during activation (Lieber 1992). Mechanical events, such as changes in length of the muscle, also affect the time course of force development (Edman and Josephson 2007). Similarly, several events involved in muscle relaxation will influence the time delay between the cessation of EMG and the end of force production, including the off-rate of acto-myosin cross-bridges and the time course of resequestration of Ca2+ by the sarcoplasmic reticulum (Marsh 1990). These electromechanical delays present a well-known challenge to the use of EMG to estimate the timing of force production.
The amplitude of EMG signal has the potential to provide a measure of the magnitude of muscle force, but this relationship is complicated by both the character of the measured EMG and the mechanics of force production in skeletal muscle. The signal measured at an EMG electrode is a voltage differential set up by the summed affect of multiple motor units depolarizing at varying distances from the electrode. The nature of the conduction of these signals through the muscle tissue, the interaction of the signals of different motor units, and variable firing rates of different motor units will all affect the voltage signal that is ultimately measured at an electrode (Hof 1984; Farina et al. 2004). Even if the EMG gives an honest signal of the relative volume of motor units active, the force actually developed by these motor units depends strongly on the dependence of muscle force output on contractile conditions, such as fiber length and velocity (Lieber 1992).
Direct measurements of muscle force are much less common than are EMG measurements, primarily owing to methodologic challenges. It is perhaps counterintuitive that it is relatively easy to measure extremely small currents set up by the movements of ions across the membranes of muscle cells, but technically difficult to measure the very large forces developed by commonly studied muscles [for example the subject of the present study, the turkey lateral gastrocnemius (LG), develops maximal forces exceeding 200 N]. Nevertheless, direct measurements of muscle force have been limited to a handful of studies and a few techniques (Gregor and Abelew 1994). Several studies have made use of measurements of muscle force to test assumptions about the relationship between EMG and force and to build mathematical models relating the two (Sherif et al. 1983; Hylander and Johnson 1993; Guimaraes et al. 1995). Results vary. Some studies indicate that the appropriate mathematical model can accurately predict the force profile of the measured muscle from the EMG. However, in most cases it is unclear to what extent a model developed for a given muscle and a fixed set of functions can be extrapolated to other muscles and other activities.
Here we use direct measurements of muscle force and EMG in the lateral LG of wild turkeys to examine some aspects of the relationship between force and EMG. We have used wild turkeys as a model for studying muscle mechanical function in part because their calcified tendons allow the direct measurement of muscle force via strain gauges on the tendon. Here, we examine the relationship between timing and magnitude of EMG and force. Specifically, we examine whether the time from the offset of EMG to the offset of force [or relaxation electromechanical delay (r-EMD)] is fixed for this muscle, or whether it varies with locomotor speed or the velocity of shortening of the muscle. We also examine the relationship between the amplitude of EMG and the amplitude of force, to determine whether it is consistent across locomotor speed as well as during the different periods of force production by the LG.
Methods
The data analyzed for this study were from measurements reported previously (Gabaldón et al. 2004, 2008) and from unpublished data. The methods have been described in detail (Gabaldón et al. 2004) and will be described briefly here.
Wild turkeys obtained from a domestic breeder were kept in indoor/outdoor pens and given food and water ad libitum. All use of animals was reviewed and approved by animal care and use committees at Oregon State University and Brown University.
Animals were trained to run on treadmills over a period of 6–10 weeks. Training sessions were ∼20 min of continuous running over a range of speeds. Animals were trained 4–5 times per week. The treadmill was a commercially available treadmill for fitness training (Keys Pro 2000, Keys Fitness Products, Dallas, Texas), supplied with a belt that provided favorable traction for turkeys.
Measurements of muscle electrical activity (EMG) and force required the surgical implantation of transducers. Turkeys were anesthetized by inhalation of isoflurane (1.5–2.5%), and heart rate, respiratory rate, and temperature were monitored. Sterile procedures were used for all surgeries. Sonomicrometer crystals, fine-wire bipolar EMG electrodes, and strain gauges were implanted in the LG. Leads from implanted transducers were routed underneath the skin to a location on the back where small connectors (Microtech, Inc., Boothwyn, Pennsylvania, USA) were sutured to the skin. Sonomicrometer crystals (2 mm, Sonometrics Inc., London, ON, Canada) were implanted in the proximal part of the LG where fascicles could be easily visualized for proper crystal alignment. Crystals were implanted 12–18 mm apart to measure a segment rather than the entire length of muscle fascicles. Bipolar EMG electrodes were inserted near the sonomicrometry crystals. The EMG leads were constructed from 0.076 mm, teflon-coated, stainless-steel wire (Cooner Wire Company, Chatsworth, CA), with 1 mm bared ends. Strain gauges (Type FLK-1-11, Tokyo Sokki Kenkyujo Co., Ltd., Tokyo, Japan) were attached to both the superficial and deep aspect of the LG calcified tendon with a small amount of cyanoacrylate adhesive. Animals were allowed to recover for 1 or 2 days following surgery.
Measurements were taken as animals walked or ran on the treadmill over a range of speeds from 1 to 4 m s−1. Measurements were also taken on inclines of +6° and +12° (uphill) and −6° and −12° (downhill) running. Fascicle lengths were recorded by sonomicrometry at a frequency of 992 Hz. EMG signals were amplified (1000X) via DAM50 differential amplifiers (World Precision Instruments, Sarasota, FL). Strain signals were recorded via Vishay strain gauge conditioners (Measurements Group, Raleigh, NC, USA). All signals were recorded by a computer at 4000 Hz with a 16 bit A/D converter (PCI-MIO-16, National Instruments, Austin, TX, USA).
Following the collection of data, sonomicrometer, EMG, and strain signals were processed using software (Igor Pro, Wavemetrics, Lake Oswego, OR, USA). Length signals from sonomicrometry were filtered with a 20 Hz low-pass FIR filter. EMG signals were filtered with a Butterworth bandpass filter with a 30 Hz low cutoff and a 1000 Hz high cutoff. Muscle force was calculated from measurements of tendon strain, with values determined from in situ contractions with independent measures of muscle force (Gabaldón et al. 2004).
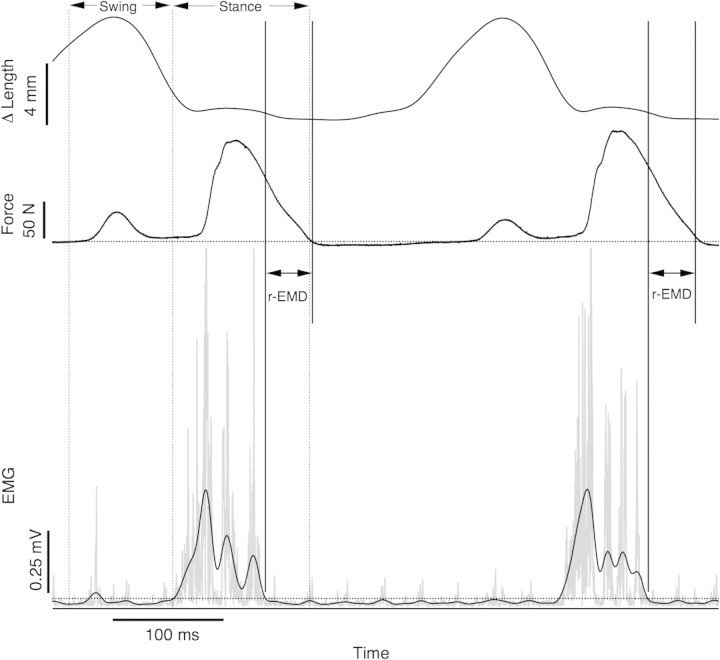
The threshold method used to identify the time of EMG offset and force offset is illustrated in Fig. 1. The filtered EMG signal was rectified and then further filtered with a low-pass, 30 Hz FIR filter to create an envelope signal. This frequency was chosen somewhat subjectively, as it produced an envelope that profiled bursts of activity but was less sensitive to artifactual bursts. A threshold level for activity was identified for EMG by measuring the standard deviation of the signal during an obvious period of inactivity (usually during early swing phase for this muscle). The threshold level was set at two times this standard deviation. The time of EMG offset was identified as the intersection of the envelope signal with the threshold level. For cases in which there were multiple intersections within a burst, the last intersection was used. A similar approach was taken to identify the timing of the offset of force with a threshold level calculated from the standard deviation of the force signal. r-EMD was measured as the time from the offset of EMG in stance to the time of the offset of force. The activation EMD was not measured in this study, as the gradual onset of force made this variable difficult to estimate reliably.
Fig. 1.
Method for determining r-EMD and mean values of EMG and force. Raw EMG signals were bandpass filtered (30,1000 Hz) and full-wave rectified (gray trace). This EMG signal was further filtered with a 30 Hz low-pass filter to produce an envelope signal (black trace, lower panel). A cutoff value (dotted line) was calculated as twice the standard deviation of the EMG over 50 ms of baseline signal. Force (middle panel) cutoff was also determined as twice the standard deviation over baseline values. The time from the end of EMG to the end of force was taken as the r-EMD. The measured length change (top trace) over this period was used to calculate the average velocity. The noisiest of the EMG signals analyzed was used for this sample to illustrate the utility of the threshold method. The regions over which values were averaged for swing phase and stance phase to obtain average values of force and EMG are indicated by vertical dotted lines.
The average velocity of shortening during the relaxation period was calculated from the difference in muscle length at force off and muscle length at EMG off, divided by the time interval. This provided a measure of velocity averaged for the r-EMD period indicated in Fig. 1.
Mean force and mean EMG amplitude were calculated separately for the stance and swing phases. Signals were averaged over the period of interest. The timing of the force pulse in swing phase was defined as the period between the time when the force trace first crossed the threshold level (2 SD of force), and the beginning of the EMG burst corresponding to stance phase force production (as identified by the EMG threshold). Force and EMG for the stance phase were measured for the period of time between the beginning of the EMG burst and the end of force production, as determined by the force threshold. For EMG, average values were calculated after subtracting the average amplitude of signal noise, as measured during muscle inactivity in early swing phase.
For each animal and running trial, 10 strides were analyzed. Means of these 10 strides were then averaged for each animal. To test for the effect of speed on r-EMD, a two-way mixed-model analysis of variance (ANOVA) was performed, with individual as the random effect and speed as the fixed effect. This test was performed in the application STATA SE. Least-squares linear regression was used to test for correlation between mean EMG amplitude and average force.
Results
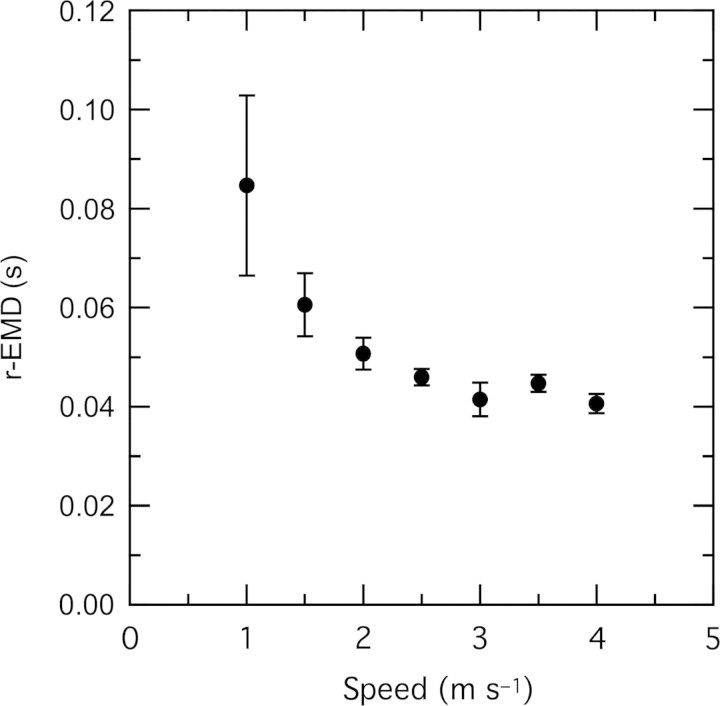
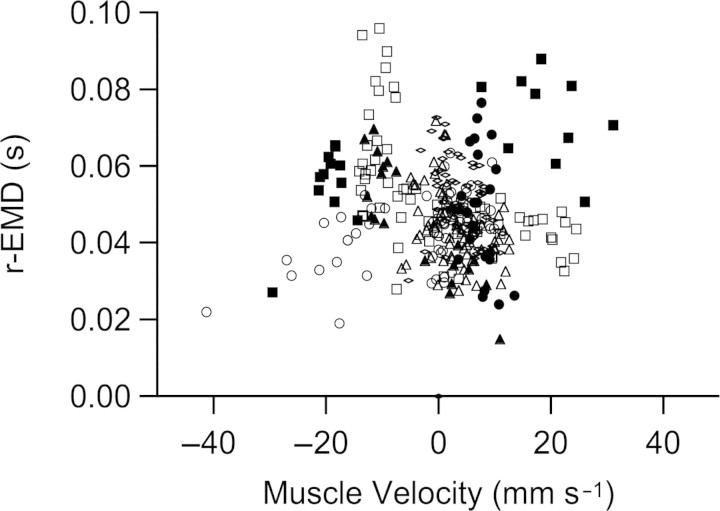
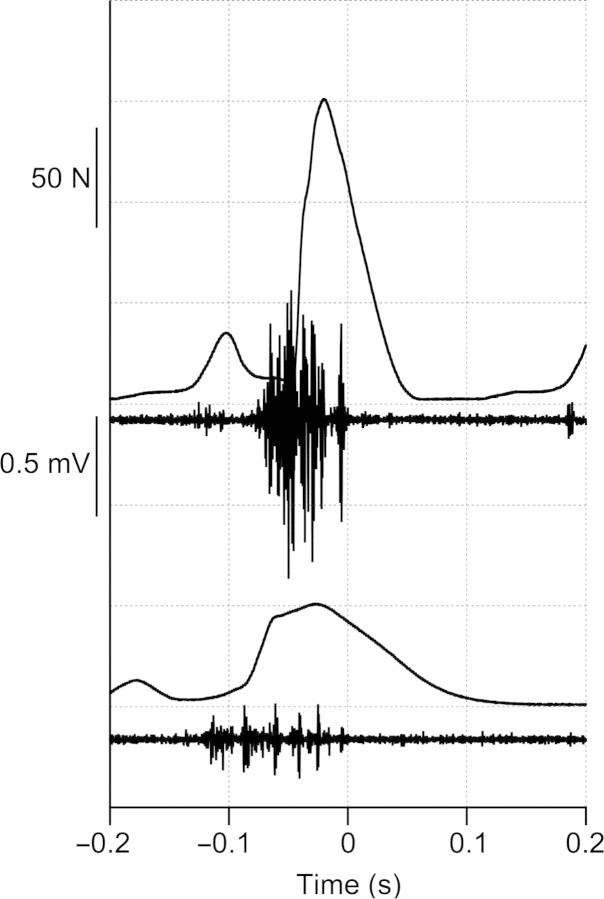
The r-EMD was correlated with speed (ANOVA, P < 0.02). This delay was greatest at walking speeds, decreasing to a relatively constant value over most of the range of running speeds (Fig. 2). For stance phase, there is a small but significant increase in the velocity of muscle shortening with running speed in this muscle (Gabaldón et al. 2008). To investigate whether the variation in r-EMD might be explained by a velocity effect on muscle forces or deactivation rates, we examined the relationship between r-EMD and average fascicle velocity during the time period between offset of EMG and offset of force. When data for level running only were included, linear regression indicated a weak relationship between velocity and r-EMD (R2 = 0.034, P = 0.004). However, this relationship was not significant when data from uphill and downhill running were included (Fig. 3). Data for uphill and downhill running provide a range of shortening and lengthening velocities independent of speed. The lack of a significant relationship between muscle velocity and r-EMD shown in Fig. 3 suggests that the variation in r-EMD with speed is not explained by the effects of muscle velocity on deactivation rates. Longer r-EMD times at slow speeds corresponded to a lower slope of muscle force decay during relaxation, as apparent in the sample strides in Fig. 4.
Fig. 2.
The delay from the offset of EMG to the offset of force (r-EMD) was correlated with running speed. n = 4, mean ± SEM.
Fig. 3.
There was no significant relationship between the velocity of muscle shortening velocity and r-EMD when all data were included across speed (filled symbols) and incline (open symbols). Different individuals are indicated by different symbols.
Fig. 4.
Sample traces for force and EMG for 4 m s−1 (top traces) and 1 m s−1 (bottom traces) illustrate the difference in relaxation rate at the two speeds. Data are aligned to the time of EMG offset (at t = 0) for both speeds.
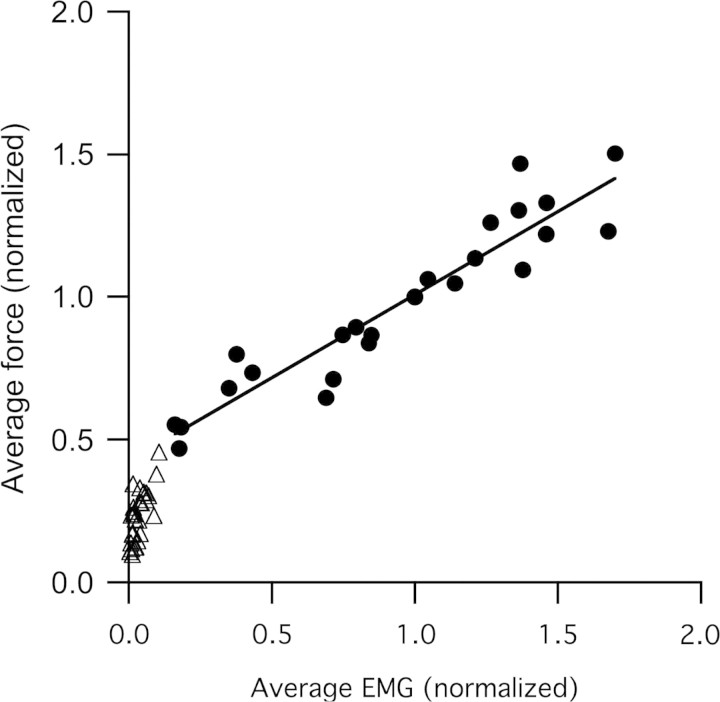
The average force during stance phase was highly correlated with the mean EMG amplitude during stance phase (Fig. 5). As expected, higher forces corresponded to greater EMG signals. Linear regression of force and EMG during the stance phase only indicates that, across the entire range of walking and running speeds, stance phase forces increased linearly with EMG magnitude (R2 = 0.84). Swing phase forces were below the value that would be predicted by the extrapolation of the relationship between stance phase force and EMG (Fig. 5). It is apparent from the sample strides shown in Figs. 1 and 4 that qualitatively, the ratio of force to EMG is very different during the stance and swing phases.
Fig. 5.
Force measured during stance across a range of walking and running speeds (from 1 to 4 m s−1) increases linearly with mean amplitude of EMG (y = 0.58x + 0.42; R2 = 0.84). The regression of stance-phase force and EMG has a positive intercept, suggesting that at low forces the relationship may become nonlinear. Both mean force and mean EMG amplitude are lower during swing than during stance. Measurements represent average values averaged over the period of interest. Force and EMG were both averaged over the period from the beginning of stance-phase EMG to the end of stance phase force. Values of both force and EMG were normalized to the values obtained at 2.5 m s−1 for each bird.
Discussion
The analysis presented here offers lessons both encouraging and cautionary for the use of EMG as an indicator of the timing and magnitude of muscle force. The r-EMD in the turkey LG is relatively constant over a range of running speeds (2–4 m s−1), suggesting that for a given muscle EMG timing variables can be constant for a relatively wide range of activities. However, the relatively large difference between the r-EMD for slow walking compared with running suggests that assuming this value to be fixed for a given muscle may be problematic. Our measurements of the relationship between mean EMG amplitude and average muscle force in the LG also indicate that an assumption of a linear relationship between these two variables is justified under some, but not all conditions. When the entire range of swing phase and stance phase forces are considered together, the relationship between mean EMG amplitude and force is nonlinear.
r-EMD—determinants and trends
Because the EMG is the only available measure of muscle activity in many studies, there has been considerable interest in the magnitude of the electromechanical delay and the factors that contribute to it. Most of this work has focused on human subjects, and most of it has investigated the delay between the onset of EMG and the beginning of force development [the activation electromechanical delay (a-EMD)]. Several steps between the depolarization of the sarcolemma (the event measured by EMG) and the production of measurable force by actomyosin cross-bridges can potentially contribute to the a-EMD. It has been suggested that the process of “taking up slack” in the series elastic component represents the majority of the delay, as processes such as the propagation of muscle action potentials and the release of Ca2+ from the sarcoplasmic reticulum are expected to be quite rapid relative to the EMD typically measured (Cavanagh and Komi 1979). This idea is supported by recent work showing a correlation between a-EMD and the degree of initial strain in the tendon of human triceps surae (Muraoka et al. 2004), and by lower measured a-EMD in the relatively stiff muscles of patients with cerebral palsy (Granata et al. 2000). Similarly, Cavanagh and Komi (1979) studied the a-EMD in forearm muscles of human subjects and found it was significantly longer for concentric contractions compared with eccentric or isometric contractions.
Fewer studies have examined the EMD for relaxation. As would be expected from the relatively shorter activation times compared with relaxation times for typical muscle, measured r-EMDs are typically longer than the EMDs for activation (Vos et al. 1990; Ferris-Hood et al. 1996). For example, Ferris-Hood and coworkers (1996) reported r-EMDs ranging from 239 to 300 ms for human knee extensors, much longer than the typically reported values for activation EMDs for voluntary contractions of 35–80 ms.
Comparative studies report a wide range of electromechanical delays for relaxation. Biewener and coworkers (1992) found an r-EMD of 17 ms in starling pectoralis during flight at 13.7 m s−1. Values for Tamar wallabies’ r-EMD are also slightly shorter than are those of the turkey LG, ranging from ∼32 ms in the plantaris to 43 ms in the LG [based on Fig. 8, (Biewener et al. 2004)]. Values for the ankle extensors of guinea fowl appear to be similar to those of turkeys (∼60 ms, based on reported offset times and stride times for running at 1.3 m s−1, (Daley and Biewener 2003). The r-EMD for a guinea fowl digital flexor appears to be shorter, ∼30 ms (Daley and Biewener 2003).
What explains the variation in measured r-EMD in different species, and what explains the variation in r-EMD across speed in the turkey LG? The variation in r-EMD within the turkey LG could be simply an artifact resulting from a limited ability to detect very low-level EMG signals. EMG amplitude is lowest at the slowest speeds. Very low-level EMG signals that occur late in force production at the slowest speeds could fall below our threshold for detection, resulting in an overestimate of the r-EMD. If this is the explanation for the trend observed here, our results may be most relevant as a caution for assumptions about a single EMD for EMG signals across a range of activities. Without measurements of force, our measurements of EMG would have led to either an overestimation of the duration of force at fast speeds, or an underestimate of the duration of force at slow speeds, depending on the value for r-EMD that was assumed.
It is also possible that the observed relationship between r-EMD and speed has a physiological basis. Just as shortening velocity (as in taking up slack) can likely influence the EMD for muscle activation, it might be expected that muscle velocity could influence the electromechanical delay for muscle relaxation. A relationship between changes in muscle length and timing of force development could result from the influence of muscle velocity on force output, and/or from the interaction between activation/relaxation processes and changes in muscle length that have been observed in vitro (Gordon et al. 2000). However, our results suggest that the variation in r-EMD with locomotor speed is not explained by variation in the pattern of shortening or lengthening of the muscle, because there is no correlation between fascicle velocity and r-EMD.
Changes in the pattern of muscle fiber recruitment might explain the observed correlation between r-EMD and locomotor speed. A longer r-EMD would be expected for slower types of fibers, as they have lower rates of Ca2+ cycling and longer relaxation times (Close 1972). This influence of excitation–contraction kinetics likely explains much of the variation in r-EMD between different muscles and different species. For example, during fast flight a starling's entire downstroke phase is <40 ms, a time course that undoubtedly requires fast fibers with very rapid rates of force onset and decay (Biewener et al. 1992). These rapid rates are apparent not only in the Starling's very short r-EMD, but also in the very short activation EMD (∼3 ms for rapid flight, Biewener et al. 1992). Within humans, fiber type has been implicated as one of the factors influencing EMD (Norman and Komi 1979). Slow fibers recruited in the turkey LG at slow walking speeds would be expected to have slower rates of relaxation (and therefore longer r-EMD) than the fast fibers that are added to the recruited pool at faster speeds. The problem with this possible explanation for the pattern of r-EMD observed here is that it would seem to violate Henneman's size principle for the order of recruitment of motor units (Henneman et al. 1974). According to the size principle, slow fibers recruited at slow speeds should continue to be recruited at fast speeds; that is, slow fibers are not derecruited as additional fast fibers are recruited. Thus, one would expect that the time from the offset of EMG activity to the offset of force would be dominated by the slow relaxation time course of slower motor units at all speeds. Other studies using arguably more refined methods for inferring motor unit recruitment from EMG signals have found evidence that the order of motor unit recruitment does not always follow the size principle (Wakeling et al. 2002; Hodson-Tole and Wakeling 2007). Further study in this area is warranted.
Integrated EMG area and force
Measures of mean amplitude or area of EMG signals are often reported in studies of muscle function during locomotion (Gillis and Biewener 2002; Konow et al. 2008; McGowan et al. 2006). It is generally assumed that EMG intensity provides a reliable estimate of the volume of recruited muscle, but not necessarily of the developed force. The difficulty in relating amplitude of EMG to amplitude of force lies in the fact that although EMG may give a reliable measure of the volume of active motor units, many factors, including muscle length, velocity, and activation/deactivation kinetics will influence the force an active motor unit produces (Hof 1984; Gabaldón et al. 2008). Indeed, even the assumption that EMG amplitude is related to the volume of muscle recruited has been challenged (Farina et al. 2004). In isometric contractions, the relationship between force and EMG amplitude is usually linear or close to linear and predictable in systems that have been measured, including mammalian masseter (Hylander and Johnson 1989) and human knee extensors (Alkner et al. 2000).
The relationship between average EMG and force during locomotion can be evaluated from comparative studies that provide direct measurements of force from individual muscles. Hedrick and coworkers (2003) reported a strong correlation (R2 = 0.91) between mean EMG and force for the cockatiel pectoralis over a range of flight speeds. In guinea fowl ankle extensor and toe flexors, the relationship between magnitude of force and EMG during running is more variable (Daley and Biewener 2003). The relationship between EMG and force was found to be significant across running speeds (R2 = 0.65 and 0.58 for level and incline running, respectively) for the LG, but weak (R2 = 0.33, level running) or not significant (incline running) for digital flexor IV (Daley and Biewener 2003). One of the determinants of the relationship between EMG and force is the contractile condition of the muscle (Hof 1984). For example, force output should be reduced for a given EMG signal in a muscle when it shortens relative to when it is isometric. This may explain some of the variability in the EMG versus force relationship in, for example, muscles during uphill versus level running (Roberts et al. 1997; Gabaldón et al. 2008).
The results presented here reinforce the challenges associated with estimating force production from the amplitude of the EMG signal. In the turkey LG, the relationship between the amplitude of EMG and the developed force is linear across the range of forces developed during the stance phase of walking and running. However, there is a clear nonlinearity when the relatively low forces and EMG levels produced during swing are included (Fig. 4). This result illustrates two points relevant to the interpretation of the amplitude of EMG signals.
Given the very low, and in many cases absent (Fig. 4) EMG trace during the swing phase, measurement of EMG only in this muscle would likely lead to the conclusion that either no or negligible force was developed in the LG during swing phase. Because the amplitude of force for a given level of EMG is much higher during the swing phase as compared with stance, the EMG is not a reliable indicator of the relative force developed during different periods of the stride cycle. The explanation for the relatively high amplitude of force for a given EMG amplitude during swing phase is not fully established, but we hypothesize that it is due to development of passive force by the LG (Roberts et al. 1997). To the extent that muscles develop forces passively during locomotion (in muscle connective tissue elements and sarcomeric spring-like proteins, such as titin), the relation between EMG and force production is further obscured. The pattern of the EMG–force relationship shown in Fig. 5 demonstrates that the conclusion that the muscle develops force passively is not necessarily well-supported by EMG data alone. Because the stance-phase force–EMG relationship during stance phase has a positive y-intercept, the force–EMG relationship must presumably depart from the observed regression at very low levels of force and EMG (as zero force is expected at zero EMG). Thus, based on EMG data alone, an alternative explanation for the very low EMG signals during the swing phase is that the force–EMG relationship at the lowest forces and activities departs from the linear relationship for force and EMG observed for stance phase. A full understanding of the mechanisms underlying these relationships will require further study.
Acknowledgments
The authors thank Nicolai Konow and Shannon Gerry for organizing the stimulating and informative symposium “Electromyography: Interpretation and limitations in functional analyses of musculoskeletal systems.” We thank Manny Azizi, Frank Nelson, and Arianne Cease for help with experiments. This work was support by grant AR46499 from the NIH.
References
- Alkner BA, Tesch PA, Berg HE. Quadriceps EMG/force relationship in knee extension and leg press. Med Sci Sports Exerc. 2000;32:459–63. doi: 10.1097/00005768-200002000-00030. [DOI] [PubMed] [Google Scholar]
- Basmajian JV, De Luca CJ. Muscles alive: their functions revealed by electromyography. Baltimore: Williams & Wilkins; 1985. [Google Scholar]
- Biewener AA, Dial KP, Goslow GE. Pectoralis-muscle force and power output during flight in the starling. J Exp Biol. 1992;164:1–18. [Google Scholar]
- Biewener AA, McGowan C, Card GM, Baudinette RV. Dynamics of leg muscle function in tammar wallabies (M. eugenii) during level versus incline hopping. J Exp Biol. 2004;207:211–23. doi: 10.1242/jeb.00764. [DOI] [PubMed] [Google Scholar]
- Cavanagh PR, Komi PV. Electromechanical delay in human skeletal muscle under concentric and eccentric contractions. Eur J Appl Physiol Occup Physiol. 1979;42:159–63. doi: 10.1007/BF00431022. [DOI] [PubMed] [Google Scholar]
- Close RI. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972;52:129–97. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- Daley MA, Biewener AA. Muscle force-length dynamics during level versus incline locomotion: a comparison of in vivo performance of two guinea fowl ankle extensors. J Exp Biol. 2003;206:2941–58. doi: 10.1242/jeb.00503. [DOI] [PubMed] [Google Scholar]
- Dial KP, Kaplan SR, Goslow GE, Jr, Jenkins FA., Jr A functional analysis of the primary upstroke and downstroke muscles in the domestic pigeon (Columba livia) during flight. J Exp Biol. 1988;134:1–16. doi: 10.1242/jeb.134.1.1. [DOI] [PubMed] [Google Scholar]
- Edman KA, Josephson RK. Determinants of force rise time during isometric contraction of frog muscle fibres. J Physiol. 2007;580:1007–19. doi: 10.1113/jphysiol.2006.119982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina D, Merletti R, Enoka RM. The extraction of neural strategies from the surface EMG. J Appl Physiol. 2004;96:1486–95. doi: 10.1152/japplphysiol.01070.2003. [DOI] [PubMed] [Google Scholar]
- Ferris-Hood K, Threlkeld AJ, Horn TS, Shapiro R. Relaxation electromechanical delay of the quadriceps during selected movement velocities. Electromyogr Clin Neurophysiol. 1996;36:157–70. [PubMed] [Google Scholar]
- Gabaldón AM, Nelson FE, Roberts TJ. Mechanical function of two ankle extensors in wild turkeys: shifts from energy production to energy absorption during incline versus decline running. J Exp Biol. 2004;207:2277–88. doi: 10.1242/jeb.01006. [DOI] [PubMed] [Google Scholar]
- Gabaldón AM, Nelson FE, Roberts TJ. Relative shortening velocity in locomotor muscles: turkey ankle extensors operate at low V/Vmax. Am J Physiol Regul Integr Comp Physiol. 2008;294:R200–10. doi: 10.1152/ajpregu.00473.2007. [DOI] [PubMed] [Google Scholar]
- Gatesy SM. An electromyographic analysis of hindlimb function in Alligator during terrestrial locomotion. J Morphol. 1997;234:197–212. doi: 10.1002/(SICI)1097-4687(199711)234:2<197::AID-JMOR6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Gillis GB, Biewener AA. Effects of surface grade on proximal hindlimb muscle strain and activation during rat locomotion. J Appl Physiol. 2002;93:1731–43. doi: 10.1152/japplphysiol.00489.2002. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev. 2000;80:853–924. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- Goslow GE, Seeherman HJ, Taylor CR, McCutchin MN, Heglund NC. Electrical activity and relative length changes of dog limb muscles as a function of speed and gait. J Exp Biol. 1981;94:15–42. doi: 10.1242/jeb.94.1.15. [DOI] [PubMed] [Google Scholar]
- Granata KP, Ikeda AJ, Abel MF. Electromechanical delay and reflex response in spastic cerebral palsy. Arch Phys Med Rehabil. 2000;81:888–94. doi: 10.1053/apmr.2000.5578. [DOI] [PubMed] [Google Scholar]
- Gregor RJ, Abelew TA. Tendon force measurements and movement control: a review. Med Sci Sports Exerc. 1994;26:1359–72. [PubMed] [Google Scholar]
- Guimaraes AC, Herzog W, Allinger TL, Zhang YT. The EMG-force relationship of the cat soleus muscle and its association with contractile conditions during locomotion. J Exp Biol. 1995;198:975–87. doi: 10.1242/jeb.198.4.975. [DOI] [PubMed] [Google Scholar]
- Hedrick TL, Tobalske BW, Biewener AA. How cockatiels (Nymphicus hollandicus) modulate pectoralis power output across flight speeds. J Exp Biol. 2003;206:1363–78. doi: 10.1242/jeb.00272. [DOI] [PubMed] [Google Scholar]
- Henneman E, Clamann HP, Gillies JD, Skinner RD. Rank order of motoneurons within a pool: law of combination. J Neurophysiol. 1974;37:1338–49. doi: 10.1152/jn.1974.37.6.1338. [DOI] [PubMed] [Google Scholar]
- Hodson-Tole EF, Wakeling JM. Variations in motor unit recruitment patterns occur within and between muscles in the running rat (Rattus norvegicus) J Exp Biol. 2007;210:2333–45. doi: 10.1242/jeb.004457. [DOI] [PubMed] [Google Scholar]
- Hof AL. EMG and Muscle Force - an introduction. Hum Mov Sci. 1984;3:119–53. [Google Scholar]
- Hylander WL, Johnson KR. The relationship between masseter force and masseter electromyogram during mastication in the monkey Macaca fascicularis. Arch Oral Biol. 1989;34:713–22. doi: 10.1016/0003-9969(89)90078-2. [DOI] [PubMed] [Google Scholar]
- Hylander WL, Johnson KR. Modelling relative masseter force from surface electromyograms during mastication in non-human primates. Arch Oral Biol. 1993;38:233–40. doi: 10.1016/0003-9969(93)90033-i. [DOI] [PubMed] [Google Scholar]
- Jayne BC, Lauder GV. Red and white muscle activity and kinematics of the escape response of the bluegill sunfish during swimming. J Comp Phys A. 1993;173:495–508. [Google Scholar]
- Konow N, Camp A, Sanford C. Congruence between muscle activity and kinematics in a convergently derived prey-processing behavior. Int Comp Biol. 2008 doi: 10.1093/icb/icn045. doi: 10.1093/icb/icn045. [DOI] [PubMed] [Google Scholar]
- Lieber RL. Skeletal muscle structure and function. Baltimore: Williams and Wilkins; 1992. [Google Scholar]
- Marsh RL. Deactivation rate and shortening velocity as determinants of contractile frequency. Am J Physiol. 1990;259:R223–30. doi: 10.1152/ajpregu.1990.259.2.R223. [DOI] [PubMed] [Google Scholar]
- McGowan CP, Duarte HA, Main JB, Biewener AA. The effects of load carrying on metabolic cost and hindlimb muscle dynamics in guinea fowl (Numida meleagris) J Appl Physiol. 2006;101:1060–9. doi: 10.1152/japplphysiol.01538.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraoka T, Muramatsu T, Fukunaga T, Kanehisa H. Influence of tendon slack on electromechanical delay in the human medial gastrocnemius in vivo. J Appl Physiol. 2004;96:540–4. doi: 10.1152/japplphysiol.01015.2002. [DOI] [PubMed] [Google Scholar]
- Norman RW, Komi PV. Electro-mechanical delay in skeletal-muscle under normal movement conditions. Acta Physiol Scand. 1979;106:241–8. doi: 10.1111/j.1748-1716.1979.tb06394.x. [DOI] [PubMed] [Google Scholar]
- Roberts TJ, Marsh RL, Weyand PG, Taylor CR. Muscular force in running turkeys: the economy of minimizing work. Science. 1997;275:1113–5. doi: 10.1126/science.275.5303.1113. [DOI] [PubMed] [Google Scholar]
- Sherif MH, Gregor RJ, Liu LM, Roy RR, Hager CL. Correlation of myoelectric activity and muscle force during selected cat treadmill locomotion. J Biomech. 1983;16:691–701. doi: 10.1016/0021-9290(83)90079-9. [DOI] [PubMed] [Google Scholar]
- Stern JT, Jr, Larson SG. Telemetered electromyography of the supinators and pronators of the forearm in gibbons and chimpanzees: implications for the fundamental positional adaptation of hominoids. Am J Phys Anthropol. 2001;115:253–68. doi: 10.1002/ajpa.1080. [DOI] [PubMed] [Google Scholar]
- Vos EJ, Mullender MG, van Ingen Schenau GJ. Electromechanical delay in the vastus lateralis muscle during dynamic isometric contractions. Eur J Appl Physiol Occup Physiol. 1990;60:467–71. doi: 10.1007/BF00705038. [DOI] [PubMed] [Google Scholar]
- Wakeling JM, Kaya M, Temple GK, Johnston IA, Herzog W. Determining patterns of motor recruitment during locomotion. J Exp Biol. 2002;205:359–69. doi: 10.1242/jeb.205.3.359. [DOI] [PubMed] [Google Scholar]